Abstract
Objectives:
To assess complete gross resection (CGR) rates and survival outcomes in patients with advanced ovarian cancer who underwent primary debulking surgery (PDS) during a 13-year period in which specific changes to surgical paradigm were implemented.
Methods:
We identified all patients with stage IIIB-IV high-grade ovarian carcinoma who underwent PDS at our institution, with the intent of maximal cytoreduction, from 1/2001–12/2013. Patients were categorized by year of PDS based on the implementation of surgical changes to our approach to ovarian cancer debulking (Group 1, 2001–2005; Group 2, 2006–2009; Group 3, 2010–2013).
Results:
Among 978 patients, 78% had stage IIIC disease and 89% had disease of serous histology. Carcinomatosis was found in 81%, and 60% had bulky upper abdominal disease (UAD). Compared to Group 1, those who underwent PDS during the latter 2 time periods had higher ASA scores (p<0.001), higher-stage disease (p<0.001), and more often had carcinomatosis (p=0.015) and bulky UAD (p=0.009). CGR rates for Groups 1–3 increased from 29% to 40% to 55%, respectively (p<0.001). Five-year progression-free survival (PFS) rates increased over time (15%, 16%, and 20%, respectively; p=0.199), as did 5-year overall survival (OS) rates (40%, 44%, and 56%, respectively; p<0.001). On multivariable analysis, CGR was independently associated with PFS (p<0.001) and OS (p<0.001).
Conclusions:
Despite higher-stage disease and greater tumor burden, CGR rates, PFS and OS for patients who underwent PDS increased over a 13-year period. Surgical paradigm shifts implemented specifically to achieve more complete surgical cytoreduction are likely the reason for these improvements.
Keywords: ovarian cancer, complete gross resection, primary debulking surgery, surgical paradigm, progression-free survival, overall survival
Introduction
Ovarian cancer is the leading cause of death among gynecologic malignancies. Approximately 22,240 women in the U.S. will be diagnosed with ovarian cancer in 2018, and an estimated 14,070 will die from this disease [1]. According to the National Cancer Institute, the 5-year survival rate for all stages of ovarian cancer is 47%. The rate, however, falls to 29% for those with distant metastases at the time of diagnosis [2].
Cytoreductive surgery with maximal tumor debulking is a key component of ovarian cancer treatment. In 1975, Griffiths published a landmark study demonstrating an inverse relationship between residual tumor burden and survival [3]. The greatest survival benefit was seen when resection of all visible tumor was achieved. Since then, multiple studies have confirmed that complete cytoreduction, also referred to as complete gross resection (CGR), is an important prognostic factor for survival [4–10]. In the absence of existing randomized controlled trials, a large systematic review of patients with stage III or IV ovarian cancer treated with primary debulking surgery (PDS) showed that complete cytoreduction was associated with significantly prolonged progression-free survival (PFS) and overall survival (OS) [11].
Most patients with ovarian cancer are diagnosed with advanced-stage disease, commonly with metastases to the omentum, small and large bowel, diaphragm, and upper abdominal (UAB) organs [2]. As the goal of PDS has evolved from debulking with <1–2 cm of residual disease to complete resection of all visible tumor, the role of complex surgery has become increasingly important. Several institutions have incorporated extensive surgical procedures into their practices, thereby successfully increasing CGR rates [12–16].
In pursuit of continual improvement in ovarian cancer outcomes, we questioned whether advances in preoperative and perioperative practices, in addition to expanding surgical extent, would further increase CGR rates and improve survival. Our institution implemented multiple advancements in our approach to ovarian cancer debulking surgery from 2001 to 2013. These included a shift in cytoreductive goal from ≤1 cm residual disease to no gross residual disease (CGR), performance of extensive UAB surgery and cardiophrenic lymph node dissection, alterations in patient selection, and modifications in operative start times. The primary objective of this study was to assess the changes in CGR rates and survival outcomes over the 13-year period during which these changes took place. Our secondary objective was to analyze whether the observed changes in CGR rates were independently associated with PFS and OS.
Methods
After obtaining Institutional Review Board approval for this single-institution, retrospective cohort study, we used the Memorial Sloan Kettering Cancer Center (MSK) Gynecology Service database to identify all patients with FIGO 2009 stage IIIB-IV ovarian, fallopian tube or primary peritoneal carcinoma, who underwent PDS at our institution with the intent of maximal cytoreduction between 1/1/2001 and 12/31/2013. Patients who underwent exploratory laparotomy for anticipated debulking but who were ultimately declared unresectable due to extensive disease burden, were still included in the analysis. The study was restricted to high-grade epithelial histologies. Those who received neoadjuvant chemotherapy (NACT) or presented for management of recurrent disease were excluded. We limited our analysis to patients who underwent PDS prior to 12/31/2013 to ensure adequate follow-up for calculation of 5-year survival.
Records for individual patients were reviewed and clinical variables were abstracted. BRCA mutation status was determined to be positive (genetic testing with evidence of deleterious BRCA mutation), negative (genetic testing without evidence of deleterious BRCA mutation), or unknown with/without a family history suggestive of hereditary breast and ovarian cancer (HBOC) syndrome. Patients meeting 1 or more of the following National Comprehensive Cancer Network (NCCN) criteria were deemed as having a positive family history: 1) one relative (1st, 2nd or 3rd degree) with either breast cancer diagnosed ≤ age 50, 2 primary breast cancers, or male breast cancer; 2) one relative with ovarian, fallopian tube, or primary peritoneal cancer; 3) 2 relatives (same side of the family) with breast, pancreatic, and/or prostate cancer; or 4) known mutation within the family of a gene that increases susceptibility to cancer [17].
