Abstract
Background:
Posttraumatic stress disorder often does not resolve after conventional psychotherapies or pharmacotherapies. Pilot studies have reported that 3,4-methylenedioxymethamphetamine (MDMA) combined with psychotherapy reduces posttraumatic stress disorder symptoms.
Aims:
This pilot dose response trial assessed efficacy and safety of MDMA-assisted psychotherapy across multiple therapy teams.
Methods:
Twenty-eight people with chronic posttraumatic stress disorder were randomized in a double-blind dose response comparison of two active doses (100 and 125 mg) with a low dose (40 mg) of MDMA administered during eight-hour psychotherapy sessions. Change in the Clinician-Administered PTSD Scale total scores one month after two sessions of MDMA served as the primary outcome. Active dose groups had one additional open-label session; the low dose group crossed over for three open-label active dose sessions. A 12-month follow-up assessment occurred after the final MDMA session.
Results:
In the intent-to-treat set, the active groups had the largest reduction in Clinician-Administered PTSD Scale total scores at the primary endpoint, with mean (standard deviation) changes of −26.3 (29.5) for 125 mg, −24.4 (24.2) for 100 mg, and −11.5 (21.2) for 40 mg, though statistical significance was reached only in the per protocol set (p=0.03). Posttraumatic stress disorder symptoms remained lower than baseline at 12-month follow-up (p<0.001) with 76% (n=25) not meeting posttraumatic stress disorder criteria. There were no drug-related serious adverse events, and the treatment was well-tolerated.
Conclusions:
Our findings support previous investigations of MDMA-assisted psychotherapy as an innovative, efficacious treatment for posttraumatic stress disorder.
Keywords: 3,4-Methylenedioxymethamphetamine (MDMA); posttraumatic stress disorder; depression; sleep disturbance; oxytocin; serotonin
Introduction
Posttraumatic stress disorder (PTSD) is a disabling condition characterized by overwhelming negative emotions, panic, anxiety, intrusive re-experiencing of traumatic events, avoidance, negative alterations in cognition, and hyperarousal symptoms. Approximately 8% of the US population (24 m individuals) will suffer from PTSD in their lifetime (Kessler et al., 1995), and existing treatment approaches have limited effectiveness. Nonresponse and treatment dropout rates are high (Eftekhari et al., 2013; Goetter et al., 2015; Sareen et al., 2007), and at least 35% are left with debilitating symptoms that significantly impact quality of life. Individuals often feel fragmented and disconnected from self and others (Sareen et al., 2006). They seem to be continuously living a story that is in the distant past, and consequentially are not fully living in present time. The social impact includes alienation, loneliness, and functional impairment (Sareen et al., 2007). There is an immense need for innovative treatment options that improve outcomes, especially for PTSD refractory to psychotherapy and/or pharmacotherapies (Krystal et al., 2017).
3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy is a novel approach that combines psychotherapy with limited administration of MDMA in a controlled setting to enable people suffering from PTSD to process trauma more effectively. Early reports of use in psychotherapy have suggested the potential utility of MDMA in psychotherapy (Bouso et al., 2008; Greer and Tolbert, 1986). Two previous randomized controlled trials showed promising safety and efficacy results for treatment of PTSD (Mithoefer et al., 2011, 2013, 2018; Oehen et al., 2013). MDMA’s unique subjective and therapeutic effects induce an optimal state that complements the process of working through traumatic memories while reducing the fear response (Mithoefer et al., 2016; Yazar-Klosinski and Mithoefer, 2017). Trauma theorists have asserted that emotional engagement is necessary for processing traumatic experiences (Foa, 2007; Jaycox et al., 1998) and, under the influence of MDMA, people are able to remain emotionally connected while working with difficult traumatic material.
This article presents results from a blinded, phase 2 dose response trial designed to evaluate the safety and efficacy of MDMA (40, 100, or 125 mg) as an adjunct to psychotherapy in 28 participants with chronic PTSD. PTSD symptom severity, dissociation, depression symptoms, and sleep quality were assessed at baseline, one-month after the second blinded session, after open-label sessions, and at 12-month follow-up. Safety outcomes were collected throughout the treatment period.
Methods
Participants and study overview
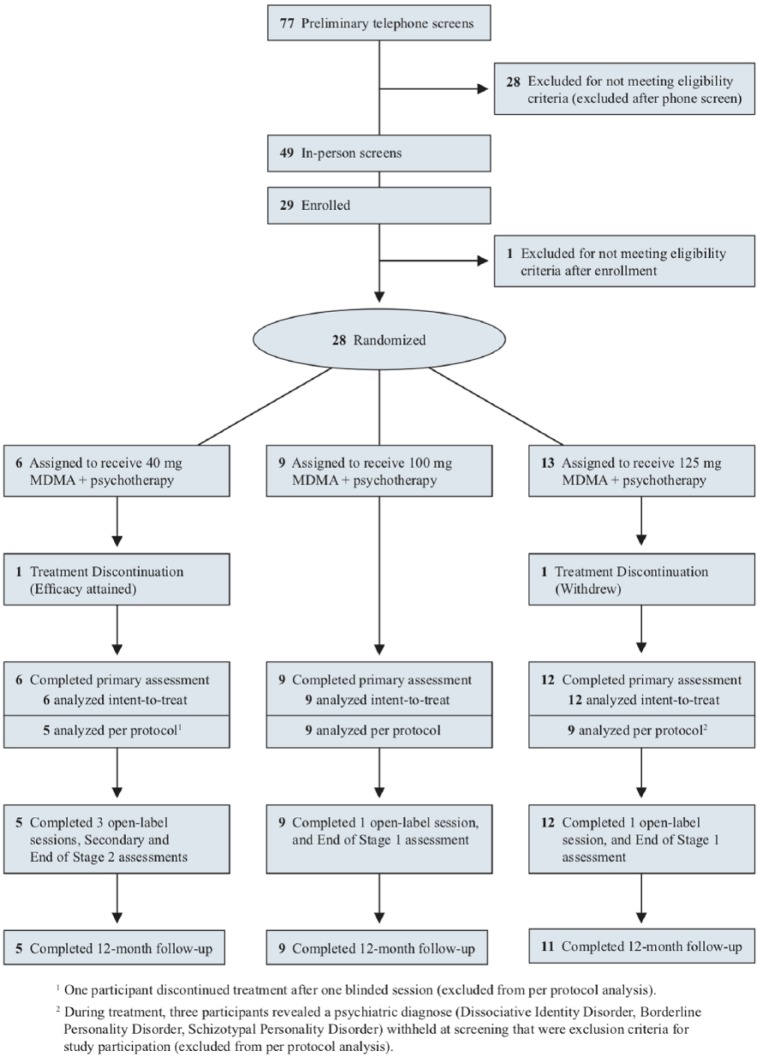
Candidates were recruited via Internet advertisements and referrals from mental health professionals and were screened using a scripted telephone interview (see Figure 1). Potential participants, men and women 18 years or older, underwent in-person psychological assessment, electrocardiogram, and physical examinations. Inclusion criteria required PTSD for at least six months, and a score of ⩾50 on the Clinician Administered PTSD Scale (CAPS-IV). Candidates had failed to respond to at least one course of pharmacotherapy and/or psychotherapy. Participants were otherwise physically healthy and free of psychiatric or medical contraindications for receiving MDMA. Women could not be pregnant or lactating. The study was conducted at an outpatient clinic in Boulder, Colorado between October 2012–February 2017. This trial was reviewed by the Copernicus Group Independent Review Board (Research Triangle Park, North Carolina, USA), and was designed and conducted in accordance with good clinical practices and Consolidated Standards of Reporting Trials (CONSORT) guidelines (Moher et al., 2010). The trial was registered at clinicaltrials.gov (NCT #NCT01793610).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Procedures
After written informed consent and enrollment, participants completed outcome measures and were interviewed by an independent rater to assess baseline PTSD severity, functioning, and psychological history (see Figure 2). To establish a safe setting and therapeutic alliance before MDMA sessions, participants underwent three 90-minute preparatory sessions with a male/female therapy team. Psychiatric medications were tapered by the study physician and discontinued at least five half-lives before MDMA administration. Each participant was assigned to one of nine therapy teams (eight therapists in total).
Figure 2.
