Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a well-known environmental teratogenic agent for cleft palate. But transforming growth factor β3 (TGF-β3) is an essential growth factor for palatogenesis. This study is to clarify effects of TCDD and TGF-β3 in mouse embryonic palatal mesenchymal (MEPM) cells. The result showed that with increase of TCDD (0.5 nM-10 nM), the expression of TGF-β3 increased, but after 10 nM TCDD, the expression of TGF-β3 reduced. The viabilities of MEPM cells decreased in 10 nM TCDD-treated group. But the viabilities increased in 10 ng/mL TGF-β3-treated group, or the viabilities were between that of them in combination of 10 nM TCDD and 10 ng/mL TGF-β3-treated group. This phenomenon was the same as the motilities. In addition, we found that the expression of phosphorylated Smad2/3 and Smad7 was increased by 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3 induced, but the expression of Smad4 was decreased. These data revealed that the TGF-β/Smad signaling pathway affected TCDD and TGF-β3 in MEPM cells.
Keywords: TCDD, MEPM cells, TGF-β3, TGF-β/Smad signaling
Introduction
Palatogenesis is a tightly regulated process, in which multipotential mesenchymal cells can differentiate into chondrocytes to form cartilage.1,2 The secondary palates of the palatal shelves grow and fuse in mesenchymal cell proliferation. Interestingly, transforming growth factor β3 (TGF-β3) is expressed in the palatal mesenchyme during palatal growth and elevation.3 Transforming growth factor β3 plays a very important role in a variety of cellular processes including cell proliferation, differentiation, apoptosis, migration, invasion, matrix synthesis, and the immune response.4-6 It was also thought that TGF-β3 played a very important role during palatogenesis.7 Transforming growth factor β3 was an important regulator of palatal fusion, and TGF-β3-knockout mice exhibited cleft palate, but exogenous TGF-β3 treated TGF-β3-knockout mice can rescue palatal fusion.8 Moreover, TGF-β3 was also the most effective for human palatal mesenchymal cell proliferation.9 Transforming growth factor β3 can initiate diverse cellular responses by binding and activating specific cell surface receptors, which also can activate TGF-β receptors to stimulate the phosphorylation of receptor-regulated Smad proteins, such as Smad2 and Smad3. Phospho-Smad2/3 (p-Smad2/3) formed complexes with Smad4, which accumulated in the nucleus and regulated the transcription of target genes. The actions of TGF-β were antagonized by Smad7, which can prevent phosphorylation of Smad2/3, thereby blocking TGF-β/Smad signaling.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a well-known teratogenic agent of cleft palate. Morphological studies revealed that TCDD caused cleft palate by disturbing palatal shelf growth and inhibiting the fusion of palatal shelves.10 Many genes played important roles in palatogenesis such as TGF-β3, KLF4.7,11 But there were few reports about the relationships of TCDD and TGF-β3 in mouse embryonic palatal mesenchymal (MEPM) cells. In this study, we found the possible effects of TCDD and TGF-β3 in MEPM cells.
Materials and Methods
Cell Culture and Treatment
Mouse embryonic palatal mesenchymal cells were derived from palatal tissue on 13-day C57BL/6 mice embryos (Henan Laboratory Animal Center of Zhengzhou University, China). All experiments were performed in accordance with the Experimental Animal Center Guide for the Care and Use of Laboratory Animals, and the Institutional Ethical Guidelines for Experiments with Animals. The method of MEPM cell culture was according to the detail by Feng et al.12 The MEPM cells were cultured in flasks with DMEM (Dulbecco's Modified Eagle's medium)/F12 medium (Hyclon, Logan, Utah) supplemented with 10% fetal calf serum (Sijiqing, Hangzhou, China). The MEPM cells were placed in a humidified incubator at 37°C with 5% CO2 atmosphere with media replaced every other day. The third passage cells were seeded. Some cells were treated with 0.5 nM, 1 nM, 5 nM, 10 nM, 20 nM, and 50 nM TCDD, and TCDD concentration was selected according to some reports,13,14 others were treated with 10 nM TCDD (DD-2378-S, Sigma, Saint Louis, Missouri), or 10 ng/mL TGF-β3 (cyt-143, PROSPEC, Zion, Israel), or combination of 10 nM TCDD and 10 ng/mL TGF-β3 for further analysis. Control cells were treated with DMSO (Dimethyl sulfoxide) (D2650; Sigma).
