Abstract
A previous study showed that continuous low-dose-rate irradiation promoted the growth of silkworm larvae. This study aimed to confirm that finding, determine the optimal dose rate for growth promotion, and compare low- and high-dose-rate irradiation in silkworms, while also investigating the effects of the radiation-emitting sheet on growth and tumor transplantability in mice. Silkworm eggs were placed on low-dose-emitting sheets with 4 different dose rates (γ-ray rate: 1.7 -22.4 μSv/hour) or on control sheets. The other groups of silkworm larvae received single whole-body X-irradiation (0.1-50 Gy), and subsequent body weight changes were monitored. Starting at 3 weeks old, Balb/c mice were bred on the same sheets, and body weight change was measured. Seven weeks later, the mice were used to investigate the transplantability of EMT6 tumor cells cultured in vitro. The silkworms bred on the 13.4- and 22.4-μSv/hour sheets became larger than the control. Single 50-Gy irradiation suppressed the growth of silkworms. An increase in the time to EMT6 tumor development was observed in low-dose-rate-irradiated mice. This study confirmed growth promotion of silkworms by continuous low-dose radiation and demonstrated growth suppression at a high dose rate. Growth promotion was not observed in mice; further studies using higher dose-rate sheets may be warranted.
Keywords: radiation hormesis, continuous low-dose irradiation, growth promotion, tumor transplantability
Introduction
There is an increasing number of studies addressing the issue of biological effects of low-dose radiation. While some studies have suggested bionegative effects of low-dose radiation, especially in animals with radiosensitive genetic backgrounds,1 many studies have suggested biopositive and beneficial effects. Among the beneficial effects of low-dose radiation, our group has been interested in growth promotion of silkworms, Bombyx mori, and suppression of tumor development in mice. Recently, Shibamoto et al2 reported growth promotion of silkworm larvae bred on low-dose radiation-emitting sheets. They used a sheet with a γ-ray dose rate of 3.8 μSv/hour (h), but no other dose rates were investigated. In particular, effects of much higher radiation doses were not investigated, so the possible existence of a “biphasic response,” that is, promotion at low doses and suppression at high doses, was not explored.
Suppression of tumorigenesis and metastases by low-dose radiation has been reported by several groups.3–10 Our group has also investigated tumor cell transplantability after single doses of X-irradiation.11 In that study, 100 or 1000 tumor cells cultured in vitro were transplanted to syngeneic mice that had been preirradiated with 0 to 1500 mGy to the whole body; subsequent tumor development was then monitored. An increase in the mean time to tumor appearance was observed in Balb/c mice receiving 100 or 200 mGy, although overall transplantation rates were not different.
To extend the previous investigations from our group, we performed the present study to address multiple research questions. We aimed to investigate: (1) the optimal dose rate for growth promotion of silkworms; (2) the difference between continuous low-dose irradiation and single irradiation in silkworms; (3) the biphasic response to irradiation in silkworm growth; (4) growth promotion by low-dose irradiation in mice; and (5) suppression of tumor transplantability by low-dose irradiation in mice.
Materials and Methods
Low-Dose-Radiation-Emitting Sheets
The sheets used in this study were manufactured at Aoyama Stein Co, Ltd (Kobe, Japan). The methods for making the sheets have been described previously.2 Briefly, monozites containing 228Ac and 77Br were rubbed into sheets made of polyvinyl chloride (PVC) materials. For this study, 2 kinds of sheets containing different amounts of isotopes were made (“very-low-dose” sheet and “low-dose” sheet). For control groups, PVC sheets with similar colors and physical properties containing no radioisotopes were also manufactured. Both sheets had a thickness of 3 mm and were cut to 20 × 15 cm to fit the breeding cages. Higher dose rates were obtained by stacking multiple sheets. The sheet types and number of stacked sheets employed for the silkworm and mouse experiments are summarized in Table 1. Radiation dose rates at the surface of combinations of the sheets were measured with a Geiger-Mueller counter (Inspector USB, Measure Works, Tokyo, Japan) for α, β, and γ rays, and an ion chamber survey meter (ICS-321B; Aloka, Tokyo, Japan) for γ rays. Table 1 also shows the dose rates for the various combinations of sheets used in the experiment. When the sheets were stacked, the α, β, and γ rays from the lower sheets were considered to be absorbed or attenuated by the upper sheets, so the dose rates of the sheets were not in proportion to the number of the sheets.
Table 1.