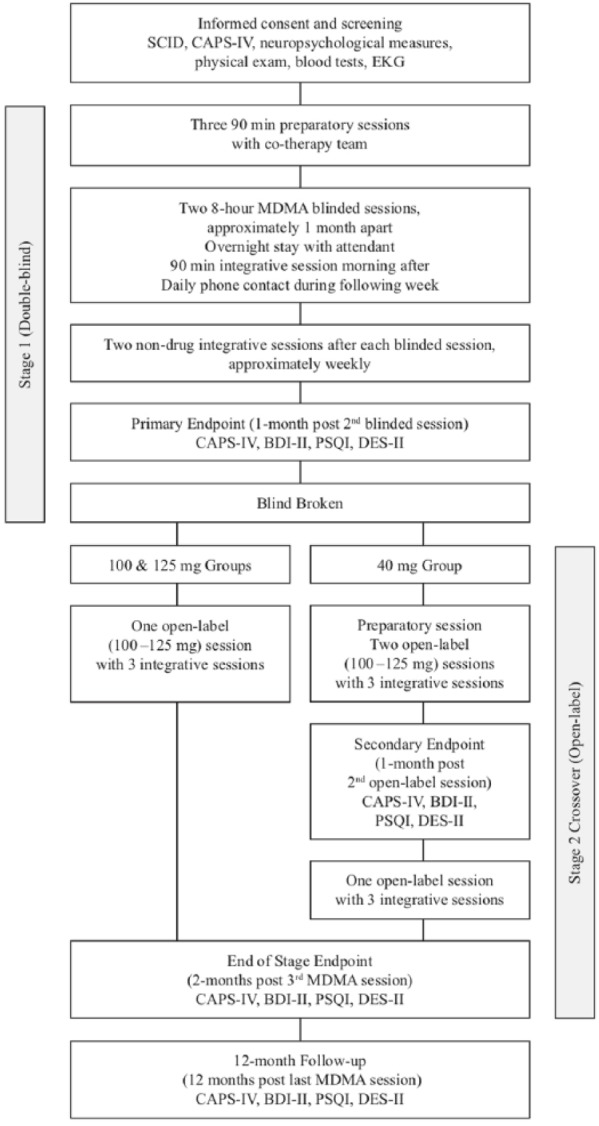
Study design.
BDI-II: Beck Depression Inventory-II; CAPS-IV: Clinician Administered PTSD Scale; DES-II: Dissociative Experience Scale-II; EKG: electrocardiogram; MDMA: 3,4-methylenedioxymethamphetamine; PSQI: Pittsburgh Sleep Quality Index; PTSD: posttraumatic stress disorder; SCID: Structured Clinical Interview for Diagnoses Axis I Research Version.
Participants were randomized to receive an active (125 or 100 mg) or a comparator (40 mg) dose of MDMA during two double-blind eight-hour experimental sessions spaced a month apart. Participants were randomized through a Web-based system that was blinded to site staff, study monitors, and statistical analysts. A supplemental dose half the quantity of the initial dose (62.5, 50 or 20 mg) was available approximately 90 min after the first dose, if not contraindicated. The MDMA was synthesized by David E Nichols (Purdue University) per applicable regulations, and compounded with lactose to make equivalent-weight gelatin capsules across doses as in prior studies conducted within this development program (Mithoefer et al., 2011).
The manualized therapeutic approach (Mithoefer, 2016) represents a modification of earlier work with psychedelics (Grof, 2001; Pahnke et al., 1971), which was subsequently adapted for use with MDMA (Greer and Tolbert, 1998; Metzner and Adamson, 2001). Therapists presented neither agendas nor solutions, and remained curious, open, and attentive to the participant’s developing experience. As much as possible, they followed the participant’s process and respected their pace, creating a sense of safety and communicating trust in the participant’s innate capacity for healing. Eyeshades and headphones were available during periods of focused inward attention. Participants could listen to a playlist of largely instrumental music intended to support the participant’s process. After the effects of the MDMA subsided, participants could eat dinner, and remained overnight in the clinic with a night attendant in the adjacent room.
The therapy room was carefully furnished to resemble a comfortable living area. Curtains created privacy and allowed for natural light to come in through the top so that participants could see the sky and treetops. The room was furnished with lamps that provided a low glow. The room had plants, fresh flowers, a couch that could be transformed into a bed during the eight-hour experimental sessions, two end tables, and two comfortable upholstered chairs for the two therapists. Colorful rugs covered part of the wooden floor and several paintings decorated the walls. A small desk and bookcase were placed in one corner, and there was a safe for secure drug storage.
On the morning following each experimental session, the first of three integrative sessions was conducted. The purpose of this session was to assess the participant’s mental state and stability, and to facilitate assimilation of experiences and insights gained during the experimental session. Daily 15–60-minute telephone contact occurred for seven days following each experimental session. Two more integrative sessions took place before the next experimental session.
At the primary endpoint, one month after the second blinded experimental session, each participant was assessed by the same blinded independent rater and completed self-report measures, after which the blind was broken. Participants in the 40 mg group crossed over to have one preparatory session and three open-label sessions (100–125 mg MDMA) with associated integrative sessions. Participants in the 100 mg and 125 mg groups underwent a third, open-label session (100–125 mg MDMA). Outcome measures were administered a month after the second open-label session and two months after the third open-label session. A 12-month follow-up assessment occurred 12 months (±one month) after the final active dose MDMA session.
Assessments
The CAPS-IV served as the primary outcome measure. This gold-standard clinician-administered PTSD measure includes symptom subscales and CAPS-IV total score. The dichotomous diagnostic score (Weathers et al., 2001), and ⩾30% drop in CAPS-IV total scores were used to evaluate clinically significant changes in PTSD symptoms. The same blinded independent rater who was not present during any therapy sessions administered the CAPS-IV.
Secondary outcome measures assessed symptoms of depression via Beck Depression Inventory-II (BDI-II) (Beck and Steer, 1984; Beck et al., 1996), dissociation with the Dissociative Experiences Scale-II (DES-II) (Bernstein and Putnam, 1986; Carlson and Putnam, 1993), and sleep quality with the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989).
Safety
Adverse events, reactions, vital signs, and suicidal ideation and behavior were closely monitored. Treatment emergent adverse events (TEAEs) were collected until two months following the last open-label session; serious adverse events (SAEs) and TEAEs that represented a change in psychiatric status were recorded until 12-month follow-up. Spontaneously reported reactions, based on reports in phase 1 and prior phase 2 studies (Mithoefer et al., 2011, 2013, 2018; Oehen et al., 2013), were collected during experimental sessions and the seven days following.
Heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP) and body temperature were monitored regularly during MDMA sessions. Blood pressure and heart rate (Welch Allyn, Skaneateles Falls, New York, USA) were measured before drug administration, and approximately every half-hour for the first four hours, then hourly until six hours after ingestion. Tympanic temperature was measured hourly (Welch Allyn, Skaneateles Falls, New York, USA).
Suicidal ideation and behavior were assessed with the clinician-administered Columbia Suicide Severity Rating Scale (C-SSRS) at each visit and on two of seven contact days (Posner et al., 2007, 2011). Data is summarized as positive ideation (PI; >0 for suicidal ideation score), serious ideation (SI; =4 or 5 for ideation score), and positive behavior (PB, >0 behavior score).
Statistical analyses
Power calculations were performed using results from a completed randomized, inactive-placebo controlled study of MDMA-assisted psychotherapy (Mithoefer et al., 2011). The current pilot study was underpowered to detect small to medium effect sizes, but could possibly detect a large effect. Efficacy analyses for all measures were performed on the intent-to-treat (ITT) set, consisting of all who had at least one MDMA session and completed an outcome assessment after baseline. CAPS-IV data were also analyzed in the per protocol (PP) set, which included all participants who completed both blinded sessions, primary outcome assessment, and did not experience a major protocol deviation. Participants who completed the open-label crossover were included in the crossover ITT set. Safety analyses included all participants who received at least one dose of MDMA.
The primary efficacy outcome was the change in CAPS-IV Total scores from baseline to one month after the second blinded session, analyzed using an analysis of variance (ANOVA) with α=0.05. Preplanned t-tests compared dose groups when significant main effects were found. Secondary measures (BDI-II, PSQI, DES-II) were analyzed with the same method. Cohen’s d independent-groups pretest-posttest design was used for comparator-subtracted effect size estimates (Kadel and Kip, 2012). Descriptive statistics display the percentage of participants not meeting PTSD criteria on CAPS-IV (diagnostic score) and those attaining a ⩾30% decrease in scores post-treatment.
For the open-label crossover, within-subjects t-tests compared scores on all measures at one-month after two open-label sessions to the primary endpoint. To explore whether a third active dose MDMA session produced further benefit, within-subjects t-tests of individual treatment groups compared scores of two vs three sessions. Peak vital signs averaged across the two blinded MDMA sessions were analyzed by a one-way ANOVA with t-tests to comparing groups. All statistical analyses were conducted using SPSS, version 20.0 (IBM Corporation, Armonk, New York, USA).
