Abstract
Background:
Previously, the authors have developed a model of how reward-seeking and distress- avoiding behaviour is regulated by the human brain. The forebrain’s evolution in vertebrates was taken as a starting point.
Aims:
The authors want to inspire colleagues to study in particular the pharmacological effects on the described ancient forebrain structures in order to modify specific symptoms of mental disorders.
Methods:
Compilation of data and ideas of previous articles, with examples to illustrate.
Results:
A primary (lamprey-like), secondary (frog-like) and tertiary (mammal-like) forebrain can be distinguished, organized according to a Russian doll model. The first constituent is primarily involved in producing the emotional response, while the last is principally concerned with constructing conscious cognitive behaviour (including verbal and written communication). Mental disorders comprise (partly related and partly unrelated) biological and rational phenomena. The secondary system regulates the intensity of reward-seeking and distress-avoiding behaviour. An essential component of the primary forebrain evaluates the results of behavioural actions: the lateral habenula-projecting pallidum. These neurons regulate the activity of ascending dopaminergic pathways. The authors suggest that these habenula-projecting pallidum neurons are targeted by subanaesthetic dosages of ketamine. The medial habenula is enriched with nicotinergic acetylcholine receptors and regulates the activity of ascending adrenergic and serotonergic neurons. This may link varenicline-induced hostility to selective serotonin reuptake inhibitor-induced aggression.
Conclusions:
Studying the effects of new compounds on the primary and secondary brains in lampreys and frogs may yield interesting new treatments of mental disorders.
Keywords: Amygdala, habenula, GPh, ketamine, varenicline
Introduction
Translational neuroscience is voguish; this scientific discipline tries to translate understanding of the basic biological background of central nervous system disorders to theories of how these disorders can be prevented or treated. At the same time, functional neuroimaging techniques have become the most important tools in determining the effects of diseases and their treatment on the brain systems related to mental functions and behaviour. An important limitation of common functional magnetic resonance imaging (fMRI) is its relatively low spatial and temporal resolution which hampers the study of small and complex structures, for example, the habenuloid complex, when these are not specifically addressed (Batalla et al., 2017). Approximately three-quarters of the human brain consists of cerebral neocortex (Voogd et al., 1998), and since the cerebral cortex shows intricate functional connectivity which is easily accessible for fMRI research, huge efforts have been made to establish its role in the pathogenesis and treatment mechanisms of mental disorders (e.g. Rolls, 2016; Rolls et al., 2018). The human cerebral cortex and corresponding parts of the dorsal thalamus appeared very late during vertebrate evolution: in early mammals evolving about 145 million years ago (MYA) (Voogd et al., 1998). The habenuloid complex was already an important component of the brains of ancestors preceding the earliest vertebrates of about 560 MYA, and this structure maintained its output connectivity with the ascending monoaminergic systems of the midbrain during the entirety of evolution from these earliest vertebrates right up to modern humans (see Loonen and Ivanova, 2015, 2016b). These early vertebrates had brains comparable to those of the modern lamprey, and since they must have been capable of obtaining food, offspring and safety, their brains must also have been capable of regulating the corresponding reward-seeking and distress-avoiding behaviours. Dysregulation of these two types of behaviour can be considered to represent the fundamental origin of the majority of mood, anxiety, addictive and psychotic disorders. The lamprey brain can, therefore, be applied to the study of the basic biological processes causing such disorders, and, moreover, those parts of the human brain corresponding to the ancient vertebrate brain may well be considered to be primarily involved in causing these disorders.
Evolution of the forebrain in vertebrates
The lamprey forebrain consists of a two-sided hemisphere with a medial pallium (cortex-like structure) connected to the thalamus (hypothalamus, subthalamus, (dorsal) thalamus and epithalamus), which is in line and continuous with the brainstem and spinal cord (see Loonen and Ivanova, 2015, 2016b). The hemisphere is small, comprising pallium and subpallium; the subpallium contains an extrapyramidal system with similar components and structure to its human equivalent (Grillner and Robertson, 2016; Stephenson-Jones et al., 2012). The pallidal part of this extrapyramidal system contains a glutamatergic nucleus with terminals running to the lateral division of the habenuloid complex: the habenula-projecting globus pallidus (GPh). These GPh neurons are a component of a distinct reward-evaluation circuit which select actions by (dis)inhibiting ascending dopaminergic mesostriatal projections (Stephenson-Jones et al., 2013).
A later evolutionary stage of the human cerebrum is represented by the frog brain. The forebrain of the humans’ amphibian-like ancestor contains an amygdaloid as well as a striatal extrapyramidal system (Moreno and Gonzalez, 2006). The ganglionic amygdaloid complex is principally homologous with the original lamprey striatopallidal complex, consisting of ‘striatal’ central and medial amygdaloid nuclei and a ‘pallidal’ bed nucleus of the stria terminalis (Moreno et al., 2012; Loonen, 2013). The new amphibian striatal complex consists of a large part which corresponds to the human shell part of the accumbens nucleus, and a small part giving rise to its core part and the dorsal striatum (caudate nucleus and putamen). It should be realized, however, that the cortex-like pallium of these amphibian-like ancestors has not yet achieved the input analysing and output generating role of the human cerebral cortex (see Loonen, 2013), but is still part of a more extensive ‘limbic’ behavioural control system including almost all of the pallial and subpallial regions (Laberge and Roth, 2007; Laberge et al., 2008; Roth et al., 2003).