Experimental Parameters Including Dose Rate, Number of Radiation-Emitting or Control Sheets, and Number of Silkworms and Mice (Inoculation Site).
| Dose Rate | |||
|---|---|---|---|
| Type of experiment | n | α, β, γ (μSv/hour) | γ (μSv/hour/mSv/year) |
| Silkworm | |||
| Body weight change | |||
| 1) Very-low-dose × 1 | 50 | 39.8 | 1.7/14.9 |
| 2) Very-low-dose × 4 | 50 | 45.0 | 4.1/35.9 |
| 3) Low-dose × 1 | 50 | 105.0 | 13.4/117.4 |
| 4) Low-dose × 2 | 50 | 131.0 | 22.4/196.2 |
| Mouse | |||
| Body weight change | |||
| 5) Very-low-dose × 1 | 50 | 39.8 | 1.7/14.9 |
| 6) Very-low-dose × 5 | 25 | 45.4 | 5.2/45.6 |
| 7) Low-dose × 1 | 30 | 105.0 | 13.4/117.4 |
| Tumor transplantability | |||
| 8) Very-low-dose × 5 | 50 | 45.4 | 5.2/45.6 |
| 9) Low-dose × 1 | 60 | 105.0 | 13.4/117.4 |
| 10) Control × 1 | 0.3 | 0.1 / 0.9 | |
Silkworms
Approval by the Animal Ethics Committee of Nagoya City University was unnecessary for this aspect of the study because silkworms are nonmammalian. Silkworm eggs and feed containing mulberry powder, vitamins, and minerals were purchased from Kougensha Co, Ltd (Matsumoto, Japan). They were randomly divided into 5 groups and placed on low-dose-radiation-emitting sheets and control sheets. Fifty eggs were placed on the sheets in each group. The eggs were incubated at 25°C to 27°C under a humidified atmosphere (60%-70% humidity). After about 14 days, the eggs hatched and young silkworms emerged, with no apparent difference between the low-dose radiation groups and the control groups. Thereafter, feed was placed on the sheets and the young silkworms moved freely on the sheets. The young silkworms were very small and fragile immediately after hatching. Therefore, the body weights of all silkworms were measured 3 times a week from about 14 days after hatching.
In experiments investigating the effect of single-dose X-ray irradiation, the silkworm larvae were irradiated immediately after hatching, on the day all eggs hatched. Larvae were whole-body irradiated in a plastic chamber with an X-ray machine (CAX-210; Chubu Medical Co. Ltd, Yokkaichi, Japan; 210 kV, 10 mA, 2-mm Al filter) without physical restraint or anesthesia. This machine has been used in our previous biological studies, and radiation procedures have been described previously in greater detail.12 Silkworms were irradiated at a dose rate of 1 Gy/min to 0.1, 0.6, 3.6, 20, and 50 Gy.
Mice
Experimental studies using mice were approved by the Animal Ethics Committee of Nagoya City University. Balb/c female mice at the age of 3 weeks were purchased from Chubu Kagaku Shizai Co. Ltd (Nagoya, Japan). They were maintained under specific pathogen-free conditions at 24°C ± 2°C and 60% ± 10% relative humidity and were provided with nutritional chow (CRF-1; Clarles River Laboratories Japan Inc., Yokohama, Japan) and reverse osmosis (RO) water ad libitum. The RO water rejects approximately 95% to 99% of all ionic materials including bacteria, viruses, and pyrogens. On the day of purchase, mice were randomly divided into 2 groups and placed on the low-dose radiation-emitting and control sheets. The body weights of all mice were measured once a week thereafter.
Tumor Transplantability Study
The characteristics of the EMT6 cells and tumors used in this experiment have been described in detail previously.13 The experiments were performed after breeding the mice on the sheets for 7 weeks. Exponentially growing EMT6 cells cultured in vitro were trypsinized and suspended in phosphate-buffered saline (PBS). Cell density was measured with a Coulter counter and the suspension was diluted with PBS to a density of 105 cells/mL. Then 10 μL of the suspensions of EMT6 (ie, containing approximately 1000 cells) were injected subcutaneously into both hind legs of 10-week-old Balb/c mice in the low-dose sheet and control sheet groups. After tumor cell inoculation, the mice were returned to the same cages with radiation-emitting or control sheets. Thereafter, development of tumor masses was checked 3 times a week until day 50. Tumor transplantability was judged separately in the right and left legs. The 3 dimensions of each tumor were measured using a caliper and tumor volume was estimated using the formula π/6 × product of the 3 dimensions. Tumors were judged as grown when the volume of palpable nodules exceeded 200 mm3.