Results
Sample
The overall participant flow is depicted in the CONSORT diagram (Figure 1 and Supplementary Material Checklist), with n=28 randomized, n=27 completing the primary assessment, and n=25 assessed at 12-month follow-up. Nine men (32%) and 19 women (68%), average age 42.0 years (standard deviation (SD)=12.9) ranging from 22–66, and mostly White/Caucasian (92.9%) ethnicity, enrolled in the study (Table 1). The majority had experienced two or more traumatic events, such as childhood sexual or physical abuse, combat, ritual abuse, assaults, accidents or witnessing a crime. All had a diagnosis of PTSD; all but one met the criteria for PTSD on the CAPS-IV. The average duration of PTSD before enrollment was 29.4 years. All participants had undergone at least one form of psychotherapy, 20 participants (71.4%) had been prescribed drugs for depression, and 15 participants (53.6%) had been prescribed drugs for anxiety, as classified in accordance with Neuroscience-based Nomenclature and not by the reason they were prescribed. Nearly half of the participants (42.9%) had been diagnosed with major depression, and another quarter with depression (Table 1). Self-injurious behavior prior to enrollment was reported by 10 participants (35.7%). Lifetime C-SSRS showed 27/28 (96.4%) of participants had suicidal ideation and 8/28 (28.6%) reported suicidal behavior.
Table 1.
Demographics and baseline characteristics.
| Characteristic | 40 mg MDMA (n=6) |
100 mg MDMA (n=9) |
125 mg MDMA (n=13) |
Total (n=28) |
|---|---|---|---|---|
| Age, mean (SD), years | 40.0 (11.7) | 39.6 (9.8) | 44.6 (15.4) | 42.0 (12.9) |
| Sex, no. (%) | ||||
| Male | 1 (16.7) | 3 (33.3) | 5 (38.5) | 9 (32.1) |
| Female | 5 (83.3) | 6 (66.7) | 8 (61.5) | 19 (67.9) |
| Ethnicity, no. (%) | ||||
| White/Caucasian | 5 (83.3) | 8 (88.9) | 13 (100.0) | 26 (92.9) |
| Latino/Hispanic | 0 | 1 (11.1) | 0 | 1 (3.6) |
| Native American | 1 (16.7) | 0 | 0 | 1 (3.6) |
| BMI, mean (SD) | 24.7 (7.3) | 23.7 (3.2) | 25.7 (6.5) | 24.9 (5.7) |
| Duration of PTSD, mean (SD), months | 260.3 (163.1) | 337.7 (197.7) | 406.5 (276.2) | 353.0 (231.9) |
| Pre-study therapy, no. (%) | ||||
| EMDR | 5 (83.3) | 6 (66.7) | 7 (53.8) | 18 (64.3) |
| Group psychotherapy | 1 (16.7) | 4 (44.4) | 3 (23.1) | 8 (28.6) |
| PE | 0 | 2 (22.2) | 1 (7.7) | 3 (10.7) |
| CPT | 0 | 0 | 1 (7.7) | 1 (3.6) |
| CBT, not otherwise specified | 3 (50.0) | 4 (44.4) | 4 (30.8) | 11 (39.3) |
| Holotropic breathwork | 0 | 0 | 1 (7.7) | 1 (3.6) |
| Psychodynamic | 1 (16.7) | 0 | 1 (7.7) | 2 (7.1) |
| IPT | 0 | 1 (11.1) | 0 | 1 (3.6) |
| Other | 6 (100.0) | 8 (88.9) | 13 (100.0) | 27 (96.4) |
| None | 0 | 0 | 0 | 0 |
| Pre-study psychiatric medications by drug class,a no. (%) | ||||
| Drugs for depression | 2 (33.3) | 7 (77.8) | 11 (84.6) | 20 (71.4) |
| Drugs for anxiety | 3 (50.0) | 5 (55.6) | 7 (53.8) | 15 (53.6) |
| Drugs for psychosis | 1 (16.7) | 2 (22.2) | 3 (23.1) | 6 (21.4) |
| Drugs for insomnia | 0 | 2 (22.2) | 3 (23.1) | 5 (17.9) |
| Drugs for stimulation | 1 (16.7) | 1 (11.1) | 2 (15.4) | 3 (10.7) |
| Other drugs | 1 (16.7) | 3 (33.3) | 6 (66.7) | 9 (32.1) |
| Psychiatric medical history,b no. (%) | ||||
| Alcohol abuse | 0 | 1 (11.1) | 1 (7.7) | 2 (7.1) |
| ADHD | 2 (33.3) | 2 (22.2) | 2 (15.4) | 6 (21.4) |
| Eating disorder | 0 | 0 | 2 (15.4) | 2 (7.1) |
| Depression | 1 (16.7) | 4 (44.4) | 2 (15.4) | 7 (25.0) |
| Dissociative disorders | 0 | 1 (11.1) | 1 (7.7) | 2 (7.1) |
| Major depressionc | 2 (33.3) | 3 (33.3) | 7 (53.8) | 12 (42.9) |
| Panic attack | 1 (16.7) | 1 (11.1) | 1 (7.7) | 3 (10.7) |
| Self-injurious behavior | 1 (16.7) | 4 (44.4) | 5 (38.5) | 10 (35.7) |
| Substance abuse | 0 | 3 (33.3) | 0 | 3 (10.7) |
| Lifetime C-SSRS,d no. (%) | ||||
| Positive ideation | 6 (100.0) | 8 (88.9) | 13 (100.0) | 27 (96.4) |
| Serious ideation | 2 (33.3) | 6 (66.7) | 5 (38.5) | 13 (46.4) |
| Positive behavior | 1 (16.7) | 3 (33.3) | 4 (30.8) | 8 (28.6) |
ADHD: attention deficit/hyperactivity disorder; BMI: body mass index; CBT: cognitive behavioral therapy; CPT: cognitive processing therapy; C-SSRS: Columbia Suicide Severity Rating Scale; EMDR: eye movement desensitization reprocessing; IPT: interpersonal therapy; MDMA: 3,4-methylenedioxymethamphetamine; PE: prolonged exposure; PTSD: posttraumatic stress disorder; SD: standard deviation.
Pre-study psychiatric medications are listed by the type of drug according to the Neuroscience based Nomenclature and not by the reason they were prescribed; bMedical Dictionary for Regulatory Activities (MedDRA) preferred terms; data coded with MedDRA v17.1; cMedDRA preferred term ‘major depression’ encompasses both major depressive disorder and major depression; dlifetime accounts for all suicidal ideation and behavior prior to study, according to participant recall and medical records. According to the C-SSRS scoring guide, scores of four or five on the suicidal ideation category are considered serious ideation, and scores of one or greater are considered positive behavior or ideation.
Primary outcome
The primary outcome was change in CAPS-IV total scores from baseline to one month after the second blinded session (Table 2 and Figure 3). In the ITT set, the active dose groups had the largest reduction in PTSD symptom severity with mean (SD) changes of −26.3 (29.5) for 125 mg, −24.4 (24.2) for 100 mg, and −11.5 (21.2) for 40 mg. Although there was no significant overall effect (F2,26=0.68, p=0.52). Cohen’s d effect sizes with 40 mg subtracted was 0.42 (–0.57, 1.42) for 125 mg and 0.37 (–0.57, 1.42) for 100 mg.
Table 2.
Outcome measures for blinded segment.