The mammalian extrapyramidal system is, largely, a converging cortico-striato-thalamo-cortical (CSTC) circuit (see Loonen and Ivanova, 2013). The cerebral cortex, including the hippocampus and corticoid amygdala (Heilbronner et al., 2016), is connected with the input ganglia of the extrapyramidal system in a topographically arranged fashion (Alexander et al., 1986; Heimer, 2003; Voogd et al., 1998). This includes the frontal cerebral cortex, which is at the same time target of all the CSTC circuits; this organization results in the existence of several re-entry circuits starting from and ending in the same frontal area (Alexander et al., 1986). This suggests the existence of two re-entry circuits starting from and ending at the anterior cingulate cortex (BA24) and subgenual cingulate cortex (BA25), and including the core respectively shell part of the accumbens nucleus as extrapyramidal entry stations (see Loonen and Ivanova, 2016a). These circuits regulate the motivation to obtain reward or to avoid distress, and being successful results in feelings of pleasure and happiness. The activity of these re-entry circuits is regulated by ascending monoaminergic tracts originating within the midbrain, and which are, in turn, controlled by the habenuloid complex (see Batalla et al., 2017; see Loonen and Ivanova, 2016b). In mammals, the amygdaloid and hippocampal complexes (which are considered to have evolved directly from the forebrain of humans’ earliest vertebrate ancestors) may regulate the habenuloid activity via the hypothalamus, septal area and mammalian homologue of GPh.
The human-like cerebral cortex is of a relatively recent evolutionary date and is therefore unlikely to have been primarily involved in regulating essential behavioural processes. However, due to its size and structure, the cerebral cortex may be much more capable of interpreting input and generating suitable output than the amygdaloid and hippocampal complexes (see Loonen and Ivanova, 2017a). Reciprocal connectivity between primary brain structures (amygdaloid, hippocampal, and habenuloid complex, as well as structures connecting these) and the neuronal networks of the cerebral cortex could enable a far more sophisticated behavioural response to environmental circumstances. This reciprocal connectivity also directly and indirectly modulates the activity of the ventral striatal re-entry circuits and therefore the motivation for reward-seeking and distress-avoiding behaviour.
To conclude, a primary (lamprey-like), secondary (amphibian-like) and tertiary (mammalian-like) forebrain can be distinguished according to a Russian doll model. The amygdaloid, hippocampal and habenuloid complexes (primary forebrain) regulate essential reward-seeking and distress-avoiding behaviour, the ventral striatal extrapyramidal complex (secondary forebrain) regulates the intensity of these behaviours and the cerebral neocortex (tertiary forebrain) enables the sophisticated translation of sensory input to complex behavioural output.
Evolution of human culture and technology from a biological perspective
With an estimated age of about 200,000 years, the modern human is a relatively young animal species (McDougall et al., 2005). Humans have a far larger brain compared with body weight than other apes, but the percentage of the human brain accounted for by the cerebral cortex is about the same as in other apes (Van Dongen, 1998; Voogd et al., 1998). No part of the cerebral cortex, including the prefrontal cortex, has become disproportionally larger in humans. It has been suggested that the larger size of the human brain in comparison with body weight, which enables many more elaborate neuronal networks, is the only basis of higher intelligence in humans compared with other apes (Gabi et al., 2016).
Modern humans may have ‘occurred’ relatively recently; human culture has existed for no more than a fraction of that period. The control of fire is considered to represent one of our most important technological achievements, but the cooking of vegetables was actually displayed by early hominins living about 780,000 years ago (Melamed et al., 2016). Other investigations support the existence of burning sites from about 1.5 MYA onwards, and there is evidence of actual hearths from around 700,000–400,000 years ago (Gowlett, 2016). Such behaviour, therefore, was displayed long before the first modern humans appeared, and certainly cannot be considered a typical modern human accomplishment. Examples of ancient human artistic forms are the hand stencils found in cave sites in the Maros karsts of Sulawesi, Indonesia (Aubert et al., 2014). The earliest dated images found at these sites had a minimum age of 39,900 years (Aubert et al., 2014). The oldest evidence of deep-sea fishing is dated at about 42,000 years ago (O’Connor et al., 2011), while agriculture appears far later in the history of humans (Larson et al., 2014). Plant and animal domestication started with the taming of dogs about 15,000 years ago (MacHugh et al., 2017). Animal domestication resulted in ancient settlements at oases, and some of these, for example Jericho, later developed into cities. Regulating daily living and trading within and between these communities probably necessitated the development of writing, which occurred within the time frame of 10,000–5000 years ago (Gross, 2012; Schmandt-Besserat, 2014). The Mesopotamian cuneiform script, which was invented circa 3200 bc, can be traced without any discontinuity over a period of 10,000 years, from a prehistoric antecedent to the present-day alphabet, and is therefore considered the oldest form of writing (Schmandt-Besserat, 2014). Writing is an absolute necessity for technological evolution because it allows communication between persons without any contact in place and time. Particularly, after the invention of printing and quite recently digitalization, writing can be considered a prerequisite for the founding of our current and technologically advanced society. However, it should be realized that genuine urbanization started somewhere in the 19th century, mass human transportation at the beginning of the 20th century and the internet in the 1970s. In summary, the most primitive human cultural developments started about 40,000 years ago, the art of human philosophy (which depends upon writing) lasts for only up to five millennia, major scientific progress dates from only the last few centuries and the most important technological advancements so far are only a few decades old at the most.
