Abstract
Purpose
Peripapillary vascular alterations have been classically described as hallmarks of Leber's Hereditary Optic Neuropathy (LHON). We recently demonstrated microvascular pathology involving the macula in patients affected with chronic LHON using optical coherence tomography angiography (OCTA). Macular vascular pathology in acute LHON has not previously been reported.
Methods
The macular superficial vasculature of an asymptomatic carrier and an affected patient with acute LHON, mitochondrial DNA mutation 3460, was assessed by OCTA.
Results
Similar findings of peripapillary microangiopathy and vascular telangiectasias were seen the affected patient, but in the parafoveal macula. These changes were most prominent nasally and inferiorly, corresponding to the proximal portions of the papillomacular bundle. The foveal avascular zone was markedly enlarged in the affected patient relative to the asymptomatic mother.
Conclusions
These findings in acute LHON further supports the clinical utility of vascular parameters and suggest that further studies focused on macular pathology may be warranted to assess the natural history of LHON.
Keywords: Leber's hereditary optic neuropathy, Optical coherence tomography angiography, Macular vascular pathology, Subclinical vasculopathy
1. Introduction
Peripapillary vascular alterations, historically described on fuduscopy, have been classically described as hallmarks of Leber's Hereditary Optic Neuropathy (LHON).1,2 Our laboratory recently demonstrated microvascular pathology involving the macula in patients affected with chronic LHON using optical coherence tomography angiography (OCTA).3 We hereby report the presence of macular vascular pathology even in the early stages of disease.
2. Case report
A 22-year-old male with LHON, genetically confirmed for a 3460 mitochondrial DNA (mtDNA) mutation presented with a 2 month history of vision loss beginning with the left eye, followed by vision loss in the right eye one month later. Family history was significant for bilateral vision loss in the mother's cousin at age 30. The patient endorsed alcohol consumption amounting to two to three bottles of beer per day. The patient also had a one-year history of exposure to kitchen smoke, having an occupation as a restaurant server. The patient began Idebenone 250 mg PO TID.
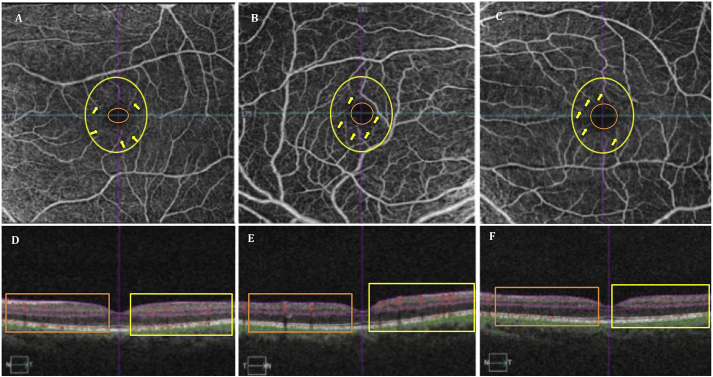
Best-corrected visual acuities (BCVA) were 20/200 in the right eye (OD) and 20/400 in the left eye (OS). Fundus examination revealed temporal pallor, peripapillary telangiectasias, and pseudo-edema in both eyes (OU). Pupils were equal and reactive to light with no afferent pupillary defects. The patient exhibited dyschromatopsia on Ishihara plate testing, scoring 5/14 OD and 2/14 OS. Humphrey visual field (HVF) testing showed mild, bilateral cecocentral scotomas. Structural OCT displayed bilateral retinal nerve fiber layer (RNFL) pseudoedema and ganglion cell complex (GCC) thinning. OCTA images of the macular superficial vasculature were obtained using Spectral Domain-OCT (Cirrus HD-OCT, software V.6.0; Carl Zeiss Meditec, Inc., Dublin, CA, USA). The scan size of the macular area was 6 × 6 mm. The same set of images were acquired for the patient's 38-year-old mother, who had no significant past medical history and was asymptomatic though sharing the mtDNA 3460 mutation, and hence a normal for comparison (Fig. 1).
Fig. 1.
