Short abstract
Subcutaneous formalin injections are used as a model for tissue injury-induced pain where formalin induces pain and inflammation indirectly by crosslinking proteins and directly through activation of the transient receptor potential A1 receptor on primary afferents. Activation of primary afferents leads to both central and peripheral release of neurotransmitters. Mast cells are found in close proximity to peripheral sensory nerve endings and express receptors for neurotransmitters released by the primary afferents, contributing to the neuro/immune interface. Mast cell proteases are found in large quantities within mast cell granules and are released continuously in small amounts and upon mast cell activation. They have a wide repertoire of proposed substrates, including Substance P and calcitonin gene-related peptide, but knowledge of their in vivo function is limited. We evaluated the role of mouse mast cell proteases (mMCPs) in tissue injury pain responses induced by formalin, using transgenic mice lacking either mMCP4, mMCP6, or carboxypeptidase A3 (CPA3), or mast cells in their entirety. Further, we investigated the role of mast cells in heat hypersensitivity following a nerve growth factor injection. No statistical difference was observed between the respective mast cell protease knockout lines and wild-type controls in the formalin test. Mast cell deficiency did not have an effect on formalin-induced nociceptive responses nor nerve growth factor-induced heat hypersensitivity. Our data thus show that mMCP4, mMCP6, and CPA3 as well as mast cells as a whole, do not play a significant role in the pain responses associated with acute tissue injury and inflammation in the formalin test. Our data also indicate that mast cells are not essential to heat hypersensitivity induced by nerve growth factor.
Keywords: Pain formalin, transgenic, mast cell, protease
Introduction
When tissue injury occurs, a cascade of responses including inflammation and pain sensation is initiated to minimize harm and facilitate healing. Subcutaneous formalin injection serves as a translational model for tissue injury-induced pain where formalin causes injury and inflammation by interacting with the transient receptor potential (TRP) A11 and by covalently crosslinking proteins in a nonspecific fashion, thus disrupting cell membranes.2 TRPA1 is expressed on a subpopulation of TRPV1-positive primary afferents,3,4 and activation leads to action potential formation and subsequent transmitter release in both the central and peripheral terminals of the afferents, thereby causing pain sensation and activation of immune cells, including mast cells. In the skin, this process is generally referred to as cutaneous neurogenic inflammation5 (also reviewed in the study by Gouin et al.6)
Nerve growth factor (NGF) is a neurotrophic factor essential for the development and survival of sympathetic and sensory neurons.7 NGF injections have been used in a translational model of inflammation-induced hypersensitivity toward both thermal and mechanical stimuli, a hypersensitivity that persist for hours and days, respectively, after injection.8,9 NGF activates the high-affinity tropomysin kinase A receptor (TrkA) on nociceptive neurons, promoting upregulation and intracellular modifications of TRPV1 receptors via the mitogen-activated protein kinase and phosphatidylinositide 3-kinase signaling pathways,10,11 which subsequently leads to thermal and mechanical hypersensitivity.
Mast cells are immune cells that are considered to be of particular importance in tissue injury responses, as they are widely distributed throughout the body, express receptors capable of recognizing various different substances of exogenous and endogenous origin, and can respond quickly by producing and releasing appropriate mediators, many of which are inflammatory, such as cytokines.12,13 Besides cytokines, mast cells also contain proteases that are stored in their active form in large amounts within the mast cell secretory granules, which are released upon mast cell activation.14–16 Furthermore, granular content is continuously released at baseline conditions by the so-called piecemeal degranulation.17 Consequently, mast cell proteases are found in the circulation18 as well as in biologically active form19,20 on mast cell surfaces at baseline conditions and can have an impact in vivo in the absence of mast cell stimuli. This has for instance been demonstrated for chymase, which has been shown to increase intestinal permeability at homeostatic conditions21 and to decrease bone mass in the absence of any mast cell activation stimulus.22 Hence, mast cell proteases are biologically active in the tissue both in inflammatory conditions as described below and in the absence of stimuli that cause mast cell degranulation.
The mast cell proteases are generally divided into three groups based on their peptide bond cleavage specificities: chymases, tryptases, and carboxypeptidases.23 The mouse mast cell proteases (mMCPs) mMCP4 (chymase), tryptase mMCP6, and carboxypeptidase A3 (CPA3) are considered the closest functional homologs to the human mast cell chymase, tryptase, and carboxypeptidase and are all expressed in connective tissue mast cells.24 Chymases and tryptases have an extensive repertoire of proposed substrates, mostly based on in vitro experiments, while the cleavage profile of CPA3 is more limited.23 Several of the mast cell protease substrates are pro-inflammatory mediators, which are inactivated upon protease cleavage. Mast cell protease substrates also include pro-inflammatory precursor proteins, which the proteases activate by enzymatic cleavage. For instance, mast cell chymase has been shown to degrade the neuropeptides Substance P (SP) and vasoactive intestinal peptide (VIP),25 pro-inflammatory mediators that are released from primary afferents upon their activation, and can induce mast cell degranulation.26 Chymase also degrades bradykinin,27 the interleukin (IL) precursors Pro-IL-18 and Pro-IL-1β28,29 as well as IL-6, IL-13, IL-33, HMGB1 (high mobility group protein B1), and heat shock protein 70.30,31 Mast cell tryptase cleaves VIP25,32 and is efficient in degrading the neuropeptide calcitonin gene-related peptide (CGRP).32 Additionally, tryptase activates the protease-activated receptor 2 (PAR2), which mediates inflammation as well as itch33,34 and has been reported to generate NGF by cleaving its precursor peptide pro-NGF.35 CPA3 is the least studied of the three proteases but has been shown to cleave endothelin-1 (ET-1),36 a neurotransmitter capable of transmitting pain and itch by activating the ETA receptor on sensory neurons37,38 as well as degranulating mast cells.39
Information about the exact in vivo role of the mast cell proteases is limited; however, they have been linked to both protective and harmful effects in inflammatory conditions.16,23,40 Studies in mice have shown that mMCP4 has a pro-inflammatory role in arthritis41 and contributes to skin inflammation together with the elastase mMCP5 after epidermal burn injury.42,43 In allergic airway inflammation, however, mMCP4 modulates IL-33 levels and has a protective effect.44 PAR2 activation by mMCP6 contributes to intestinal inflammation in mice,33 and human tryptase contributes to joint inflammation via the same receptor when injected in mice.45 Based on the reported roles of the mast cell-specific proteases in neurotransmitter degradation and inflammatory conditions, the individual contributions of chymase mMCP4, tryptase mMCP6, and carboxypeptidase CPA3 to tissue injury-induced pain behavior were investigated in this study by performing the formalin test on mMCP4−/−, mMCP6−/−, and CPA3−/− mice. The role of intact mast cells was studied as well by performing the formalin test on the mast cell-deficient mouse line Mcpt5Cre+;R-DTA. The same mouse line was used in investigating the role of mast cells in heat sensation and heat hypersensitivity by performing the Hargreaves test coupled with a subcutaneous NGF injection.