Considering the timeframes of biological and cultural evolutions, it is highly unlikely that the acquisition of our advanced technical and philosophical skills is reflected by important evolutionary neuroanatomical changes. Whenever such changes may have occurred, they are probably restrained to a microscopic and/or biochemical level.
Reason and knowledge
Humans can substitute almost every observation, experience and behavioural reaction by spoken or written language symbols. This offers them the opportunity to create a virtual mental world which is intermingled with the real physical world and results in the set of thoughts and memories designated by the term ‘mind’. Consciousness and communication depend upon a proper functioning of the cerebral cortex, which may indicate that the human mind depends upon the functioning of neuronal networks within or involving this part of the brain. The content of the individual’s thoughts and mental skills are not reflected by the physical construction of these neuronal networks, but their quality in certain respects is, so some people can become delusional due to dysmorphic salience (Van Os, 2009): it is important to note that the content of common delusions in Western society has changed during the last century. Mental disorders always partly consist of distorted mind activity without a strict biological background. This ‘mind’ content is usually not accessible for drug treatment.
Consequences for neuropsychopharmacology
Two interacting regulatory mechanisms of human behaviour can be distinguished: cognitive and emotional (see Loonen and Ivanova, 2016c). The first mechanism is operated by a neuronal complex consisting largely of the neocortex and its extrapyramidal circuits, and which probably also produces consciousness, thoughts and memories. The second is controlled by the amygdaloid and hippocampal complexes and the hypothalamus and upper brainstem, and produces intuitive and automatic behaviour. Human conduct consists of a mixture of both types of behaviours, each with its own rules, training, recollections and also imperfections. Mental disorders are incorporated in both regulatory mechanisms, and treatment options can be found in the cognitive as well as the emotional regulatory system. Although certain symptoms of mental disorders can be affected by a global adaptation of neocortical network activity, drugs are unlikely to be suitable to specifically address certain neocortical networks. Psychotropic drugs affect components of the emotional regulatory system and modulate the activity of the primary and secondary forebrain, as defined in the Introduction. Their impact on the activity of the primary and secondary forebrains and the interaction within and between them should, in our opinion, be the most important subject of study when investigating the psychotropic treatment potential of investigational new drugs both in animals and in humans.
Selective serotonin agonists and antagonists
At least 17 molecules (tryptophan hydroxylase, monoamine oxidase A, serotonin transporter and 14 serotonin receptors) regulate the serotonergic synapse activity (Barnes and Sharp, 1999). Serotonin plays a role in almost every integrative function of the central nervous system, including mood, anxiety, stress, aggression, feeding, cognition and sexual behaviour (Olivier, 2015). Non-selective stimulation or inhibition of serotonergic neurotransmission, therefore, has multiple, and partly opposite, effects; this is even more true for the acute and chronic effects of drugs that increase serotonin levels since this may also result in adapted serotonin (5-HT) receptor sensitivity. 5-HT1a and 5-HT2 receptors in particular play an important role in mediating, for example, part of the effects of antidepressant and antipsychotic drugs (see Loonen and Ivanova, 2016c, 2016d). Within the cortical parts of the amygdaloid complex, excitatory 5-HT2A receptors are present on stimulatory glutamatergic pyramidal neurons and on inhibitory GABAergic interneurons and GABAergic projection neurons, which results in mixed behavioural effects. The 5-HT2C receptor probably exclusively activates the amygdaloid complex (by activating cortical pyramidal cells and ganglionic GABAergic projection neurons) (Bombardi, 2014). Moreover, 5-HT2C receptors and, to a lesser extent, 5-HT2A receptors, have constitutive activity, which enables clozapine’s considerable inverse 5-HT2C agonistic activity (which is consequently inhibitory) (Aloyo et al., 2009). From neuroanatomical studies, it can be concluded that serotonin inhibits the release of dopamine within the dorsal striatum by affecting 5-HT2C receptors and also within the ventral striatum and prefrontal cortex by affecting 5-HT2A receptors (see Loonen and Ivanova, 2016d). The inverse agonistic effect within the dorsal striatum may explain clozapine’s therapeutic effects on cognitive functioning (Meltzer and McGurk, 1999; Woodward et al., 2005) by actively increasing activity within the dorsal extrapyramidal circuitry. Other atypical antipsychotics are more selectively binding 5-HT2A receptors and in combination with their relatively potent dopamine antagonistic activity this would result in less extrapyramidal stimulation of the prefrontal cortex (leaving only direct effects within it).