Quantitative Real-Time Polymerase Chain Reaction
Total RNAs were isolated from MEPM cells using Trizol reagent (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. To detect expression of TGF-β3, first strand complementary DNA (cDNA) was synthesized using a PrimeScript II 1st strand cDNA Synthesis Kit (6210A, TakaRa Biotechnology, Kyoto, Japan), and then amplified by quantitative real-time polymerase chain reaction (qRT-PCR) with the SYBR Premix Ex Taq Kit (DRR420A; TaKaRa) through ABI 7900 PRISM system (7900HT; Applied Biosystems, Carlsbad, California). GAPDH was used as an internal control. The qRT-PCR conditions were as follows: polymerase activation for 15 minutes at 95°C, 40 cycles at 95°C for 15 seconds, 56°C for 20 seconds, and 72°C for 30 seconds. Polymerase chain reaction products were identified by melting curve analysis. All primers were synthesized by Invitrogen. The primer sets were as follows: TGF-β3-forward: 5′-CCTGGCCCTGCTGAACTTG-3′, and reverse, 5′-TTGATGTGGCCGAAGTCCAAC-3′. GAPDH-forward: 5′-TGACGTGCCGCCTGGAGAAAC-3′, and reverse, 5′-CCGGCATCGAAGGTGGAAGAG-3′.
Cell Viability Assays
To evaluate the effect of TCDD, the viabilities of MEPM cells were determined by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (JT343, Genview, California) assay. The third passage of MEPM cells (5 × 103 cells per well) were seeded in 96-well plates (Nunc, Denmark). The cells were treated with 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3, or DMSO (≤0.05%). After 72 hour-incubation, the cell viabilities were detected by MTT assay according to the manufacturer’s protocols.
Scratch Wound-Healing Motility Assay
The MEPM cells were seeded on 6-well plates and allowed to grow to confluence. Confluent monolayers were scratched with a pipette tip and the cells were washed with PBS 3 times to remove cells debris. The cells were treated with 10 nM TCDD, or 10 ng/mL TGF-β3 or combination of 10 nM TCDD and 10 ng/mL TGF-β3, respectively. After maintaining under standard conditions for 24 hours, plates were washed once with fresh medium to remove nonadherent cells and then photographed. The percentages of open spaces covered by migrated cells were determined using Image J software (1.37 National Institutes of Health, Bethesda, MD, USA) (http://rsb.info.nih.gov/ij/).
Western Blot Analysis
Total lysates from different treated MEPM cells using 5× SDS-lysis buffer supplemented with proteases inhibitors (M250, Amresco, Ohio) were determined. Protein concentration was determined using a standard BSA protein assay (Dingguo, Beijing, China). A total of 40 μg proteins were fractionated on 12% SDS-PAGE, and transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, the membranes were immunoblotted with the primary antibodies: Smad2 (ab71109, Abcam, Massachusetts), p-Smad2 (sc101801, Santa Cruz, California), Smad3 (BM3559, Boster Biotech, Wuhan, China), p-Smad3 (GD-CZ5616R, Santa Cruz), Smad4 (PB0446, Boster Biotech), and Smad7 (sc-365846, Santa Cruz). β-actin was probed as a loading control. Then membranes were washed and incubated with HRP-conjugated secondary antibody (sc-2004 or sc-2005, Santa Cruz), Western blot analysis was performed using the Odyssey Infrared Imaging System (Li-Cor Lincoln, Nebraska).