CGR was defined as no visible disease remaining at the end of the surgical procedure. Minimal residual disease (MRD) was defined as one or more tumor nodules 1–10 mm in maximal dimension remaining at the completion of surgery, and suboptimal debulking was defined as any residual tumor nodule >10 mm in maximal dimension remaining at the completion of surgery. Peritoneal carcinomatosis was deemed as present or absent according to the attending surgeon’s operative note. If carcinomatosis was not specifically addressed in the operative note, it was defined as the presence of ≥20 tumor nodules within the abdominal cavity. Bulky UAB disease was defined as tumor implants ≥1 cm cephalad to the greater omentum. OR Tumor Index, a scoring system that reflects extent of disease based on a 0–2-point scale (carcinomatosis and bulky UAB disease each accounting for 1 point), was used to quantify disease burden [18]. Surgical adverse events occurring within 30 days of surgery were prospectively captured and graded according to the MSK institutional surgical secondary events grading system [19]. The date of recurrence/progression was determined by computed tomography (CT) scan. The appearance of one or more new lesions on CT scan, leading to a secondary debulking surgery or the initiation of a new chemotherapy regimen, was considered a recurrence. A change in treatment regimen due to an increase in size of an existing lesion was considered progression. The use of intraperitoneal (IP) chemotherapy was defined as administration of ≥1 cycle of IP treatment.
The study timeline was divided into 3 periods based on the implementation of changes to our institutional approach to ovarian cancer debulking, and patients were stratified into groups based on the year of their primary surgery: 2001–2005 (Group 1), 2006–2009 (Group 2), and 2010–2013 (Group 3). In 2001, we began to incorporate extensive UAB procedures into our debulking armamentarium [13]. In 2006, our goal for PDS evolved from residual disease ≤10 mm to either CGR or as minimal residual tumor as possible [7]. During 2010 to 2013, 3 additional changes were gradually adopted: routine performance of cardiophrenic lymph node resection [20], use of the selection criteria for NACT as described by Aletti et al. [21], and implementation of earlier operative start times [18]. The decision to define 2010 as the division point between Groups 2 and 3 was also influenced by the increase in our NACT rates after 2010 [22].
Statistical analysis
Differences in distribution among the patient groups were tested using the chi-square or Fisher exact test (if the cell count was <5) for categorical variables and the Kruskal-Wallis test for continuous variables. For 30-day/90-day mortality, short follow-up patients were excluded (0 excluded for 30-day mortality and 3 excluded for 90-day mortality).
PFS was defined as the time from the date of PDS to the date of recurrence/progression, death, or last follow-up, whichever occurred first. Both progression and death were considered events. OS was defined as the time from the date of PDS to the date of death or last follow-up, whichever occurred first. Death was considered an event. Median survival time and the 5-year survival rate were estimated using the Kaplan-Meier method.
The effect of residual disease on survival outcome was tested using the log-rank test. The associated hazard ration (HR) was obtained by fitting a Cox proportional hazards (PH) model without accounting for the institutional changes in the management of ovarian cancer over time. To examine the effect of PDS-year groups (reflecting the institutional changes in the management of ovarian cancer over time) on survival, we selected to test PFS and OS rates at the 5-year time point using a chi-square test with 2 degrees of freedom. The relevant point estimate and variance were obtained using the Kaplan-Meier method. To examine whether CGR was an independent predictor for PFS/OS, a stratified Cox PH model was fit with PDS-year group as the stratification factor. All variables with a p value <0.05 in the univariate setting were entered into the multivariable model. IP chemotherapy was modeled as a time-dependent covariate. Calculated p values were two-tailed, and p values <0.05 were considered statistically significant. The statistical analyses were performed using SAS 9.4.
Results
We identified 978 consecutive patients with stage IIIB-IV ovarian, fallopian tube or primary peritoneal carcinoma, based on the 2009 FIGO staging system, who underwent intended PDS with maximal cytoreductive effort between 1/1/2001 and 12/31/2013. Demographic and clinical characteristics are shown in Table 1. The median age was 61 (range, 19–95 years). Eighty-one percent (n=794) had stage III disease (stage IIIB, n=33 [3%]; stage IIIC, n=761 [78%]), and 19% (n=184) had stage IV disease. Most patients had disease of serous histology (n=869, 89%). Among those with known BRCA status, 28% (n=144) had a BRCA mutation. Eighty-one percent (n=792) had carcinomatosis and 60% (n=585) had bulky UAB disease. The median operative time was 280 min (range, 36–893 min), and the median length of hospital stay (LOS) was 8 days (range, 1–22 days). Fifteen percent (n=148) had 1 or more grade 3–5 complications. CGR was achieved in 42% (n=408), MRD in 39% (n=378), and suboptimal debulking in 20% (n=192). Almost all patients received postoperative primary platinum/taxane-based chemotherapy (n=949, 99%). IP chemotherapy was administered in 34% (n=322). Thirty-day all-cause mortality was 0.4% (4 deaths), and 90-day all-cause mortality was 1.3% (13 deaths; 3 patients excluded due to short follow-up).
Table 1.