The forebrain of the lamprey might offer a suitable model for investigating the effects of drugs affecting 5-HT2 receptors within the amygdaloid complex in the absence of functional ventral and dorsal extrapyramidal circuitry. The lamprey’s lateral and medial pallium probably correspond largely to (respectively) the human corticoid amygdala and hippocampal complex, and their subpallium to the human extended amygdala. Ocaña et al. (2015) present an example of how ‘behavioural’ consequences of neuronal stimulation can be studied in these animals: they looked at the effects of electrical microstimulation of the lateral pallium after surgical exposure of the dorsal surface of the brain whilst the trunk and tail were kept intact and allowed to move freely. When the animals had fully recovered from the effects of anaesthesia, the researchers measured how stimulation of different areas could elicit eye, orienting, swimming and mouth movements. In lampreys, the GPh also receives direct input from the lateral pallium and has an important role in evaluating motor programmes (Stephenson-Jones et al., 2013). The study of the influence of modulation of the activity of GPh neurons on movement related to reward seeking with common and novel neuropharmacological techniques (Nair et al., 2013) could possibly be translated into new treatments for certain stress-induced or habit-related disorders.
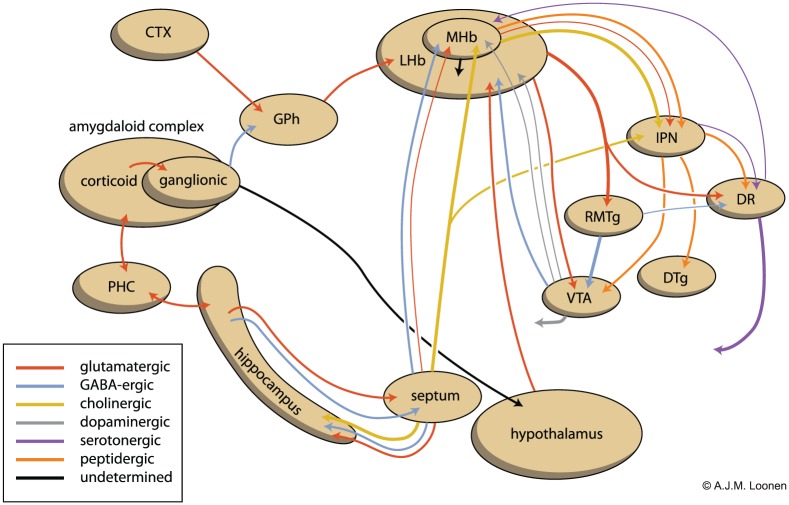
A comparison with the behavioural effects in amphibians could conceivably offer possibilities to study the influence on the ventral extrapyramidal system in the lack of the dorsal component. The activity of monoaminergic midbrain-to-forebrain projections is regulated by the habenuloid complex (Figure 1): this pathway was very well conserved during the evolution of our vertebrate ancestors, which therefore offers the possibility of study in lampreys and amphibians of how serotonergic input to the primary and secondary forebrain is originally regulated.
Figure 1.
Scheme showing the connectivity of the amygdalo-hippocampal system to the midbrain through the habenular complex (adapted from Loonen and Ivanova, 2016b).
CTX: cerebral cortex; DR: dorsal raphe nucleus; DTg: dorsal tegmental nucleus; GPh: habenula-projecting part of the globus pallidus; IPN: interpeduncular nucleus; LHb: lateral habenula; MHb: medial habenula; PHC: parahippocampal cortex; RMTg: rostromedial tegmental nucleus; VTA: ventral tegmental area.
Selective pharmacological modulation of the complex emotional response
In lampreys, motor behaviour is primarily controlled by a striatopallidal system (within the subpallium of the forebrain) which regulates the activity of specific motor centres within the hypothalamus and upper brainstem (Grillner et al., 2008; Robertson et al., 2014). Because in humans the majority of the lamprey striatopallidal system was incorporated within the extended amygdala, this control is likely to be reflected by input from the extended amygdala to hypothalamus and brainstem (Figure 2). The amygdala receives massive sensory input from the tertiary forebrain (Freese and Amaral, 2009; Pitkänen, 2000) and plays an essential role in selecting the information which is most relevant for current wellbeing: this selection process is termed ‘salience’ (Aleman and Kahn, 2005; Berridge et al., 2009; Pryce, 2018). In interaction with contextual (memorized) details supplied by the hippocampus (Chun, 2000; Lee and Lee, 2013; Kitamura, 2017), it activates hypothalamic and brainstem centres to produce a relevant emotional response (such as fear, anger, love, appetite, sexual desire or power dominance) (Sewards and Sewards, 2003). We have hypothesized that the intensity of these emotional behaviours (motivation) is regulated by two re-entry circuits starting and ending at the anterior cingulate cortex (BA24) and subgenual cingulate cortex (BA25), and including the core and shell part of the accumbens nucleus as first relay stations. In turn, these re-entry circuits are controlled by the habenuloid complex affecting the activity of monoaminergic ascending pathways from the midbrain (see Loonen and Ivanova, 2016a, 2016b, 2017b, 2018). The habenula is a complex of at least 15 nuclei in the epithalamus which can be divided into medial and lateral divisions (Aizawa et al., 2012; Andres et al., 1999; Wagner et al., 2014). Major input into the medial division comes from two medial septal nuclei and the diagonal band of Broca via the stria medullaris (Klemm, 2004). The lateral division receives first order inputs from the lateral preoptic area, the lateral hypothalamus, bed nucleus of the stria terminalis and (other) basal ganglia (Klemm, 2004; Matthews Felton et al., 1999). In addition, limbic neocortical areas – particularly anterior insular, anterior cingulate, prelimbic and infralimbic cortices – project to the lateral division (Kim and Lee, 2012), as the hippocampus does (Matthews Felton et al., 1999). These neocortical areas could modulate the input from the amygdaloid complex to the habenula, which we consider primary. The neurochemistry of this putatively primary connectivity is shown in Figure 1. The described corticohabenular pathway can be considered to represent the mechanism along which neocortical networks (cognition) influence the intensity of reward-seeking behaviour (emotional drive).