Statistical Analysis
All data were compared using either double-sided Student t test or one-way analysis of variance. The choice of tests was performed automatically using SPSS software, version 13.0 (SPSS, Chicago, Illinois). All data were presented as mean (standard deviation) of 3 independent experiments. Differences were considered to be statistically significant when *P < .05.
Results
The Effect of TGF-β3 by TCDD in MEPM Cells
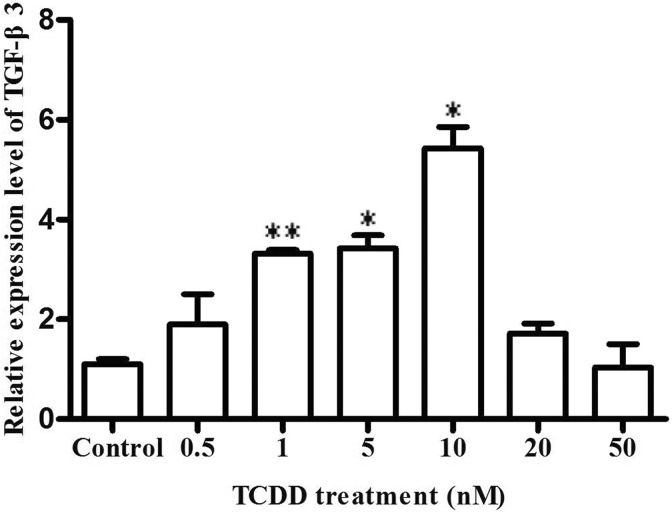
Transforming growth factor β3 was the essential growth factor for palatogenesis.15 We explored the expression of TGF-β3 in MEPM cells by 0.5 nM, 1 nM, 5 nM, 10 nM, 20 nM, and 50 nM TCDD induced for 72 hours. As shown in Figure 1, we found that the mRNA levels of TGF-β3 significantly increased (1.90 ± 0.86 fold), (3.32 ± 0.10 fold), (3.42 ± 0.37 fold), and (5.43 ± 0.61 fold) in MEPM cells for 0.5 nM, 1 nM, 5 nM, and 10 nM TCDD induced compared to the corresponding control cells, respectively. The mRNA levels of TGF-β3 decreased (1.72 ± 0.28 fold) and (1.04 ± 0.66 fold) at 20 and 50 nM TCDD compared to the corresponding control cells, respectively.
Figure. 1.
The effect of TGF-β3 by TCDD induced in MEPM cells. 0.5 nM, 1 nM, 5 nM, 10 nM, 20 nM and 50 nM TCDD, or DMSO (≤0.05%)-treated MEPM cells as the experiment group and control group, respectively. After treated for 72 hours, the expression of TGF-β3 was measured by qRT-PCR. Data were mean values ± standard deviation of 3 replicate experiments. *P < .05 or **P < .01 versus the corresponding control values. MEPM indicates mouse embryonic palatal mesenchymal; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TGF-β3, transforming growth factor β3; qRT-PCR, quantitative real-time polymerase chain reaction.
The Effects of Cell Proliferation in MEPM Cells
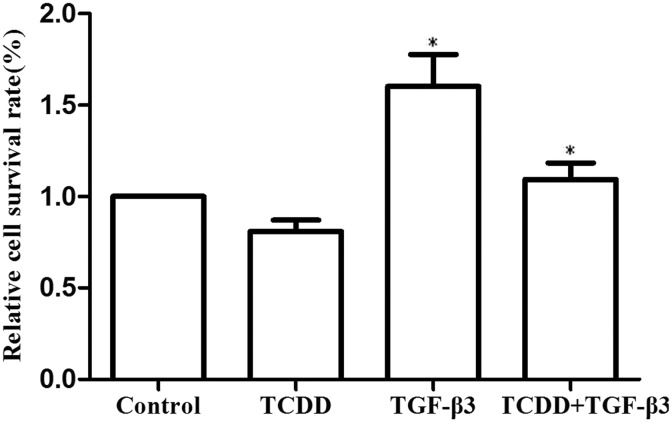
In the experiments, we evaluated the effect of cell proliferation at 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3 in MEPM cells. After treatment for 72 hours, the cell proliferation was measured by MTT assay. As shown in Figure 2, we found that the relative cell survival rate of TCDD group was decreased (13.23 ± 11.40%) compared to the corresponding control cells, but proliferation rate of TGF-β3 group was increased (56.34 ± 1.56%), while that of the combination of TCDD and TGF-β3 group of cells was increased (11.56 ± 42.41%).
