Abstract
The medicinal herb Lycium barbarum fruit has been widely used for improving and maintaining the health of the eyes in the Far East for many centuries. This study is aimed at investigating whether protective effects generated from the aqueous (LBA) and ethanol (LBE) extracts of the L. barbarum fruit existed against oxidative stress-induced apoptosis in human retinal pigment epithelial cells. L. barbarum extracts LBA and LBE exerted the activity of ROS scavenging and rescued UVB irradiation-induced growth inhibition in retinal pigment epithelial ARPE-19 cells. Compared to LBA, the ethanol extract LBE exerted a superior protective activity on UVB-induced growth arrest in ARPE-19 cells. Both L. barbarum extracts significantly reduced cell cycle G2-arrest population in ARPE-19 cells. Furthermore, the cytometer-based Annexin V/propidium iodide staining assay further showed that both L. barbarum extracts protected ARPE-19 cells from UVB-induced apoptosis. L. barbarum extracts also reduced the activation of γH2AX, a sensor of DNA damage in ARPE-19 cells in a dose-responsive manner. By using Ingenuity Pathway Analysis (IPA), the bioinformatics revealed that the protective effects of both LBA and LBE extracts might be involved in three signaling pathways, especially the Toll-like receptor (TLR) pathway associated with cellular proliferation. Our study suggests that both ethanol and aqueous extracts of L. barbarum exhibit antioxidant activity and rescue UVB-induced apoptosis of ARPE-19 cells. Collectively, the ethanol extract exerts a superior effect on rescuing UVB-induced growth arrest of ARPE-19 compared to the aqueous extract, which might be associated with the activation of TLR signaling. Our present work will benefit the preventive strategy of herbal medicine-based vision protection for treating eye diseases such as age-related macular degeneration in the future.
1. Introduction
Age-related macular degeneration (AMD), a progressive macular retinal disease with degenerative changes, can be divided into atrophic and exudative, characterized by the progressive atrophy of retinal pigment epithelial (RPE) cells and the formation of choroidal neovascularization (CNV) [1]. RPE cells are located between the layers of photoreceptor cells and provide nutrition to the latter. If oxidative damage occurs in RPE cells, the breakdown of photoreceptor cells would quickly follow and visual acuity might become damaged [2].
The fruit of Lycium barbarum (LB) wolfberry is a traditional Chinese herbal medicine that has multiple functions in pharmacology [3] like antioxidation [4–6], antiaging [7, 8], neuroprotection [9–12], cytoprotection [13, 14], and immunomodulating [5, 15]. A previous study showed that LBP (Lycium barbarum polysaccharides) extracted from the fruit of L. barbarum might be responsible for the above biological activities [16]. LBP was also shown to exert a protective effect against oxidative damage in cells [17–20]. Based on the antioxidant activity of L. barbarum, many studies have demonstrated that LBP has a protective effect against oxidative injury in cells [17–20], and many studies have focused on the bioactivities of this extract of L. barbarum. However, the effects of the ethanol fraction of LB extracts have been little addressed by previous studies. In the study, we prepared both aqueous (LBA) and ethanol (LBE) extracts of LB and investigated the protective effects of LBA or LBE on human retinal pigment epithelial (ARPE-19) cells from UVB damage, notably proliferation inhibition and apoptosis. We also discussed the possible mechanism underlying L. barbarum extract-mediated protective effect on retinal pigment epithelial cells.
2. Materials and Methods
2.1. Plant Material and Extraction
A total of 500 g of dried fruits of L. barbarum were placed in boiling 3 L water (100°C) for 4 h according to a traditional method described as in the previous study [21]. After filtration, using Whatman no. 3 filter paper, the aqueous extract of L. barbarum was lyophilized. For the ethanol extracts, 500 g of dried fruits was placed in 3 L of ethanol for 3 h at 70°C. The solution was filtrated with Whatman no. 3 filter paper and then evaporated at 35°C with reduced pressure.
2.2. Cell Culture
Arising retinal pigment epithelia cell line-19 (ARPE-19), a monolayer of polarized epithelial cells located between the sensory retina and choriocapillaris, is differentiated and mitotically inactive under normal physiological conditions. The ARPE-19 (No. 60,383), obtained from the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan), was grown in DMEM medium (Dulbecco's Modified Eagle's Medium, Invitrogen Corporation, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin in an incubator with 5% CO2 at 37°C. Cells were pretreated with L. barbarum extracts (from 0 to 200 μg/mL) for 2 h; then, cells were exposed to 50 mJ/cm2 of UVB cultured for 24 h. The experiment of different exposing dose of UVB was performed in triplicate and repeated three times to ensure reproducibility.
2.3. Assessment of Cell Viability
The cell viability was assessed using a colorimetric tetrazolium 3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide (MTT) assay. Briefly, 1 × 104 cells were seeded into a 96-well plate, and the cells were treated with different exposing doses of UVB (from 0 to 60 mJ/cm2) for indicated periods (24 and 48 hr, respectively). The final concentration of MTT in each well was 0.5 mg/mL, and the cells were further incubated. Afterward, the media containing MTT were removed, and the crystal formazan was dissolved entirely with Dimethyl sulfoxide (DMSO). The absorption length of light was measured at 570 nm using a microplate reader (Thermo, Massachusetts, USA), and the relative cell viability was presented as the percentage of absorbance values in treated cells to that of control cells.
2.4. Assessment of Cell Cycle Distribution
The assay of flow cytometer-based propidium iodide (PI) staining was used for detecting cell cycle distribution as described previously [22]. Briefly, ARPE-19 cells were pretreated with LBA and LBE for 2 h; then, the cells were irradiated with 50 mJ/cm2 UVB for 24 hr. Afterward, trypsin-suspended cells were washed and ethanol-fixed. After centrifugation, the cells were stained with 10 μg/mL PI (Sigma, St. Louis, MO) and 10 μg/mL RNase A at room temperature in the dark. The PI-stained cells were further analyzed by FACScan, a flow cytometer (Becton-Dickinson, Mansfield, MA), and WinMDI/PC-freeware V2.9 (Joseph Trotter, La Jolla, CA, USA).
2.5. Assessment of Mitochondrial Membrane Potential (ΔΨm)
The changes in the mitochondrial membrane potential (ΔΨm) were assessed using a mitochondrial permeable lipophilic cationic dye 5,5,6,6-Tetrachloro-1,1,3,3-tetraethylbenzimidazolylcarbocyanine iodide (JC-1). In healthy cells, JC-1 accumulates in the mitochondria and emits red fluorescence (560 nm); however, in mitochondrial cells with changed membrane potential, the JC-1 accumulates in the cytoplasm and emits fluorescence (530 nm). ARPE-19 cells were pretreated with LBA and LBE and incubated with 10 mg/mL JC-1 at 37°C in the dark. Cells were washed twice with serum-free medium and detected using a fluorescence microscope (Olympus IX71 CTS).