Shows OCT-A images (A–C) of the superficial vascular networks for the macular RGC-IPL in the asymptomatic LHON mt3460 carrier (A), and affected LHON mt3460 patient (B,C). Close inspection of the superficial vascular networks demonstrate visible attenuation of the parafoveal microvascular networks as seen by the increasing size of the foveal avascular zone (FAZ) (orange circle) beginning from the unaffected mother's eye (A) to the patient's most affected eye (C). Qualitative assessment of the parafoveal vasculature (yellow circle) revealed telangiectatic blood vessels and vascular tortuosity (yellow arrows) more prominent in the patient's right eye (B; more recently affected eye) relative to the patient's left eye (C; primary affected eye). Such parafoveal vascular features are less apparent (yellow arrows) in the unaffected mother (A). OCT cross-sections overlaying retinal flow (red) on OCT reflectance (gray scale) (D–F) show perfusion defects for both temporal (yellow box) and nasal (orange box) regions of the patient's left eye (F). In contrast, the patient's right eye revealed increased perfusion for both temporal (yellow box) and nasal (orange box) relative to both the patient's left eye (F) and the mother's unaffected eye (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Peripapillary vascular alterations1,2 have been classically described as hallmarks of acute LHON. Our laboratory recently investigated the vascular parameters in late disease stages in patients with chronic LHON.3 We hereby report similar findings of microangiopathy and vascular telangiectasias, but in the parafoveal macula. These changes were most prominent nasally and inferiorly, corresponding to the proximal portions of the papillomacular bundle (PMB) associated with the fibers that leave the optic nerve head inferotemporally and contain the smallest retinal ganglion cell (RGC) fibers that are the most vulnerable to mitochondrial dysfunction.4,5 Consistent with Balducci et al., our findings revealed vascular changes predominantly in the corresponding areas of mitochondrial dysfunction and early ganglion cell involvement.6 Such parafoveal vascular features were less apparent in the unaffected mother. Intriguingly, OCTA of the macular microvasculature revealed marked enlargement of the foveal avascular zone (FAZ) in the affected patient bilaterally (OS > OD) relative to the asymptomatic mother. In addition, the patient's subacutely affected eye (OS) exhibited less parafoveal microangiopathy and greater vascular attrition, seen by the larger FAZ and attenuated parafoveal microvasculature relative to the more acutely affected eye (OD). These findings in the subacutely affected eye resemble our laboratory's recent findings in chronic LHON, whereby the parafoveal vascular density was significantly reduced in the macular region corresponding to the PMB.3 The present report of acute LHON further supports the clinical utility of vascular parameters as useful biomarkers for monitoring disease and appreciating that in LHON, the process begins with the smallest axons.
4. Conclusion
These findings in acute LHON, in conjunction with our recent findings in chronic LHON, may help expand our understanding of the natural history of LHON and associated disease in the retina. Future studies focusing on the macula with longitudinal follow-up may provide valuable insight into the dynamic changes of the macular vasculature over the course of the disease. The current study provides a single reporting of the macular vascular changes in acute LHON. Additional studies may be warranted to replicate these findings and to further support the clinical utility of vascular measures as potential biomarkers.
Patient consent
The article does not contain personally identifiable patient information.
Funding
No funding or grants.
Authorship
These findings may help broaden our knowledge regarding the natural history of LHON and the evolution of vascular and neuronal loss in the retina.
Conflicts of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2018.11.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nikoskelainen E., Hoyt W.F., Nummelin K. Ophthalmoscopic findings in Leber's hereditary optic neuropathy. I. Fundus findings in asymptomatic family members. Arch Ophthalmol. 1982;100(10):1059–1068. doi: 10.1001/archopht.1982.01030040575003. [DOI] [PubMed] [Google Scholar]
- 2.Nikoskelainen E., Hoyt W.F., Nummelin K. Ophthalmoscopic findings in Leber's hereditary optic neuropathy: II. The fundus findings in the affected family members. Arch Ophthalmol. 1983;101(7):1059–1068. doi: 10.1001/archopht.1983.01040020061011. [DOI] [PubMed] [Google Scholar]
- 3.Borrelli E., Balasubramanian S., Triolo G. Topographic macular microvascular changes and correlation with visual loss in chronic leber hereditary optic neuropathy. Am J Ophthalmol. 2018;192:217–228. doi: 10.1016/j.ajo.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Sadun A.A., La Morgia C., Carelli V. Mitochondrial optic neuropathies: our travels from bench to bedside and back again. Clin Exp Ophthalmol. 2013;41(7):702–712. doi: 10.1111/ceo.12086. [DOI] [PubMed] [Google Scholar]
- 5.Pan B.X., Ross-Cisneros F.N., Carelli V. Mathematically modeling the involvement of axons in Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2012 9;53(12):7608–7617. doi: 10.1167/iovs.12-10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balducci N., Savini G., Cascavilla M.L. Macular nerve fibre and ganglion cell layer changes in acute Leber's hereditary optic neuropathy. Br J Ophthalmol. 2016;100(9):1232–1237. doi: 10.1136/bjophthalmol-2015-307326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.