Figure. 2.
The effects of cell proliferation in MEPM cells. 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3, or DMSO (≤0.05%) treated MEPM cells as the experiment group and control group, respectively. After treated for 72 hours, the cell proliferation was determined by MTT assay according to the manufacturer’s protocols. Data were mean values ± standard deviation of three replicate experiments. *P < .05 versus the corresponding control values. MEPM indicates mouse embryonic palatal mesenchymal; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
The Effects of Cell Motility in MEPM Cells
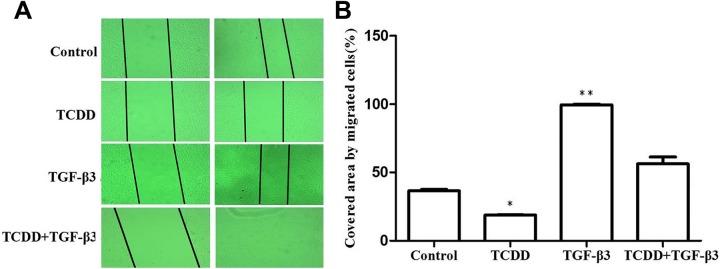
To determine whether 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3 affected the motilities of MEPM cells. We used wound-scratch healing assay to detect the cell motility. After 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3 exposed to MEPM cells for 24 hours, the scratch test was performed. As shown in figure 3, we found that the covered area by migrated cells in the control group was (36.53 ± 1.41%) compared to corresponding control cells. We found that the covered area in the 10 nM TCDD group was (18.80 ± 0.29%) compared to the corresponding control cells. We found that the covered area in the combination of 10 nM TCDD and 10 ng/mL TGF-β3 group was (56.29 ± 7.07%) compared to the corresponding control cells. We found that the covered area in the combination of 10 ng/mL TGF-β3 group was (99.50 ± 0.71%) compared to the corresponding control cells.
Figure. 3.
The effects of cell migration in MEPM cells. When MEPM cells were seeded and grew to confluence, the cells were scratched with a pipette tip, and 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3, or DMSO (≤0.05%) treated MEPM cells as the experiment group and the control group, respectively. After 24 hours treatment, the cells were photographed. The wound closure was determined using ImageJ software. Data were mean values ± standard deviation of 3 replicate experiments. *P < .05 or **P < .01 versus the corresponding control values. MEPM indicates mouse embryonic palatal mesenchymal; TGF-β3, transforming growth factor β3; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
The Effects of TGF-β/Smad in MEPM Cells
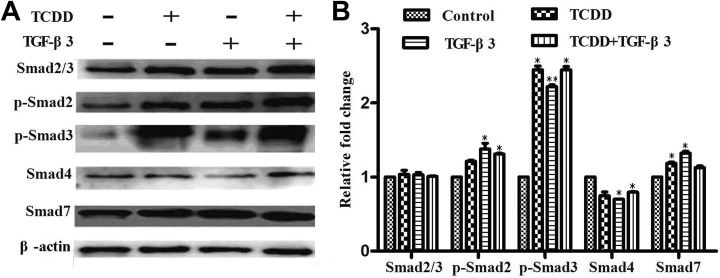
TGF-β/Smad pathway may exist in MEPM cells by TCDD and TGF-β3 induced. Smads were intracellular effectors of TGF-β/Smad signaling. Smad2 and Smad3 can be activated and form heteromeric complexes with Smad4. Smad7 played a key role in the regulation of TGF-β/Smad signaling, but it was involved in negative feedback. Therefore, we assessed the protein expression of these TGF-β/Smad pathway gene in MEPM cells. As shown in Figure 4, we found that the expression of p-Smad2 increased (1.21 ± 0.02 fold), (1.38 ± 0.11 fold), and (1.31 ± 0.01 fold) in 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3 group compared to the corresponding control cells, respectively. The expression of p-Smad3 increased (2.45 ± 0.07 fold), (2.22 ± 0.03 fold) and (2.45 ± 0.06 fold), respectively. But the expression of Smad4 decreased (0.25 ± 0.07 fold), (0.20 ± 0.001 fold), and (0.20 ± 0.006 fold), respectively. The expression of Smad7 increased (1.19 ± 0.02 fold), (1.33 ± 0.04 fold), and (1.13 ± 0.03 fold) respectively.