2.6. FITC-Annexin V/PI Apoptosis Assay
The assessment of apoptotic cells was performed according to previous work [22]. Briefly, cells were pretreated with LB extracts 2 hr, respectively, prior to UVB irradiation for 24 hr. The changes of early and late apoptosis were determined using an Annexin-V-Fluorescein isothiocyanate (FITC)/PI Apoptosis Detection kit (BioVision, CA 94043, USA). Briefly, cells were resuspended in binding buffer then incubated in the dark with FITC-labeled Annexin V and propidium iodide for 15 min at room temperature; finally, the samples were diluted with phosphate-buffered saline (PBS). Flow cytometry was carried out on a FACScan instrument (FACS Calibur; Becton Dickinson, Mountain View, CA, USA), and data were processed with WinMDI/PC-software V2.9 (written by Joseph Trotter, Scripps Research Institute, La Jolla, CA, USA). Cells labeled with annexin conjugated with the FITC fluorescence were recognized as apoptotic populations.
2.7. Determination of Intracellular ROS
We measured the changes in intracellular ROS according to the previous work with minor modifications [23]. 5 × 105 cells were seeded in a six-well plate in triplicate. The cells were pretreated with or without LBA and LBE 2 hr prior to the UVB irradiation. After 12 hr, the cells were washed twice with PBS, collected, and then further incubated with 10 μM dichloro-dihydro-fluorescein diacetate probe (DCFH-DA, Molecular Probes Inc., Eugene, OR, USA) at 37°C for 30 min. The fluorescence intensity of DCFH was quantified by flow cytometry (the length of excitation is 485 nm, and the length of emission is 525 nm). The results were presented as the percentage of the control cells (100%).
2.8. Assessment of DNA Damage
Briefly, cells were pretreated with LB extracts 2 hr, respectively, prior to UVB irradiation. Afterward, cells were harvested and fixed with 70% ethanol at −20°C overnight; then, the cells were washed twice with BSA-T-PBS solution (1% BSA and 0.2% Triton X-100 in PBS). The cells were then incubated with 0.2 μg/mL antibodies against phosphorylated-Ser139 H2AX (γH2AX), and the secondary antibody was subsequently conjugated with Alexa Fluor 488 (Jackson Laboratory, Bar Harbor, Marine, USA). The intensity of fluorescence was measured by flow cytometry (FACSCalibur, Becton-Dickinson). The results were quantified using the software Cell Quest (Becton-Dickinson).
2.9. Microarray Analysis
RNA molecules were isolated using MirVana Total RNA Isolation Kit (Applied Biosystems, Foster City, CA, USA) according to the instructions of the manufacturer. The microarray and the data analysis were performed by Welgene Biotech (Taipei, Taiwan) using the SurePrint G3 GE 8 × 60 K Microarray, 8 × 60 K, AMADID 028005 (Agilent Technologies, USA [24]). The arrays were scanned with G2505C Microarray Scanner (Agilent). The information of probes on the arrays was extracted from the image data using Feature extraction 10.5.1.1 (Agilent) for quantifying signal and the intensity of background.
2.10. Ingenuity Pathway Analysis
The molecular functions of the unique gene analysis of the UVB-induced genes were analyzed using the software Ingenuity Pathway Analysis® (IPA, Ingenuity Systems, Redwood City, California, USA). The changed genes which met the criteria and were correlated with the biological functions of the Ingenuity Pathways Knowledge Base (Ingenuity Systems) were included.
2.11. Statistical Analysis
All experiments were conducted in triplicate, and the data were represented as mean ± SD. The data were subjected to an analysis of variance (ANOVA) and Duncan's multiple range tests. p < 0.05 was considered significant.
3. Results
3.1. UVB-Induced Cell Death in Retinal Pigment Epithelial Cells
ARPE-19 cells were exposed to UVB light with indicated doses of UVB (from 0 to 60 mJ/cm2, respectively) for 24 hr, and the cell viabilities were 100 ± 2.61%, 76.97 ± 2.35%, 62.08 ± 2.40%, 59.17 ± 2.43%, 56.68 ± 3.08%, 51.98 ± 1.78%, and 47.52 ± 2.92%. At 48 hr, viabilities were 100 ± 4.22%, 80.57 ± 4.48%, 75.77 ± 6.09%, 48.06 ± 4.68%, 38.02 ± 3.27%, 35.20 ± 3.08%, and 33.66 ± 2.86% (Figure 1). The results showed that the irradiation of 50 mJ/cm2 UVB significantly induced cell death of RPE cells.
Figure 1.
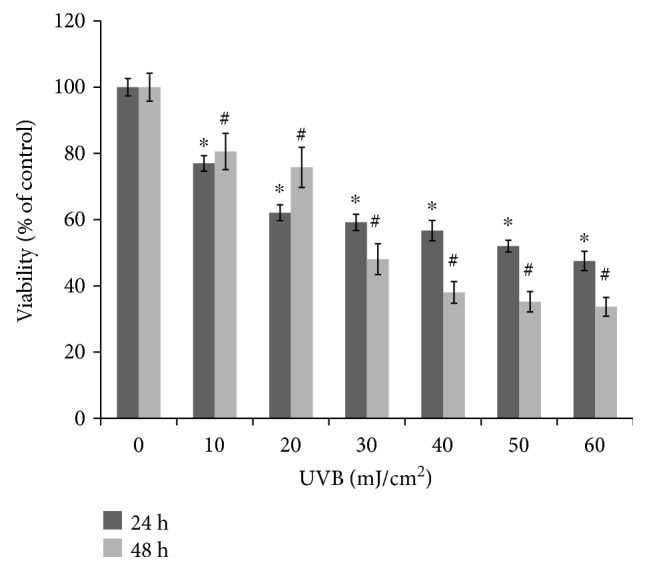
The viability of UVB irradiation on growth of ARPE-19 cells. The cells were exposed to the irradiation of UVB at indicated doses and then incubated further for 24 hr and 48 hr, respectively. The viability of cells was determined by MTT assay. The results are expressed as mean ± standard deviation (SD) (n = 3). The (∗) asterisk and (#) hash symbols indicate p < 0.05 vs. cells without UVB irradiation for 24 hr and 48 hr, respectively.
3.2. L. barbarum Extracts Reduced UVB-Induced Cell Death in Retinal Pigment Epithelial Cells
To evaluate whether LBA and LBE protected ARPE-19 cells against UVB-induced cell death, we detected the viability of ARPE-19 cells after UVB (50 mJ/cm2) incubation for 24 hr and 48 hr, with or without LBA and LBE pretreatment in 25 and 50 (μg/mL). As shown in Figure 2, the viability of cells was decreased to 44.51 ± 2.38% after being exposed to UVB (50 mJ/cm2) for 24 hr; LBA and LBE pretreatments with a variety of concentrations from 25 μg/mL to 50 μg/mL for 48 hr prevented the loss of cell viability. Finally, 50 μg/mL of both LBA and LBE increased the cell variety from 44.51 ± 2.38% to 53.54 ± 2.35% and from 44.51 ± 2.38% to 57.96 ± 6.50%, respectively.
Figure 2.