Figure 2.
Scheme showing the initiation and execution of the emotional response.
BST: bed nucleus of the stria terminalis; CA: corticoid amygdala; CM: centromedial amygdala; dPFC: dorsolateral prefrontal cortex; MC: motor cortex; mPFC: medial prefrontal cortex; PAG: periaqueductal grey; PMC: premotor cortex; SMC: supplementary motor cortex.
In order to achieve an objective, animals need to select one element of the available behavioural repertoire and evaluate its outcome in order to determine whether the goal was achieved (Stephenson-Jones et al., 2013). The basal ganglia play a key role in both action selection and action evaluation. In lampreys, the circuitry which selects and evaluates behaviour is located in separate, non-overlapping parts of the pallidum. In these ancient animals, the lateral GPh receives direct excitatory projections from the animal’s pallium, inhibitory input exclusively from striosomal striatal neurons and inhibitory dopaminergic feedback (Stephenson-Jones et al., 2013). Glutamatergic GPh neurons with a similar function have been identified in mice and non-human primates (rhesus monkeys) (Hong and Hikosaka, 2013; Stephenson-Jones et al., 2016). In humans, these neurons of the border region of the globus pallidus could be involved in terminating specific actions or thoughts, so their failure could play an important role in causing compulsions or obsessions. We have hypothesized that also the secondary and primary forebrain contains GPh neurons, which could then be localized within the ventral pallidum and bed nucleus of the stria terminalis. These would be suitable candidates, thus putative pharmacological targets, to be implicated in causing apathy and impulsivity, craving and anhedonia, as well as anxiety and panic.
Ketamine’s and varenicline’s mechanism of action
Since the randomized, double-blind, placebo-controlled trial of intravenous treatment with a subanaesthetic dose of ketamine hydrochloride in seven depressed patients by Berman and colleagues (2000) the therapeutic effects of this N-methyl-D-aspartate receptor antagonist in patients with depression has been extensively studied (Andrade, 2017a, 2017b, 2017c, 2017d, 2017e). Ketamine was also discovered to have obsession-alleviating effects in patients with obsessive–compulsive disorder (Rodriguez et al., 2013). However, although Ketamine is well known to affect the activity of the lateral habenula (Yang et al., 2018; Zanos and Gould, 2018) and the preliminary positron emission tomography findings of Carlson and co-workers also indicated that ketamine decreased the activity of the right habenula in depressed patients (Carlson et al., 2013), not a single neuroimaging study describes ketamine’s possible influence on GPh neurons (Ionescu et al., 2018). This is probably due to the habenula’s relatively small size in humans, which complicates the imaging of this pallido-habenular connectivity when this issue is not specifically addressed (Batalla et al., 2017). Nevertheless, blocking the activity of glutamatergic GPh neurons would disinhibit ascending dopaminergic neurons and increase the activity of the reward-seeking circuitry, resulting in an improvement of depressive symptoms. This would make this mechanism a very good candidate to explain ketamine’s acute antidepressant effects. Moreover, affecting the activity of GPh neurons could link ketamine’s activity to brain-derived neurotrophic factor (BDNF) (Loonen and Ivanova, 2016c; Loonen et al., 2017; Zanos and Gould, 2018). BDNF expression plays a role in, and BDNF is a biomarker in, both unipolar and bipolar depression (Fernandes et al., 2015; Jentsch et al., 2015, Munkholm et al., 2016; Wu et al., 2014). This antidepressant mechanism deserves further study, and, in our opinion, could be a suitable starting point for the development of new antidepressant treatments. This would certainly be a more selective mechanism, and preferable to affecting cortico-habenular connectivity.
Varenicline is a partial α4-β2 nicotinic acetylcholine receptor agonist which is prescribed as an aid to smoking cessation (Jiménez-Ruiz et al., 2009). Numerous reports illustrate varenicline’s potential to induce neuropsychiatric side effects (including depression) (Ahmed et al., 2013; Moore et al., 2011) and aggression towards others (Moore et al., 2010a, 2010b). The medial habenula is widely known for its abundance of nicotinic acetylcholine receptors, including α3, α4, α5, β2 and/or β4 containing receptor subtypes (Antolin-Fontes et al., 2015; Leslie et al., 2013; Viswanath et al., 2014). The medial habenula is connected via the interpeduncular nucleus to the upper raphe nuclei (Figure 3) (Antolin-Fontes et al., 2015) and affecting these nicotinic receptors may result in activation of 5-HT pathways to the amygdaloid and hippocampal complexes. This would link varenicline-induced hostility to aggression induced by selective serotonin reuptake inhibitors (Moore et al., 2010b).
Figure 3.
Simplified representation of the connectivity through the epithalamus.
GPh: habenula-projecting globus pallidus; IPN: interpeduncular nucleus; LHb: lateral habenula; MHb: medial habenula; RMTg: rostromedial tegmental nucleus; SNc: substantia nigra, pars compacta; VTA: ventral tegmental nucleus.