Figure. 4.
The effects of TGF-β/Smad signaling molecules in MEPM cells. MEPM cells were treated 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3, or DMSO (≤0.05%), respectively. After 72 hours treatment, the expression of Smad2/3, phosphorylation of Smad2 and Smad3, Smad4 and Smad7 proteins were measured by Western blot, with β-actin as loading controls, and band fluorescence intensity was analyzed and quantified using Odyssey Infrared Imaging System. The relative expression level of Smad2/3, phosphorylation of Smad2 and Smad3, and Smad7 proteins were calculated after normalization with the loading control β-actin. The data are the mean values ± standard deviation of three replicate experiments. *P < .05 or **P < .01 versus the corresponding control values. MEPM indicates mouse embryonic palatal mesenchymal; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TGF-β3, transforming growth factor β3.
Discussion
The MEPM cells are very important in palateogenesis. The MEPM cells can undergo programming cell death, migration, epithelial-mesenchymal transition and differentiation, which are coincident with the process of palatal fusion and disappearance of MEPM cells. Transforming growth factor β3 has been indicated to play an essential role in the development of palatal shelves. For example, the expression of TGF-β3 had been identified to upregulate in fetal mouse palatal shelves,16,17 and TGF-β3 was also upregulated in the palatal tissues of people with cleft palate.17 Moreover, TGF-β3 exposure completely prevented the dioxin-induced block of palatal fusion in this system.18 Transforming growth factor β3/Smad signaling pathway may medicate cleft palate.19-21 Up to present, no reports are about the relationships of TCDD and TGF-β3 in MEPM cells. We found that when different concentrations of TCDD (0.5 nM, 1 nM, 5 nM, 10 nM, 20 nM, and 50 nM) are exposed to MEPM cells, the expression of TGF-β3 gene was always higher than the control, and the expression of TGF-β3 was highest in MEPM cells by 10 nM TCDD induced. The results were also identical with TGF-β3 expression upregulates on cleft palates by low-dose TCDD induced in mice.17 The cleft palates by TCDD-induced are thought to result from a failure of the fusion of the palatal shelves, and the TGF-β3 is essential during palatal fusion stage. Therefore, high concentration of TCDD causes a decrease in TGF-β3.
The relationship between TCDD and TGF-β3 remained obscure. Low-dose TCDD can affect palatogenesis and lead to malformations in the early stages of cartilage development.22 Numerous studies demonstrated that TCDD inhibited programmed cell death of MEPM cells in palatal shelves, and TCDD can alter MEPM cell differentiation.23-25 However, few reports revealed that TCDD explored to MEPM cells. In this study, we focused on the interactive effects between TCDD and TGF-β3. We found that 10 nM TCDD inhibited the MEPM cell proliferation, which was same that TCDD inhibited urogenital sinus mesenchymal cell proliferation.26 Instead, 10 ng/mL TGF-β3 promoted the MEPM cell proliferation, which was consistent that TGF-β3 can stimulate the proliferation of mesenchymal cells in vaginal thread.27 The combination of 10 nM TCDD and 10 ng/mL TGF-β3 also promoted MEPM cell proliferation, but the rate of cell viability was between TCDD and TGF-β3 treated alone, which might be consistent with TGF-β3 as an effective antidote to dioxin-induced MEPM cells.28 This phenomenon was same as the effect of motilities in MEPM cells by 10 nM TCDD, 10 ng/mL TGF-β3, or combination of 10 nM TCDD and 10 ng/mL TGF-β3. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibited the MEPM cell motility, which concurred with the fact that TCDD-mediated the inhibition of MCF-7 cell migration.29 Transforming growth factor β3 promoted MEPM cell motility, which is aligned to TGF-β3 activating the palatal midline epithelial seam (MES) cell migration.30 And the combination of TCDD and TGF-β3 also promoted MEPM cell motility, which might be because the TGF-β3 plays an important role in MEPM cells to antagonize TCDD.