Materials and methods
Generation of transgenic animals and genotyping
Mouse lines deficient in the mast cell proteases mMCP4,46 mMCP6,47 and CPA348 as well as a mast cell-deficient mouse line (Mcpt5Cre+;R-DTA (diphtheria toxin A))49 were generated as previously described. In the Mcpt5Cre+;R-DTA mice, Cre recombinase expression is controlled by the Mcpt5 promoter, inducing diphtheria toxin gene expression through Cre-mediated deletion of a loxP-flanked stop cassette. As Mcpt5 (coding for the chymase mMCP5) is exclusively expressed by connective tissue mast cells, only those cells are affected and undergo cell death as a consequence of activated diphtheria toxin expression.49 The mMCP4−/−, mMCP6−/−, and CPA3−/− lines were all backcrossed for a minimum of 10 generations to the wild-type strain used (C57BL/6) and were maintained in a homozygous state. To avoid genetic drift, the respective knockout strains were routinely re-backcrossed to the wild-type C57BL/6 line. For detecting mast cell-deficient mice, the following primers were used: 5′- ACAGTGGTATTCCCGGGGAGTGT-3′ (forward), 5′- GTCAGTGCGTTCAAAGGCCA-3′ (reverse), and 5′- TGAGAAGGGCTATGAGTCCCA-3′ (reverse, mutant allele). For detecting mMCP4-, mMCP6-, CPA3-null genotypes, the following primers were used: mMCP4: 5′-CAAGGTCCAACTAACTCCCTTTGTGCTCC-3′ (forward), 5′-GGTGATCTCCAGATGGGCCATGTAAGGGCG-3′ (reverse), and 5′-GGGCCA GCTCATTCCTCCCACTCATGATCT-3′ (reverse, mutant allele); mMCP6: 5′-TTTAGC TGGACTCAGGCTGTGCTCCTCACT-3′ (forward), 5′-CTCCTGAATTGG AGCTAACCCTGGGATTCT-3′ (reverse), and 5′-GACCATGTGATCGCGCTTCT-3′ (reverse, mutant allele); and CPA3: 5′-GGACTGTTCATCCCCAGGAACC-3′ (forward), 5′-CTGGCGTGCTTTTCATTCTGG-3′ (reverse), and 5′-GTCCGGACACGCTGAACTTG4-3′ (reverse, mutant allele).
Behavior
All behavioral tests were performed on adult (>7 weeks old) male mice with the exception of the NGF-associated heat hypersensitivity test where one female couple was used together with five male couples. The tests were performed in the day (light) part of the cycle and by the same female investigator. The mast cell-deficient mice were observed by two female investigators in the formalin test. Controls for protease-deficient mice were age-matched wild-type mice (C57BL/6) housed in the same animal room; for the mast cell-deficient Mcpt5Cre+;R-DTA mice, littermate Mcpt5-Cre−;R-DTA mice were used as controls. All behavior analyses were performed in a controlled environment of 20°C–24°C, 45%–65% humidity, and 12-h day/night cycle. All animal procedures were approved by the local ethical committee in Uppsala and followed the Directive 2010/63/European Union of the European Parliament and of the Council, The Swedish Animal Welfare Act (SFS (Svensk författningssamlingar) 1988:534), The Swedish Animal Welfare Ordinance (SFS 1988:539), and the regulations regarding the use of animals for scientific purposes: SJVFS (Statens jordbruksverks författningssamlingar) 2017:40 (L150). The observers were blinded to the genotype when measuring pain behavior in the mast cell-deficient mice. However, this was not possible when working with the protease-deficient animals due to the organization of the animals in the facility, so the risk of observational bias cannot be excluded.
Formalin test
Each mouse was gently restrained using a paper towel before 20 µl of 5% formalin (37% formaldehyde (Sigma-Aldrich, St. Louis, MO) diluted in 0.9% saline) was injected subcutaneously into the plantar surface of the right hind paw with a microsyringe (1710 TLL (PTFE luer lock) 100 μl; Hamilton Central Europe, Ghiroda, Romania) using a 30-G needle (BD Microlance, Beckton, Dickinson & Co. Ltd., Drogheda, Ireland). The mouse was then observed in a transparent cage surrounded by mirrors on three sides for the following 60 min to measure pain behavior defined as licking and biting of the injected paw, using a stopwatch. The results were expressed as the mean amount of pain behavior (in seconds) for each group during every 5-min interval ± standard error of mean (SEM) and during the first 10 min (Phase 1), the following 50 min (Phase 2), and over the whole hour.
