Abstract
Epithelium mammary carcinoma is a cancer with a high death rate among women. One factor having a significant impact on metastasis is cell migration. The aim of this study was to compare migration rate inhibition of caffeic acid (CA) and its phenethyl ester (CAPE) on MCF-7 breast cancer cells. Microscopic evaluation was used to determine the morphology of carcinoma cells, before and after 24-hour treatment with CA and CAPE using a dose of 50 µM. The cytotoxic effect was measured by XTT-NR-SRB assay (tetrazolium hydroxide-neutral red-Sulforhodamine B) for 24-hour and 48-hour periods, using CA and CAPE, with doses of 50 and 100 µM. These doses were used to determine cell migration inhibition using a wound closure assay for 0-hour, 8-hour, 16-hour, and 24-hour periods. Both CA and CAPE treatments displayed cytotoxic activity in a dose- and time-dependent trend. CAPE displayed IC50 values more than twice as low as CA. IC50 values for the XTT assay were as follows: CA was 102.98 µM for 24 hours and 59.12 µM for 48 hours, while CAPE was 56.39 µM for 24 hours and 28.10 µM for 48 hours. For the NR assay: CA was 84.87 µM at 24 hours and 65.05 µM at 48 hours, while CAPE was 69.05 µM at 24 hours and 29.05 µM at 48 hours. For the SRB assay: At 24 hours, CA was 83.47 µM and 53.46 µM at 48 hours, while CAPE was 38.53 µM at 24 hours and 20.15 µM at 48 hours. Both polyphenols induced migration inhibition, resulting in practically halting the wound closure. CAPE produced better results than CA with the same doses and experiment times, though both CA and CAPE displayed cytotoxic activity against MCF-7 cells, as well as inhibited migration.
Keywords: propolis, caffeic acid, CAPE, migration, wound healing assay, breast cancer, epithelium mammary carcinoma
Introduction
Cell migration, a central process in the development and maintenance of multicellular organisms, requires the orchestrated formation of tissue in particular directions to specific locations. This movement property of cells is engaged in the growth of tissues and organs, embryonic development, immune response, and many other pathological processes such as inflammatory response as well as cancer metastasis.1-9 In pathology, invasion of cancer is determined by its ability to penetrate tissue barriers. Therefore, cell migration/invasion assays are used in many disciplines of biomedical sciences and related fields for this purpose. The study of cell migration in cancer research is of particular interest because the main cause of death in cancer patients is the presence of metastases (spread of cancer to secondary organs or sites throughout the body). In order for most tumor cells to proliferate and cause metastases, they must migrate and attack cells through the extracellular matrix, then pass through the vessel walls to the surrounding tissues (extravasation), where they eventually settle, proliferate, induce angiogenesis, and thereby form metastatic outbreaks.1,10-12
Various biological methods are used for detailed studies of these events. These methods include the following: cell wound closure assays,13 where a scratch is produced in a confluent cell monolayer; transwell migration assay (Boyden chamber assay),14 which is used for analysis of leukocyte response; and cell exclusion zone assay,15 by which so-called “stoppers” are removed after cell adhesion to create an area for cell migration. Other commonly used methods to quantify cell migration include the following: the fence (ring) assay, microcarrier bead assay, spheroid migration assay, and capillary chamber migration assay, as well as others, such as 3-dimensional assays.1,10,16 These tests all provide essential data to understand how aggressively specific cell types can spontaneously migrate and consequently cause metastasis. Cells can migrate in a single cellular form, such as in a mesenchymal or amoeboid-like motion, or by multicellular motion called collective cell migration or transmission.17
Breast cancer, especially epithelium mammary carcinoma, is one of the most significant causes of cancer-related deaths among women, primarily due to its high rate of metastases.18,19
The endocrine system plays an important role in development of the mammary gland. Among others, growth hormone is an important regulatory factor in the development of the mammary glands20,21 by promoting cell proliferation.22 However, as a true cytokine, growth hormone is produced locally and directly induces tumor progression.23 Furthermore, there are studies suggesting the role of GHR (growth hormone receptor) affects mediated signaling pathways in the development of human breast cancer either by autocrine or paracrine mechanisms.24,25
Studies of the NF-κB nuclear factor in breast cancer have shown its activation and consequent overexpression of antiapoptotic genes.26-28 Because the exact causes of NF-κB activation have not been fully explored, they are believed to be due to hormonal factors.