2,3,7,8-Tetrachlorodibenzo-p-dioxin increased the mRNA expression of TGF-β3 in MEPM cells, and TCDD might make function by TGF-β/Smad pathway. Phospho-Smad2/3 can bind Smad4 to form a heteromeric complex in the nucleus, where they act as transcription factors.31 Smad7 was an inhibitor of TGF-β/Smad signaling pathway.32 The current study showed that TCDD increased the expression of p-Smad2/3 and inhibited the expression of Smad4. Activation of the TGF-β/Smad signaling pathways was transfection with a dominant negative Smad4.
Taken together, we demonstrated that cellular levels of p-Smad2/3 were activated by 10 nM TCDD, and Smad4 was inhibited by 10 nM TCDD. Transforming growth factor β3 was revealed to play an important role in MEPM cells to antagonize TCDD. These suggested that TCDD and TGF-β3 mediated TGF-β/Smad signaling pathways. But some researchers detected the mRNA expression levels of Smad2, Smad3, Smad4, and Smad7 in the palates of fetuses by TCDD induced at E13.5, E14.5, and E15.5.13 The mRNA levels of Smad2, Smad3, Smad4, and Smad7 were lower in MEPM cells by TCDD induced compared to the corresponding control. They believed that TCDD did not make function by TGF-beta/Smad signaling.13,33 However, there are 2 kinds of cells in the mouse palatal shelves, such as medial edge epithelial cells and MEPM cells. But the authors did not tell about which kinds of cells making mainly function in tissues of mouse palatal shelves. In addition, the expression of gene in transcription level was different from that of the post transcriptional level. However, TCDD receptor (aryl hydrocarbon receptor [AhR]) can downregulate the TGF-β/Smad pathway in human glioblastoma cells.34 Expression of genes in the TGF-β signaling pathway was significantly deregulated in smooth muscle cells from aorta of AhR knockout mice by TCDD induced.35 Moreover, inhibition of TGF-β signaling pathway also inhibited TCDD-induced Treg activity.36
In conclusion, we found that TCDD and TGF-β3 might had negative relationship in MEPM cells by TGF-β/Smad signaling, but the precise mechanisms of TCDD and TGF-β3 mediated-cleft palate in MEPM cells require further investigation.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the grants of the National Natural Science Foundation of China (81502843), and Henan Province Xinxiang Key Laboratory of medical tissue regeneration program.