Hargreaves test
Prior to the experiment, the mice were acclimatized to the Hargreaves setup (transparent acrylic glass chambers with a glass floor) on two separate occasions for 30 min in order to minimize stress caused by new surroundings. On the day of the experiment, the mice were acclimatized in the setup for 60 min or until no exploratory behavior was observed. The Hargreaves heat source (IITC Life Science, Woodland Hills, CA) was placed with the guide light pointing toward the plantar surface of the right hind paw, and the thermal beam was started. Paw withdrawal would stop the test, and the response time was noted. The cut-off time was set to 20 s. The test was repeated two or three times per animal, allowing at least 5 min between each measurement.
NGF–evoked hypersensitivity
Baseline measurements were performed using the Hargreaves test as previously described. The mice were then injected subcutaneously with 50 ng human recombinant β-NGF (MBL International, Woburn, MA) dissolved in 20 µl 0.9% saline into the plantar surface of the right hind paw with a microsyringe (1710 TLL 100 μl, Hamilton Central Europe, Ghiroda, Romania) using a 30-G needle (BD Microlance, Beckton, Dickinson & Co. Ltd., Drogheda, Ireland). Hargreaves measurements were repeated at 30 min and at 1, 2, 4, and 24 h after NGF injection to follow the development of hypersensitivity. The test was repeated two or three times per animal at each time point, allowing at least 5 min between measurements. The results were expressed as the mean withdrawal latency time at each time point for each group ± SEM. Development of hypersensitivity for each group was evaluated as the difference between the mean response time at baseline (before the NGF injection) versus the mean response time at each time point after the injection.
Toluidine blue staining
Naïve adult Mcpt5Cre+;R-DTA mice and Cre-negative controls (one female and one male per genotype) were sacrificed and plantar surfaces of hind paws were collected and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 h. Subsequently, the skin was transferred to a stepwise gradient of sucrose solutions in PBS, ending with 30% sucrose overnight at 4°C. Skin samples were embedded in optimal cutting temperature (OCT) compound (Richard-Allan Scientific, Kalamazoo, MI) on dry ice and cut using a cryostat (CryoCut 1800, Leica Reichert-Jung, Wetzlar, Germany) into 12-µm-thick sections. Staining of tissue sections started with 10-min incubation in acetone at −20°C, followed by rehydration in PBS (twice for 5 min) and stained in toluidine blue (Sigma-Aldrich, St. Louis, MO) solution (0.1% toluidine blue in 171 mM NaCl (pH 2)) for 2 min. Tissue sections were washed in distilled water, dehydrated, and mounted. Mast cells were observed with a Nikon Eclipse 90i bright field microscope (Nikon Instruments Europe, Amsterdam, Netherlands) using a 10× and 20× objective.
Statistics
For all sets of data, normality of variance was assessed by a Shapiro–Wilks test. When comparing two groups, parametric calculations were conducted with two-tailed Student’s t test, and nonparametric calculations were performed using a two-tailed Mann–Whitney test. Nonparametric calculations of p values (>2 groups) were conducted with Kruskal–Wallis (one-way analysis of variance (ANOVA)) followed by Dunn’s post hoc test. For the NGF/Hargreaves test, development of hypersensitivity for each group was compared using repeated measurement ANOVA with Dunnett’s post hoc multiple comparison. All calculations were performed using Prism version 5.04 (GraphPad Software, Inc., San Diego, CA). Values of p < 0.05 were considered significant.
Results
mMCP4−/−, mMCP6−/−, and CPA3−/− mice do not differ significantly from controls in pain behavior at any time point of the formalin test
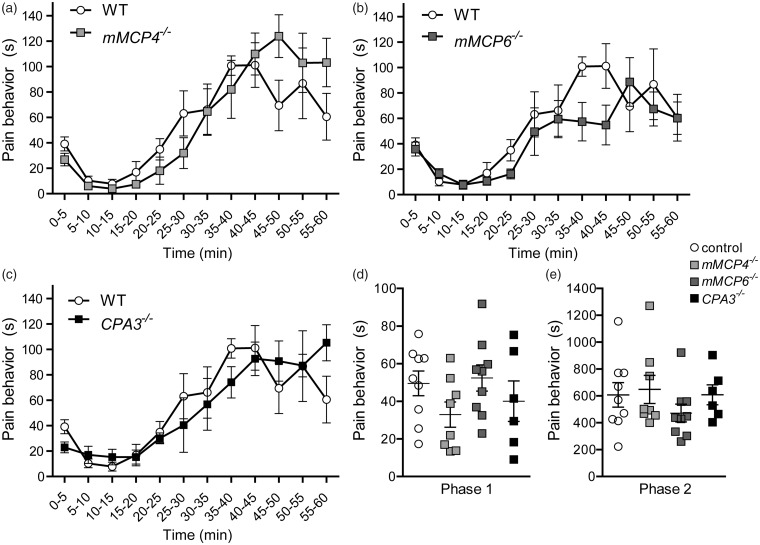
To evaluate if single mast cell protease deficiency plays a role in the pain responses to tissue injury and acute inflammation, the formalin test was performed on mast cell protease knock-out mice mMCP4−/−, mMCP6−/−, and CPA3−/−, together with age-matched wild-type controls (Figure 1). The accuracy of the three knockout lines has been evaluated in previous analyses where each null mutation was shown to result in absence of the respective protein.46–48 The mMCP4−/− mice did not differ significantly from the controls at any 5-min interval, but a slight trend toward higher pain responses during the last 15 min of the formalin test was observed; at 45–50 min, they demonstrated 124.0 ± 17 s of pain behavior versus 69.5 ± 20 s for controls (Figure 1(a)). The mMCP6−/− mice did not differ significantly from controls either at any time point, but contrary to the mMCP4−/− mice, the mMCP6−/− mice had a tendency to show lower pain responses than controls during the inflammatory phase; at 40–45 min, they exhibited on average 54.8 ± 16 s of pain behavior versus 101.2 ± 18 s for controls (Figure 1(b)). The pain behavior of CPA3−/− mice did not differ from controls in either direction (Figure 1(c)). The total pain responses in Phase 1 and Phase 2 were also compared, with no difference observed between genotypes (Figure 1(d)).