Demographic and clinical characteristics stratified by PDS-Year Group: 2001–2005 (Group1), 2006–2009 (Group 2), 2010–2013 (Group 3)
| PDS-Year Group | |||||
|---|---|---|---|---|---|
| Characteristic | All patients (N = 978) | 2001–2005 (N = 315) | 2006–2009 (N = 320) | 2010–2013 (N = 343) | p |
| Age, years | |||||
| Median (range) | 61 (19–95) | 62 (25–95) | 61 (29–88) | 62 (19–86) | 0.429 |
| BMI, kg/m2 | |||||
| Median (range) | 25.7 (16.3–58.5) | 24.9 (16.7–42.9) | 26 (16.3–54.6) | 26 (16.7–58.5) | 0.030 |
| ASA score (n=970) | |||||
| 1–2 | 520 (54%) | 207 (66%) | 203 (64%) | 110 (32.5%) | <0.001 |
| 3–4 | 450 (46%) | 106 (34%) | 116 (36%) | 228 67.5%) | |
| Preoperative serum albumin, g/dL | |||||
| Median (range) | 4.1 (2–5.1) | 4.1 (2–5.1) | 4.1 (2.5–4.9) | 4.1 (2.3–5) | 0.424 |
| BRCA status (n=971) | |||||
| Positive | 144 (15%) | 27 (9%) | 47 (15%) | 70 (20.5%) | <0.001 |
| Unk, FHx suggestive | 90 (9%) | 48 (15%) | 29 (9%) | 13 (4%) | |
| Unk, FHx not suggestive | 371 (38%) | 189 (61%) | 120 (37.5%) | 62 (18%) | |
| Negative | 366 (38%) | 47 (15%) | 123 (72%) | 196 (74%) | |
| BRCA status (excluding BRCA unk) | |||||
| Positive | 144 (28%) | 27 (36.5%) | 47 (28%) | 70 (26%) | |
| Negative | 366 (72%) | 47 (63.5%) | 123 (38.5%) | 196 (57.5%) | |
| FIGO Stage | |||||
| IIIB-C | 794 (81%) | 283 (90%) | 261 (82%) | 250 (73%) | <0.001 |
| IIIB | 33 (3%) | 14 (4%) | 12 (4%) | 7 (2%) | |
| IIIC | 761 (78%) | 269 (85%) | 249 (78%) | 243 (71%) | |
| IV | 184 (19%) | 32 (10%) | 59 (18%) | 93 (27%) | |
| Histology | |||||
| Serous | 869 (89%) | 269 (85%) | 289 (90%) | 311 (91%) | 0.06 |
| Other | 109 (11%) | 46 (15%) | 31 (10%) | 32 (9%) | |
| Carcinomatosis (n=976) | |||||
| No | 184 (19%) | 76 (24%) | 52 (16%) | 56 (16%) | 0.015 |
| Yes | 792 (81%) | 239 (76%) | 266 (84%) | 287 (84%) | |
| Bulky UAB disease | |||||
| No | 393 (40%) | 146 (46%) | 110 (34%) | 137 (40%) | 0.009 |
| Yes | 585 (60%) | 169 (54%) | 210 (66%) | 206 (60%) | |
| OR Tumor Index (n=976) | |||||
| 0 | 133 (14%) | 57 (18%) | 35 (11%) | 41 (12%) | 0.008 |
| 1 | 309 (32%) | 108 (34%) | 90 (28%) | 111 (32%) | |
| 2 | 534 (55%) | 150 (48%) | 193 (61%) | 191 (56%) | |
| Operative time, min | |||||
| Median (range) | 280 (36–893) | 239 (36–750) | 295 (44–893) | 318 (47–773) | <0.001 |
| EBL, mL | |||||
| Median (range) | 700 (5–8000) | 700 (5–8000) | 700 (50–7000) | 600 (10–5200) | 0.286 |
| Residual disease | |||||
| CGR (0 mm) | 408 (42%) | 92 (29%) | 129 (40%) | 187 (55%) | <0.001 |
| Minimal residual (1–10 mm) | 378 (39%) | 151 (48%) | 120 (38%) | 107 (31%) | |
| Suboptimal (>10 mm) | 192 (20%) | 72 (23%) | 71 (22%) | 49 (14%) | |
| Hospital LOS, days | |||||
| Median (range) | 8 (1–122) | 8 (3–122) | 8 (1–86) | 7 (1–36) | <0.001 |
| Major (Grade 3–5) complication | |||||
| No | 830 (85%) | 274 (87%) | 269 (84%) | 287 (84%) | 0.440 |
| Yes | 148 (15%) | 41 (13%) | 51 (16%) | 56 (16%) | |
| Upfront chemotherapy | |||||
| No | 9 (1%) | 4 (1%) | 2 (1%) | 3 (1%) | 0.710 |
| Yes | 949 (99%) | 305 (99%) | 308 (99%) | 336 (99%) | |
| Upfront IP chemotherapy (n=955) | |||||
| No | 633 (66%) | 290 (94%) | 173 (56%) | 170 (51%) | <0.001 |
| Yes | 322 (34%) | 19 (6%) | 137 (44%) | 166 (49%) | |
| 30-day mortality | |||||
| Alive | 974 (99.6%) | 313 (99.4%) | 318 (99.4%) | 343 (100%) | --* |
| Dead | 4 (0.4%) | 2 (0.6%) | 2 (0.6%) | 0 (0%) | |
| 90-day mortality (n=975) | |||||
| Alive | 962 (98.7%) | 305 (97.1%) | 314 (98.7%) | 343 (100%) | 0.002 |
| Dead | 13 (1.3%) | 9 (2.9%) | 4 (1.3%) | 0 (0%) | |
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; Unk, unknown; FHx, family history; FIGO, International Federation of Gynecology and Obstetrics; UAB, upper abdominal; EBL, estimated blood loss; CGR, complete gross resection; LOS, length of stay; IP, intra-peritoneal.
No p value provided for 30-day mortality due to one category level count less than 5.