Deregulation of the NF-κB signal transduction pathway in various types of human cancers was observed. When breast cancer was taken into consideration, a high level of NF-κB activation was reached in 86% of HER2-positive but estrogen receptor (ER)-negative cancers, a value of 33% in the basal-like cancers (HER2-negative and ER-negative), and only weakly evident in breast cancer with ER-positive phenotype.29 Frasor et al30 observed a positive crosstalk between ER and NF-κB in breast cancer MCF-7 cells. It is noteworthy that CAPE (phenethyl ester of caffeic acid) acts as a specific inhibitor of NF-κB in breast cancer cells.31-33
CAPE is a derivative of CA, and both occur naturally in propolis. Many research studies have shown that the properties of these polyphenols and propolis itself display antibacterial, antioxidative, antiviral, antibacterial, anti-inflammatory, antiplatelet, antineoplastic, as well as antitumor activities.34-40 Moreover, a diet rich in polyphenols and flavonoids from natural sources (plant foods and bee products) may represent a protective lifestyle, which can promote (breast) cancer chemoprevention as well as show promise that anticancer phytotherapeutics might be developed from food.41
In our previous study, we showed that CAPE exhibited higher cytotoxic and proapoptotic activity, as well as increased cell arrest induction capability in comparison to CA against breast cancer cells MDA-MB-231.42 We also studied the influence of CA and CAPE on migration rate and found that CA reduced the migration rate in oral carcinoma cells (SCC-25).43 In recent research, we compared the migration rate of CAPE and CA against triple-negative breast cancer cells, and CAPE displayed a higher inhibitory effect on migration against MDA-MB-231 cells than CA.44
Active cancer cells’ motility is an essential characteristic of most metastatic stages, starting with local invasion and penetration through the basal membrane, through migration in the 3-dimensional space of the extracellular matrix, as well as the ability to overcome blood vessel walls or the lymph wall barrier, and to migrate within a colonized body, and consequently, to develop metastases. At present, the importance of active migration in the metastasis process is not questioned.45 Today, the mechanism of breast cancer cells migration is under investigation by the researchers.
In this study, we compared the migration rate inhibition of breast cancer MCF-7 cells when treated with either CA or CAPE, accompanied by their cytotoxicity comparison. The MCF-7 cell line is one of few breast cancers that expresses ER-α and progesterone receptor. MCF-7 cells are of particular interest to us, as they maintain a number of characteristics similar to mammary epithelium. To the best of our knowledge, this is the first time such a study was carried out.
Materials and Methods
Cancer Cell Line
For this research, the MCF-7 line (human breast adenocarcinoma, No. 86012803 SIGMA from Sigma-Aldrich, Poznań, Poland) was used, as it is a model of human epithelium mammary carcinoma. The manufacturer’s recommendations for preparations were precisely followed. The MCF-7 cells were cultured with Leibovitz’s L-15 medium, with 10% of inactivated fetal bovine serum (Sigma-Aldrich, Poznań, Poland), and kept at 37°C without CO2.
All cultured cells were supplemented with antibiotics of the following concentrations: penicillin—100 U mL−1, streptomycin—100 µg mL−1, and fungistatic amphotericin B with a concentration of 0.25 µg mL−1. The medium was changed every 48 to 72 hours, with the passage carried out with a confluence of 80% to 90%.
Reagents
Both CA (Sigma No. C0625) and CAPE (Sigma No. C8221) were purchased from Sigma-Aldrich, Poznań, Poland, and were collected, stored, and used specifically according to the manufacturer’s instructions. Both compounds were dissolved in ethanol.
Microscopic Morphology Evaluation of Carcinoma Cells: Hematoxylin and Eosin Staining Protocol
Preparation of samples for morphological evaluation was performed following the same standard method, as described previously.44
Briefly, the MCF-7 cells were inoculated into 2-chamber microscopic culture vessels (Lab-Tek, Waltham, MA), at a count of 1000 cells/well. The cells were hydrated in the following series of dilutions: 99.6%, 96%, 90%, 80%, 70%, and 50% and stained with hematoxylin for 7 minutes (standard hematoxylin and eosin staining protocol). Next, the plates were washed with phosphate-buffered saline solution for approximately 30 minutes until blue and were then incubated for 30 seconds with eosin. The plates were then washed with phosphate-buffered saline solution and then dehydrated. The plates were then mounted and analyzed under an optical microscope. A 50-µM dosage of both analyzed substances was used with a 24-hour incubation time.
Cell Viability by Mitochondrial Activity, XTT Assay
Viable cells depend on an intact mitochondrial membrane and respiratory chain. Activity of the measured substances were determined using mitochondrial dehydrogenases from the viable cells. The substance XTT (2,3-bis[2-methoxy-4-nitro-5-sulfopheny]-2H-tetrazolium-5-carboxyanilide inner salt) is a tetrazolium salt that cleaves to formazan. The XTT assay was performed as described previously.44 Cells were inoculated on 96-well plates, at an amount of 104 cells/well to measure cytotoxicity. A fresh medium was added and left for 72 hours to obtain the rate of cell growth. After the medium was decanted, separate culture mediums were added, which contained 10 µM, 25 µM, 50 µM, or 100 µM of either CA or CAPE, respectively. Enzyme activity was measured at 480 nm. The XTT assay was obtained from Xenometrix AG, Allschwil, Switzerland. We performed the assay procedure in exact accordance with the manufacturer’s instructions and protocol.