Conclusions
The present paper describes two interacting brain mechanisms independently regulating rational and emotional behaviours. Motivation to exhibit these behaviours depends upon the activity of two ventral extrapyramidal re-entry circuits, including the core and shell part of the nucleus accumbens, respectively. These are hypothesized to belong to the secondary forebrain. According to this model, the primary forebrain is essentially similar to the brain of the earliest vertebrate ancestors of humans, which, in turn, is similar to the forebrain of the lamprey. The cerebral neocortex evolved relatively late, and is the essential part of the tertiary brain producing thoughts and language. As the neuronal networks interact with the primary and secondary forebrain, they can modulate the emotional response which is generated by the primary brain. An essential component of the primary brain is the habenuloid complex, which regulates the activity of ascending dopaminergic, adrenergic and serotonergic pathways, hence the activity of the three components of the forebrain. Whether the goal of the behavioural response is reached is evaluated by a system including glutamatergic GPh neurons which regulates the activity of ascending dopaminergic pathways. It is postulated that these GPh neurons are targeted by a subanaesthetic dosage of ketamine. The medial habenular division is enriched with nicotinergic receptors and primarily regulates the activity of ascending adrenergic and serotonergic pathways. It is suggested that new selective treatment strategies can be developed by studying the modulatory mechanism in relatively ancient non-mammalian animal species with specifically addressing mechanisms to affect the functioning of GPh neurons.
Acknowledgments
The authors greatly appreciate the help of Mrs Kate Barker (BA, PGCE) who has proofread the manuscript. This work resulted from a collaboration between the Mental Health Research Institute in Tomsk and the Groningen Research Institute of Pharmacy (GRIP) of the University of Groningen. The Russian part is carried out within the framework of Tomsk Polytechnic University Competitiveness Enhancement Programme.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anton JM Loonen  https://orcid.org/0000-0003-4942-6195
https://orcid.org/0000-0003-4942-6195
References
- Ahmed AI, Ali AN, Kramers C, et al. (2013) Neuropsychiatric adverse events of varenicline: A systematic review of published reports. J Clin Psychopharmacol 33: 55–62. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Kobayashi M, Tanaka S, et al. (2012) Molecular characterization of the subnuclei in rat habenula. J Comp Neurol 520: 4051–4066. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS. (2005) Strange feelings: Do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol 77: 283–298. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Aloyo VJ, Berg KA, Spampinato U, et al. (2009) Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther 121: 160–173. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2017. a) Ketamine for depression, 1: Clinical summary of issues related to efficacy, adverse effects, and mechanism of action. J Clin Psychiatry 78: e415–e419. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2017. b) Ketamine for depression, 2: Diagnostic and contextual indications. J Clin Psychiatry 78: e555–e558. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2017. c) Ketamine for depression, 3: Does chirality matter? J Clin Psychiatry 78: e674–e677. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2017. d) Ketamine for depression, 4: In what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry 78: e852–e857. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2017. e) Ketamine for depression, 5: Potential pharmacokinetic and pharmacodynamic drug interactions. J Clin Psychiatry 78: e858–e861. [DOI] [PubMed] [Google Scholar]
- Andres KH, von Düring M, Veh RW. (1999) Subnuclear organization of the rat habenular complexes. J Comp Neurol 407: 130–150. [DOI] [PubMed] [Google Scholar]
- Antolin-Fontes B, Ables JL, Görlich A, et al. (2015) The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology 96(Pt B): 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert M, Brumm A, Ramli M, et al. (2014) Pleistocene cave art from Sulawesi, Indonesia. Nature 514: 223–227. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152. [DOI] [PubMed] [Google Scholar]
- Batalla A, Homberg JR, Lipina TV, et al. (2017) The role of the habenula in the transition from reward to misery in substance use and mood disorders. Neurosci Biobehav Rev 80: 276–285. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. (2009) Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardi C. (2014) Neuronal localization of the 5-HT2 receptor family in the amygdaloid complex. Front Pharmacol 5: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson PJ, Diazgranados N, Nugent AC, et al. (2013) Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: A preliminary positron emission tomography study. Biol Psychiatry 73: 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM. (2000) Contextual cueing of visual attention. Trends Cogn Sci 4: 170–178. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Molendijk ML, Köhler CA, et al. (2015) Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: A meta-analysis of 52 studies. BMC Med 13: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. (2009) Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA. (eds) The Human Amygdala. New York: The Guildford Press, pp.3–42. [Google Scholar]