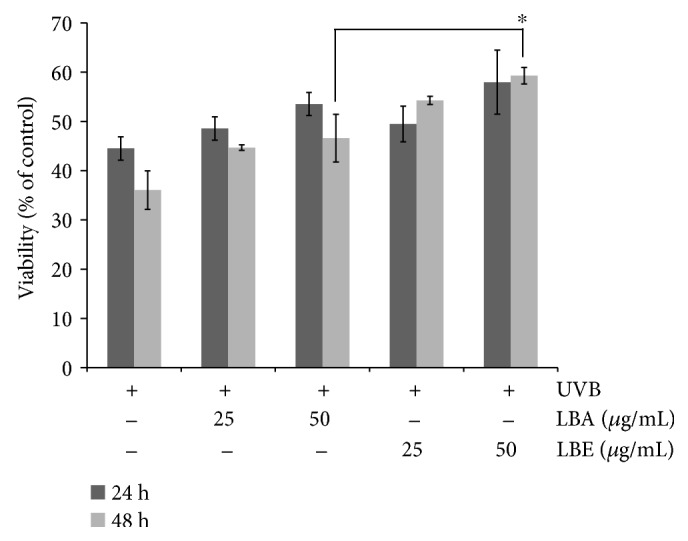
L. barbarum extracts rescue the viability of UVB-treated cells in an appropriate concentration. ARPE-19 cells were pretreated with either LBA or LBE for 2 hr before being exposed to UVB irradiation. The cell viability was determined by MTT assay 24 hr and 48 hr after UVB irradiation (50 mJ/cm2). The control group was the UVB-irradiated cells without L. barbarum extracts. The results are expressed as a percentage of control and are represented by mean ± SD (n = 3). The asterisk indicates p < 0.05 vs. UVB-exposed cells without LBA and LBE pretreatments.
3.3. LBA and LBE Reduce Endogenous ROS Level after Irradiated ARPE-19
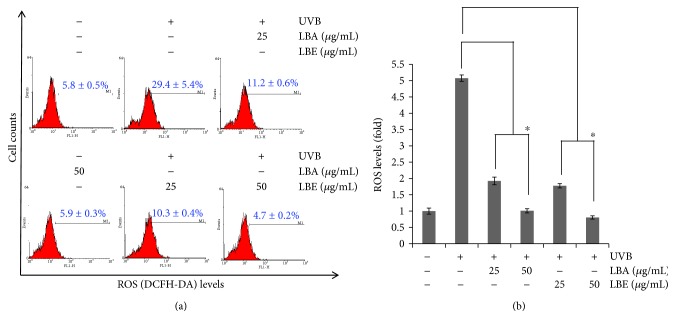
As shown in Figure 2, the pretreatments of LBA and LBE rescue the viability of ARPE-19 cells following the irradiation of UVB. Under normal conditions, reactive oxygen species (ROS) could act as a second messenger in cell signaling and regulates various biological functions [25–27]. However, excess ROS production could inhibit proliferation of cells and cause cell death [26–29]. A high level of endogenous ROS is highly correlated with the pathophysiology of retinal degeneration diseases including AMD. After exposure to UVB, levels of ROS will reach 29.4 ± 5.4%, compared to pretreatment with LBA and LBE in a high dose of 50 (μg/mL) then down to 5.9 ± 0.3% and 4.7 ± 0.2%, respectively. As shown in Figure 3, LBA and LBE reduced the levels of endogenous ROS in human ARPE-19 cells after the irradiation of UVB (p < 0.05).
Figure 3.
Effect of L. barbarum extracts on reactive oxygen species (ROS) production of ARPE19 cells after UVB irradiation exposure. (a) ARPE-19 cells pretreated with PBS or L. barbarum extracts LBA and LBE 25 and 50 μg/mL for 2 hr. After being exposed to 50 mJ/cm2 UVB irradiation, cells were harvested and subjected to a flow cytometer-based DCFDA staining assay, which is oxidized by ROS to the high fluorescence of DCF for detecting the changes of H2O2 in cells. (b) The quantitative analysis of (a). The asterisk symbols indicate p < 0.05 vs. UVB-irradiated cells without LBA or LBE pretreatments.
3.4. LBA and LBE Significantly Reduced UVB-Induced Apoptosis of ARPE-19
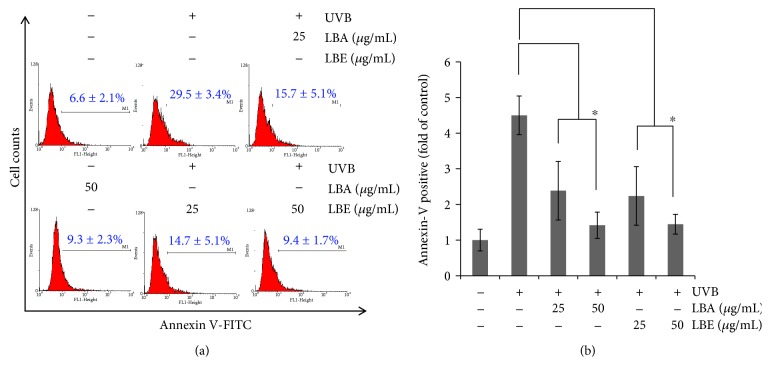
To determine whether LBA and LBE mitigated cell apoptosis in UVB-irradiated ARPE-19 cells, the cytometer-based Annexin V/PI dual staining assay was conducted. ARPE-19 cells were cultured with 0, 25, and 50 μg/mL of LBA and LBE for 24 hr before being irradiated with UVB 50 mJ/cm2 for 24 hr. Data are represented as (mean ± SD) of five individual experiments, with p < 0.05 compared with control. Following UVB-induced apoptosis of ARPE-19 cells, incubation of two treatments for 24 hr demonstrated levels of apoptotic cells decreased from 29.5 ± 3.4% to 15.7 ± 5.1% and 9.3 ± 2.3% by LBA and down to 14.7 ± 5.1% and 9.4 ± 1.7% by LBE, respectively. As shown in Figure 4, the results showed that both treatments protect apoptosis in the ARPE-19 cells, suggesting the protective effects of LBA and LBE on ARPE-19 cell.
Figure 4.
Pretreatment with L. barbarum extracts rescues UVB-induced apoptosis of retinal pigment epithelial cells. (a) ARPE-19 cell was pretreated with vehicle control or L. barbarum extracts LBA and LBE for 2 hr, respectively, prior to the irradiation of 50 mJ/cm2 UVB. Afterward, cells were harvested and assessed using a flow cytometer-based Annexin V staining. Results are represented as mean ± SD. (b) The quantitative analysis of (a). The asterisk indicates p < 0.05 vs. UVB-irradiated cells without LBA and LBE pretreatments.