Cytotoxicity by Lysosomal Activity, NR Assay
Cell survival rate can also be determined by using the ability of viable cells to bind to neutral red (NR), a weak cationic dye that binds to anionic sites of the lysosomal matrix. NR readily percolates through the cell membrane and accumulates intracellularly in lysosomes. The NR assay was performed as described previously.44 Briefly, the quantity of dye incorporated into cells was measured by spectrometry at 540 nm, which is directly proportional to the number of cells of an intact membrane. The NR assay was obtained from Xenometrix AG, Allschwil, Switzerland. Both CA and CAPE were used at concentrations of 10 µM, 25 µM, 50 µM, or 100 µM, respectively, with 24 hours and 48 hours of incubation. All test procedures were performed exactly, following the instructions and protocol of the manufacturer.
Cell Proliferation by SRB Assay
One very sensitive toxicological marker is the total amount of synthesized proteins. Sulforhodamine B (SRB; Acid Red 52) is another anionic dye used for this purpose, as it binds electrostatically to cellular proteins. The assay was performed according to the previously described method.44 Briefly, a fixed dye was solubilized and measured photometrically at optical density 540 nm, with a reference filter of 690 nm. The optical density values were correlated with total protein content and therefore the cell number. Concentrations of 10 µM, 25 µM, 50 µM, or 100 µM of CA and CAPE, respectively, were used to perform the experiments for 24 hours and 48 hours of incubation. The SRB test was obtained from Xenometrix AG, Allschwil, Switzerland. The test procedure was performed exactly in accordance to the instructions and protocol of the manufacturer.
Carcinoma Cell Migration: Cell Wound Closure Assay
The ability of CA and CAPE to modify cell migration was assessed using the cell wound closure assay. This method, which we previously used and described,44 was implemented to evaluate the migration activity rate of MCF-7 cells exposed to CAPE and CA.
Briefly, the MCF-7 cells (4 ×106 cells/well) were plated in 6-well plates for 48 hours to achieve an 80% confluence, then wounded by scratching with a p200 pipette tip. The cells were then incubated with DMEM (Dulbecco’s modified Eagle’s medium) containing 0.5% fetal bovine serum and treated with 50 µM and 100 µM doses of CA/CAPE. The control sample was without any treatment. To quantify the migration rate in MCF-7 cells, we used a monolayer gap closure migration assay, embedded using ImageJ software (version 1.50i, National Institute of Health, Bethesda, MD), with a wound healing tool macro (Montpellier RIO Imaging, CNRS, Montpellier, France). The area of the initial wound was measured, followed by the gap area measurements after 8 hours, 16 hours, and 24 hours. The migration factor was presented as the gap area value compared with the initial scratch area.
Statistical Analysis
All results are expressed as means ± SD and were obtained from 3 separate experiments and performed in quadruplicate (n = 12). The results were performed with independent sample t tests. The experimental means were compared with the mean values of untreated cells harvested in a parallel manner. Differences between 24-hour, 48-hour, and control sample results were tested for significance using the 1-way Friedman analysis of variance test. P < .05 was considered statistically significant.
Results
In this research, we conducted a quantitative assessment of breast cancer cells’ viability. To obtain comparative results, we chose the XTT-NR-SRB (Tetrazolium hydroxide-Neutral Red-Sulforhodamine B) assay. In parallel, we evaluated the impact of CAPE and CA on MCF-7 breast cancer cells’ morphological features. Cancer cell motility and migration were evaluated using a wound healing assay, after treatments of CA and CAPE.
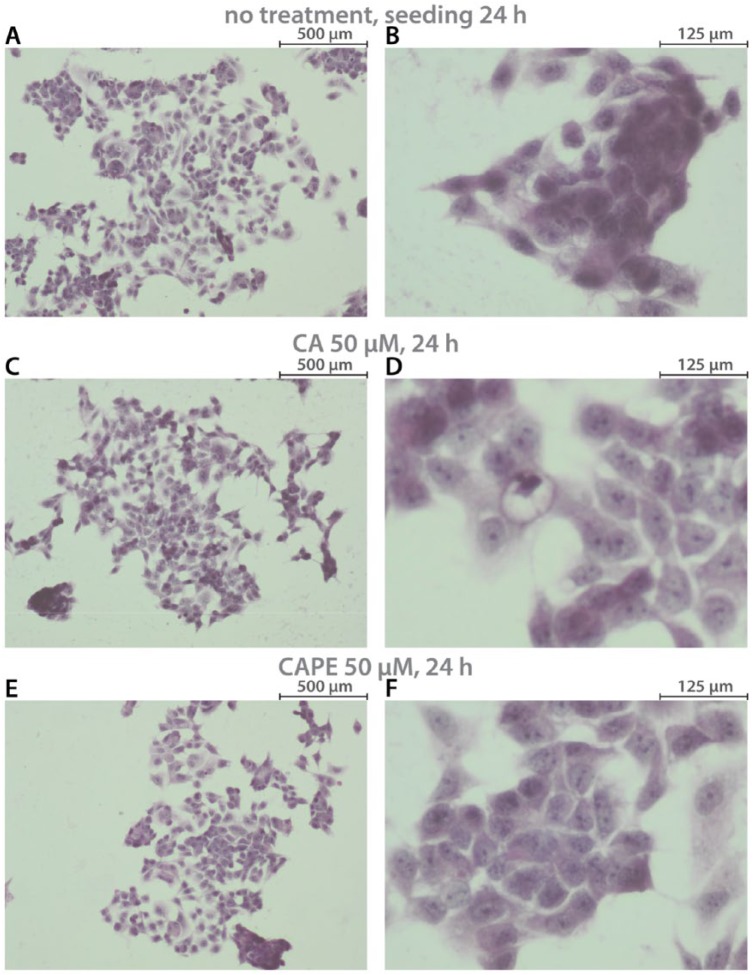
A cytomorphological view of MCF-7 cells is presented in Figure 1. Phenotypically, the examined cells were relatively large adherent cells, formed into a mass, and exhibited robust cell-cell adhesion. Changes were observed in MCF-7 cells’ morphological view, after CA and CAPE treatment. That is, after CA treatment, MCF-7 cells began to cluster in islands. Cancer cells displayed pleomorphism of size and shape and a thin rim of cytoplasm. Pleomorphism of nuclei coloration was also observed. Successively, after CAPE treatment, we clearly saw lower cell-cell contact, karyopyknosis, as well as changes in cytoplasm density and shape. Invasive processes of the cell body were observed.