Changes by PDS-year group are listed in Supplemental Table S1. A total of 315 patients underwent PDS during 2001–2005 (Group 1), 320 during 2006–2009 (Group 2), and 343 during 2010–2013 (Group 3). Comparisons of demographic and clinical variables are shown in Table 1 and Supplemental Table S1. Compared to Group 1, there were almost twice as many patients in Group 2 and three times as many in Group 3 with stage IV disease (stage IV disease: Group 1, n=32 [10%; Group 2, n=59 [18%]; and Group 3, n=93 [27%]; p<0.001). Higher American Society of Anesthesiologists (ASA) score, carcinomatosis, bulky UAB disease, and OR Tumor Index of 2 were significantly more common in Groups 2 and 3. Despite higher-stage disease and greater tumor burden, CGR rates rose from 29% to 40% to 55% over the 3 time periods (p<0.001). Median operative times also rose from 239 min (range, 36–750 min) to 295 min (range, 44–893 min) to 318 min (range, 47–773 min), respectively. Median LOS was 8 days (range, 3–122 days) for patients in Group 1, 8 days (range, 1–86 days) for those in Group 2, and 7 days (range, 1–36) for those in Group 3 (p<0.001). There was no significant difference in age, preoperative serum albumin, histology, estimated blood loss (EBL), major complication rates, or rates of postoperative chemotherapy use between groups. Significantly more patients received IP chemotherapy in Group 2 (n=137/310, 44%) and Group 3 (n=166/336, 49%) compared to Group 1 (n=19/309, 6%; p<0.001). However, among patients who underwent CGR, the use of IP chemotherapy decreased from 62.8% in Group 2 to 40.1% in Group 3 (Supplemental Table S2). Thirty-day mortality rates decreased over time (0.6% to 0.6% to 0%, respectively), as did 90-day mortality rates (2.9% to 1.3% to 0%, respectively) (p=0.002).
CGR rates increased with increasing PDS-year group across all OR Tumor Indices (Table 2). For patients with an OR Tumor Index of 0, CGR rates rose from 58% (Group 1) to 76% (Group 3); for patients with an OR Tumor Index of 2, CGR rates rose from 32% (Group 1) to 65% (Group 3); for patients with an OR Tumor Index of 2, CGR rates rose from 17% (Group 1) to 44% (Group 3). Notably, suboptimal debulking rates decreased over time in those with the highest tumor burden (OR Tumor Index of 2) from 36% (Group 1) to 18% (Group 3).
Table 2.
Residual disease by OR Tumor Index and PDS-Year group
| Residual disease | 2001–2005 (Group 1) | 2006–2009 (Group 2) | 2010–2013 (Group 3) | |
|---|---|---|---|---|
| OR Tumor Index 0 | CGR | 33 (58%) | 25 (71%) | 31 (76%) |
| Minimal residual | 21 (37%) | 8 (23%) | 8 (19%) | |
| Suboptimal | 3 (5%) | 2 (6%) | 2 (5%) | |
| OR Tumor Index 1 | CGR | 34 (31%) | 45 (50%) | 72 (65%) |
| Minimal residual | 59 (55%) | 34 (38%) | 26 (23%) | |
| Suboptimal | 15 (14%) | 11 (12%) | 13 (12%) | |
| OR Tumor Index 2 | CGR | 25 (17%) | 59 (31%) | 84 (44%) |
| Minimal residual | 71 (47%) | 77 (40%) | 73 (38%) | |
| Suboptimal | 54 (36%) | 57 (29%) | 34 (18%) |
Abbreviations: CGR, complete gross resection
Definitions: CGR, residual disease 0 mm; Minimal residual, residual disease 1–10 mm; suboptimal, residual disease >10 mm
Median follow-up for the entire cohort was 77.7 months (range, 1.3–198 months). Median PFS was 18.2 months (95% CI, 17.3–19.9 months), and the 5-year PFS rate was 16.9% (95% CI, 14.6–19.4%). Median OS was 55.4 months (95% CI, 52–59.6 months), and the 5-year OS rate was 46.5% (95% CI, 43.3–49.7%).
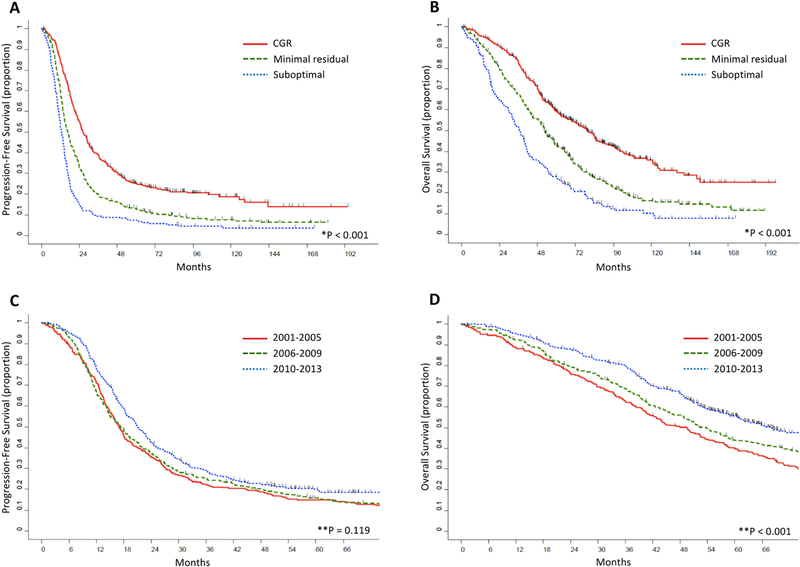
We also examined the effect of residual disease on survival. Kaplan-Meier curves for PFS and OS are shown in Figures 1A and 1B, respectively, and results are summarized in Table 3. As expected, residual disease was inversely correlated with survival. For CGR versus MRD versus suboptimal debulking, survival outcomes were as follows: median PFS, 26.5 months, 16.5 months, and 12.6 months, respectively; 5-year PFS rate, 25%, 13%, and 7%, respectively (p<0.001); median OS, 79.1 months, 52.5 months, and 36.6 months, respectively; 5-year OS rate, 59%, 43%, and 27%, respectively (p<0.001).
Figure 1.
(A) Progression-free survival and (B) overall survival stratified by residual disease. (C) Progression-free survival and (D) overall survival stratified by PDS-Year group.