References
- 1. Moghadam FH, Tayebi T, Dehghan M, et al. Differentiation of bone marrow mesenchymal stem cells into chondrocytes after short term culture in alkaline medium. Int J Hematol Oncol Stem Cell Res. 2014;8(4):12–19. [PMC free article] [PubMed] [Google Scholar]
- 2. Rider DA, Nalathamby T, Nurcombe V, Cool SM. Selection using the alpha-1 integrin (CD49a) enhances the multipotentiality of the mesenchymal stem cell population from heterogeneous bone marrow stromal cells. J Mol Histol. 2007;38(5):449–458. [DOI] [PubMed] [Google Scholar]
- 3. Hill CR, Jacobs BH, Brown CB, Barnett JV, Goudy SL. Type III transforming growth factor beta receptor regulates vascular and osteoblast development during palatogenesis. Dev Dyn. 2015;244(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cichon MA, Radisky DC. Extracellular matrix as a contextual determinant of transforming growth factor-beta signaling in epithelial-mesenchymal transition and in cancer. Cell Adh Migr. 2014;8(6):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. [DOI] [PubMed] [Google Scholar]
- 6. Guo P, Zhao KW, Dong XY, Sun X, Dong JT. Acetylation of KLF5 alters the assembly of p15 transcription factors in transforming growth factor-beta-mediated induction in epithelial cells. J Biol Chem. 2009;284(27):18184–18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu H, Leslie EJ, Jia Z, et al. Irf6 directly regulates Klf17 in zebrafish periderm and Klf4 in murine oral epithelium, and dominant-negative KLF4 variants are present in patients with cleft lip and palate. Hum Mol Genet. 2016;25(4):766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozturk F, Li Y, Zhu X, Guda C, Nawshad A. Systematic analysis of palatal transcriptome to identify cleft palate genes within TGFbeta3-knockout mice alleles: RNA-Seq analysis of TGFbeta3 Mice. BMC Genomics. 2013;14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu X, Ozturk F, Liu C, Oakley GG, Nawshad A. Transforming growth factor-beta activates c-Myc to promote palatal growth. J Cell Biochem. 2012;113(10):3069–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burns FR, Peterson RE, Heideman W. Dioxin disrupts cranial cartilage and dermal bone development in zebrafish larvae. Aquat Toxicol. 2015;164:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Mori T, Iseki K, et al. Differential expression of decorin and biglycan genes during palatogenesis in normal and retinoic acid-treated mice. Dev Dyn. 2003;226(4):618. [DOI] [PubMed] [Google Scholar]
- 12. Feng C, Xu Z, Li Z, Zhang D, Liu Q, Lu L. Down-regulation of Wnt10a by RNA interference inhibits proliferation and promotes apoptosis in mouse embryonic palatal mesenchymal cells through Wnt/beta-catenin signaling pathway. J Physiol Biochem. 2013;69(4):855–863. [DOI] [PubMed] [Google Scholar]
- 13. Pu YL, Liu LL, Gan LQ, He XM, Fu YX. Mechanism of cleft palate in mice induced by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Zhonghua Zheng Xing Wai Ke Za Zhi. 2011;27(6):448–453. [PubMed] [Google Scholar]
- 14. Cho SJ, Jung JS, Jin I, et al. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of synaptic proteins in dissociated rat cortical cells. Molecules Cells. 2002;14(2):238–244. [PubMed] [Google Scholar]
- 15. Nawshad A, LaGamba D, Hay E. Transforming growth factor β (TGFβ) signalling in palatal growth, apoptosis and epithelial mesenchymal transformation (EMT). Arch Oral Biol. 2004;49(9):675–689. [DOI] [PubMed] [Google Scholar]
- 16. Nogai H, Rosowski M, Grun J, et al. Follistatin antagonizes transforming growth factor-beta3-induced epithelial-mesenchymal transition in vitro: implications for murine palatal development supported by microarray analysis. Differentiation. 2008;76(4):404–416. [DOI] [PubMed] [Google Scholar]
- 17. Gan LQ, Fu YX, Liu X, et al. Transforming growth factor-beta3 expression up-regulates on cleft palates induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Toxicol Ind Health. 2009;25(7):473–478. [DOI] [PubMed] [Google Scholar]
- 18. Thomae TL, Stevens EA, Bradfield CA. Transforming growth factor-beta3 restores fusion in palatal shelves exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 2005;280(13):12742–12746. [DOI] [PubMed] [Google Scholar]