Figure 1.
MCF-7 breast cancer—cytomorphological view of cells: (A, B) without any treatment; (C, D) after 24 hours with 50 µM of caffeic acid (CA); (E, F) after 24 hours of 50 µM caffeic acid phenethyl ester (CAPE). Samples were prepared with hematoxylin and eosin staining. Exposition: optical magnification ×100 (A, C, and E), ×400 (B, D, and F). Main features: (A) hyperchromasia, fairly large adherent cells, forming dome-like structures, irregular nuclear shapes; (B) cells formed as a mass, disorganized nuclei, robust cell-cell adhesion; (C) cells grouped in clusters/islands; (D) pleomorphism of coloration, size, and shape (of nuclei and whole cells), thin rim of cytoplasm; (E) lower cell-cell contact, regularly dispersed chromatin, cells grouped in one place; (F) cells formed as grape-like, karyopyknosis, cytoplasm density and shape change, disorganized nuclei, cell body with invasive processes.
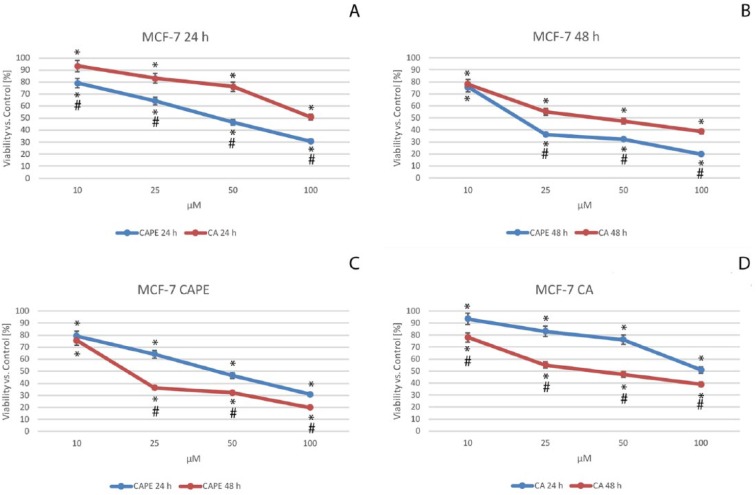
Cell viability of MCF-7 cells after CA and CAPE treatments was measured using a triple cytotoxic assay. First, an XTT assay was performed. Cell viability by XTT is based on enzyme’s mitochondrial activity on live cells, which become inactive just after cell death. The data were presented after their normalization as the percentage of control values (Figure 2).
Figure 2.
Viability of the MCF-7 cells after caffeic acid phenethyl ester (CAPE) (C) and caffeic acid (CA) (D) treatment, both with dosages of from 10 to 100 µM with 24-hour (A) and 48-hour (B) incubation periods. Cytotoxic activity was measured by XTT Cell Proliferation Assay. The results are presented as the mean and standard deviation of 3 independent experiments, with 12 wells each (P < .05; Friedman ANOVA test; *Significant difference vs control; #Significant difference 48 hours vs 24 hours).
In the case of CA treatment of MCF-7 cells (Figure 2A and D), cell viability decreased in a dose-dependent manner, falling from 93.5% for a dose of 10 µM, 83.1% for 25 µM, 76.2% for 50 µM, finally reaching a value of 50.9% with a dose of 100 µM after 24 hours. Similarly, CAPE values (Figure 2A and C) followed a dose-dependent manner, namely, 79.2% for a dose of 10 µM, 64.3% for 25 µM, 46.6% for 50 µM, and 30.8% with 100 µM of CAPE after 24 hours. The effect displayed a dose-dependent trend for both polyphenols; however, a higher cytotoxic effect was clearly achieved with CAPE at 24 hours.