Figure 1.
mMCP4−/−, mMCP6−/−, and CPA3−/− mice do not differ from controls in the formalin test. Twenty microliters of 5% formalin were injected subcutaneously into the right hind paw and the mouse was then observed for pain behavior for 60 min. (a) mMCP4−/− mice (n = 8) show similar pain behavior to controls (n = 9) over the time course of the formalin test, except with a slight trend for higher pain responses in the last 15 min of the test. (b) In contrast to the mMCP4−/− mice, the mMCP6−/− mice (n = 9) had a slight tendency to show lower pain responses than controls during the inflammatory phase, but the difference was not significant (p > 0.05). (c) The pain behavior of CPA3−/− mice (n = 6) was not significantly different from controls. (d) and (e) No differences were observed between genotypes in the total pain behavior in Phase 1 (0–10 min) or in the inflammatory Phase 2 (10–60 min). The same set of control mice (n = 9) was used in all comparisons and presented in (a), (b), and (c) to facilitate visual presentation. Kruskal–Wallis and Dunn’s post hoc tests were used. Data are presented as mean ± SEM. WT: wild type.
Mast cell deficiency does not affect pain responses in the formalin test, Hargreaves test, or inflammation-induced heat hypersensitivity
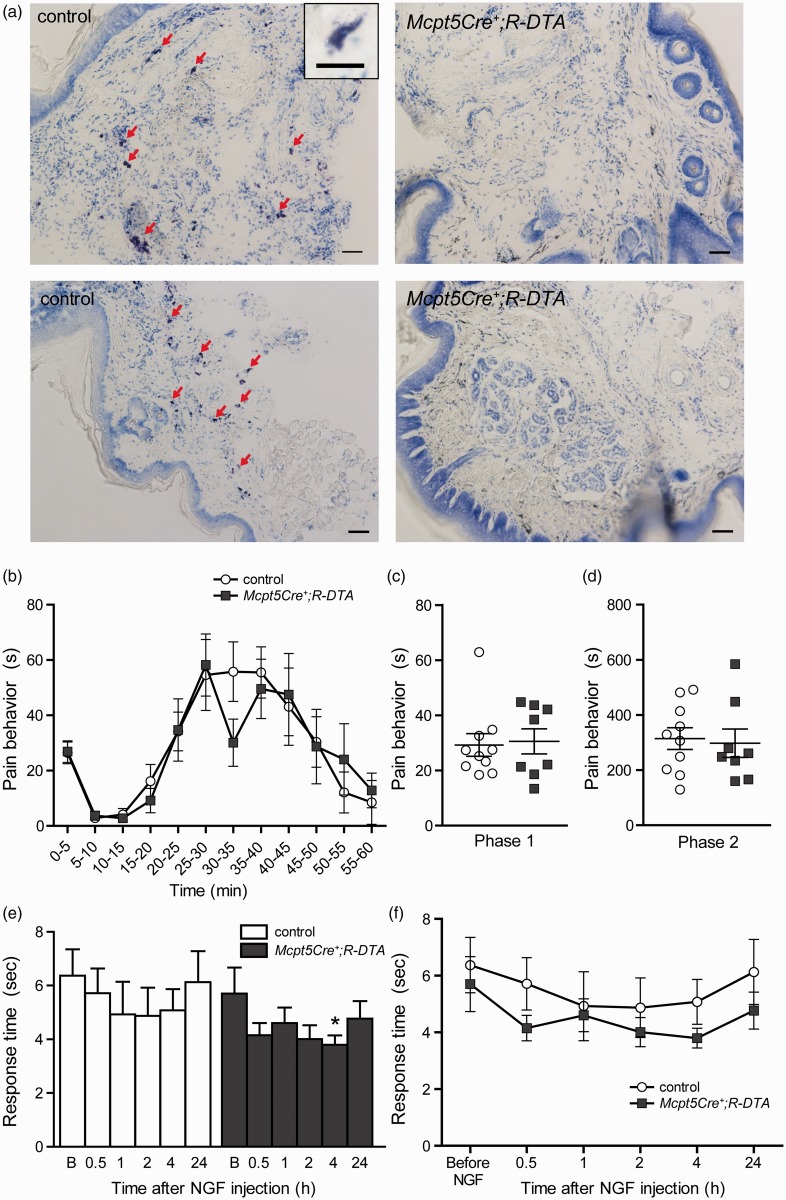
After establishing that the individual mast cell proteases do not contribute significantly to the behavioral phenotype observed in the formalin test, we wanted to investigate if mast cell deficiency would have an effect. To study the role of mast cells in formalin-induced nociception, the connective tissue mast cell-deficient line (Mcpt5Cre+;R-DTA) was used. Previous experiments have shown that Mcpt5Cre+;R-DTA mice have an almost complete depletion of mast cells in the peritoneal cavity as well as in the skin of the abdomen, back, and ears.49 In this study, it was confirmed that mast cells are also absent in plantar hind paw skin (Figure 2(a)). Moreover, an earlier study has shown that Mcpt5Cre+;R-DTA animals completely lack mMCP6, which is exclusively expressed by connective tissue type mast cells.50 The pain responses of mast cell-deficient mice (Mcpt5Cre+;R-DTA) and controls were not significantly different at any time point (Figure 2(b)). However, slightly lower pain responses for mast cell-deficient mice were observed at 30–35 min, where they demonstrated on average 30.1 ± 8.5 s of pain behavior versus 55.8 ± 11 s for controls, but the difference was not significant. When the total pain responses in Phase 1 and Phase 2 were compared between genotypes, no differences were seen (Figure 2(c) and (d)).