3.5. L. barbarum Extracts Attenuate UVB-Induced Loss of Mitochondrial Membrane Potential
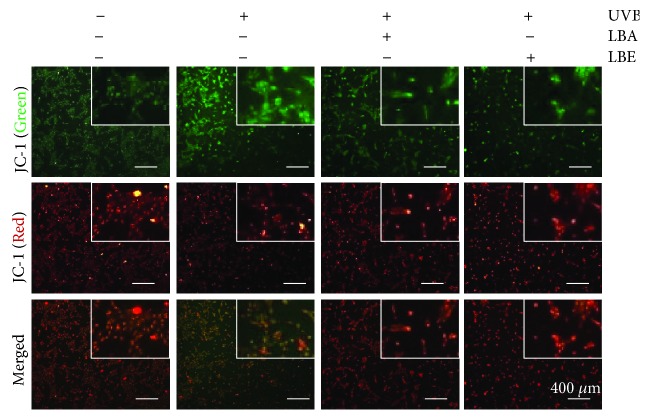
Mitochondria-mediated signaling is mainly responsible for apoptosis; we further determined whether LB extract protects UVB-induced apoptosis through the regulation of mitochondrial signaling. The JC-1 fluorescence dye was used for detecting the loss of mitochondrial membrane potential (MMP), a marker of mitochondrial-mediated apoptosis [30]; therefore, we assessed the MMP membrane potential (ΔΨm) in UVB-irradiated cells using JC-1 staining. As shown in Figure 5, the results show that L. barbarum extract pretreatment had strong intensity of red fluorescence (J-aggregation) and weak intensity of green fluorescence (JC-1 monomer) compared to UVB irradiation alone, indicating that both L. barbarum extracts LBA and LBE prevent the loss of MMP in UVB-irradiated cells.
Figure 5.
The effect of L. barbarum extracts on the UVB-induced loss of mitochondrial membrane potential (MMP). ARPE-19 cells pretreated with 50 μg/mL LBA or LBE, respectively, prior to the irradiation of 50 mJ/cm2 UVB light. Afterward, the changes of MMP (ΔΨm) were detected by a fluorescence microscope-based JC-1 staining assay. The wavelengths of emission are 580 nm (red, upper panels) and 530 nm (green, lower panels). Scale bars: 400 μm.
3.6. LBA and LBE Protected UVB-Induced DNA Damage of ARPE-19 Cells
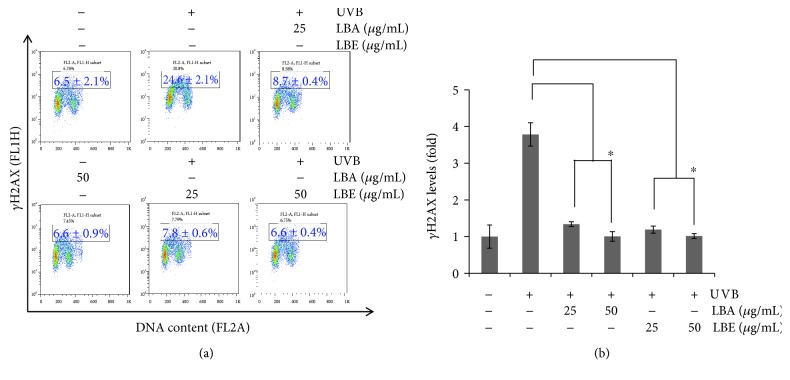
γH2AX, a phosphorylated histone variant H2AX at site Ser139, is a marker of DNA damage [31]. The assessment of γH2AX is widely used for detecting DNA damage. As shown in Figure 6, the protective ability of both LBA and LBE pretreated with various concentrations at 25 and 50 μg/mL shows 24.6 ± 2.1% of UVB-irradiated, 8.7 ± 0.4% and 6.6 ± 0.9% of LBA, and 7.8 ± 0.6% and 6.6 ± 0.4% of LBE significantly attenuated the activation of γH2AX in a dose-responsive manner, suggesting a protective role of both LBA and LBE in UVB-induced apoptosis of human ARPE-19 cells.
Figure 6.
The protective effect of LB extracts on UVB-induced DNA damage of ARPE-19 cells. (a) ARPE-19 cells pretreated with LBA and LBE 25 and 50 μg/mL for 2 hr prior to the exposure to 50 mJ/cm2 UVB irradiation, cells were harvested and subjected to the flow cytometer-based γH2AX immunostaining assay and repeated in three independent experiments. (b) The quantitative analyses of γH2AX level in ARPE-19 cells (n = 3). The asterisk indicates p < 0.05 vs. UVB-exposed cells without the pretreatment of LB extracts.
4. Discussion
Chronic photooxidative stress from the environment could cause various damages including the integrity of the membrane, increased ROS, DNA damage, and cell death of RPE. Dysfunction and degeneration of RPE cells are crucially involved in the pathogenesis of AMD or other RPE degeneration-associated diseases [32, 33]. The apoptotic death of RPE cells, followed by photoreceptor cell death, is thought to mainly contribute to the pathogenesis of the dry form of AMD [33]. The progression of AMD pathogenesis is highly correlated with various oxidative stresses and could be prevented or delayed by supplying vitamin C or other antioxidants [34, 35]. L. barbarum (wolfberry) has been used as a traditional antiaging herb in Chinese pharmacopeia over a long history [36]. LBA, the primary active ingredient of lycium barbarum, isolated from the aqueous extracts of lycium barbarum, has multibioactivity properties on modulating cellular physiology, and LBA could exert protective effects on hepatocytes and neurons of the eye [37–40]. However, little is known regarding ethanol extracts of LB.
To confirm whether LB extracts LBA and LBE could protect ARPE-19 cells against UVB-induced cell damage, we first examine the viability of ARPE-19 cells after the irradiation of UVB. Our results showed that the pretreatments of both LBA and LBE significantly mitigated the proliferation of ARPE-19 cells. Under normal conditions, reactive oxygen species (ROS) could act as a second messenger in cell signaling and regulates various biological functions [25–27]; however, a high level of endogenous ROS is highly correlated with the pathophysiology of retinal degeneration diseases including AMD. Here, we demonstrated that both aqueous and ethanol extracts of L. barbarum could enhance antioxidant activities and attenuate the levels of endogenous ROS in ARPE-19 cells. Furthermore, both aqueous and ethanol extracts of L. barbarum have potent antioxidant activities and rescue cells from UVB-induced apoptosis. Moreover, ethanol extracts of L. barbarum display stronger antioxidant effects than the aqueous extract of L. barbarum. For bioactive constituents, L. barbarum is rich in antioxidants, including hydrophilic polysaccharides [41] and the hydrophobic flavonoids carotenoid and riboflavin [42]. Despite polysaccharide fractions, LBP has been shown to have antioxidant activity, and a previous study revealed that crude polysaccharide extracts exhibited stronger antioxidant activity than purified polysaccharide fractions (LBP) because antioxidants such as carotenoids, riboflavin, ascorbic acid, thiamine, and nicotinic acid are more abundant in crude extracts. In our study, the results showed that both LBA and LBE reduced the level of ROS in human ARPE-19 cells after UVB irradiation. Moreover, the JC-1 staining for detecting the loss of mitochondrial membrane potential (MMP) as a marker of mitochondrial-mediated apoptosis was conducted, and the results showed that the pretreatment of both LB extracts prevented the loss of MMP following UVB irradiation.