Even the smallest doses of these 2 agents had similar viability values on MCF-7 cells after 48 hours. That is, the values were 78.1% for CA and 75.6% for CAPE, for a dose of 10 µM. For CAPE (Figure 2B and C), the values reached 36.2%, 32.2%, and 19.9% for respective doses of 25 µM, 50 µM, and 100 µM after 48 hours. CA values (Figure 2B) were as follows: for a 25-µM dose 55.1%, for 50 µM 47.3%, and finally, for 100 µM, CA was 38.7%. For both substances, after a 48-hour period, the effect of cytotoxicity showed a dose-dependent trend. When CAPE activity was compared with that of CA, viability of cells was lower for CAPE using the same doses, after an incubation time of 48 hours. A dose-dependent (Figure 2A and B) and time-dependent (Figure 2C and D) trend was seen for both examined substances.
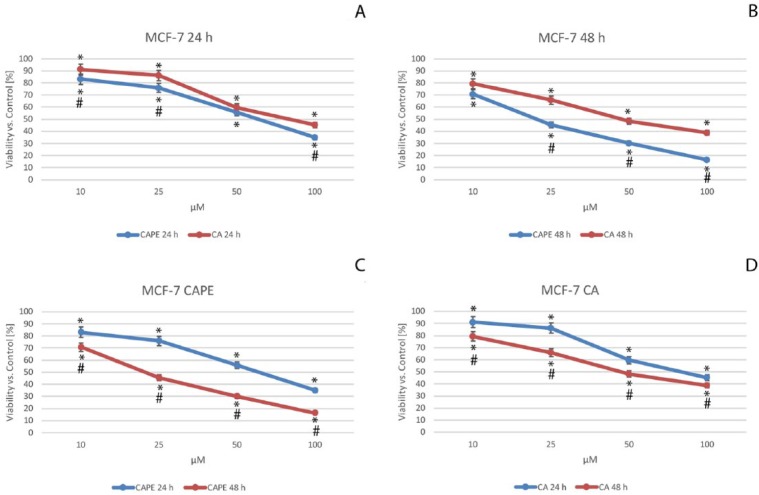
NR viability assay was a key component for the next part of the experiment. Cells that are viable take up NR dye and incorporate it into lysosomes. Undoubtedly, cells that are not viable do not take up the dye. Based on this property, we measured the cytotoxic effect of CAPE and CA against MCF-7 cells, using a NR lysosomal assay. The data were presented after their normalization as the percentage of control values (Figure 3).
Figure 3.
Viability of the MCF-7 cells after caffeic acid phenethyl ester (CAPE) (C) and caffeic acid (CA) (D) treatments, both with dosages from 10 to 100 µM with 24-hour (A) and 48-hour (B) incubation periods. NR lysosomal activity assay was used to measure the cytotoxic activity. The results are presented as mean and standard deviation of 3 independent experiments, with 12 wells each (P < .05; Friedman ANOVA test; *Significant difference vs control; #Significant difference 48 hours vs 24 hours).
Using CA against MCF-7 cells (Figure 3A and D), cell mortality displayed a dose-dependent trend; the viability values fell from 91.2% for a dose of 10 µM, to 86.2% for 25 µM, 59.7% for 50 µM, and 45.2% with a dose of 100 µM after 24 hours. When CAPE’s cytotoxic activity was compared with that of CA against MCF-7 cells (Figure 3A and B), we clearly observed that cell viability values for a dose of 10 µM were highest. At 24 hours, CA was 91.2% and CAPE was 83.1%, while at 48 hours, CA was 79.3% and CAPE was 70.8%. For the CAPE treatment (Figure 3A and C), at 24 hours, the values reached 75.9%, 55.6%, and 34.9% for respective doses of 25 µM, 50 µM, and 100 µM. When using CA against MCF-7 cells (Figure 3A and D), at 24 hours, the values were as follows: 86.2%, 59.7%, and 45.2% for respective doses of 25 µM, 50 µM, and 100 µM. These results show that CAPE achieved a slightly stronger cytotoxic effect at 24 hours.
Both CA and CAPE treatments displayed an explicit impact on cell viability in a dose-dependent trend, after a 48-hour period (Figure 3B). The values were the following: at 25 µM, CAPE was 45.4% and CA was 65.8% for 50 µM. CAPE was 30.1% and CA was 48.4%, and finally, for 100 µM, CAPE was 16.3% and CA was 38.8%. It can be clearly observed that CAPE induced greater cell mortality than CA at the same dosage. Both CA and CAPE displayed cytotoxic activity against MCF-7 cells in a dose- and time-dependent manner.