- Gabi M, Neves K, Masseron C, et al. (2016) No relative expansion of the number of prefrontal neurons in primate and human evolution. Proc Natl Acad Sci USA 113: 9617–9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowlett JA. (2016) The discovery of fire by humans: A long and convoluted process. Philos Trans R Soc London B Biol Sci 371: 10.1098/rstb.2015.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Robertson B. (2016) The basal ganglia over 500 million years. Curr Biol 26: R1088–R1100. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P, Saitoh K, et al. (2008) Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res Rev 57: 2–12. [DOI] [PubMed] [Google Scholar]
- Gross M. (2012) The evolution of writing. Curr Biol 22: R981–R984. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, et al. (2016) Circuit-based corticostriatal homologies between rat and primate. Biol Psychiatry 80: 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L. (2003) A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry 160: 1726–1739. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. (2013) Diverse sources of reward value signals in the basal ganglia nuclei transmitted to the lateral habenula in the monkey. Front Hum Neurosci 7: 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Felicione JM, Gosai A, et al. (2018) Ketamine-associated brain changes: A review of the neuroimaging literature. Harv Rev Psychiatry 26: 320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch MC, Van Buel EM, Bosker FJ, et al. (2015) Biomarker approaches in major depressive disorder evaluated in the context of current hypotheses. Biomark Med 9: 277–297. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ruiz C, Berlin I, Hering T. (2009) Varenicline: A novel pharmacotherapy for smoking cessation. Drugs 69: 1319–1338. [DOI] [PubMed] [Google Scholar]
- Kim U, Lee T. (2012) Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur J Neurosci 35: 1253–1269. [DOI] [PubMed] [Google Scholar]
- Kitamura T. (2017) Driving and regulating temporal association learning coordinated by entorhinal-hippocampal network. Neurosci Res 121: 1–6. [DOI] [PubMed] [Google Scholar]
- Klemm WR. (2004) Habenular and interpeduncularis nuclei: Shared components in multiple-function networks. Med Sci Monit 10: RA261–RA273. [PubMed] [Google Scholar]
- Laberge F, Roth G. (2007) Organization of the sensory input to the telencephalon in the fire-bellied toad, Bombina orientalis. J Comp Neurol 502: 55–74. [DOI] [PubMed] [Google Scholar]
- Laberge F, Mühlenbrock-Lenter S, Dicke U, et al. (2008) Thalamo-telencephalic pathways in the fire-bellied toad Bombina orientalis. J Comp Neurol 508: 806–823. [DOI] [PubMed] [Google Scholar]
- Larson G, Piperno DR, Allaby RG, et al. (2014) Current perspectives and the future of domestication studies. Proc Natl Acad Sci USA 111: 6139–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Lee CH. (2013) Contextual behavior and neural circuits. Front Neural Circuits 7: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. (2013) Nicotinic receptors in addiction pathways. Mol Pharmacol 83: 753–758. [DOI] [PubMed] [Google Scholar]
- Loonen AJM. (2013) Hoofdstuk 11. Herkennen, kennen, weten en geheugen. In: Het Beweeglijke Brein. De Neurowetenschappelijke Achtergronden van de Psychische Functies (2nd Edition). Haarlem, NL: Uitgeverij Mension, pp. 279–319. [Google Scholar]
- Loonen AJM, Ivanova SA. (2013) New insights into the mechanism of drug-induced dyskinesia. CNS Spectr 18: 15–20. [DOI] [PubMed] [Google Scholar]
- Loonen AJM, Ivanova SA. (2015) Circuits regulating pleasure and happiness: The evolution of reward-seeking and misery-fleeing behavioral mechanisms in vertebrates. Front Neurosci 9: 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonen AJM, Ivanova SA. (2016. a) Circuits regulating pleasure and happiness in major depression. Med Hypotheses 87: 14–21. [DOI] [PubMed] [Google Scholar]
- Loonen AJM, Ivanova SA. (2016. b) Circuits regulating pleasure and happiness: The evolution of the amygdalar-hippocampal-habenular connectivity in vertebrates. Front Neurosci 10: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonen AJM, Ivanova SA. (2016. c) Circuits regulating pleasure and happiness-mechanisms of depression. Front Hum Neurosci 10: 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonen AJM, Ivanova SA. (2016. d) Role of 5-HT2C receptors in dyskinesia. Int J Pharm Pharm Sci 8: 5–10. [Google Scholar]
- Loonen AJM, Ivanova SA. (2017. a) Commentary on “A non-reward attractor theory of depression”: A proposal to include the habenula connection. Neurosci Biobehav Rev 83: 736–741. [DOI] [PubMed] [Google Scholar]
- Loonen AJM, Ivanova SA. (2017. b) Evolution of circuits regulating pleasure and happiness with the habenula in control. CNS Spectr. Epub ahead of print 1 November 2017. DOI: 10.1017/S1092852917000748. [DOI] [PubMed] [Google Scholar]
- Loonen AJM, Ivanova SA. (2018) Circuits regulating pleasure and happiness: Evolution and role in mental disorders. Acta Neuropsychiatr 30: 29–42. [DOI] [PubMed] [Google Scholar]
- Loonen AJM, Kupka RW, Ivanova SA. (2017) Circuits regulating pleasure and happiness in bipolar disorder. Front Neural Circuits 11: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall I, Brown FH, Fleagle JG. (2005) Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature 433: 733–736. [DOI] [PubMed] [Google Scholar]
- MacHugh DE, Larson G, Orlando L. (2017) Taming the past: Ancient DNA and the study of animal domestication. Ann Rev Anim Biosci 5: 329–351. [DOI] [PubMed] [Google Scholar]
- Matthews Felton T, Linton L, Rosenblatt JS, et al. (1999) First and second order maternal behavior related afferents of the lateral habenula. NeuroReport 10: 883–887. [DOI] [PubMed] [Google Scholar]