Statistical Analysis
Body weight changes of the silkworms and mice were compared using factorial analysis of variance followed by the Bonferroni/Dunn post hoc test. Tumor transplantability curves were compared by the logrank test. Times to tumor development in the mice developing EMT6 tumors were compared by t test. All statistical analyses were performed using StatView version 5.0 (SAS Institute Inc., Cary, North Carolina).
Results
Growth Promotion of Silkworms on Radiation-Emitting Sheets
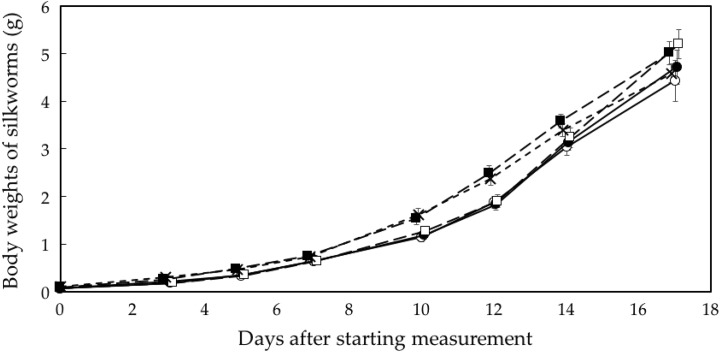
Figure 1 shows changes in the body weight of the silkworm larvae. The silkworms grown on the 2 low-dose-rate sheets (γ-ray dose rate: 13.4 and 22.4 μSv/h) became larger than those of the control group (P < .001), whereas the silkworms grown on the lower dose-rate sheets (Table 1, No. 1 and 2) did not show accelerated growth.
Figure 1.
Changes in body weights of silkworms. ^, control; •, 1 very-low-dose sheet (γ-ray rate: 1.7 μSv/h); □, 4 very-low-dose sheets (4.1 μSv/h); ▪, 1 low-dose sheet (13.4 μSv/h); ×, 2 low-dose sheets (22.4 μSv/h). n = 50 for each group. Data represent mean and standard errors.
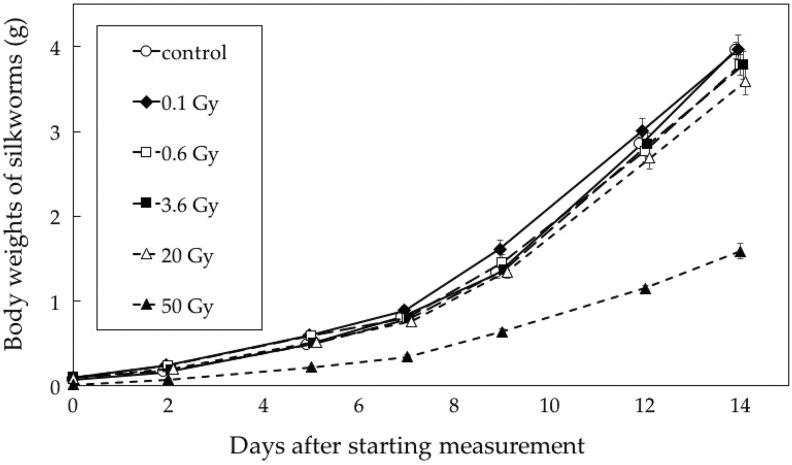
Growth of Silkworms Receiving Single Irradiation at High Dose Rates
Figure 2 shows changes in the body weight of silkworm larvae after single low-dose-rate X-irradiation. The 50-Gy group showed significant growth retardation. Otherwise, there were no differences between the groups receiving 0.1 to 20 Gy and the control group.
Figure 2.
Changes in body weights of silkworms irradiated at a high dose rate. n = 50 for each group except for the 50-Gy group (n = 25). Data represent mean and standard errors.