Therefore, we further determined whether LBA and LBE occluded cell apoptosis under UVB-irradiated ARPE-19 cells using a cytometer-based Annexin V/PI double staining assay. Consistently, our results showed that UVB-induced apoptosis of ARPE-19 cell at 24 hr; however, the percentages of UVB-induced apoptotic cells were decreased with both LB extract pretreatments. Both extracts exerted protective effects on UVB-induced apoptosis in an ARPE-19 cell line. It is well known that irradiation of UVB or visible light causes photochemical lesions or other damages through the induction of DNA damage [43]; therefore, examination of whether LB extracts could protect ARPE-19 cells from DNA damage using the assessment of γH2AX, a marker of DNA damage [31], is desirable.
Our results showed that the pretreatments of both LBE and LBA significantly attenuated the activation of γH2AX in a dose-responsive manner, suggesting a protective role of LB extracts in UVB-induced DNA damage of human ARPE-19 cells. Because of the great benefits of L. barbarum, many studies have been conducted extensively on the preventive effects of LB extracts against eye diseases [44–46]. For example, the work of Li et al. suggested that LBP is neuroprotective and could delay secondary degeneration of retinal ganglion cells (RGCs), which might be associated with the downregulation of oxidative stress and the MAPK JNK/c-Jun pathway in retinal tissue [46]. Furthermore, the work of Liu et al. demonstrated that the treatment of LBP not only improves morphology and function of retinal tissue in rd1 mice, an in vivo photoreceptor degenerating model of retinitis pigmentosa, but also delays the functional degeneration of RGCs [45]. However, little is known regarding the bioactivity and mechanism of L. barbarum against DNA damage; consequently, our study is the first to reveal L. barbarum extracts as protective agents for UVB-induced DNA damage.
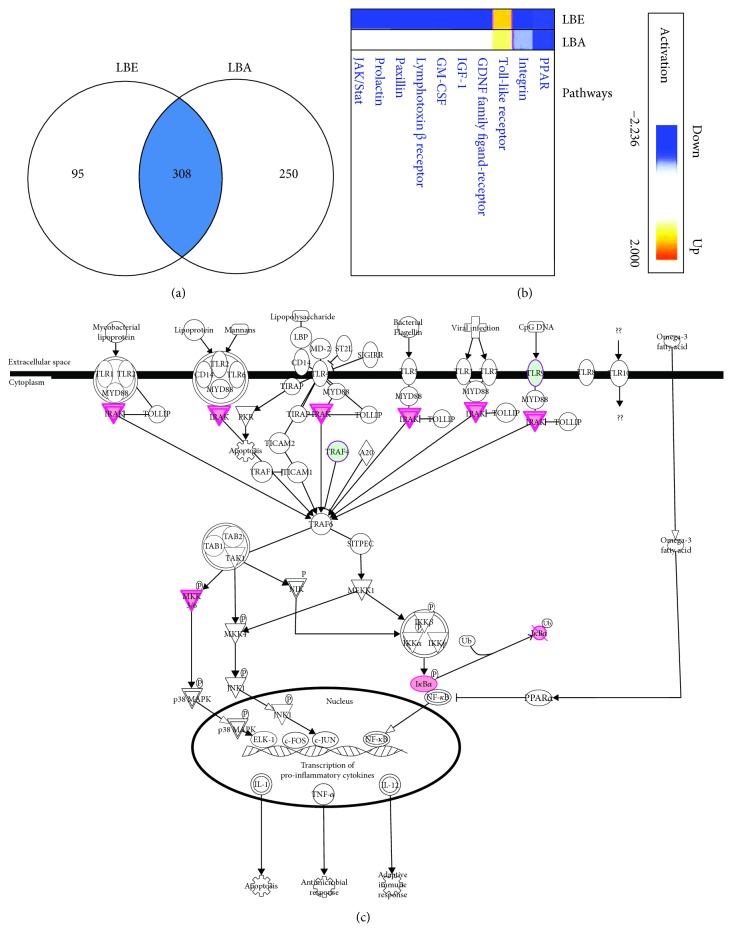
Previously, Lin et al.'s work analyzed the interrelationship between Lycium barbarum and gene expression in mouse spleen using oligo-microarray, and their result showed that three genes, Bcl-2, NFκB, and TNF, were upregulated whereas two proapoptotic genes (apoptotic protease-activating factor-1 (apaf-1) and caspase-3) were downregulated by the treatment of Lycium barbarum [47]. Given the superior cytoprotection on UVB irradiation-induced growth arrest by ethanol extract LBE, we further investigated the mechanism using human global gene expression profiles of RNA isolated from ARPE-19 cells pretreated with L. barbarum extracts LBE or LBA prior to the irradiation of UVB analyzed by a cDNA microarray at 24 hr. We identified that a total of 403 genes in LBE-treated cells and a total of 558 genes in LBA-treated cells were changed, and 308 genes might be involved in the signaling pathways associated with the protective effect of L. barbarum extracts using analysis by IPA bioinformatics software (Figure 7(a)). The analysis of IPA further suggested that three signaling pathways of the top-level functional annotation categories of genes including PPAR, Integrin survival signaling, and Toll-like receptor (TLR) signaling pathways were affected by the pretreatment of both L. barbarum extracts LBE and LBA (Figures 7(b) and 7(c)).
Figure 7.
Activation of genes involved in L. barbarum extract-mediated protective effect on UVB irradiation. The human global gene expression profiles of RNA were isolated from ARPE-19 cells pretreated with L. barbarum extracts LBE or LBA prior to the irradiation of UVB then analyzed by a cDNA microarray at 24 hr. (a) A total of 403 genes in LBE and a total of 558 genes in LBA were changed, and 308 genes might be involved in the signaling pathways associated with the protective effect of L. barbarum extracts using the analysis of IPA bioinformatics software. (b). Top-level functional annotation categories of genes including peroxisome proliferator-activated receptor (PPAR), the nuclear receptor as a transcription factor, Integrin, and Toll-like receptor (TLR) signaling pathways were affected by the pretreatment of both L. barbarum extracts LBE and LBA. Remarkably, the activation level of the TLR pathway is higher in the LBE pretreatment than in the LBA pretreatment. (c) Genes might be involved in the activation of the TLR pathway. A pink color indicates the upregulation of the genes. A green color indicates the downregulation of the genes.
Peroxisome proliferator-activated receptors (PPARs) are important nuclear transcription factors for oxidative defense system in eukaryotic cells. The PPAR family has at least three members including PPARα, PPARβ/δ, and PPARγ [48]. For example, PPARγ has been shown to have an antioxidant function through transcriptional regulating a number of antioxidation-associated gene catalase (CAT), glutathione peroxidase (GPX-3), heme oxygenase-1 (HO-1), and manganese superoxide dismutase (MnSOD) [49].