CGR, residual disease 0 mm; Minimal residual, residual disease 1–10 mm; suboptimal, residual disease >10 mm
*Log-rank p value
**Chi-square p value at 5-year time point
PDS, primary debulking surgery; CGR, complete gross resection
Table 3.
Overall and progression-free survival stratified by residual disease
| PFS | ||||
| Residual disease | Median PFS, montds (95% CI) | 5-year PFS rate (85% CI) | HR (95% CI) | p value |
| CGR | 26.5 (24–29.1) | 25.3% (21–29.7%) | 1 | <0.001 |
| Minimal residual | 16.5 (14.9–18.2) | 12.9% (9.7–16.5%) | 1.66 (1.42–1.94) | |
| Suboptimal | 12.6 (11.2–14.2) | 7.3% (4.2–11.5%) | 2.61 (2.17–3.14) | |
| OS | ||||
| Residual disease | Median OS, months (95% CI) | 5-year OS rate (85% CI) | HR (95% CI) | p value |
| CGR | 79.1 (68.7–87.1) | 59.3% (54.1–64%) | 1 | <0.001 |
| Minimal residual | 52.5 (48.8–58.4) | 42.8% (37.6%−47.8%) | 1.68 (1.41–2) | |
| Suboptimal | 36.6 (32.1–40.2) | 27.4% (21.2%−33.9%) | 2.58 (2.11–3.17) | |
Abbreviations: PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; CGR, complete gross resection
Definitions: CGR, residual disease 0 mm; Minimal residual, residual disease 1–10 mm; suboptimal, residual disease >10 mm
PFS and OS increased for each PDS-year group over time (Table 4). The 5-year PFS rate was highest for the most recent PDS-year group, although this did not reach statistical significance. Median PFS for Group 3, Group 2 and Group 1 was 21.1 months, 17.3 months and 16.9 months, respectively, and 5-year PFS rates were 20%, 16%, and 15%, respectively (Figure 1C; p=0.199). Median OS and 5-year OS rates followed similar trends to those of PFS; however, the results were statistically significant. Median OS for Group 3, Group 2 and Group 1 was 68 months, 53.7 months and 49.4 months, respectively, and 5-year OS rates were 56%, 44 % and 40%, respectively (Figure 1D; p<0.001).
Table 4.
CGR rates with corresponding PFS and OS stratified by PDS-Year group
| All patients | 2001–2005 (Group 1) | 2006–2009 (Group 2) | 2010–2013 (Group 3) | p value | |
|---|---|---|---|---|---|
| CGR rate (%) | 41.7% (n=408) | 29.2% (n=92) | 40.3% (n=129) | 54.5% (n=187) | |
| Median follow-up (months) | 77.7 (1.3–198) | 152.7 (1.3–198) | 103.5 (1.3–143.5) | 64.7 (6.5–102.3) | |
| Median PFS (months; range) | 18.2 (17.3–19.9) | 16.9 (15.4–18.2) | 17.3 (15–19.4) | 21.1 (18.7–23.1) | |
| Median OS (months; range) | 55.4 (52–59.6) | 49.4 (42.1–53.5) | 53.7 (48.2–59) | 68.0 (58.8–82.1) | |
| 5-year PFS rate (95% CI) | 16.9% (14.6–19.4%) | 14.9% (11.2–19.1%) | 15.8% (12–20%) | 20.0% (15.9–24.6%) | 0.199 |
| 5-year OS rate (95% CI) | 46.5% (43.3–49.7%) | 39.7% (34.3–45.2%) | 44.0% (38.4–49.4%) | 55.6% (49.9–60.9%) | <0.001 |
Abbreviations: CGR, complete gross resection; PFS, progression-free survival; OS, overall survival; CI, confidence interval
Multivariable Cox PH models for OS and PFS are presented in Table 5. To best account for the evolving changes in ovarian cancer treatment, these models were adjusted for PDS-year group using stratification. After further controlling for potential confounding variables, CGR was found to be independently associated with both improved PFS and OS. Compared to CGR, MRD was associated with a 39% higher risk of recurrence/progression (adjusted HR 1.393; 95% CI, 1.174–1.654) and a 36% higher risk of death (adjusted HR 1.36; 95% CI, 1.118–1.653). Compared to CGR, a suboptimal debulking was associated with a 92% increased risk of recurrence/progression (adjusted HR 1.921; 95% CI, 1.547–2.386) and a 75% increased risk of death (adjusted HR 1.751; 95% CI, 1.378–2.224). Additional factors independently associated with an increased risk of recurrence/progression included stage IV disease compared to stage III disease, non-serous histology, unknown BRCA status (with or without a family history suggestive of HBOC syndrome) or negative BRCA status compared to confirmed BRCA mutation, and an OR Tumor Index of 2 compared to 0. Higher serum albumin and the use of IP chemotherapy were associated with a significantly decreased risk of recurrence/progression. Factors associated with OS were similar. Stage IV disease, non-serous histology, BRCA unknown status, and an OR Tumor Index of 2 were independently associated with an increased risk of death, while IP chemotherapy use was associated with a significantly decreased risk of death.
Table 5.