| 40 mg MDMA (n=6) |
100 mg MDMA (n=9) |
125 mg MDMA (n=12)a |
|
|---|---|---|---|
| Primary efficacy variable, ITT set | |||
| CAPS-IV total score, mean (SD) | |||
| Baseline | 84.8 (8.0) | 94.4 (20.2) | 93.5 (20.0) |
| Post 2 blinded sessions | 73.3 (24.5) | 70.0 (28.2) | 64.3 (33.6) |
| Changeb | –11.5 (21.2) | –24.4 (24.2) | –26.3 (29.5) |
| p Valuec | – | 0.36 | 0.27 |
| Primary efficacy variable, PP set | |||
| CAPS-IV total score, mean (SD) | |||
| Baseline | 84.6 (9.0) | 94.4 (20.2) | 91.6 (19.7) |
| Post 2 blinded sessions | 80.6 (18.8) | 70.0 (28.2) | 54.6 (31.9) |
| Changeb | –4.0 (11.9) | –24.4 (24.2) | –37.0 (20.9) |
| p Valuec | – | 0.10 | 0.01 |
| Secondary efficacy variables, ITT set | |||
| CAPS-IV PTSD diagnostic criteria met, no. (%) | |||
| Baseline | |||
| Yes | 6 (100.0) | 8 (88.9) | 13 (100.0) |
| No | 0 | 1 (11.1) | 0 |
| Post 2 blinded sessions | |||
| Yes | 4 (66.7) | 5 (55.6) | 7 (58.3) |
| No | 2 (33.3) | 4 (44.4) | 5 (41.7) |
| ⩾30% CAPS-IV total score decrease (post 2 blinded sessions), no. (%) | |||
| Yes | 1 (16.7) | 5 (55.6) | 6 (50.0) |
| No | 5 (83.3) | 4 (44.4) | 6 (50.0) |
| BDI-II, mean (SD) | |||
| Baseline | 23.8 (6.2) | 28.2 (13.6) | 29.3 (11.7) |
| Post 2 blinded sessions | 12.3 (6.3) | 18.3 (16.2) | 17.3 (16.7) |
| Changeb | –11.5 (7.8) | –9.9 (13.3) | –11.0 (13.7) |
| p Valuec | – | 0.81 | 0.94 |
| PSQI, mean (SD) | |||
| Baseline | 12.0 (3.2) | 13.0 (5.1) | 11.7 (4.0) |
| Post 2 blinded sessions | 11.2 (3.3) | 9.4 (4.9) | 9.1 (4.0) |
| Changeb | –0.8 (2.5) | –3.6 (6.2) | –2.0 (4.7) |
| p Valuec | – | 0.31 | 0.64 |
| DES-II, mean (SD) | |||
| Baseline | 14.4 (6.5) | 28.4 (18.0) | 21.5 (10.7) |
| Post 2 blinded sessions | 14.2 (4.4) | 15.2 (9.8) | 15.1 (14.5) |
| Changeb | –0.2 (6.9) | –13.3 (15.3) | –5.9 (12.0) |
| p Valuec | – | 0.06 | 0.37 |
BDI-II: Beck Depression Inventory-II; CAPS-IV: Clinician Administered PTSD Scale; DES-II: Dissociative Experience Scale-II; ITT: intention to treat; MDMA: 3,4-methylenedioxymethamphetamine; PP: per protocol; PSQI: Pittsburgh Sleep Quality Index; PTSD: posttraumatic stress disorder; SD: standard deviation.
Includes n=13 at baseline; bchange from baseline; ccompared to 40 mg MDMA.
Figure 3.
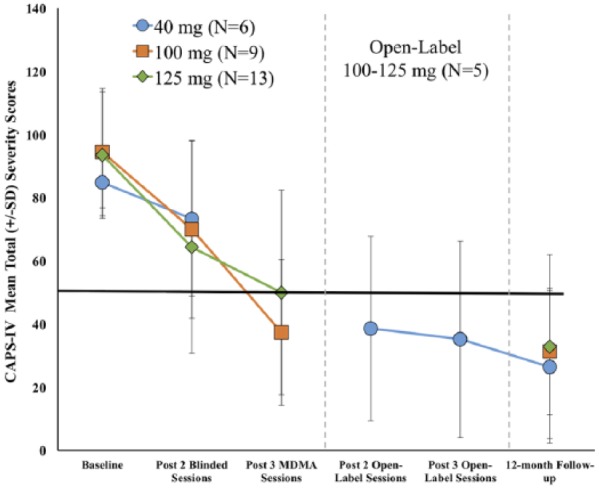
Change over time in Clinician Administered PTSD Scale (CAPS-IV) total scores in the intent-to-treat set. The primary endpoint occurred one month after the second blinded 3,4-methylenedioxymethamphetamine (MDMA) session. After assessment, the blind was broken. The active dose groups (100 and 125 mg) had one additional open-label MDMA session and completed an assessment two months after the third session. The comparator group (40 mg) crossed over to receive three open-label (100–125 mg) sessions, with assessments after the second and third sessions. The 12-month follow-up visit occurred after the final open-label MDMA session.
PTSD: posttraumatic stress disorder; SD: standard deviation.
In the PP set (Table 2), there was a significant main effect in change of CAPS-IV total scores (F2,22=4.01, p=0.03). Compared to the 40 mg group (mean change (SD) –4.0 (11.9)), the 125 mg group had a significant reduction (–37.0 (20.9), p=0.01) and the 100 mg group trended towards significance (–24.4 (24.2), p=0.10). Cohen’s d effect sizes with 40 mg subtracted was 1.12 (–0.10, 2.35) for 125 mg and 0.73 (–0.45, 1.90) for 100 mg.
Secondary outcomes
All secondary outcomes are reported for the ITT set (Table 2). More participants in the active dose groups did not meet PTSD diagnostic criteria according to the CAPS-IV at the primary endpoint (33.3% (40 mg), 44.4% (100 mg), and 41.7% (125 mg)). As a measure of clinical significance, the percentage of participants who attained a ⩾30% decrease in CAPS-IV total scores was substantially greater for active dose groups (16.7% (40 mg), 55.6% (100 mg), and 50.0% (125 mg)). Change in depressive symptoms, determined by the BDI-II, was approximately equivalent across groups (F2,26=0.03, p=0.97). Mean (SD) change in PSQI total scores was −0.8 (2.5) for 40 mg, −3.6 (6.2) for 100 mg, −2.0 (4.7) for 125 mg, indicating some improvement in sleep quality for all groups, yet this failed to reach significance (F2,26=0.583, p=0.57). Fewer dissociative experiences were reported by active dose groups (mean (SD) change in DES-II total scores, −13.3 (15.3) for 100 mg, and −5.9 (12.0) for 125 mg) compared to 40 mg −0.2 (6.9), although the difference was not significant (F2,26=2.09, p=0.15).
Open-label sessions
Two months after the final open-label session, CAPS-IV total scores significantly declined compared to the primary endpoint for both groups (ITT set: blind 100 mg/open-label, t8=6.82, p<0.0001; blind 125 mg/open-label t11=2.62, p=0.02), and four additional participants no longer met criteria for PTSD, indicating that the third MDMA session further improved treatment outcomes in this sample (Tables 3 and 4). Scores also generally improved on other measures, with some reaching significance (BDI-II (blind 100 mg/open-label, t8=2.74, p=0.03; blind 125 mg/open-label t11=1.14, p=0.28), PSQI (blind 100 mg/open-label, t8=1.97, p=0.08; blind 125 mg/open-label t8=1.59, p=0.15), and DES-II (blind 100 mg/open-label, t8=2.36, p=0.046; blind 125 mg/open-label t10=2.21, p=0.05)).
Table 3.
Outcome measures for open-label crossover, intent-to-treat set.
| Variable | 40 mg blinded MDMA/ 100–125 mg open-label (n=5) |
|---|---|
| CAPS-IV total score, mean (SD) | |
| Post 2 blinded sessions | 73.3 (24.5)a |
| Post 2 open-label sessions | 38.6 (29.2) |
| p Valueb | 0.01 |
| CAPS-IV PTSD diagnostic criteria met (post 2 open-label sessions), no. (%) | |
| Yes | 1 (20.0) |
| No | 4 (80.0) |
| ⩾30% CAPS-IV total score decrease (post 2 open-label sessions),c no. (%) | |
| Yes | 4 (80.0) |
| No | 1 (20.0) |
| BDI-II, mean (SD) | |
| Post 2 blinded sessions | 12.3 (6.3) |
| Post 2 open-label sessions | 6.8 (6.2) |
| p Valueb | 0.01 |
| PSQI, mean (SD) | |
| Post 2 blinded sessions | 11.2 (3.3) |
| Post 2 open-label sessions | 7.6 (4.0) |
| p Valueb | 0.24 |
| DES-II, mean (SD) | |
| Post 2 blinded sessions | 14.2 (4.4) |
| Post 2 open-label sessions | 7.2 (3.7) |
| p Valueb | 0.04 |
BDI-II: Beck Depression Inventory-II; CAPS-IV: Clinician Administered PTSD Scale-IV; DES-II: Dissociative Experience Scale-II; MDMA: 3,4-methylenedioxymethamphetamine; PSQI: Pittsburgh Sleep Quality Index; PTSD: posttraumatic stress disorder; SD: standard deviation.
Includes n=6 at post two blinded sessions; bwithin-subjects t-tests; cparticipants attaining a ⩾30% CAPS-IV total score decrease from post two blinded sessions (primary endpoint) to post two open-label sessions (secondary endpoint).
Table 4.