Integrins are transmembrane proteins and the heterodimerization of integrin receptors have been shown to regulate cell survival, differentiation, and migration of metazoa through communicating signals via the plasma membrane [50]. Neuronal survival is exhibited by olfactory ensheathing cell, a type of glia that supports axon outgrowth olfactory system through integrin/milk fat globule-EGF factor 8 ((MFG-E8) a secreted glycoprotein) signaling pathway [51]. Moreover, a recent study demonstrated that the sensory axons of the spinal cord can regenerate by expressing tenascin-binding α9-integrin together with kindlin-1, the integrin activator [52].
Toll-like receptors (TLRs) are single and noncatalytic receptors with membrane-spanning domains and the TLRs can recognize microbial pathogen and initiate signal transduction pathways of innate immunity [53]. Beyond the role of TLRs in activating innate immunity, TLR seems to play a critical role in neuron survival [54–57]. Patel and Hackam's work suggested that the oxidative stress-activated TLR3 triggers neuron protection rather than pathogenic signaling in the retina tissue of mice [58]. Bsibsi et al.'s work showed that inflammation-induced TLR-3 activation triggers a neuroprotective response rather than a proinflammatory response in human astrocytes [59]. Furthermore, Jeong et al.'s work showed that polyinosinic poly-cytidylic acid- (poly(I:C)-) induced activation of TLR3 might favor the survival of microglia after cerebral ischemia, suggesting the neuroprotective role of TLR3 [60]. Controversially, the activation of TLR might also create a degenerative effect in neuron cells; for example, Chintala et al.'s work showed that poly(I:C)-induced TLR3 activation upregulated the protein level of MAPK JNK3 and promoted the degeneration of RGCs in mouse eye [61]. Similarly, Gao et al.'s work showed that poly(I:C)-induced TLR3 evoked an inflammatory response and cell death both in murine photoreceptor 661W cells and an in vivo model [62]. Therefore, the role of TLR in prosurvival or pro-cell death of neuron cells may depend on the types [55] of stimuli, the level [60] of stimuli, and the downstream of TLR signaling [58].
In the results of microarray analysis combining the IPA bioinformatics approach, the activation level of the TLR pathway is higher in LBE pretreatment than that in LBA pretreatment (Figures 7(b) and 7(c)). Given the pivotal role of the TLR pathway in cell proliferation and cell survival [63], it is worth further investigating the three signaling pathways, especially the TLR pathway in LB extract-mediated cytoprotection in human retinal pigment epithelial cells.
4.1. LBA and LBE Rescue UVB-Induced G2-Arrest after Irradiated ARPE-19
In the study, 50 mJ/cm2 of UVB irradiation significantly caused the accumulation of cell cycle G2/M population in human ARPE-19 cells. Interestingly, Chou et al.'s work showed that 10 to 30 mJ/cm2 UVB caused S-arrest in ARPE-19 cells [64]; we suggest that different doses of UVB irradiation might result in the discrepancy in the arrest of cell cycle S and G2/M phases. Furthermore, the pretreatment of both LBA and LBE rescues UVB irradiation-induced G2/M-arrest in ARPE-19 (Figure S1).
5. Conclusions
We demonstrated that both aqueous and ethanol extracts of L. barbarum exhibit antioxidant activities and prevent UVB irradiation-induced DNA damage and apoptosis of ARPE cells. Interestingly, ethanol extracts of L. barbarum exert stronger antioxidant than aqueous extract, suggesting that polyphenolic components might enhance the antioxidant activities of the ethanol extract of L. barbarum. Based on the results of the present study, we conclude that both aqueous and ethanol extracts of L. barbarum protect ARPE-19 cells from UVB exposure-induced growth and apoptosis through reducing endogenous ROS and DNA damage (Figure 8). This study is the first to depict the cytoprotective activity of L. barbarum extracts on cytoprotection in UVB-induced DNA damage in ARPE-19 retinal cells that might be correlated with three signaling pathways including PPAR, Integrin, and TLR. Further investigation on the ethanol extracts of L. barbarum will benefit the prevention of retinal degenerative-associated diseases such as AMD in the future.
Figure 8.
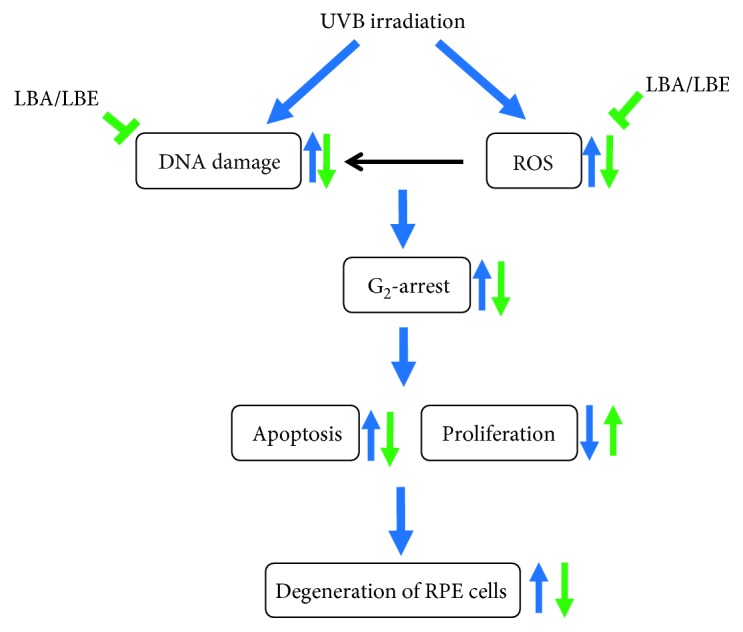
The proposed mechanism of Lycium barbarum extract-mediated protection against UVB-induced damage in human retinal pigment epithelial (RPE) cells. The irradiation of UVB causes the increase of endogenous ROS and the induction of DNA damage, resulting in the damage and degeneration of RPE cells. The extracts of L. barbarum (wolfberry) LBA and LBE exert the potent antioxidant activity and attenuate the DNA damage induced by UVB irradiation in RPE cells, therefore, protecting the RPE cells from UVB-induced cell cycle G2/M-phase arrest, growth inhibition, and apoptosis, further delaying or attenuating the degeneration of retinal pigment epithelial cells. The blue arrows indicate the impacts of UVB exposure. Green arrows indicate the bioactivities of L. barbarum extracts.
Acknowledgments
The study was supported financially by the grants MOST106-2320-B-037-012, MOST107-2320-B-037-023, and MOST107-2311-B-037-001 from the Ministry of Science and Technology, Taiwan; by the grants NSYSU-KMU106-P019 and NSYSU-KMU107-P002 from the National Sun Yat-sen University-KMU Joint Research Project, Taiwan; by the grant from Yuan's General Hospital, Kaohsiung (RG15-002); by the grants KMU-SH000151, KMU-M104008, and KMU-TP105A07 from the Kaohsiung Medical University, Taiwan; and by the grants kmhk-105-018 and kmhk-106-022 from the Kaohsiung Municipal Hsiaokang Hospital, Taiwan. We are also grateful for the Center for Research Resources and Development (Kaohsiung Medical University) for the instrumental support of IPA analysis, flow cytometer, and confocal microscopy.
Contributor Information
Wangta Liu, Email: liuwangta@kmu.edu.tw.