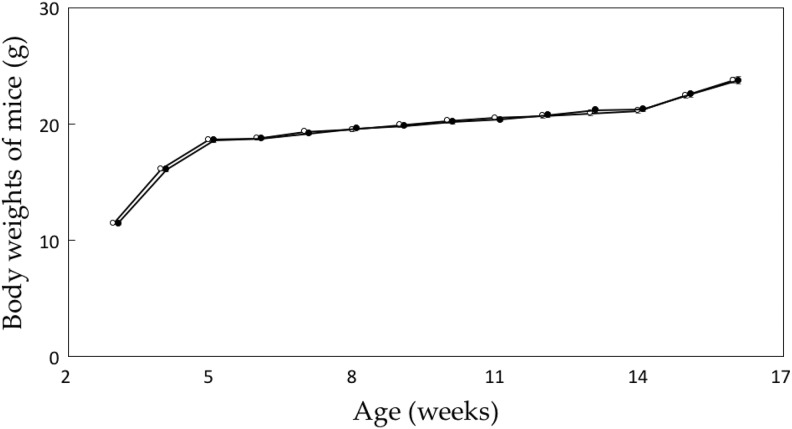
Growth of Mice Bred on Radiation-emitting Sheets
Figure 3 shows changes in the body weight of Balb/c mice bred on the low-dose sheet (Table 1, No. 7; γ-ray rate, 13.4 μSv/h) and those bred on the control sheet; there was no difference between the 2 groups. In the other groups of mice, breeding on the lower dose-emitting sheets (Table 1, No. 5 and 6) also did not promote an increase in body weight (data not shown).
Figure 3.
Changes in body weights of Balb/c mice. ^, control; •, radiation-emitting sheet (13.4 μSv/hour). n = 30 for each group. Data represent mean and standard errors.
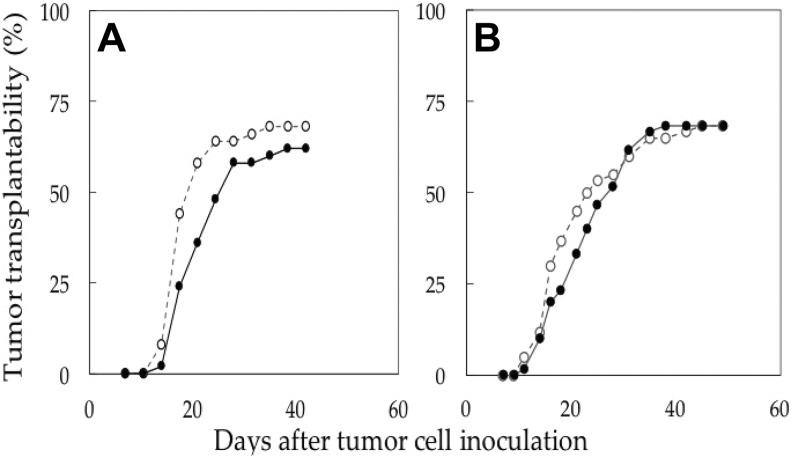
Tumor Transplantability in Mice
In these experiments, there were no differences in the overall transplantability curves between the mice bred on the radiation-emitting sheets and those on the control sheet (Figure 4). However, when the time to tumor appearance was compared in mice developing EMT6 tumors, the mean period to tumor development was longer in mice receiving low-dose irradiation (Figure 4A: γ-ray rate: 5.2 μSv/h; P = .02 by t test). The differences were also significant when analyzed by the logrank test in the groups developing EMT6 tumors (P = .016).
Figure 4.
EMT6 tumor transplantability in Balb/c mice. ^, control; •, radiation-emitting sheet (A, 5.2 μSv/h; B, 13.4 μSv/h). Each group consisted of 50 (A) or 60 (B) inoculation sites.
In the mice bred on the higher dose sheets (13.4 μSv/h), a similar pattern appeared to be present but the difference was not significant (P = .34 by t test and .61 by the logrank test; Figure 4B).
Discussion
In this study, we confirmed the growth promotion effects of low-dose continuous irradiation on silkworms that had been reported previously.2 The dose rates for which we observed growth promotion were higher than those used in the preceding study (γ-ray rate: 13.4 and 22.4 μSv/h vs 3.8 μSv/h) and we did not observe growth promotion at lower dose rates. We speculate that this discrepancy might have resulted from the differences in the methods adopted for selection of silkworm eggs. In the former study, 55 young silkworms were randomly chosen from 200 silkworms for each group; by contrast, we studied all 50 eggs in each group in this study. There might have been a selection bias in the previous study, and the selected well-grown larvae might have been more likely to respond to the lower dose stimuli. The γ-ray dose rates at which we observed growth promotion were 117 and 196 mSv/year. The mechanisms underlying this effect should be investigated in future studies. It is currently unclear whether these are the increased production of growth-hormone-like substances, increased appetite, or other mechanisms. Consumption of feed tended to be faster in the silkworms bred on the radiation-emitting sheets (Table 1, No. 3 and 4), but we were unable to quantify this parameter.