- Melamed Y, Kislev ME, Geffen E, et al. (2016) The plant component of an Acheulian diet at Gesher Benot Ya’aqov, Israel. Proc Natl Acad Sci USA 113: 14674–14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25: 233–255. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Furberg CD, Glenmullen J, et al. (2011) Suicidal behavior and depression in smoking cessation treatments. PLoS One 6: e27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TJ, Glenmullen J, Furberg CD. (2010. a) Prescription drugs associated with reports of violence towards others. PLoS One 5: e15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TJ, Glenmullen J, Furberg CD. (2010. b) Thoughts and acts of aggression/violence toward others reported in association with varenicline. Ann Pharmacother 44: 1389–1394. [DOI] [PubMed] [Google Scholar]
- Moreno N, Gonzalez A. (2006) The common organization of the amygdaloid complex in tetrapods: New concepts based on developmental, hodological and neurochemical data in anuran amphibians. Progr Neurobiol 78: 61–90. [DOI] [PubMed] [Google Scholar]
- Moreno N, Morona R, López JM, et al. (2012) Characterization of the bed nucleus of the stria terminalis in the forebrain of anuran amphibians. J Comp Neurol 520: 330–363. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Vinberg M, Kessing LV. (2016) Peripheral blood brain-derived neurotrophic factor in bipolar disorder: A comprehensive systematic review and meta-analysis. Mol Psychiatry 21: 216–228. [DOI] [PubMed] [Google Scholar]
- Nair SG, Strand NS, Neumaier JF. (2013) DREADDing the lateral habenula: A review of methodological approaches for studying lateral habenula function. Brain Res 1511: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaña FM, Suryanarayana SM, Saitoh K, et al. (2015) The lamprey pallium provides a blueprint of the mammalian motor projections from cortex. Curr Biol 25: 413–423. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Ono R, Clarkson C. (2011) Pelagic fishing at 42,000 years before the present and the maritime skills of modern humans. Science 334: 1117–1121. [DOI] [PubMed] [Google Scholar]
- Olivier B. (2015) Serotonin: A never-ending story. Eur J Pharmacol 753: 2–18. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. (2000) Connectivity of the rat amygdaloid complex. In: Aggleton JP. (ed.) The Amygdala. A Functional Analysis. Oxford, UK: Oxford University Press, pp.31–115. [Google Scholar]
- Pryce CR. (2018) Comparative evidence for the importance of the amygdala in regulating reward salience. Curr Opin Behav Sci 22: 76–81. [Google Scholar]
- Robertson B, Kardamakis A, Capantini L, et al. (2014) The lamprey blueprint of the mammalian nervous system. Progr Brain Res 212: 337–349. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Kegeles LS, Levinson A, et al. (2013) Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: Proof-of-concept. Neuropsychopharmacology 38: 2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. (2016) A non-reward attractor theory of depression. Neurosci Biobehav Rev 68: 47–58. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Cheng W, Gilson M, et al. (2018) Effective connectivity in depression. Biol Psychiatry Cogn Neurosci Neuroimaging 3: 187–197. [DOI] [PubMed] [Google Scholar]
- Roth G, Grunwald W, Dicke U. (2003) Morphology, axonal projection pattern, and responses to optic nerve stimulation of thalamic neurons in the fire-bellied toad Bombina orientalis. J Comp Neurol 461: 91–110. [DOI] [PubMed] [Google Scholar]
- Schmandt-Besserat D. (2014) The evolution of writing. Available at: https://sites.utexas.edu/dsb/tokens/the-evolution-of-writing/ (2014, accessed 20 April 2018).
- Sewards TV, Sewards MA. (2003) Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull 61: 25–49. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Ericsson J, Robertson B, et al. (2012) Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. J Comp Neurol 520: 2957–2973. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Kardamakis AA, Robertson B, et al. (2013) Independent circuits in the basal ganglia for the evaluation and selection of actions. Proc Natl Acad Sci USA 110: E3670–E3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson-Jones M, Yu K, Ahrens S, et al. (2016) A basal ganglia circuit for evaluating action outcomes. Nature 539: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen PAM. (1998) Chapter 23. Brain size in vertebrates. In: Nieuwenhuys R, ten Donkelaar HJ, Nicholson C. (eds) The Central Nervous System of Vertebrates. Berlin; Heidelberg: Springer-Verlag, pp.2099–2134. [Google Scholar]
- Van Os J. (2009) A salience dysregulation syndrome. Br J Psychiatry 194: 101–103. [DOI] [PubMed] [Google Scholar]
- Viswanath H, Carter AQ, Baldwin PR, et al. (2014) The medial habenula: Still neglected. Front Hum Neurosci 7: 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Nieuwenhuys R, van Dongen PAM, et al. (1998) Mammals. In: Nieuwenhuys R, ten Donkelaar HJ, Nicholson C. (eds) The Central Nervous System of Vertebrates. Berlin; Heidelberg: Springer-Verlag, pp.1637–2097. [Google Scholar]
- Wagner F, Stroh T, Veh RW. (2014) Correlating habenular subnuclei in rat and mouse by using topographic, morphological, and cytochemical criteria. J Comp Neurol 522: 2650–2662. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, et al. (2005) A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 8: 457–472. [DOI] [PubMed] [Google Scholar]
- Wu R, Fan J, Zhao J, et al. (2014) The relationship between neurotrophins and bipolar disorder. Exp Review Neurother 14: 51–65. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, et al. (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554: 317–322. [DOI] [PubMed] [Google Scholar]
- Zanos P, Gould TD. (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]