Chien-Chih Chiu, Email: cchiu@kmu.edu.tw.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interests.
Supplementary Materials
Figure S1: the effect of L. barbarum extract pretreatments on UVB-induced G2-arrest in ARPE-19 cells. ARPE-19 cells were pretreated with 50 μg/mL L. barbarum extracts LBA or LBE, respectively, for 2 hr prior to the irradiation of UVB described in the section of Materials and Methods.
References
- 1.Macular Photocoagulation Study Group, Maguire M. G., Fine S. L., et al. Visual outcome after laser photocoagulation for subfoveal choroidal neovascularization secondary to age-related macular degeneration: the influence of initial lesion size and initial visual-acuity. Archives of Ophthalmology. 1994;112(4):480–488. doi: 10.1001/archopht.1994.01090160056023. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson S. E. G., Sundelin S. P., Wihlmark U., Brunk U. T. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Documenta Ophthalmologica. 2003;106(1):13–16. doi: 10.1023/A:1022419606629. [DOI] [PubMed] [Google Scholar]
- 3.Lim T. K. Lycium barbarum. In: Lim T. K., editor. Edible Medicinal and Non-Medicinal Plants: Volume 6, Fruits. Dordrecht: Springer Netherlands; 2013. pp. 240–266. [Google Scholar]
- 4.Capetandes A., Gerritsen M. E. Simplified methods for consistent and selective culture of bovine retinal endothelial cells and pericytes. Investigative Ophthalmology and Visual Science. 1990;31(9):1738–1744. [PubMed] [Google Scholar]
- 5.Yue B., Kawa J., Chang I., Sawaguchi S., Fishman G. Effects of chondroitin sulfate on cultured human retinal pigment epithelial cells. Cell Biology International Reports. 1991;15(5):365–376. doi: 10.1016/0309-1651(91)90125-3. [DOI] [PubMed] [Google Scholar]
- 6.Tang J., Kern T. S. Inflammation in diabetic retinopathy. Progress in Retinal and Eye Research. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivak J. M., Fini M. E. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Progress in Retinal and Eye Research. 2002;21(1):1–14. doi: 10.1016/S1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z., Chen H., Ke G., et al. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009;58(4):954–964. doi: 10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung N., Mitchell P., Wong T. Y. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 10.Dupont C., Armant D., Brenner C. Epigenetics: definition, mechanisms and clinical perspective. Seminars in Reproductive Medicine. 2009;27(5):351–357. doi: 10.1055/s-0029-1237423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller-Kasprzak E., Jagodzinski P. P. 5-Aza-2′-deoxycytidine increases the expression of anti-angiogenic vascular endothelial growth factor 189b variant in human lung microvascular endothelial cells. Biomedicine and Pharmacotherapy. 2008;62(3):158–163. doi: 10.1016/j.biopha.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Chabane N., Zayed N., Afif H., et al. Histone deacetylase inhibitors suppress interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthritis and Cartilage. 2008;16(10):1267–1274. doi: 10.1016/j.joca.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Gao G., Li Y., Zhang D., Gee S., Crosson C., Ma J. X. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Letters. 2001;489(2-3):270–276. doi: 10.1016/S0014-5793(01)02110-X. [DOI] [PubMed] [Google Scholar]
- 14.Pons M., Marin-Castano M. E. Nicotine increases the VEGF/PEDF ratio in retinal pigment epithelium: a possible mechanism for CNV in passive smokers with AMD. Investigative Ophthalmology and Visual Science. 2011;52(6):3842–3853. doi: 10.1167/iovs.10-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao G., Li Y., Fant J., Crosson C. E., Becerra S. P., Ma J. X. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium--derived factor in brown Norway and Sprague Dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002;51(4):1218–1225. doi: 10.2337/diabetes.51.4.1218. [DOI] [PubMed] [Google Scholar]
- 16.Bharadwaj A. S., Appukuttan B., Wilmarth P. A., et al. Role of the retinal vascular endothelial cell in ocular disease. Progress in Retinal and Eye Research. 2013;32:102–180. doi: 10.1016/j.preteyeres.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simo R., Villarroel M., Corraliza L., Hernandez C., Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. Journal of Biomedicine & Biotechnology. 2010;2010:15. doi: 10.1155/2010/190724.190724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elner V. M., Scales W., Elner S. G., Danforth J., Kunkel S. L., Strieter R. M. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Experimental Eye Research. 1992;54(3):361–368. doi: 10.1016/0014-4835(92)90048-W. [DOI] [PubMed] [Google Scholar]
- 19.Broadhead M. L., Becerra S. P., Choong P. F. M., Dass C. R. The applied biochemistry of PEDF and implications for tissue homeostasis. Growth Factors. 2010;28(4):280–285. doi: 10.3109/08977191003604513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X., Chen H. H., Zhang L. W. Potential therapeutic effects of pigment epithelium-derived factor for treatment of diabetic retinopathy. International Journal of Ophthalmology. 2013;6(2):221–227. doi: 10.3980/J.ISSN.2222-3959.2013.02.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui B. K., Liu S., Lin X. J., et al. Effects of Lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules. 2011;16(11):9116–9128. doi: 10.3390/molecules16119116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu C. C., Liu P. L., Huang K. J., et al. Goniothalamin inhibits growth of human lung cancer cells through DNA damage, apoptosis, and reduced migration ability. Journal of Agricultural and Food Chemistry. 2011;59(8):4288–4293. doi: 10.1021/jf200566a. [DOI] [PubMed] [Google Scholar]
- 23.Yen C. Y., Chiu C. C., Haung R. W., et al. Antiproliferative effects of goniothalamin on Ca9-22 oral cancer cells through apoptosis, DNA damage and ROS induction. Mutation Research. 2012;747(2):253–258. doi: 10.1016/j.mrgentox.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Kan J. Y., Yen M. C., Wang J. Y., et al. Nesfatin-1/nucleobindin-2 enhances cell migration, invasion, and epithelial-mesenchymal transition via LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget. 2016;7(21):31336–31349. doi: 10.18632/oncotarget.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Autreaux B., Toledano M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M., Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Letters. 2008;266(1):37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clerkin J. S., Naughton R., Quiney C., Cotter T. G. Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Letters. 2008;266(1):30–36. doi: 10.1016/j.canlet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Geest C. R., Buitenhuis M., Groot Koerkamp M. J. A., Holstege F. C. P., Vellenga E., Coffer P. J. Tight control of MEK-ERK activation is essential in regulating proliferation, survival, and cytokine production of CD34+-derived neutrophil progenitors. Blood. 2009;114(16):3402–3412. doi: 10.1182/blood-2008-08-175141. [DOI] [PubMed] [Google Scholar]
- 29.Wu X., Hua X. Targeting ROS: selective killing of cancer cells by a cruciferous vegetable derived pro-oxidant compound. Cancer Biology & Therapy. 2014;6(5):646–647. doi: 10.4161/cbt.6.5.4092. [DOI] [PubMed] [Google Scholar]