Outcome measures for two vs three 3,4-methylenedioxymethamphetamine (MDMA) sessions, intent-to-treat set.
| Variable | 40 mg blinded MDMA/ 100–125 mg open-label (n=6)a |
100 mg MDMA (n=9) |
125 mg MDMA (n=12) |
|---|---|---|---|
| CAPS-IV total score, mean (SD) | |||
| Post 2 blinded sessions | 73.3 (24.5) | 70.0 (28.2) | 64.3 (33.6) |
| Post 3 MDMA sessions | — | 37.3 (23.1) | 50.0 (32.4) |
| p Valueb | <0.0001 | 0.02 | |
| Post 2 open-label sessions | 38.6 (29.2) | — | — |
| Post 3 open-label sessions | 35.2 (31.1) | — | — |
| p Valueb | 0.78 | — | — |
| CAPS-IV PTSD diagnostic criteria met, no. (%) Post 3 MDMA sessions |
|||
| Yes | — | 2 (22.2) | 6 (50.0) |
| No | — | 7 (77.8) | 6 (50.0) |
| Post 3 open-label sessions | |||
| Yes | 2 (66.7) | — | — |
| No | 3 (60.0) | — | — |
| ⩾30% CAPS-IV total score decrease | |||
| Post 3 MDMA sessionsc | |||
| Yes | — | 8 (88.9) | 5 (41.7) |
| No | — | 1 (11.1) | 7 (58.3) |
| Post 3 open-label sessionsd | |||
| Yes | 2 (66.7) | — | — |
| No | 3 (60.0) | — | — |
| BDI-II, mean (SD) | |||
| Post 2 blinded sessions | 12.3 (6.3) | 18.3 (16.2) | 17.3 (16.7) |
| Post 3 MDMA session | — | 10.2 (9.3) | 13.6 (13.6) |
| p Valueb | — | 0.03 | 0.28 |
| Post 2 open-label sessions | 6.8 (6.2) | — | — |
| Post 3 open-label sessions | 9.6 (9.0) | — | — |
| p Valueb | 0.19 | — | — |
| PSQI, mean (SD) | |||
| Post 2 blinded sessions | 11.2 (3.3) | 9.4 (4.9) | 9.2 (4.2) |
| Post 3 MDMA session | — | 7.4 (4.1) | 7.7 (1.9) |
| p Valueb | — | 0.08 | 0.15 |
| Post 2 open-label sessions | 7.6 (4.0) | — | — |
| Post 3 open-label sessions | 4.8 (4.4) | — | — |
| p Valueb | 0.24 | — | — |
| DES-II, mean (SD) | |||
| Post 2 blinded sessions | — | 15.2 (9.8) | 15.1 (14.5) |
| Post 3 MDMA session | — | 10.6 (9.7) | 7.0 (5.2) |
| p Valueb | — | 0.046 | 0.05 |
| Post 2 open-label sessions | 7.2 (3.7) | — | — |
| Post 3 open-label sessions | 6.6 (4.6) | — | — |
| p Valueb | 0.58 | — | — |
BDI-II: Beck Depression Inventory-II; CAPS-IV: Clinician Administered PTSD Scale-IV; DES-II: Dissociative Experience Scale-II; PSQI: Pittsburgh Sleep Quality Index; PTSD: posttraumatic stress disorder; SD: standard deviation.
For open-label sessions (n=5); bwithin-subjects t-tests; cparticipants attaining a ⩾30% CAPS-IV total score decrease from post two blinded sessions (primary endpoint) to post three MDMA sessions (end of stage 1); dparticipants attaining a ⩾30% CAPS-IV total score decrease from post two open-label sessions (secondary endpoint) to post three open-label sessions (end of stage 2).
After two blinded sessions, the 40 mg group crossed over for three open-label MDMA (100–125 mg) sessions. One month after the second open-label session, PTSD symptom severity improved significantly compared to the primary endpoint (CAPS-IV total scores (t4=4.49, p=0.01)), as did symptoms of depression (BDI-II scores (t4=4.60, p=0.01)) and dissociation (DES-II scores (t4=2.96, p=0.04)). Sleep quality (PSQI scores (t4=1.39, p=0.24)) presented no significant changes. Scores did not significantly change further two-months after the third open-label session for this group.
12-Month follow-up
Twelve months after the last active dose of MDMA (Table 5), PTSD symptom severity was evaluated again. CAPS-IV total scores for the ITT set at baseline and 12-month follow-up mean (SD) were 92.0 (18.0) and 31.0 (24.2), respectively. PTSD severity was significantly lower compared to baseline (t24=11.30, p<0.0001). CAPS-IV total scores declined on average −9.6 (19.5) from treatment exit to the 12-month assessment. The majority (76%) did not meet PTSD diagnostic criteria, demonstrating enduring positive effects of MDMA-assisted psychotherapy. Analysis of secondary outcomes also found significant improvement at the 12-month follow-up compared to baseline for depression (BDI-II: t23=8.15, p<0.0001), sleep quality (PSQI: t22=6.46, p<0.0001), and dissociation (DES-II: t22=5.7, p<0.0001), indicating sustained gains well after the active treatment period ended.
Table 5.
Outcome measures for 12-month follow-up, intent-to-treat set.
| Variable | 12-month follow-up completers (n=25)a |
|---|---|
| CAPS-IV total score, mean (SD) | |
| Baseline | 92.0 (18.0) |
| 12-Month follow-up | 31.0 (24.2) |
| p Valuea | <0.0001 |
| CAPS-IV PTSD diagnostic criteria met (12-month follow-up), no. (%) | |
| Yes | 6 (24.0) |
| No | 19 (76.0) |
| BDI-II, mean (SD) | |
| Baseline | 27.8 (11.3) |
| 12-Month follow-up | 7.3 (8.5)b |
| p Valuec | <0.0001 |
| PSQI, mean (SD) | |
| Baseline | 12.2 (4.2) |
| 12-Month follow-up | 5.4 (3.5)b |
| p Valuec | <0.0001 |
| DES-II, mean (SD) | |
| Baseline | 22.2 (13.5) |
| 12-Month follow-up | 5.5 (5.2)b |
| p Valuec | <0.0001 |
BDI-II: Beck Depression Inventory-II; CAPS-IV: Clinician Administered PTSD Scale-IV; DES-II: Dissociative Experience Scale-II; PSQI: Pittsburgh Sleep Quality Index; PTSD: posttraumatic stress disorder; SD: standard deviation.
Includes n=28 at baseline; bn=23, one participant completed 12-month CAPS-IV, but not BDI-II, PSQI, or DES-II; cwithin-subjects t-tests.
Safety
Reactions reported by ⩾40% in any group on day of blinded sessions were anxiety and jaw clenching/tight jaw (Table 6), followed by headache, muscle tension, dizziness, fatigue, and low mood. The most commonly reported reactions on one or more of the seven days following blinded MDMA sessions, included sleep-related reactions (insomnia, need more sleep) and low mood, increased irritability, and ruminations. Most were mild to moderate, with frequency decreasing across the week following the experimental sessions.
Table 6.
Treatment-emergent adverse events (TEAEs) and expected reactions during two blinded 3,4-methylenedioxymethamphetamine (MDMA) sessions and seven days following, intent-to-treat set.
| 40 mg MDMA (n=6) |
100 mg MDMA (n=9) |
125 mg MDMA (n=13) |
Total (n=28) |
|
|---|---|---|---|---|
| Top reactions during experimental sessions, no. (%)a | ||||
| Anxiety | 2 (33.3) | 6 (66.7) | 7 (53.8) | 17 (60.7) |
| Dizziness | 1 (16.7) | 2 (22.2) | 7 (53.8) | 12 (42.9) |
| Fatigue | 2 (33.3) | 4 (44.4) | 4 (30.8) | 11 (39.3) |
| Headache | 4 (66.7) | 4 (44.4) | 3 (23.1) | 13 (46.4) |
| Jaw clenching, tight jaw | 2 (33.3) | 5 (55.6) | 8 (61.5) | 18 (64.3) |
| Low mood | 0 | 5 (55.6) | 2 (15.4) | 7 (25.0) |
| Muscle tension | 2 (33.3) | 4 (44.4) | 7 (53.8) | 13 (46.4) |
| Top reactions during 7 days of contact, no. (%)a | ||||
| Anxiety | 2 (33.3) | 8 (88.9) | 10 (76.9) | 20 (71.4) |
| Difficulty concentrating | 2 (33.3) | 5 (55.6) | 2 (15.4) | 9 (32.1) |
| Fatigue | 2 (33.3) | 7 (77.8) | 9 (69.2) | 18 (64.3) |
| Headache | 4 (66.7) | 3 (33.3) | 5 (38.5) | 12 (42.9) |
| Increased irritability | 2 (33.3) | 5 (55.6) | 6 (46.2) | 13 (46.4) |
| Insomnia | 3 (50.0) | 7 (77.8) | 6 (46.2) | 16 (57.1) |
| Lack of appetite | 1 (16.7) | 1 (11.1) | 8 (61.5) | 10 (35.7) |
| Low mood | 2 (33.3) | 6 (66.7) | 9 (69.2) | 17 (60.7) |
| Muscle tension | 2 (33.3) | 1 (11.1) | 6 (46.2) | 9 (32.1) |
| Nausea | 1 (16.7) | 3 (33.3) | 8 (61.5) | 12 (42.9) |
| Need more sleep | 2 (33.3) | 5 (55.6) | 8 (61.5) | 15 (53.6) |
| Ruminations | 1 (16.7) | 5 (55.6) | 6 (46.2) | 12 (42.9) |
| Psychiatric TEAEs, no. (%)b | ||||
| Anxiety | 0 | 3 (33.3) | 4 (30.8) | 7 (25.0) |
| Depressed mood | 0 | 2 (22.2) | 2 (15.4) | 4 (14.3) |
| Irritability | 0 | 2 (22.2) | 1 (7.7) | 3 (10.7) |
| Obsessive rumination | 0 | 1 (11.1) | 1 (7.7) | 2 (7.1) |
| Panic attack | 0 | 0 | 1 (7.7) | 1 (3.6) |
| Restlessness | 0 | 1 (11.1) | 0 | 1 (3.6) |
Frequency of participants who reported an expected, spontaneously reported reaction collected during and seven days following blinded experimental sessions 1 and 2 (only reactions reported by ⩾40% of participants in any group are displayed).