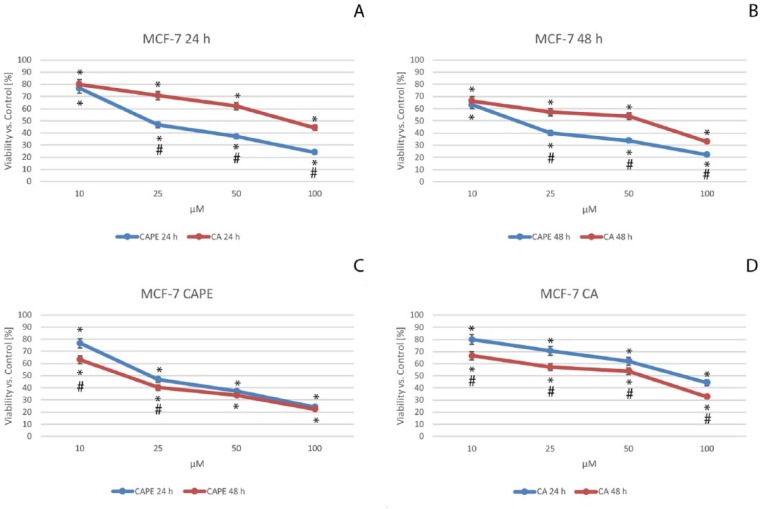
The final part of the cytotoxic tests was a cell proliferation assay based on its key component—the dye, SRB (Acid Red 52). The cytotoxicity was measured as the result of total change in the amount of dye incorporated by the cells in the culture. The data were presented after their normalization as the percentage of control values (Figure 4).
Figure 4.
Viability of the MCF-7 cells after caffeic acid phenethyl ester (CAPE) (C) and caffeic acid (CA) (D) treatments, both with dosages from 10 to 100 µM with 24-hour (A) and 48-hour (B) incubation periods. Cytotoxic activity was measured by SRB (Sulforhodamine B) Cell Proliferation Assay. The results are presented as mean and standard deviation of 3 independent experiments of 12 wells each (P < .05; Friedman ANOVA test; *Significant difference vs control; #Significant difference 48 hours vs 24 hours).
In the case of CA treatment of MCF-7 cells (Figure 4A and D), cell viability declined and displayed a dose-dependent trend, dropping after 24 hours from 80.0% for a dose of 10 µM to 70.6% for 25 µM, and 62.0% for 50 µM to a value of 44.3% using a dose of 100 µM of CA. Comparing CAPE with CA (Figure 4A and B), the cytotoxic effect for a dose of 10 µM of CAPE was similar to those of CA. At 24 hours, CA was 80.0% and CAPE was 76.7%; and at 48 hours, CA was 66.5% while CAPE was 63.2%. At a dosage of 10 µM, both agents manifested a similar cytotoxic effect on the MCF-7 cells. The viability displayed a dose- and time-dependent trend. For CAPE, at 24 hours (Figure 4A and C), the values reached 70.6%, 62.0%, and 44.3% for doses of 25 µM, 50 µM, and 100 µM, respectively. After 24 hours, CAPE displayed significant stronger cytotoxic activity than CA.
After 48 hours of incubation, for both polyphenols (Figure 4B), cell viability exposed a dose-dependent trend, with the following values: for 25 µM, CAPE was 40.1% and CA was 57.1%; for 50 µM, CAPE was 33.8% and CA was 53.8%; and finally, for 100 µM, CAPE reached its minimal value of 22.2% and CA was 32.9%. When CAPE cytotoxic activity was compared with CA against MCF-7 cells after 48 hours, it was again higher for CAPE than CA using the same concentrations. However, the lowest dose resulted in a similar cytotoxicity value for both CA and CAPE treatments of the MCF-7 cells. Dependent trends for the dose (for both CA and CAPE) and time (only CA) domains were confirmed, whereas the time impact on cytotoxicity (Figure 4C and D) was not significant for the highest doses of CAPE (50 and 100 µM).
During the experiment, we calculated the half maximal inhibitory concentration (IC50) for both CA and CAPE. It is noteworthy that the 50% mortality of breast cancer cells (MCF-7) was lower for CAPE than CA for all cytotoxic assays. For both the 24-hour and 48-hour experiments, this confirmed that CAPE displayed a stronger cytotoxic effect on MCF-7 cells than CA. All obtained IC50 values are shown in Table 1.
Table 1.
IC50 (µM) Values of Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Against Breast Cancer MCF-7a.
| Method | Compound | Time of Incubation |
|
|---|---|---|---|
| 24 hours | 48 hours | ||
| XTT | CA | 102.98 | 59.12 |
| CAPE | 56.39 | 28.10 | |
| NR | CA | 84.87 | 65.05 |
| CAPE | 69.05 | 29.05 | |
| SRB | CA | 83.47 | 53.46 |
| CAPE | 38.53 | 20.15 | |
Experiments were performed using 3 methods (XTT, 2,3-bis[2methoxy-4-nitro-5-sulfopheny]-2H-tetrazolium-5-carboxyanilide inner salt; NR, neutral red; SRB, Sulforhodamine B, respectively), for 24-hour and 48-hour incubation times. All data confirmed a higher cytotoxic effect of CAPE than CA on MCF-7 cells.