Figure 2.
Mast cell-deficient mice do not differ from controls in the formalin test or in heat hypersensitivity induced by NGF. (a) Toluidine blue-stained cryo sections of hind paws. Plantar surfaces of hind paws of naïve Mcpt5Cre-;R-DTA (controls, n = 2) and Mcpt5Cre+;R-DTA (n = 2) were fixed with PFA, embedded in OCT compound, cut into 12-µm-thick sections, and subsequently stained with toluidine blue. Red arrows point to metachromatically stained mast cells in control tissue, which are absent in tissue of Mcpt5Cre+;R-DTA mice. 10× objective, scale bar = 50 µm. Inset represents metachromatically stained mast cell, 20× objective, scale bar = 20 µm. (b) The pain behavior of mast cell-deficient mice (Mcpt5Cre+;R-DTA, n = 8) does not differ from controls (n = 10) over the time course of the formalin test.(Continued)Slightly lower responses were observed at 30–35 min, but the difference was not significant (Mann–Whitney: p = 0.10). (c) and (d) When total pain behavior in Phase 1 (0–10 min) or in the inflammatory Phase 2 (10–60 min) was analyzed, no differences were observed between genotypes. (e–f) Hargreaves test coupled with NGF injection on Mcpt5Cre+;R-DTA mice (n = 7), and controls (n = 6). (e) The baseline Hargreaves values before NGF injection (“B” on the x-axis) did not differ between the two genotypes (Student’s t test: p = 0.64). After NGF injection, only Mcpt5Cre+;R-DTA mice developed heat hypersensitivity compared with their baseline values, at 4-h post-injection (one-way repeated measurement ANOVA: p < 0.05). (f) Despite the absence of significant hypersensitivity in the controls, there was no difference observed between the two genotypes at any time point before or after the injection (Student’s t test: p > 0.05). All data are presented as mean ± SEM. NGF: nerve growth factor.
To investigate if mast cell deficiency has an effect on heat sensation and inflammation-induced heat hypersensitivity, the Hargreaves test coupled with an injection of the TrkA ligand βNGF was performed on Mcpt5Cre+;R-DTA mice and controls. No differences between genotypes were observed in the baseline response times before injection; 5.7 ± 1.0 s for mast cell-deficient mice and 6.4 ± 1.0 s for controls (Figure 2(e) and (f)). To follow the development of heat hypersensitivity, Hargreaves measurements were repeated at 30 min, 1 h, 2 h, 4 h, and 24 h after the injection and compared with the baseline values. The controls did not develop significant hypersensitivity, though there was a trend toward shorter response times post-injection, with the shortest response time measured at 2 h, 4.9 ± 1.0 s (Figure 2(e)). The Mcpt5Cre+;R-DTA mice did develop hypersensitivity at 4 h, with a response time of 3.8 ± 0.3 s (Figure 2(e)). Although the Mcpt5Cre+;R-DTA mice had a trend toward shorter response times than controls (Figure 2(f)) and developed significant heat hypersensitivity at one time point, no statistically significant differences were observed between the genotypes.
Discussion
Here, we have investigated the role of mast cell-specific proteases and mast cells in the pain response associated with the first (acute) phase and second (inflammatory) phase of tissue injury induced by formalin, as well as the role of mast cells in heat sensation and inflammation-induced heat hypersensitivity. No significant differences in pain responses following the formalin test were observed in neither mast cell protease-deficient nor mast cell-deficient mice, compared with control mice. In addition, noxious heat sensation and inflammation-induced heat hypersensitivity as measured by the Hargreaves test coupled with NGF injection were not affected by mast cell deficiency.
Formalin as a translational model for tissue injury-induced pain
Formalin, an aqueous solution of formaldehyde, is a tissue fixative that covalently crosslinks proteins in a nonspecific fashion, thus disrupting cell membranes,2 causing irreversible tissue injury. Furthermore, it has been suggested that it can cause mast cell degranulation directly.51 When formalin is injected into the mouse hind paw, the paw quickly becomes inflamed and a two-phased pain response can be observed. The pain response is usually measured by the amount of time mice spend licking or biting their paw52,53 as was done in this study, or by quantifying the flinching of the injected paw.1 Moreover, in the initial phase, it has been shown that formalin immediately causes pain by directly activating TRPA1,1 which is highly expressed in a subset of C-fiber nociceptors that also express TRPV1.3,4 The secondary pain phase usually appears between 20 and 30 min after formalin injection52 where cells injured by formalin crosslinking release damage-associated molecular patterns that can activate immune cells to initiate inflammation.13,54,55 Inflammatory mediators released from immune cells or directly from damaged tissue continue to activate TRPA1-expressing primary afferents, contributing to pain sensitization of the central nervous system.1,56 The formalin response is also dependent on SP transmission. For instance, Tachykinin 1 (SP precursor gene)-deficient mice show markedly reduced responses to formalin in both the first and the second phase57 and the SP antagonist sendide attenuates the formalin response.58 SP is stored in and released from primary afferents59 and contributes to the second/inflammatory phase of the formalin response by relaying the nociceptive signal to the central nervous system and by interacting with immune cells such as mast cells,5,60 thus promoting the inflammation.