Frequency of participants who self-reported psychiatric adverse events after first drug administration until the primary endpoint.
During the blinded segment, at least one TEAE was reported by 1/6 (40 mg), 5/9 (100 mg,) and 9/13 (125 mg) participants. TEAEs were reported more frequently in both active dose groups (100 mg, 42.1% of 38 adverse events (AEs) during this period; 125 mg, 52.6%) compared to the 40 mg group (5.3%). Most TEAEs reported beyond an experimental session and the seven days afterward were psychiatric in nature (Table 6). Most psychiatric TEAEs occurred in active dose groups (see Supplementary Material Table S1), including anxiety (33.3% of 100 mg, 30.8% of 125 mg participants), depressed mood (22.2% of 100 mg, 15.4% of 125 mg participants), and irritability (22.2% of 100, 7.7% of 125 mg participants). No psychiatric TEAEs were reported after 40 mg MDMA.
Fifteen TEAEs were reported during the open-label segment, and were also mostly psychiatric (Supplementary Material Table S1), including anxiety and obsessive rumination, each occurring in 3.8% of participants, and suicidal ideation, occurring in 7.7% of participants. Nine AEs were reported during the 12-month follow-up by four people (Supplementary Material Table S1). Three SAEs occurred – two during the 12-month follow up segment (ruptured ovarian cyst, and fractured lower limb), and one during the open-label segment (stage 1 breast cancer) – none were deemed related to MDMA (Supplementary Material Table S1).
During the study, PI in 20/28 (71.4%) and SI in 3/28 (10.7%) was reported by participants on the C-SSRS. At the 12-month follow-up, only 3/25 (12.0%) had PI with no SI (Supplementary Material Table S3). There were no reports of suicidal behavior during the study or long-term follow-up.
For peak vital sign measurements across two blinded MDMA sessions (Supplementary Material Table S2), a dose effect was significant for heart rate (F2,53=4.2, p=0.02), and nearly significant for SBP (F2,53=3.2, p=0.05). Heart rate and SBP increased as dose ascended. No dose effects were found for body temperature (F2,53=1.7, p=0.20) or DBP (F2,53=3.0, p=0.06), but peak DBP after 125 mg was observed to be higher than after 40 or 100 mg (92 vs 86 and 84 mm Hg). No medical interventions were needed for the small to moderate increases in vital signs. Results from vital sign measurements are consistent with reports in healthy controls and in other samples of people with PTSD (Kirkpatrick et al., 2014a; Mithoefer et al., 2011; Vizeli and Liechti, 2017).
Discussion
Consistent with prior research, this study provides supportive evidence that MDMA-assisted psychotherapy can be safe and efficacious in individuals with chronic PTSD refractory to medication and/or psychotherapy. This is the first trial to employ multiple therapy teams with newly trained therapists implementing the manualized approach, which is encouraging regarding the likelihood that other newly-trained providers may replicate these findings in phase 3 trials. Although significant group differences were detected only in the PP set for the primary outcome, over half of participants in the ITT set who received active MDMA doses reached a 30% or greater drop in CAPS-IV total scores compared to 16.7% in the 40 mg group. After two blinded MDMA sessions, active dose groups had the largest reductions in CAPS-IV total scores with more participants attaining clinically significant improvements in PTSD symptoms relative to the 40 mg group, supporting a dose response.
To understand if three experimental sessions were more beneficial than two sessions, outcomes were evaluated again two months after the third (last) MDMA session. After the third experimental session, both the 100 mg and 125 mg groups showed further reductions in CAPS-IV scores, providing evidence that an additional session significantly improved PTSD outcomes. On the other hand, after the 40 mg group crossed over, a large treatment response resulted after two open-label sessions with little change after the third. The difference in time to respond is likely due to individual variation in the small samples, although there may have been a small additional therapeutic effect from the initial two low-dose sessions. Importantly, the gains were maintained over a 12-month follow-up after all groups had received active doses of MDMA in either blinded or open-label sessions, with 76% (n=25) of individuals not meeting the criteria for a diagnosis of PTSD. The fact that CAPS scores continued to improve between the two-month and 12-month follow-up visits lends support to the hypothesis that MDMA helps to catalyze a therapeutic process that continues long after the last drug administration. Moreover, the secondary outcome measures (depression, sleep, and dissociation) all showed significant reduction of symptoms at 12 months compared to baseline. At the 12-month visit, only one participant was taking a medication for PTSD; nine others were taking medications for insomnia, depression, generalized anxiety disorder, attention deficit/hyperactivity disorder (ADHD), and anxiety.
These findings are noteworthy given that participants had moderate to extreme PTSD and had previously failed to benefit from psychotherapy, including approaches thought to be relatively effective (cognitive behavioral therapy (CBT) and eye movement desensitization reprocessing (EMDR)), and pharmacological treatment, including medications for depression and anxiety. At baseline, 96.4% of participants reported suicidal thinking at some point in the past; for 46.4% the suicidal ideations were serious, and 28.6% reported a history of suicidal behavior. Thus, the participants were severely impacted by symptoms before study participation, and the sample was not restricted to exclude people who had previously experienced suicidal thinking, as is common practice in many clinical trials of psychiatric drugs.
Safety outcomes for MDMA-assisted psychotherapy in a controlled clinical setting strongly suggest a favorable benefit to risk ratio. Frequency and intensity of adverse events, reactions, and suicidal ideation were similar to previous reports (Mithoefer et al., 2011, 2018; Oehen et al., 2013). The greater number of psychiatric symptoms in active dose groups, such as anxiety, depression, or suicidal ideation, could be caused by the psychotherapeutic process of recalling and discussing experiences, thoughts, and emotions related to traumatic events, and also possibly be a direct pharmacological effect of MDMA. Available data is not adequate to identify a causal relationship, but increased transient anxiety has been detected in studies of healthy individuals after MDMA and is likely due to the MDMA-stimulated release of cortisol (Baggott et al., 2016; Dolder et al., 2018; Kirkpatrick et al., 2014b; Liechti et al., 2001). The most common time for mild to moderate anxiety related to drug onset to occur is in the first hour after administration; anxiety associated with painful or stressful memories typically occurred later in the session. Therapists encourage diaphragmatic breathing and other stress inoculation techniques that are discussed during the non-drug preparatory sessions. Participants may be able to continue the therapeutic processing of trauma memories, even when facing anxiety, because of the support of two therapists, and reduced amygdalar activity (Gamma et al., 2000) through the pharmacological effects of MDMA. Vital signs after MDMA generally increased in a dose-dependent manner to values similar during moderate exercise, and were well tolerated in these participants. There were no SAEs related to the treatment, adding to the evidence that MDMA can safely be administered to patients with PTSD.