- 19. Potchinsky M, Nugent P, Lafferty C, Greene RM. Effects of dexamethasone on the expression of transforming growth factor-beta in mouse embryonic palatal mesenchymal cells. J Cell Physiol. 1996;166(2):380–386. [DOI] [PubMed] [Google Scholar]
- 20. Liu X, Zhang H, Gao L, et al. Negative interplay of retinoic acid and TGF-beta signaling mediated by TG-interacting factor to modulate mouse embryonic palate mesenchymal-cell proliferation. Birth Defects Res B Dev Reprod Toxicol. 2014;101(6):403–409. [DOI] [PubMed] [Google Scholar]
- 21. Yao Z, Chen D, Wang A, et al. Folic acid rescue of ATRA-induced cleft palate by restoring the TGF-beta signal and inhibiting apoptosis. J Oral Pathol Med. 2011;40(5):433–439. [DOI] [PubMed] [Google Scholar]
- 22. Guo H, Zhang L, Wei K, et al. Exposure to a continuous low dose of tetrachlorodibenzo-p-dioxin impairs the development of the tooth root in lactational rats and alters the function of apical papilla-derived stem cells. Arch Oral Biol. 2015;60(1):199–207. [DOI] [PubMed] [Google Scholar]
- 23. Abbott BD, Birnbaum LS. TCDD exposure of human embryonic palatal shelves in organ culture alters the differentiation of medial epithelial cells. Teratology. 1991;43(2):119–132. [DOI] [PubMed] [Google Scholar]
- 24. Takagi TN, Matsui KA, Yamashita K, Ohmori H, Yasuda M. Pathogenesis of cleft palate in mouse embryos exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD). Teratog Carcinog Mutagen. 2000;20(2):73–86. [DOI] [PubMed] [Google Scholar]
- 25. Fujiwara K, Yamada T, Mishima K, Imura H, Sugahara T. Morphological and immunohistochemical studies on cleft palates induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Congenit Anom(Kyoto). 2008;48(2):68–73. [DOI] [PubMed] [Google Scholar]
- 26. Ko K, Theobald HM, Moore RW, Peterson RE. Evidence that inhibited prostatic epithelial bud formation in 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed C57BL/6 J fetal mice is not due to interruption of androgen signaling in the urogenital sinus. Toxicol Sci. 2004;79(2):360–369. [DOI] [PubMed] [Google Scholar]
- 27. Dohr O, Vogel C, Abel J. Modulation of growth factor expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Exp Clin Immunogenet. 1994;11(2-3):142–148. [DOI] [PubMed] [Google Scholar]
- 28. Zhao SF, Chai MZ, Wu M, He YH, Meng T, Shi B. Effect of vitamin B12 on cleft palate induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin and dexamethasone in mice. J Zhejiang Univ Sci B. 2014;15(3):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seifert A, Taubert H, Hombach-Klonisch S, Fischer B, Navarrete Santos A. TCDD mediates inhibition of p53 and activation of ERalpha signaling in MCF-7 cells at moderate hypoxic conditions. Int J Oncol. 2009;35(2):417–424. [PubMed] [Google Scholar]
- 30. Ahmed S, Liu CC, Nawshad A. Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3. Developmental Biol. 2007;309(2):193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu L, Liu X, Ren X, et al. Smad2 and Smad3 have differential sensitivity in relaying TGFbeta signaling and inversely regulate early lineage specification. Sci Rep. 2016;6:21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gudey SK, Landstrom M. the role of ubiquitination to determine non-smad signaling responses. Methods Mol Biol. 2016;1344:355–363. [DOI] [PubMed] [Google Scholar]
- 33. He XM, Liu CP, Gan LQ, et al. [The antagonistic effect of folic acid and resveratrol on cleft palate in mice induced by TCDD]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2013;29(3):197–201. [PubMed] [Google Scholar]
- 34. Gramatzki D, Pantazis G, Schittenhelm J, et al. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28(28):2593–2605. [DOI] [PubMed] [Google Scholar]
- 35. Guo J, Sartor M, Karyala S, et al. Expression of genes in the TGF-β signaling pathway is significantly deregulated in smooth muscle cells from aorta of aryl hydrocarbon receptor knockout mice. Toxicol Appl Pharmacol. 2004;194(1):79–89. [DOI] [PubMed] [Google Scholar]
- 36. Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127(3):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]