Multivariable analysis of factors associated with PFS and OS
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Variable | Adjusted HR | 95% CI | p value | Adjusted HR | 95% CI | p value |
| Age | ||||||
| By 5-year increase | 1.000 | (0.966–1.036) | 0.992 | 1.023 | (0.982–1.065) | 0.282 |
| Albumin | ||||||
| By 1 g/dL increase | 0.849 | (0.725–0.994) | 0.042 | 0.848 | (0.711–1.01) | 0.065 |
| FIGO Stage | ||||||
| IIIB-C | Reference | -- | 0.003 | Reference | -- | <0.001 |
| IV | 1.349 | (1.106–1.644) | 1.466 | (1.171–1.833) | ||
| ASA score | ||||||
| 1–2 | Reference | -- | 0.300 | Reference | -- | 0.063 |
| 3–4 | 1.089 | (0.927–1.278) | 1.186 | (0.991–1.42) | ||
| Histology | ||||||
| Serous | Reference | -- | 0.010 | Reference | -- | <0.001 |
| Other | 1.359 | 1.076–1.716) | 1.714 | (1.337–2.198) | ||
| BRCA status | ||||||
| Positive | Reference | -- | <0.001 | Reference | -- | <0.001 |
| Unk, FHx suggestive | 1.776 | (1.3–2.428) | 2.091 | (1.475–2.964) | ||
| Unk, FHx not suggestive | 1.737 | (1.371–2.199) | 2.081 | (1.576–2.748) | ||
| Negative | 1.259 | (1.004–1.578) | 1.159 | (0.878–1.531) | ||
| OR Tumor Index | ||||||
| 0 | Reference | -- | <0.001 | Reference | -- | <0.001 |
| 1 | 1.150 | (0.891–1.483) | 1.049 | (0.784–1.404) | ||
| 2 | 1.541 | (1.194–1.988) | 1.564 | (1.176–2.081) | ||
| Residual disease | ||||||
| CGR | Reference | -- | <0.001 | Reference | -- | <0.001 |
| Minimal residual | 1.393 | (1.174–1.654) | 1.36 | (1.118–1.653) | ||
| Suboptimal | 1.921 | (1.547–2.386) | 1.751 | (1.378–2.224) | ||
| Postop IP chemotherapy | ||||||
| No | Reference | -- | <0.001 | Reference | -- | <0.001 |
| Yes | 0.715 | (0.593–0.863) | 0.672 | (0.537–0.841) | ||
Abbreviations: PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; ASA, American Society of Anesthesiologists; Unk, unknown; FHx, family history; CGR, complete gross resection; IP, intraperitoneal
Discussion
Adoption of the philosophy of continuous improvement, through the 3 essential steps of “creating a standard, following the standard, and finding a better way” [23], has led to multiple advances in our operative approach to advanced ovarian cancer. Over a 13-year period, our CGR rates improved from 29% to 55%, and our suboptimal debulking rates decreased from 23% to 14%. When comparing the earliest to the most recent PDS-year group, median OS and the 5-year OS rate improved by almost 40% (49.5 to 68 months and 40% to 56%, respectively), while median PFS rose by 25% and the 5-year PFS rate rose by 33% (16.9 to 21.1 months and 15% to 20%, respectively).
Other studies have shown similar improvements in CGR as a result of dedicated changes in surgical practice. In 2009, our institution published on the effects of incorporating extensive UAB procedures into our surgical armamentarium [24]. CGR rates improved significantly, as did PFS and OS. Harter et al. reported on the outcomes of implementing a specific ovarian cancer quality management program [15]. This included the formation of designated teams for ovarian cancer surgery, routine use of preoperative and intraoperative consultations to ensure maximal cytoreduction, and conferences to assess morbidity and outcomes. Among the 726 patients with FIGO stage IIB-IV disease, CGR rates increased from 33% to 62%, while OS improved from 26 months to 45 months. A recent publication from the Mayo Clinic also demonstrated improved outcomes after introducing measures to maximize cytoreductive efforts and standardize their approach to ovarian cancer surgery [25]. A significant increase in the use of higher complexity surgery (from 24% to 41%) resulted in a greater proportion of patients with completely resected disease (from 32.7% to 54.3%) and better median OS (from 36 to 40 months). These publications, along with our current work, show that continuous improvement and shifts in surgical paradigm can lead to improved ovarian cancer survival.
In our current study, increasing CGR rates were associated with limited morbidity and mortality. Not surprisingly, median operative times increased as a greater proportion of patients underwent complete cytoreduction. EBL remained stable, however, and there was no significant difference in the rate of major complications (13% to 16%). Thirty-day mortality decreased over time from 0.6% to 0%, and 90-day mortality also decreased from 2.9% to 0%. Several studies have shown similar results, citing major complication rates of 18.8% to 20.8% and 30-day mortality rates of 0.2% to1.3% [15, 25–26]. Performing more extensive surgery to achieve better surgical outcomes appears to confer improved survival without compromising patient safety.
Some may question whether changes in our CGR rates and survival were accomplished largely due to more favorable patient selection. We believe that patient selection had minimal influence on these outcomes for several reasons. First, in 2010, a study by Aletti et al. reported that widespread tumor dissemination or stage IV disease, ASA ≥3 or preoperative albumin ≤3.0 g/dL, and age ≥75 years, were associated with high morbidity and limited PDS survival benefit [21]. Based on the data of this report, patients at our institution who met these criteria began being triaged to NACT instead of PDS at the discretion of the primary surgeon. Around the same time, results of the European Organisation for Research and Treatment of Cancer (EORTC) trial showed that NACT in advanced-stage ovarian cancer was non-inferior to PDS [9]. Our NACT use increased from 22% to 30% thereafter [22]. Despite the increase in NACT use, our patients continued to have higher ASA scores and higher-stage disease with increasing PDS-year group, while median age, serum albumin, and the presence of carcinomatosis remained unchanged. Second, CGR rates increased from 2001–2005 to 2006–2009, prior to the increase in NACT use. Third, we achieved more complete cytoreductions with increasing PDS-year group, regardless of disease burden. CGR rates rose from 17% to 44% in patients with carcinomatosis and bulky UAB disease. Therefore, while we acknowledge that patient selection may have had some influence on the increase in CGR rates, changes in surgical paradigm likely had the greatest effect on our increased CGR rates and better survival outcomes.