Possible mechanisms for the treatment effect demonstrated in this sample are theorized based on the pharmacological effects of MDMA and its actions in the context of psychotherapy. Subjective effects of MDMA that bolster prosocial feelings and behaviors (Bedi et al., 2010; Hysek et al., 2014; Kamilar-Britt and Bedi, 2015) make unpleasant memories more tolerable (Carhart-Harris et al., 2014), and enhance empathy, self-compassion, (Baggott et al., 2015; Kamboj et al., 2015), and trusting in the pace of processing the experience, could all be beneficial in promoting a strong therapeutic alliance and inducing an optimal state of engagement for effectively processing traumatic memories. Healthy volunteers also report that MDMA can change the significance or meaning of perceptions (Liechti et al., 2001). MDMA-assisted psychotherapy is meant to maintain the optional “window of tolerance” (Mithoefer, 2016; Ogden et al., 2006). An enhanced therapeutic alliance combined with reduced anxiety or discomfort around difficult memories, increased self-compassion, and openness to expanding meaning of thoughts, feelings or experiences may all contribute toward therapeutic effects. Similar therapeutic procedures that include attention to setting, a pair of therapists offering nondirective, supportive care and substances that alter consciousness, are also used in psilocybin and ayahuasca research in people with depression (Carhart-Harris et al., 2018; Sanches et al., 2016).
Other proposed models include an explicit role for experiential “regression” and re-examination of past experiences, and non-ordinary or transpersonal experiences in MDMA-assisted psychotherapy (Passie, 2012).
MDMA-stimulated decrease in amygdala (Carhart-Harris et al., 2015; Gamma et al., 2000) and insular cortex activity (Walpola et al., 2017) may allow for emotional engagement without overwhelming anxiety during processing of painful traumatic memories. In healthy humans, MDMA acutely modulates brain circuitry important for memory and affective processing, and implicated in the pathophysiology of PTSD (Lanius et al., 2010), including increased resting state functional connectivity between the hippocampus and amygdala and decreased coupling of the medial prefrontal cortex with the hippocampus and posterior cingulate cortex (Carhart-Harris et al., 2015). Given that MDMA modulates emotional memory, neural pathways, fear extinction and memory reconsolidation might play a role in the underlying mechanisms for the positive treatment response (Feduccia and Mithoefer, 2018).
Limitations
Measurement of the primary variable was done by an independent rater using the CAPS-IV, which has good reliability and validity, and attrition was minimal (7%). Nevertheless, certain limitations constrain both the generalizability of findings and the ability to draw inferences concerning interactions among independent variables. The sample was relatively homogeneous, with the majority being predominantly female (68%) and White (93%). Thus, it was not possible to assess gender- or ethnicity-specific differences in outcomes.
The primary limitation was the relatively small sample size, especially in the low-dose study arm; however, this was a phase 2 trial intended to assess efficacy in a preliminary manner and to evaluate outcomes across multiple therapy teams. One participant in the 40 mg group experienced large drops in CAPS-IV total scores after one blinded session, affecting average scores in this small sample. Since this individual declined additional MDMA sessions after the first one due to an efficacious response, she was excluded from the PP analyses that showed little change in CAPS-IV scores from baseline to the primary endpoint (84.6 to 80.6) for the low dose group.
Establishing an effective blind for a psychoactive drug like MDMA is difficult. Prior phase 1 studies conducted by various groups have explored blinding MDMA with comparators of amphetamine (Bedi et al., 2010, 2014; Bershad et al., 2016; Cami et al., 2000; Kirkpatrick et al., 2014a; Mas et al., 1999; Tancer and Johanson, 2003) or methylphenidate (Dolder et al., 2018; Kuypers and Ramaekers, 2007; Ramaekers et al., 2006). Previous phase 2 clinical trials used inactive placebo (Mithoefer et al., 2011) and a low dose MDMA as comparator (25 mg or 30 mg) (Mithoefer et al., 2018; Oehen et al., 2013). This study utilized a dose-response design, comparing active doses to 40 mg MDMA, in order to enhance masking of MDMA-stimulated effects. To gauge the effectiveness of blinding procedures, participants and therapists were asked to guess the dose received after each blinded session. Therapists guessed correctly 77.3% of the time for 40 mg sessions, and 86.0% of the time for the 100 mg or 125 mg sessions. Participants also guessed correctly often, 72.7% in the 40 mg sessions, but mistakenly guessed (41.9% of the time) a low dose when in fact they had received an active dose. The research team had additional clues about group assignment from intra-session measurements of vital signs, but importantly, blinded independent raters who administered the CAPS were not present during therapy sessions and had no access to vital sign data. A limitation to the open-label crossover and 12-month follow-up was that the assessments were made under non-blinded conditions and there was no control group for comparison at these time points.
Conclusion
The promising efficacy and safety results from this dose response study, along with findings from five other phase 2 trials form the basis for expansion into multi-site phase 3 trials. In addition, the FDA granted "Breakthrough therapy" designation for MDMA-assisted psychotherapy for PTSD treatment, which may expedite the drug development process. Both short-term (one and two month) and long-term (12 month) follow-up results were positive across numerous therapy teams, demonstrating generalizability of the approach. The treatment was safe and well-tolerated. More research is needed to determine the optimal number of MDMA sessions needed to achieve symptom remission. Upcoming phase 3 trials, with a planned enrollment of 200–300 participants will evaluate the time to response and other factors that influence outcomes. If findings are replicated in phase 3 trials, MDMA-assisted psychotherapy will become an available treatment option for people suffering with PTSD.
Supplemental Material
Supplemental material, JOP806297__supplemental_material for 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial by Marcela Ot’alora G, Jim Grigsby, Bruce Poulter, Joseph W Van Derveer, Sara Gael Giron, Lisa Jerome, Allison A Feduccia, Scott Hamilton, Berra Yazar-Klosinski, Amy Emerson, Michael C Mithoefer and Rick Doblin in Journal of Psychopharmacology
Acknowledgments
The authors wish to express their gratitude to the individuals who participated in this study for committing to the deep and difficult journey of healing; the therapists who supported participants throughout their time in the study, Allison McQueen, Saj Razvi, and Sandra Van Derveer; Peggy Ivers for her organization and support; Annie Mithoefer for training of therapists; independent raters Carla Clements and Kathryn Kaye; independent rater intern Matthew Campeau; study physician Michael Levine; study pharmacist Kimm Singer; for their careful oversight of data collection and management including Colin Hennigan, Ben Shechet, Rebecca Matthews; Allison Wilens for coordinating video data collection; Lance Alster for performing data quality control analyses; Sarah Jordan for formatting figures; adherence rater team for assessing adherence to the manualized therapy; night attendants who cared for participants during their overnight stays; and all other volunteers who tirelessly supported the study.
The following author contributions were made. S Hamilton is responsible for integrity of the data and accuracy of data analysis. Study concept and design: M Ot’alora G, M Mithoefer, L Jerome, A Emerson, B Yazar-Klosinski, R Doblin. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: M Ot’alora G, A Feduccia, L Jerome, J Grigsby, B Poulter. Critical revision of the manuscript for important intellectual content: all authors. Obtained funding: R Doblin. Study supervision: M Ot’alora G
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M Ot’alora G received research funds from MAPS Public Benefit Corporation (MPBC) as the clinical trial principal investigator as well as for training and supervision of research psychotherapists. J Grigsby received research funds from MPBC as a clinical trial sub-investigator. B Poulter received research funds from MPBC as a clinical trial sub-investigator and as study administrator. JW Van Derveer III received research funds from MPBC as a clinical trial sub-investigator and as a study physician. SG Giron received research funds from MPBC as a clinical trial sub-investigator and from MAPS for directing the Zendo Project. S Hamilton received research funds from MPBC as a biostatistician. A Feduccia, A Emerson and L Jerome received salary support for full-time employment with MPBC. B Yazar-Klosinski and R Doblin received salary support for full-time employment with MAPS. M Mithoefer received research funds from MPBC as clinical trial medical monitor as well as for training of research psychotherapists.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MAPS provided all funding for this study.
ORCID Id: Lisa Jerome  https://orcid.org/0000-0001-6209-8750
https://orcid.org/0000-0001-6209-8750
Supplemental material: Supplemental material for this article is available online.