Considering the effects of cytotoxicity of CA and CAPE, which were measured in this study by triple cytotoxic assay, we observed that these 2 polyphenols are active against MCF-7 breast cancer cells. It is noteworthy that CAPE exhibited IC50 values more than twice as low as CA. There was one exception, the IC50 value for CAPE was about 18% lower than CA, when measured by NR assay at 24-hour incubation time. Nonlinear absorbance of the dye could explain this phenomenon. For the 48-hour experiment, once again, CAPE displayed significantly stronger cytotoxic activity compared with CA, with CAPE again being twice as low as CA.
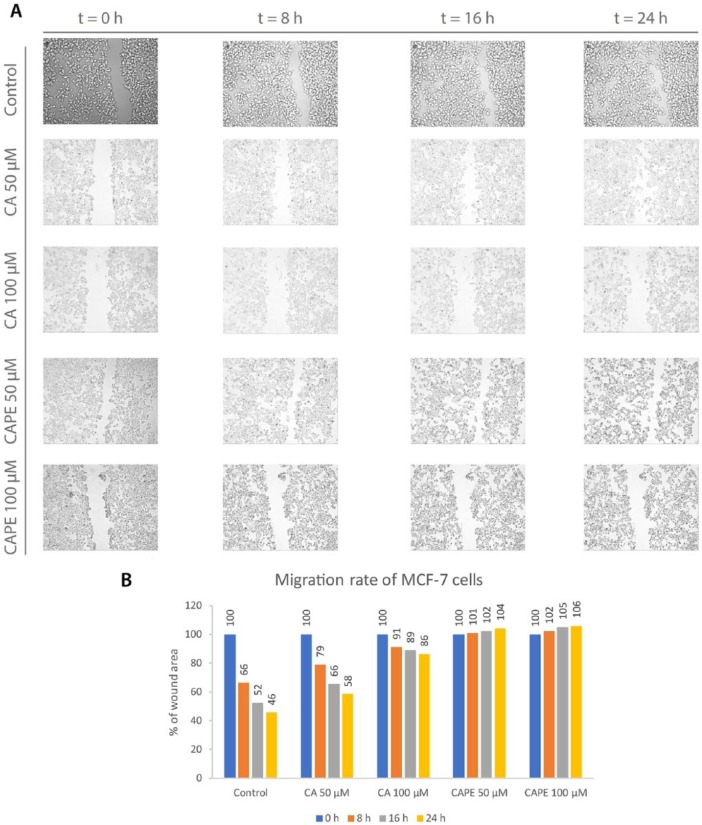
Determining the influence of the examined polyphenols on MCF-7 migration cells was the next stage of the experiment, in which we employed a wound healing assay. Wound healing occurs as a result of different processes of cell movement and replacement of missing cells. Microscopic observation of live cells’ motility is an efficient method to assess the migration rate into the gap created as a scratch at the beginning of experiment. The desirable outcome is when the wound decreases as little as possible, so the value of the gap area over the area of the primary scratch remains as high as possible, ideally for a longer time. This means the tested substance suppresses the migration of tumor cells. All results obtained in the wound healing experiment are presented in Figure 5. In the control group, where no treatment was provided, cell migration was quite dynamic. Cells started moving and “healing” the wound. After 8 hours, the gap area achieved a value of 66%. The migration continued to proceed, reaching a value of 52%, after the 16-hour period. A maximum wound closure value of 46% was achieved at the end of the observation period, after 24 hours. Using CA with a dose of 50 µM, the motility of the MCF-7 cells was halted. That is, the rate was 79% after 8 hours, meaning the process was much slower than in the control group. An area of 66% was obtained after 16 hours, and finally reached 58% after 24 hours. A dose of 50 µM of CA promoted migration inhibition. When the dose of CA was increased to 100 µM, the motility of the MCF-7 was inhibited, resulting in expressively higher values of inhibition. The gap area value was 91% in relation to the original wound after 8 hours. After 16 hours of observation, a value of 89% was obtained, and finally reached 86% after 24 hours. CA clearly promoted migration inhibition, in a dose-dependent manner. Exclusively, for a dose of 100 µM of CA, the migration process was practically halted. Interestingly, we observed “white spots” in the morphological view, meaning the MCF-7 cells started grouping in clusters, which was also observed in the morphological assessment (Figure 1C and D). It could be assumed that this was due to CA’s cytotoxic activity against MCF-7 cells.
Figure 5.
Caffeic acid (CA) and caffeic acid phenethyl ester (CAPE) at concentrations of 50 µM and 100 µM promoted an inhibitory migration effect on MCF-7 cells. The dose-dependent effect was clearly visible (A). To obtain the cell migration factor (B), a monolayer gap closure migration assay was implemented using ImageJ software. The results are presented as the gap area in relation to the area value of the initial scratch, after 8 hours, 16 hours, and 24 hours of observation.