- 30.Li H. H., Su J. H., Chiu C. C., et al. Proteomic investigation of the sinulariolide-treated melanoma cells A375: effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade. Marine Drugs. 2013;11(7):2625–2642. doi: 10.3390/md11072625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valdiglesias V., Giunta S., Fenech M., Neri M., Bonassi S. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutation Research. 2013;753(1):24–40. doi: 10.1016/j.mrrev.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Roth F., Bindewald A., Holz F. G. Keypathophysiologic pathways in age-related macular disease. Graefes Archive for Clinical and Experimental Ophthalmology. 2004;242(8):710–716. doi: 10.1007/s00417-004-0976-x. [DOI] [PubMed] [Google Scholar]
- 33.Nowak J. Z. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacological Reports. 2006;58(3):353–363. [PubMed] [Google Scholar]
- 34.The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 35.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho Y.-S., So K.-F., Chang R. Drug discovery from Chinese medicine against neurodegeneration in Alzheimer’s and vascular dementia. Chinese Medicine. 2011;6(1):p. 15. doi: 10.1186/1749-8546-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan H. C., Chuen-Chung Chang R., Koon-Ching Ip A., et al. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Experimental Neurology. 2007;203(1):269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 38.Chiu K., Chan H. C., Yeung S. C., et al. Modulation of microglia by wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. Journal of Ocular Biology, Diseases, and Informatics. 2009;2(2):47–56. doi: 10.1007/s12177-009-9023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu K., Zhou Y., Yeung S. C., et al. Up-regulation of crystallins is involved in the neuroprotective effect of wolfberry on survival of retinal ganglion cells in rat ocular hypertension model. Journal of Cellular Biochemistry. 2010;110(2):311–320. doi: 10.1002/jcb.22539. [DOI] [PubMed] [Google Scholar]
- 40.Li S. Y., Yang D., Yeung C. M., et al. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS One. 2011;6(1):p. e16380. doi: 10.1371/journal.pone.0016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ming M., Guanhua L., Zhanhai Y., Guang C., Xuan Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chemistry. 2009;113(4):872–877. doi: 10.1016/j.foodchem.2008.03.064. [DOI] [Google Scholar]
- 42.Luo Q., Cai Y., Yan J., Sun M., Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sciences. 2004;76(2):137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 43.Sparrow J. R., Nakanishi K., Parish C. A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investigative Ophthalmology and Visual Science. 2000;41(7):1981–1989. [PubMed] [Google Scholar]
- 44.Tang L., Bao S., du Y., et al. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomedicine and Pharmacotherapy. 2018;103:829–837. doi: 10.1016/j.biopha.2018.04.104. [DOI] [PubMed] [Google Scholar]
- 45.Liu F., Zhang J., Xiang Z., et al. Lycium barbarum polysaccharides protect retina in rd 1 mice during photoreceptor degeneration. Investigative Ophthalmology and Visual Science. 2018;59(1):597–611. doi: 10.1167/iovs.17-22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H., Liang Y., Chiu K., et al. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS One. 2013;8(7):p. e68881. doi: 10.1371/journal.pone.0068881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin N. C., Lin J. C., Chen S. H., Ho C. T., Yeh A. I. Effect of goji (Lycium barbarum) on expression of genes related to cell survival. Journal of Agricultural and Food Chemistry. 2011;59(18):10088–10096. doi: 10.1021/jf2021754. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal S., Yadav A., Chaturvedi R. K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochemical and Biophysical Research Communications. 2017;483(4):1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 49.Lee C. Collaborative power of Nrf 2 and PPARγ activators against metabolic and drug-induced oxidative injury. Oxidative Medicine and Cellular Longevity. 2017;2017:14. doi: 10.1155/2017/1378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau T. L., Kim C., Ginsberg M. H., Ulmer T. S. The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. EMBO Journal. 2009;28(9):1351–1361. doi: 10.1038/emboj.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y., Zou T., Xue L., Yin Z. Q., Huo S., Xu H. TGF-β1 enhances phagocytic removal of neuron debris and neuronal survival by olfactory ensheathing cells via integrin/MFG-E8 signaling pathway. Molecular and Cellular Neuroscience. 2017;85:45–56. doi: 10.1016/j.mcn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Fawcett J. W. An integrin approach to axon regeneration. Eye. 2017;31(2):206–208. doi: 10.1038/eye.2016.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda K., Akira S. Toll-like receptors in innate immunity. International Immunology. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 54.Dvoriantchikova G., Barakat D. J., Hernandez E., Shestopalov V. I., Ivanov D. Toll-like receptor 4 contributes to retinal ischemia/reperfusion injury. Molecular Vision. 2010;16:1907–1912. [PMC free article] [PubMed] [Google Scholar]
- 55.Lehnardt S., Massillon L., Follett P., et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Z., Stratton C., Francis P. J., et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. New England Journal of Medicine. 2008;359(14):1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanisch U. K., Johnson T. V., Kipnis J. Toll-like receptors: roles in neuroprotection? Trends in Neurosciences. 2008;31(4):176–182. doi: 10.1016/j.tins.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Patel A. K., Hackam A. S. A novel protective role for the innate immunity Toll-like receptor 3 (TLR3) in the retina via stat 3. Molecular and Cellular Neuroscience. 2014;63:38–48. doi: 10.1016/j.mcn.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bsibsi M., Persoon-Deen C., Verwer R. W. H., Meeuwsen S., Ravid R., van Noort J. M. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53(7):688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 60.Jeong S. Y., Jeon R., Choi Y. K., et al. Activation of microglial Toll-like receptor 3 promotes neuronal survival against cerebral ischemia. Journal of Neurochemistry. 2016;136(4):851–858. doi: 10.1111/jnc.13441. [DOI] [PubMed] [Google Scholar]
- 61.Chintala S. K., Putris N., Geno M. Activation of TLR3 promotes the degeneration of retinal ganglion cells by upregulating the protein levels of JNK3. Investigative Ophthalmology and Visual Science. 2015;56(1):505–514. doi: 10.1167/iovs.14-15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao M.-L., Wu K.-C., Deng W.-L., et al. Toll-like receptor 3 activation initiates photoreceptor cell death in vivo and in vitro. Investigative Opthalmology & Visual Science. 2017;58(2):p. 801. doi: 10.1167/iovs.16-20692. [DOI] [PubMed] [Google Scholar]
- 63.Li X., Jiang S., Tapping R. I. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;49(1):1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chou W. W., Chen K. C., Wang Y. S., Wang J. Y., Liang C. L., Juo S. H. H. The role of SIRT1/AKT/ERK pathway in ultraviolet B induced damage on human retinal pigment epithelial cells. Toxicology In Vitro. 2013;27(6):1728–1736. doi: 10.1016/j.tiv.2013.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: the effect of L. barbarum extract pretreatments on UVB-induced G2-arrest in ARPE-19 cells. ARPE-19 cells were pretreated with 50 μg/mL L. barbarum extracts LBA or LBE, respectively, for 2 hr prior to the irradiation of UVB described in the section of Materials and Methods.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.