One of our study aims was to determine whether changes in CGR rates were independently associated with improved survival. We performed a multivariable Cox PH analysis with stratification by PDS year to account for changes over time that were not specified as covariates in the model (for example, incorporation of extensive UAB surgery or the use of NACT). After controlling for additional potential confounding factors, including BRCA status and the use of IP chemotherapy, we found that CGR was independently associated with OS and PFS. Patients who underwent CGR had a 26% decreased risk of death and a 28% decreased risk of recurrence compared to those left with 1–10 mm of residual disease at the completion of surgery. The risk of death and disease recurrence were reduced by 43% and 48%, respectively, for patients with no visible disease compared to those with >1 cm residual disease. These results corroborate the findings of a recent study from Canada, in which CGR is cited as an independent prognostic factor for improved OS [26]. An important study by Chang et al. further supports the significance of CGR on survival [10]. In this meta-analysis of more than 13,000 patients with stage IIB-IV ovarian cancer, median survival ranged from 27.6 months to 66.9 months. The authors observed an independent 2.3-month increase in median survival with each 10% increase in the proportion of patients who underwent complete cytoreduction. Comparatively, the increase in median survival for each 10% increase in the proportion of patients with residual disease ≤10 mm was limited to 1.8 months. We, therefore, believe that residual disease is one of the most important prognostic determinants of survival in patients with advanced ovarian cancer, and complete resection of all visible tumor should be the goal of PDS.
The role of cytoreduction to ≤10 mm of residual disease when CGR is not feasible, especially in the setting of high tumor burden, has been questioned [27–28]. Several studies have shown a significant survival benefit with residual disease ≤10 mm compared to suboptimal debulking [7, 15, 25, 29–30]. In our study, MRD conferred a 4-month median PFS and a 16-month median OS advantage over suboptimal debulking. Moreover, a prior publication from our institution showed a significant survival benefit with MRD in patients with large-volume disease [31]. Cytoreductive efforts should be maximized to achieve residual disease ≤10 mm when CGR cannot be attained, regardless of tumor burden.
The utility of NACT may be the greatest area of controversy in the management of advanced ovarian cancer. Proponents of NACT argue that the use of chemotherapy prior to surgical cytoreduction decreases morbidity and mortality without compromising survival. The 2 randomized controlled trials comparing NACT to PDS in patients with stage III-IV ovarian cancer both found NACT to be non-inferior to PDS [9,32]. In the EORTC study, however, only 42% of patients in the PDS group underwent debulking to residual disease ≤10 mm. In the CHORUS trial, 42% of patients had residual disease ≤10 mm after primary surgery. These low rates of MRD/CGR likely contributed to the low survival outcomes in the PDS arms, with a median OS of 30 months versus 29 months in the EORTC trial and 25.8 months versus 23.7 months in the CHORUS trial when comparing NACT versus PDS. Our study was composed of stage IIIB-IV patients, although only 3.3% of patients had stage IIIB disease. We also included all patients who underwent suboptimal debulking as long as maximal cytoreduction was intended preoperatively. Our MRD/CGR rate in 2010–2013 was 86%, with a median OS of 68 months. Although it is difficult to compare survival between studies, it is also hard to dismiss such drastic differences in outcomes. We certainly acknowledge that NACT is more appropriate than PDS for patients who are likely to be left with residual disease >10 mm; however, the selection criteria for these patients are still under investigation.
The limitations of this study include the inherent biases associated with its retrospective nature. Its single-institution source may limit the external validity of our results. Additionally, although postoperative chemotherapy regimens were all platinum/taxane-based, exact regimens were variable. However, this is one of the largest studies of its kind, spanning a 13-year period, with a long median follow-up of 77.7 months. Patients across all groups received standardized perioperative care and postoperative treatment. Furthermore, in our multivariable survival analysis, we accounted for changes over time by stratifying by PDS-year group.
There is no question that complete cytoreduction during PDS remains one of the most important prognostic factors in the treatment of ovarian cancer. Continued improvement in CGR rates and survival outcomes by increasing the extent of surgery, as well as evolving changes in surgical practice, without increasing morbidity or mortality, is feasible. Additionally, increased CGR rates can independently lead to better PFS and OS. Meanwhile, the role of NACT still needs to be more clearly defined. The Trial of Radical Upfront Surgical Therapy (TRUST trial), an international, multicenter, randomized controlled trial, is currently investigating outcomes of PDS versus NACT followed by interval debulking surgery. While still many years away, we hope that the results of this trial will shed some light on this debate. Until then, PDS with maximal cytoreductive effort to achieve CGR or as minimal residual tumor as possible, followed by platinum-based chemotherapy, should remain the cornerstone of treatment for advanced ovarian cancer.
Supplementary Material
Highlights.