mMCP4 in formalin-induced pain
Mast cell chymase (canine version of mMCP4) has been shown to degrade the neuropeptides SP and VIP,25 which are pro-inflammatory mediators released by primary afferents that can induce mast cell degranulation.26 Mast cell chymase has also been shown to degrade bradykinin in vitro,27 an oligopeptide which also activates TRPA1.61,62 Tissue injury activates the kallikrein-kinin cascade, where the precursor kininogen is converted to the active pain mediator bradykinin by the serine protease kallikrein.53 Bradykinin has been shown to mediate pain in the formalin test by acting through the bradykinin 1 and 2 receptors expressed on peripheral nociceptors.63 Furthermore, it has been reported that mMCP4 degrades IL-33,31,44 which has been shown to have a role in mediating formalin-induced pain.64 Taken together, the slight trend toward an increase in nociceptive behavior observed in the later stages of the inflammatory phase in mMCP4−/− mice, although not significant, may be explained by the reported roles of mMCP4 in degradation of pro-inflammatory mediators.
mMCP6 in formalin-induced pain
Tryptase also has a role in the kallikrein-kinin pathway, as it has been demonstrated that human tryptase can cleave prekallikrein, generating kallikrein and thus contributing to bradykinin formation.65 It has been shown that pain responses and paw edema in mice in both phases of the formalin test can be greatly diminished by inhibiting kallikrein.53 Also, human tryptase can directly generate bradykinin by cleaving kininogen.65 The involvement of mMCP6 in the kallikrein-kinin pathway might explain the statistically nonsignificant trend of mMCP6−/− mice having lower pain responses in the later phase of the formalin test. Despite the capabilities of mast cell tryptase to cleave inflammatory neuropeptides CGRP and VIP in vitro,32 it has not been shown that tryptase can have protective properties in inflammatory conditions in vivo; it mainly has pro-inflammatory effects in that context.66
CPA3 has no apparent effect in formalin-induced pain responses
It has been suggested that IL-33 can initiate a hypernociceptive signaling cascade, by upregulating the production of TNFα which in turn triggers IL-1β → interferon γ (IFNγ) → ET-1 → prostaglandin E2 (PGE2) production.67 CPA3 can cleave ET-1,36 and ET-1 has been shown to induce sensitization to formalin-induced nociception in mice, as well as contributing to paw edema.68 In this study, however, there was no indication that CPA3 deficiency had any effect on formalin-induced pain behavior, suggesting that the role of CPA3 in ET-1 cleavage is of little consequence in the pain responses observed in the formalin test. Studies of the in vivo function of CPA3 are currently limited66 and mainly indicate that it has protective effects by degrading toxins found in bee and snake venom69 but no apparent connection to inflammation. As previously mentioned, CPA3−/− mice also lack the elastase mMCP5,48 which has been shown to contribute to skin inflammation after burn injury in mice.42,43 This might suggest that lacking mMCP5 would have protective effects in inflammation resulting from formalin-induced tissue injury, However, no such effect was observed here.
Mast cells are not essential for tissue injury-induced pain by formalin nor NGF-induced heat hypersensitivity
Given the central role for mast cells in inflammatory conditions,70,71 the close proximity between mast cells and primary afferents and the ability of primary afferent transmitters to activate mast cells, it was surprising to find that mast cell deficiency did not affect the behavioral phenotype induced by formalin injection. However, mast cell-deficient mice were recently shown to develop normal levels of heat and mechanically evoked hypersensitivity associated with NGF or complete Freund’s adjuvant-induced inflammation.72 This supports the theory that mast cells do not significantly contribute to pain-associated inflammatory processes originating in the skin. In this study, mast cell-deficient mice (Mcpt5Cre+;R-DTA) and their Cre-negative littermates were also tested for their sensitivity to heat using the Hargreaves test followed by NGF injection. Hargreaves testing was then repeated to follow the development of heat hypersensitivity. No difference was detected between the two genotypes at any time point. The fact that the mast cell-deficient mice did eventually develop heat hypersensitivity following the NGF injection but their littermates did not indicates that mast cells are not essential for the development of inflammation-induced heat hypersensitivity. Furthermore, other studies have found that mast cells do not express NGF receptors72,73 and would thus have a negligible role in NGF-evoked hypersensitivity.
Conclusion
Here, we have tested for the first time how acute inflammation and tissue injury pain responses in a formalin-based model are affected by the deficiency of mMCPs: mMCP4, mMCP6, and CPA3, as well as mast cell deficiency. Additionally, mast cell-deficient mice were tested in a model for noxious heat sensation and inflammation-induced heat hypersensitivity. Based on the results, neither the proteases mMCP4, mMCP6, CPA3 nor mast cells as a whole were found to be essential for the defined pain behavior.
Acknowledgments
We acknowledge David M Lee (Harvard Medical School, Boston) for providing the mMCP6−/− strain and to Hans-Reimer Rodewald and Thorsten Feyerabend (German Cancer Research Center, Heidelberg, Germany) for providing the Cpa3−/− mice. We also acknowledge Katarzyna Rogoz and Jannice Lyberg for technical support, Magnus Peterson for initial discussions, and the staff at National Veterinary Institute for animal care. MCL is a Ragnar Söderberg Fellow in Medicine.
Author Contributions
EIM performed the behavioral experiments. MG performed histological analysis, genotyping and handled the mouse colony. AR, KH and GP contributed the transgenic mouse lines. EIM, GP and MCL designed the study and wrote the manuscript. All authors have approved the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Swedish Research Council, Uppsala University and the Foundation of Ragnar Söderberg.