References
- Baggott MJ, Coyle JR, Siegrist JD, et al. (2016) Effects of 3,4-methylenedioxymethamphetamine on socioemotional feelings, authenticity, and autobiographical disclosure in healthy volunteers in a controlled setting. J Psychopharmacol 30: 378–387. [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Kirkpatrick MG, Bedi G, et al. (2015) Intimate insight: MDMA changes how people talk about significant others. J Psychopharmacol 29: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. (1984) Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol 40: 1365–1367. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, et al. (1996) Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67: 588–597. [DOI] [PubMed] [Google Scholar]
- Bedi G, Cecchi GA, Slezak DF, et al. (2014) A window into the intoxicated mind? Speech as an index of psychoactive drug effects. Neuropsychopharmacology 39: 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. (2010) Is ecstasy an “empathogen”? Effects of +/-3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry 68: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein EM, Putnam FW. (1986) Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 174: 727–735. [DOI] [PubMed] [Google Scholar]
- Bershad AK, Miller MA, Baggott MJ, et al. (2016) The effects of MDMA on socio-emotional processing: Does MDMA differ from other stimulants? J Psychopharmacol 30: 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouso JC, Doblin R, Farre M, et al. (2008) MDMA-assisted psychotherapy using low doses in a small sample of women with chronic posttraumatic stress disorder. J Psychoactive Drugs 40: 225–236. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. (1989) The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Cami J, Farre M, Mas M, et al. (2000) Human pharmacology of 3,4-methylenedioxymethamphetamine (“ecstasy”): Psychomotor performance and subjective effects. J Clin Psychopharmacol 20: 455–466. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day CMJ, et al. (2018) Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology (Berl) 235: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Murphy K, Leech R, et al. (2015) The effects of acutely administered 3,4-methylenedioxymethamphetamine on spontaneous brain function in healthy volunteers measured with arterial spin labeling and blood oxygen level-dependent resting state functional connectivity. Biol Psychiatry 78: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Wall MB, Erritzoe D, et al. (2014) The effect of acutely administered MDMA on subjective and BOLD-fMRI responses to favourite and worst autobiographical memories. Int J Neuropsychopharmacol 17: 527–540. [DOI] [PubMed] [Google Scholar]
- Carlson EB, Putnam Fw. (1993) An update on the dissociative experiences scale. Dissociation 6: 16–27. [Google Scholar]
- Dolder PC, Muller F, Schmid Y, et al. (2018) Direct comparison of the acute subjective, emotional, autonomic, and endocrine effects of MDMA, methylphenidate, and modafinil in healthy subjects. Psychopharmacology (Berl) 235: 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A, Ruzek JI, Crowley JJ, et al. (2013) Effectiveness of national implementation of prolonged exposure therapy in Veterans affairs care. JAMA Psychiatry 70: 949–955. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Mithoefer MC. (2018) MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Prog Neuropsychopharmacol Biol Psychiatry 84: 221–228. [DOI] [PubMed] [Google Scholar]
- Foa EB. (2007) Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. New York: Oxford University Press. [Google Scholar]
- Gamma A, Buck A, Berthold T, et al. (2000) 3,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H(2)(15)O]-PET in healthy humans. Neuropsychopharmacology 23: 388–395. [DOI] [PubMed] [Google Scholar]
- Goetter EM, Bui E, Ojserkis RA, et al. (2015) A systematic review of dropout from psychotherapy for posttraumatic stress disorder among Iraq and Afghanistan combat Veterans. J Trauma Stress 28: 401–409. [DOI] [PubMed] [Google Scholar]
- Greer G, Tolbert R. (1986) Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs 18: 319–327. [DOI] [PubMed] [Google Scholar]
- Greer GR, Tolbert R. (1998) A method of conducting therapeutic sessions with MDMA. J Psychoactive Drugs 30: 371–379. [DOI] [PubMed] [Google Scholar]
- Grof S. (2001) LSD Psychotherapy. 4th ed. Ben Lomond, CA: Multidisciplinary Association for Psychedelic Studies. [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, et al. (2014) MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci 9: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaycox LH, Foa EB, Morral AR. (1998) Influence of emotional engagement and habituation on exposure therapy for PTSD. J Consult Clin Psychol 66: 185–192. [DOI] [PubMed] [Google Scholar]
- Kadel R, Kip K. (2012) A SAS macro to compute effect size (Cohen’s d) and its confidence interval from raw survey data. In: Proceedings of the Annual Southeast SAS Users Group Conference, Durham, NC, USA, 14–16 October 2012, paper SD-06.. [Google Scholar]
- Kamboj SK, Kilford EJ, Minchin S, et al. (2015) Recreational 3,4-methylenedioxy-N-methylamphetamine (MDMA) or ‘ecstasy’ and self-focused compassion: Preliminary steps in the development of a therapeutic psychopharmacology of contemplative practices. J Psychopharmacol 29: 961–970. [DOI] [PubMed] [Google Scholar]
- Kamilar-Britt P, Bedi G. (2015) The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals. Neurosci Biobehav Rev 57: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, et al. (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, Baggott MJ, Mendelson JE, et al. (2014. a) MDMA effects consistent across laboratories. Psychopharmacology (Berl) 231: 3899–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, et al. (2014. b) Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology 39: 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Davis LL, Neylan TC, et al. (2017) It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: A consensus statement of the PTSD psychopharmacology working group. Biol Psychiatry 82: e51–e59. [DOI] [PubMed] [Google Scholar]
- Kuypers KP, Ramaekers JG. (2007) Acute dose of MDMA (75 mg) impairs spatial memory for location but leaves contextual processing of visuospatial information unaffected. Psychopharmacology (Berl) 189: 557–563. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, et al. (2010) Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 167: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. (2001) Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 154: 161–168. [DOI] [PubMed] [Google Scholar]
- Mas M, Farre M, de la, Torre R, et al. (1999) Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 290: 136–145. [PubMed] [Google Scholar]
- Metzner R, Adamson S. (2001) Using MDMA in healing, psychotherapy and spiritual practice. In: Holland J. (ed.) Ecstasy, A Complete Guide: A Comprehensive Look at the Risks and Benefits of MDMA. Rochester VT: Inner traditions, pp. 182–207. [Google Scholar]
- Mithoefer M. (2016) A Manual for MDMA-Assisted Psychotherapy in the Treatment of Posttraumatic Stress Disorder. Version 8. http://www.maps.org/research/mdma/mdma-research-timeline/4887-a-manual-for-mdma-assisted-psychotherapy-in-the-treatment-of-ptsd [DOI] [PMC free article] [PubMed]
- Mithoefer MC, Grob CS, Brewerton TD. (2016) Novel psychopharmacological therapies for psychiatric disorders: Psilocybin and MDMA. Lancet Psychiatry 3: 481–488. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Mithoefer AT, Feduccia AA, et al. (2018) 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 5: 486–497. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. (2011) The safety and efficacy of {+/-}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J Psychopharmacol 25: 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. (2013) Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: A prospective long-term follow-up study. J Psychopharmacol 27: 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, et al. (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340: c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, et al. (2013) A randomized, controlled pilot study of MDMA (+/- 3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD). J Psychopharmacol 27: 40–52. [DOI] [PubMed] [Google Scholar]
- Ogden P, Minton K, Pain C. (2006) Trauma and the Body: A Sensorimotor Approach to Psychotherapy (Norton Series on Interpersonal Neurobiology). New York: WW Norton & Company. [Google Scholar]
- Pahnke WN, Kurland AA, Unger S, et al. (1971) The experimental use of psychedelic (LSD) psychotherapy. Int Z Klin Pharmakol Ther Toxikol 4: 446–454. [PubMed] [Google Scholar]
- Passie T. (2012) Healing with Entactogens: Therapist and Patient Perspectives on MDMA-assisted Group Psychotherapy. Santa Cruz, CA: Multidisciplinary Association for Psychedelic Studies. [Google Scholar]
- Posner K, Brown GK, Stanley B, et al. (2011) The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168: 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, et al. (2007) Columbia classification algorithm of suicide assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 164: 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KP, Samyn N. (2006) Stimulant effects of 3,4-methylenedioxymethamphetamine (MDMA) 75 mg and methylphenidate 20 mg on actual driving during intoxication and withdrawal. Addiction 101: 1614–1621. [DOI] [PubMed] [Google Scholar]
- Sanches RF, de Lima Osorio F, Dos Santos RG, et al. (2016) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A SPECT study. J Clin Psychopharmacol 36: 77–81. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Stein MB, et al. (2007) Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med 69: 242–248. [DOI] [PubMed] [Google Scholar]
- Sareen J, Jacobi F, Cox BJ, et al. (2006) Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med 166: 2109–2116. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. (2003) Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend 72: 33–44. [DOI] [PubMed] [Google Scholar]
- Vizeli P, Liechti ME. (2017) Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol 31: 576–588. [DOI] [PubMed] [Google Scholar]
- Walpola IC, Nest T, Roseman L, et al. (2017) Altered insula connectivity under MDMA. Neuropsychopharmacology 42: 2152–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. (2001) Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety 13: 132–156. [DOI] [PubMed] [Google Scholar]
- Yazar-Klosinski BB, Mithoefer MC. (2017) Potential psychiatric uses for MDMA. Clin Pharmacol Ther 101: 194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JOP806297__supplemental_material for 3,4-Methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial by Marcela Ot’alora G, Jim Grigsby, Bruce Poulter, Joseph W Van Derveer, Sara Gael Giron, Lisa Jerome, Allison A Feduccia, Scott Hamilton, Berra Yazar-Klosinski, Amy Emerson, Michael C Mithoefer and Rick Doblin in Journal of Psychopharmacology