From 2001 to 2013, specific changes to our primary debulking surgery paradigm were implemented
Despite greater stage and tumor burden, complete gross resection (CGR) rates rose from 29% to 55%
5-year progression-free survival (PFS) rates increased from 15% to 20%
Overall survival (OS) rates increased from 40% to 56%
CGR was independently associated with PFS and OS on multivariable analysis
Acknowledgments
Funding: This study was funded in part through the National Institutes of Health/National Cancer Institute (NIH/NCI) Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest Statement
Outside the submitted work, Dr. Dennis Chi is on the Medical Advisory Boards of Bovie Medical Co. and Verthermia Inc. The other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018. January;68(1):7–30. PMID [DOI] [PubMed] [Google Scholar]
- 2.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 3.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975. October;42:101–4. PMID [PubMed] [Google Scholar]
- 4.Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: A prospective study. Gynecol Oncol 1998. May;69(2):103–8. PMID [DOI] [PubMed] [Google Scholar]
- 5.Zivanovic O, Eisenhauer EL, Zhou Q, Iasonos A, Sabbatini P, Sonoda Y, Abu-Rustum NR, Barakat RR, Chi DS. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2008. February;108(2):287–92. PMID [DOI] [PubMed] [Google Scholar]
- 6.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol. 2006. February;100(2):283–7. PMID [DOI] [PubMed] [Google Scholar]
- 7.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M, Barakat RR. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006. November;103(2):559–64. PMID . [DOI] [PubMed] [Google Scholar]
- 8.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009. March 15;115(6):1234–44. PMID [DOI] [PubMed] [Google Scholar]
- 9.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010. September;363(10):943–53. PMID [DOI] [PubMed] [Google Scholar]
- 10.Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013. September;130(3):493–8. PMID . [DOI] [PubMed] [Google Scholar]
- 11.Elattar A, Bryant A, Winter-Roach BA, Hatem M, Maik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syste Rev. 2011. August 10;(8):CD007565 PMID . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol 2006;107:77–85. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauser EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol 2006;103:1083–90. [DOI] [PubMed] [Google Scholar]
- 14.Colombo PE, Mourregot A, Fabbro M, Gutowski M, Saint-Aubert B, Quenet F, et al. Aggressive surgical strategies in advanced ovarian cancer: a monocentric study of 203 stage IIIC and IV patients. Eur J Surg Oncol 2009;35:135–43. [DOI] [PubMed] [Google Scholar]
- 15.Harter P, Muallem ZM, Buhrmann C, Lorenz D, Kaub C, Hils R, et al. Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer. Gynecol Oncol 2011;121:615–9. [DOI] [PubMed] [Google Scholar]
- 16.Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, DeMatteo R, Poynor EA, Abu-Rustum NR, Barakat RR. Chi DS, Franklin CC, Levine DA, Akselrod F, Sabbatini P, Jarnagin WR, DeMatteo R, Poynor EA, Abu-Rustum NR, Barakat RR. Gynecol Oncol. 2004;94(3):650–4. PMID [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian (Version 1.2018). https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed April 1, 2018.
- 18.Tanner EJ, Long KC, Zhou Q, Brightwell RM, Gardner GJ, Abu-Rustum NR, Leitao MM Jr, Sonoda Y, Barakat RR, Iasonos A, Chi DS. Impact of operative start time on surgical outcomes in patients undergoing primary cytoreduction for advanced ovarian cancer. Gynecol Oncol. 2012. July;126(1):58–63. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strong VE, Selby LV, Sovel M, Disa JJ, Hoskins W, Dematteo R, Scardino P, Jaques DP. Development and assessment of Memorial Sloan Kettering Cancer Center’s Surgical Secondary Events grading System. Ann Surg Oncol. 2015. April;22(4):1061–7. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowan RA, Tseng J, Murthy V, Srivastava R, Long Roche KC, Zivanovic O, Gardner GJ, Chi DS, Park BJ, Sonoda Y. Feasibility, safety and clinical outcomes of cardiophrenic lymph node resection in advanced ovarian cancer. Gynecol Oncol. 2017. November;147(2):262–266. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, Chi DS, Bristow RE, Cliby WA. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol Oncol. 2011. January;120(1):23–8. PMID [DOI] [PubMed] [Google Scholar]
- 22.Mueller JJ, Zhou QC, Iasonos A, O’Cearbhaill RE, Alvi FA, El Haraki A, Eriksson AG, Gardner GJ, Sonoda Y, Levine DA, Aghajanian C, Chi DS, Abu-Rustum NR, Zivanovic O. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol. 2016. March;140(3);436–42. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bristow RE. Predicting surgical outcome for advanced ovarian cancer, surgical standards of care, and the concept of kaizen. Gynecol Oncol. 2009. January;112(1):1–3. PMID [DOI] [PubMed] [Google Scholar]
- 24.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, Guile MW, Bristow RE, Aghajanian C, Barakat RR. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009. July;114(1):26–31. PMID [DOI] [PubMed] [Google Scholar]
- 25.Wallace S, Kumar A, Mc Gree M, Weaver A, Mariani A, Langstraat C, Dowdy S, Bakkum-Gamez J, Cliby W. Efforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasible. Gynecol Oncol. 2017. April;145(1):21–26. PMID [DOI] [PubMed] [Google Scholar]
- 26.May T, Altman A, McGee J, Lu L, Xu W, Lane K, Ghatage P, Rosen B. Examining Survival Outcomes of 852 Women With Advanced Ovarian Cancer: A Multi-institutional Cohort Study. Int J Gynecol Cancer. 2018. April 4 Epub ahead of print. PMID [DOI] [PubMed] [Google Scholar]
- 27.Horowitz NS, Miller A, Rungruang B, Richard SD, Rodriguez N, Bookman MA, Hamilton CA, Krivak TC, Maxwell GL. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015. March 10;33(8):937–43. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005. December 1;23(34):8802–11. PMID: [DOI] [PubMed] [Google Scholar]
- 29.Chiva LM, Castellanos T, Alonso S, Gonzalez-Martin A. Minimal macroscopic residual disease (0.1–1cm). Is it still a surgical goal in advanced ovarian cancer? Int J Gynecol Cancer. 2016. June;26(5):906–11. [DOI] [PubMed] [Google Scholar]
- 30.Sioulas VD, Schiavone MB, Kadouri D, Zivanovic O, Roche KL, O’Cearbhaill R, Abu-Rustum NR, DA, Sonoda Y, Gardner GJ, Leitao MM Jr., Chi DS. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol Oncol. 2017. April;145(1):15–20. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zivanovic O, Sima CS, Iasonos A, Hoskins WJ, Pingle PR, Leitao MM Jr., Sonoda Y, Abu-Rustum NR, Barakat RR, Chi DS. The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by initial tumor burden in the upper abdomen cephalad to the greater omentum. Gynecol Oncol. 2010. March;116(3):351–7. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, Dobbs S, Essapen S, Twigg J, Herod J, McCluggage G, Parmar M, Swart AM. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015. July 18;386(9990):249–57. PMID [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.