References
- 1.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A 2007; 104: 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox ML, Schray CL, Luster CN, Stewart ZS, Korytko PJ, M Khan KN, Paulauskis JD, Dunstan RW. Assessment of fixatives, fixation, and tissue processing on morphology and RNA integrity. Exp Mol Pathol 2006; 80: 183–191. [DOI] [PubMed] [Google Scholar]
- 3.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112: 819–829. [DOI] [PubMed] [Google Scholar]
- 4.Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci 2010; 43: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siebenhaar F, Magerl M, Peters EMJ, Hendrix S, Metz M, Maurer M. Mast cell–driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol 2008; 121: 955–961. [DOI] [PubMed] [Google Scholar]
- 6.Gouin O, L’Herondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhé V, Plée-Gautier E, Carré J-L, Lefeuvre L, Misery L, Le Garrec R. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017; 8: 644–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman RS, Burch RL, Crowder RJ, Lomb DJ, Schoell MC, Straub JA, Xie L. NGF deprivation-induced gene expression: after ten years, where do we stand? Prog Brain Res 2004; 146: 111–126. [DOI] [PubMed] [Google Scholar]
- 8.Hathway GJ, Fitzgerald M. Time course and dose-dependence of nerve growth factor–induced secondary hyperalgesia in the mouse. J Pain 2006; 7: 57–61. [DOI] [PubMed] [Google Scholar]
- 9.Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK, Bannon AW. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain 2013; 17: 469–479. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 2005; 24: 4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci 2007; 34: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997; 77: 1033–1079. [DOI] [PubMed] [Google Scholar]
- 13.Enoksson M, Lyberg K, Möller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol 2011; 186: 2523–2528. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz LB, Bradford TR, Irani AM, Deblois G, Craig SS. The major enzymes of human mast cell secretory granules. Am Rev Respir Dis 1987; 135: 1186–1189. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem 1981; 256: 11939–11943. [PubMed] [Google Scholar]
- 16.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol 2014; 14: 478–494. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak AM. Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem Immunol Allergy 2005; 85: 135–184. [DOI] [PubMed] [Google Scholar]
- 18.Vitte J. Human mast cell tryptase in biology and medicine. Mol Immunol 2015; 63: 18–24. [DOI] [PubMed] [Google Scholar]
- 19.Tchougounova E, Forsberg E, Angelborg G, Kjéllen L, Pejler G. Altered processing of fibronectin in mice lacking heparin. A role for heparin-dependent mast cell chymase in fibronectin degradation. J Biol Chem 2001; 276: 3772–3777. [DOI] [PubMed] [Google Scholar]
- 20.Tchougounova E, Pejler G. Regulation of extravascular coagulation and fibrinolysis by heparin-dependent mast cell chymase. FASEB J 2001; 15: 2763–2765. [DOI] [PubMed] [Google Scholar]
- 21.Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M, Finkelman FD, Pejler G, Hogan SP. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. PNAS 2009; 106: 22381–22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lind T, Gustafson A-M, Calounova G, Hu L, Rasmusson A, Jonsson KB, Wernersson S, Åbrink M, Andersson G, Larsson S, Melhus H, Pejler G. Increased bone mass in female mice lacking mast cell chymase. PLoS One 2016; 11: e0167964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pejler G, Åbrink M, Ringvall M, Wernersson S. Mast Cell Proteases. Adv Immunol 2007; 95: 167–255. [DOI] [PubMed] [Google Scholar]
- 24.Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Chapter 2—Approaches for analyzing the roles of mast cells and their proteases in vivo Adv Immunol 2015, 126, 45–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther 1988; 244: 133–137. [PubMed] [Google Scholar]
- 26.Lowman MA, Benyon RC, Church MK. Characterization of neuropeptide-induced histamine release from human dispersed skin mast cells. Br J Pharmacol 1988; 95: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reilly CF, Schechter NB, Travis J. Inactivation of bradykinin and kallidin by cathepsin G and mast cell chymase. Biochem Biophys Res Commun 1985; 127: 443–449. [DOI] [PubMed] [Google Scholar]
- 28.Omoto Y, Tokime K, Yamanaka K, Habe K, Morioka T, Kurokawa I, Tsutsui H, Yamanishi K, Nakanishi K, Mizutani H. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J Immunol 2006; 177: 8315–8319. [DOI] [PubMed] [Google Scholar]
- 29.Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med 1991; 174: 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol 2005; 175: 2635–2642. [DOI] [PubMed] [Google Scholar]
- 31.Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagälv A, Schlenner SM, Feyerabend T, Rodewald H-R, Kjellén L, Hellman L, Åbrink M. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem 2014; 289: 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam EK, Caughey GH. Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cell Mol Biol 1990; 3: 27–32. [DOI] [PubMed] [Google Scholar]
- 33.Cenac N, Coelho A-M, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol 2002; 161: 1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ui H, Andoh T, Lee J-B, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol 2006; 530: 172–178. [DOI] [PubMed] [Google Scholar]
- 35.Spinnler K, Fröhlich T, Arnold GJ, Kunz L, Mayerhofer A. Human tryptase cleaves pro-nerve growth factor (pro-NGF): hints of local, mast cell-dependent regulation of NGF/pro-NGF action. J Biol Chem 2011; 286: 31707–31713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metsa¨Rinne KP, Vehmaan-Kreula P, Kovanen PT, Saijonmaa O, Baumann M, Wang Y, Nyman T, Fyhrquist FY, Eklund KK. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted Peptide. Arterioscler Thromb Vasc Biol 2002; 22: 268–273. [DOI] [PubMed] [Google Scholar]
- 37.Plant TD, Zöllner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, Furkert J, Stein C, Oksche A. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain 2007; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McQueen DS, Noble MAH, Bond SM. Endothelin-1 activates ETA receptors to cause reflex scratching in BALB/c mice. Br J Pharmacol 2007; 151: 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamura H, Nabe T, Kohno S, Ohata K. Endothelin-1, one of the most potent histamine releasers in mouse peritoneal mast cells. Eur J Pharmacol 1994; 265: 9–15. [DOI] [PubMed] [Google Scholar]