In this study, we confirmed the existence of a biphasic response to irradiation in silkworms, that is, growth promotion at low doses and suppression at high doses. This finding supports the conclusion that the linear-no-threshold hypothesis is inappropriate, as has been emphasized recently.14–16 Chronic irradiation promoted growth, whereas acute irradiation at similar doses did not. Although it is not certain whether the timing of single-dose X-irradiation was optimal or not, the results of the present study suggest that chronic low-dose-rate irradiation is more likely to offer beneficial effects than single low-dose irradiation.
On the other hand, no growth promotion was observed in Balb/c mice using the same low-dose emitting sheets. Mouse experiments were different from silkworm experiments in several ways. Firstly, the body size of the mice was considerably larger than the size of the silkworm larvae, so that radiation doses to the whole body was correspondingly lower for mice. Since no sheets with a higher dose rate were available at the time of the experiments, using much higher dose rates was difficult. Therefore, our group is trying to make higher dose-rate sheets. In addition, the mice moved more actively in the cages than the silkworms, even climbing to the top wire mesh of the cage. This behavior could further reduce the effective dose. Furthermore, it was only possible to purchase mice at the age of 3 weeks, at which point experiments were started. By contrast, in silkworm experiments, eggs could be placed on the sheet. Therefore, further studies using higher dose-rate sheets and self-bred mice from birth may be warranted.
Nevertheless, a significant delay in EMT6 tumor development in mice was observed using the higher dose-rate sheets. Overall transplantability rates were not different. In a previous study from our laboratory, similar results were obtained using a single low-dose X-irradiation. Ito et al11 found that in groups inoculated with 100 or 1000 EMT6 cells, the mean time to tumor appearance was significantly longer in mice receiving 100 or 200 mGy at 6 or 24 hours before tumor cell inoculation compared with mice receiving sham-irradiation. The single dose of 100 or 200 mGy is higher than the total dose used in the present study. This phenomenon of delay in tumor appearance has been observed in other studies,9,10 and would imply a manifestation of stimulation of immune responses. Some molecular level evidences for such low-dose effects have been reported17–22; low-dose radiation was found to stimulate cell proliferation via the activation of the mitogen-activated protein kinase (MAPK) pathway,17–19 and p53 mutation was found to be involved in low-dose radiation-induced hormesis, adaptive response, radioresistance and genomic instability.20,21 These mechanisms are related to each other and might cause an immunological response. Under more optimal conditions including stronger stimulation, tumor transplantability rates may be lowered by low-dose-rate irradiation.
A limitation of this study was the absence of an investigation into the mechanism for the observed effects due to the absence of a method for doing so in silkworms. This study suggests that further investigations under various conditions, including higher dose rates and longer durations of radiation exposure, are warranted. Studies with cultured cells have been carried out, and the results will be published in the near future. If we succeed in clarifying the conditions for observing growth promotion and for reducing tumor transplantability in mice, we plan to examine the immunological parameters of the mice, since the stimulatory effects of low-dose radiation on the immune system have been reported.22
In conclusion, hormetic effects by continuous low-dose irradiation were demonstrated using low-dose-radiation-emitting sheets. These effects included promotion of growth in silkworms and an increase in the time to tumor development in mice.
Acknowledgments
The authors would like to thank Dr Masato Ito and Dr Tatsuya Kawai for their helpful advice on materials and methods and Takahiro Tsuchiya for radiation dosimetry.