- 40.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol 2011; 716: 212–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magnusson SE, Pejler G, Kleinau S, Abrink M. Mast cell chymase contributes to the antibody response and the severity of autoimmune arthritis. FASEB J 2009; 23: 875–882. [DOI] [PubMed] [Google Scholar]
- 42.Younan G, Suber F, Xing W, Shi T, Kunori Y, Abrink M, Pejler G, Schlenner SM, Rodewald H-R, Moore FD, Stevens RL, Adachi R, Austen KF, Gurish MF. The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. J Immunol 2010; 185: 7681–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bankova LG, Lezcano C, Pejler G, Stevens RL, Murphy GF, Austen KF, Gurish MF. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J Immunol 2014; 192: 2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waern I, Lundequist A, Pejler G, Wernersson S. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal Immunol 2013; 6: 911–920. [DOI] [PubMed] [Google Scholar]
- 45.Kelso EB, Lockhart JC, Hembrough T, Dunning L, Plevin R, Hollenberg MD, Sommerhoff CP, McLean JS, Ferrell WR. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther 2005; 316: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 46.Tchougounova E, Pejler G, Åbrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med 2003; 198: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin K, Watts GFM, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis Infection. J Immunol 2008; 180: 4885–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, Takahashi HK, Morgan ES, Dvorak AM, Fehling HJ, Rodewald H-R. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol 2005; 25: 6199–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Köhler A, Peschke K, Vöhringer D, Waskow C, Krieg T, Müller W, Waisman A, Hartmann K, Gunzer M, Roers A. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011; 34: 973–984. [DOI] [PubMed] [Google Scholar]
- 50.Öhrvik H, Grujic M, Waern I, Gustafson A-M, Ernst N, Roers A, Hartmann K, Pejler G. Mast cells promote melanoma colonization of lungs. Oncotarget 2016; 7: 68990–69001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zouikr I, Ahmed AF, Horvat JC, Beagley KW, Clifton VL, Ray A, Thorne RF, Jarnicki AG, Hansbro PM, Hodgson DM. Programming of formalin-induced nociception by neonatal LPS exposure: maintenance by peripheral and central neuroimmune activity. Brain Behav Immun 2015; 44: 235–246. [DOI] [PubMed] [Google Scholar]
- 52.Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Meth 1985; 14: 69–76. [DOI] [PubMed] [Google Scholar]
- 53.da S Emim JA, Souccar C, de A Castro MS, Godinho RO, Cezari MH, Juliano L, Lapa AJ. Evidence for activation of the tissue kallikrein-kinin system in nociceptive transmission and inflammatory responses of mice using a specific enzyme inhibitor. Br J Pharmacol 2000; 130: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–195. [DOI] [PubMed] [Google Scholar]
- 55.Eigenbrod T, Park J-H, Harder J, Iwakura Y, Núñez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol 2008; 181: 8194–8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Rivkah Isseroff R, Soulika AM, Chigbrow M, Eddinger KA, Yaksh TL. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun 2016; 56: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmer A, Zimmer AM, Baffi J, Usdin T, Reynolds K, Konig M, Palkovits M, Mezey E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc Natl Acad Sci U S A 1998; 95: 2630–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakurada T, Katsumata K, Yogo H, Tan-No K, Sakurada S, Ohba M, Kisara K. The neurokinin-1 receptor antagonist, sendide, exhibits antinociceptive activity in the formalin test. Pain 1995; 60: 175–180. [DOI] [PubMed] [Google Scholar]
- 59.Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science 1975; 190: 889–890. [DOI] [PubMed] [Google Scholar]
- 60.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015; 519: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004; 41: 849–857. [DOI] [PubMed] [Google Scholar]
- 62.Meotti FC, Figueiredo CP, Manjavachi M, Calixto JB. The transient receptor potential ankyrin-1 mediates mechanical hyperalgesia induced by the activation of B1 receptor in mice. Biochem Pharmacol 2016; 125: 1–9. [DOI] [PubMed] [Google Scholar]
- 63.Corrêa CR, Calixto JB. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol 1993; 110: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han P, Zhao J, Liu S-B, Yang C-J, Wang Y-Q, Wu G-C, Xu D-M, Mi W-L. Interleukin-33 mediates formalin-induced inflammatory pain in mice. Neuroscience 2013; 241: 59–66. [DOI] [PubMed] [Google Scholar]
- 65.Imamura T, Dubin A, Moore W, Tanaka R, Travis J. Induction of vascular permeability enhancement by human tryptase: dependence on activation of prekallikrein and direct release of bradykinin from kininogens. Lab Invest 1996; 74: 861–870. [PubMed] [Google Scholar]
- 66.Caughey GH. Mast cell proteases as pharmacological targets. Eur J Pharmacol 2016; 778: 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verri WA, Guerrero ATG, Fukada SY, Valerio DA, Cunha TM, Xu D, Ferreira SH, Liew FY, Cunha FQ. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci U S A 2008; 105: 2723–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piovezan AP, D’Orleans-Juste P, Tonussi CR, Rae GA. Endothelins potentiate formalin-induced nociception and paw edema in mice. Can J Physiol Pharmacol 1997; 75: 596–600. [PubMed] [Google Scholar]
- 69.Metz M, Piliponsky AM, Chen C-C, Lammel V, Åbrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science 2006; 313: 526–530. [DOI] [PubMed] [Google Scholar]
- 70.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 2013; 13: 362–375. [DOI] [PubMed] [Google Scholar]
- 71.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 2008; 8: 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopes DM, Denk F, Chisholm KI, Suddason T, Durrieux C, Thakur M, Gentry C, McMahon SB. Peripheral inflammatory pain sensitisation is independent of mast cell activation in male mice. Pain 2017; 158: 1314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008; 123: 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]