Footnotes
Declaration of Conflicting Interests: The author(s) declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Tang FR, Loke WK, Khoo BC. Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J Radiat Res. 2017;58(2):165–182. doi:10.1093/jrr/rrw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shibamoto Y, Kamei Y, Kamei K, Tsuchiya T, Aoyama N. Continuous low-dose-rate irradiation promotes growth of silkworms. Dose Response. 2017;15(4). doi:10.1177/1559325817735252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26(2):177–179. doi:10.1016/0167-8140(93)90101-D. [DOI] [PubMed] [Google Scholar]
- 4. Sakai K, Hoshi Y, Nomura T, et al. Suppression of carcinogenic processes in mice by chronic low dose rate gamma-irradiation. Int J Low Radiat. 2003;1(1):142–146. doi:10.1504/IJLR.2003.003485. [Google Scholar]
- 5. Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by life-long low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163(2):153–158. doi:10.1667/RR3289. [DOI] [PubMed] [Google Scholar]
- 6. Cheda A, Wrembel-Wargocka J, Lisiak E, Nowosielska EM, Marciniak M, Janiak MK. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat Res. 2004;161(3):335–340. doi:10.1667/RR3123. [DOI] [PubMed] [Google Scholar]
- 7. Ishii K, Hosoi Y, Yamada S, Ono T, Sakamoto K. Decreased incidence of thymic lymphoma in AKR mice as a result of chronic, fractionated low-dose total-body X irradiation. Radiat Res. 1996;146(5):582–585. doi:10.2307/3579560. [PubMed] [Google Scholar]
- 8. Sakamoto K, Myojin M, Hosoi Y, et al. Fundamental and clinical studies on cancer control with total or upper half body irradiation. J Jpn Soc Ther Radiol Oncol. 1997;9(3):161–175. doi:10.11182/jastro1989.9.161. [Google Scholar]
- 9. Lemon JA, Phan N, Boreham DR. Single CT scan prolongs survival by extending cancer latency in Trp53 heterozygous mice. Radiat Res. 2017;188(4.2):505–511. doi:10.1667/RR14576.1. [DOI] [PubMed] [Google Scholar]
- 10. Lemon JA, Phan N, Boreham DR. Multiple CT scans extend lifespan by delaying cancer progression in cancer-prone mice. Radiat Res. 2017;188(4.2):495–504. doi:10.1667/RR14575.1. [DOI] [PubMed] [Google Scholar]
- 11. Ito M, Shibamoto Y, Ayakawa S, Tomita N, Sugie C, Ogino H. Effect of low-dose total-body irradiation on transplantability of tumor cells in syngeneic mice. J Radiat Res. 2008;49(2):197–201. doi:10.1269/jrr.07094. [DOI] [PubMed] [Google Scholar]
- 12. Shibamoto Y, Ito M, Sugie C, Ogino H, Hara M. Recovery from sublethal damage during intermittent exposures in cultured tumor cells: implications for dose modification in radiosurgery and IMRT. Int J Radiat Oncol Biol Phys. 2004;59(5):1484–1490. doi:10.1016/j.ijrobp.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 13. Shibamoto Y, Streffer C, Fuhrmann C, Budach V. Tumor radiosensitivity prediction by the cytokinesis-block micronucleus assay. Radiat Res. 1991;128(3):293–300. doi:10.2307/3578052. [PubMed] [Google Scholar]
- 14. Marcus CS. Destroying the linear no-threshold basis for radiation regulation: a commentary. Dose Response. 2016;14(4). doi:10.1177/1559325816673491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabrese EJ. The mistaken birth and adoption of LNT: an abridged version. Dose Response. 2017;15(4). doi:10.1177/1559325817735478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacks B, Siegel JA. Preserving the anti-scientific linear no-threshold myth: authority, agnosticism, transparency, and the standard of care. Dose Response. 2017;15(3). doi:10.1177/1559325817717839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasaki M, Ejima Y, Tachibana A, et al. DNA damage response pathway in radioadaptive response. Mutat Res. 2002;504(1-2): 101–118. doi:10.1016/S0027-5107(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 18. Kavitha P, Shankar BS. Involvement of MAPK signaling in radioadaptive response in BALB/c mice exposed to low dose ionizing radiation. Int J Radiat Biol. 2016;92(5):249–262. doi:10.3109/09553002.2016.1146829. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi A, Asakawa I, Yuki K, et al. Radiation-induced apoptosis in the scid mouse spleen after low dose-rate irradiation. Int J Radiat Biol. 2002;78(8):689–693. doi:10.1080/09553000210132306. [DOI] [PubMed] [Google Scholar]
- 20. Feng RT, Weng KL. Molecular mechanisms of low dose ionizing radiation-induced hormesis, adaptive responses, radioresistance, bystander effects, and genomic instability. Int. J. Radiat. Biol. 2015;91(1):13–27. doi:10.3109/09553002.2014.937510. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi A, Ohnishi K, Asakawa I, et al. Radiation response of apoptosis in C57BL/6 N mouse spleen after whole-body irradiation. Int J Radiat Biol. 2001;77(9):939–945. doi:10.1080/09553000110062873. [DOI] [PubMed] [Google Scholar]
- 22. Shibamoto Y, Nakamura H. Overview of biological, epidemiological, and clinical evidence of radiation hormesis. Int J Mol Sc. 2018;19(8):2387 doi:10.3390/ijms19082387. [DOI] [PMC free article] [PubMed] [Google Scholar]