When compared, using CAPE in a wound healing assay resulted in stronger inhibition of cell migration than CA. For the CAPE treatment dose of 50 µM, the gap area value was 101% for 8 hours. The wound remained at 102% at 16 hours and increased to 104% after 24 hours. At this point, we observed a specific phenomenon, where the gap did not decrease, but rather increased slightly after CAPE treatment, therefore becoming a barrier. Again, referring to the morphological view (Figure 1E and F), the cells started grouping in a “grape-like” form. The necrosis of the MCF-7 cells might be suspect as well, as “white spots” were more visible in the sample. The process described above even intensified. Increasing the CAPE dose to 100 µM produced similar results, as was expected. CAPE halted breast cancer cells from migrating at 102% after 8 hours. The shape of the gap held stable, even increasing slightly, achieving a value of 105% after 16 hours, followed by a value of 106% after 24 hours. The loosing of cell adhesion and cluster grouping were again specific for this dose.
CA and CAPE both promoted migration inhibition of MCF-7 cells in a visible dose-dependent trend. Better results were achieved with CAPE, as the wound area held, and therefore practically halted the motility of the MCF-7 cells.
Discussion
Classification of breast cancer cells is mainly related to the presence of the ER. MCF-7 cell line is useful for in vitro breast cancer research, because it retains features specific to the mammary epithelium, particularly its ability to process estrogen via ER in the MCF-7 cell cytoplasm. Consequently, the line acts as an ER-positive cell line. Other main features are progesterone receptor positive and HER2 negative. The survival and proliferation of breast cancer cells are dependent on ER signaling.46,47
Although ER-positive breast cancers are assumed to be less aggressive and more discriminated, they still cause metastases.
Natural substances, such as polyphenols and flavonoids, are still of great interest to researchers, due to their effects on cancer cells and their having practically no impact on healthy cells.48,49 Estrogen-related substances seem to act better on estrogen-positive neoplasm cells.50
Wu et al37 showed that CAPE reduced tumor volume in MCF-7 xenografts and cell culture. A dose-dependent cytotoxic effect of CAPE on MCF-7 cells was observed with the IC50 value of approximately 15 µM, after 72-hour incubation time (MTT assay). Our results exhibited IC50 values in the range of 20 to 29 µM (depending on the assay used), for 48-hour experiment, which are not far from their results. We confirmed dose dependency (both for CAPE and CA) and dependency in the time domain (except CAPE with the doses higher than 25 µM for SRB test). It is worth noting that CAPE did not affect healthy cells, which we confirmed in our previous research.40 In the above-mentioned study, Wu et al confirmed that the growth of MCF-10A cells also was not affected when treated by CAPE. There are 2 subtypes of MCF-10 cell line: MCF-10A and MCA-10F (immortal but non-tumorigenic). MCF-10A is adherent, and MCA-10F is floating. Hence, we believe that they could be considered in our further research, to be used both in cytotoxicity and migration rate inhibition assays, as control, together with specific migration markers. In the same research, CAPE did not show a significant effect on normal cells. Also in our previous research,40 we reported that CAPE reduced the growth of breast cancer cells, in opposition to the noncancerous IMR-90 fibroblast control line.
An interesting study on CAPE against MCF-7 cells was conducted by Watabe et al.31 They observed nuclear factor NF-κB was inhibited by CAPE. Moreover, CAPE induced cycle arrest and apoptosis in MCF-7 cells. This was the result of Fas death-inducing receptors being aggregated through the FasL-independent mechanism. They observed 2 pathways that were involved in this process, namely, FADD/caspase-8 and JNK/p38. They also reported that CAPE inhibits MCF-7 cells growth, which is in line with our research.
Rosendahl et al51 tested the impact of coffee intake on the course of breast cancer and found that higher coffee consumption was associated with a lesser invasion of primary tumors. They also performed in vitro research, in which the activity of caffeine and CA against MCF-7 breast cancer cells was measured. They observed that CA inhibited the cancer cells’ proliferation; moreover, CA reduced the growth of breast cancer cells due to modulation of ER and IGFIR levels. They found that CA (in doses of from 2 to 50 µM) and also caffeine alone induced cell death among MCF-7 cells. Our cytotoxic results of CA on MCF-7 cells confirm their conclusion.
In our study, we compared the cytotoxic effect of CA and CAPE. We observed that CAPE displayed stronger cytotoxic effect than CA, which is consistent with other studies. We performed a morphological assessment, where we clearly observed the impact of CA and CAPE on behavior of the MCF-7 cells, for example, cell-cell adhesion, cells grouping and clustering after treatment, changes in colorization and both cytoplasm, nuclei, and pleomorphism.
Also in our previous study,42,44 we found better activity of CAPE than CA, on the MDA-MB-231 cells.
In our research, the cytotoxic effect of CA and CAPE was assessed for 24-hour and 48-hour incubation times, while a migration assay was done up to 24 hours (included), with check points at 8 and 16 hours. A high cytotoxic effect could affect interpretation of high migration rate inhibition (especially by CAPE, at 24 hours). Cell adhesion plays a crucial role in such processes as do growth, differentiation, and migration. On the other hand, one of the features of necrotic cells is a loss of adhesion to the substrate, which should be clearly visible in a microscopic view (detachment of cells from the substrate). In our experiment, the assessed cells wholly adhered to the substrate, except when mechanically removed by the initial scratch. We found this to be a limitation of this work. To assess the cytotoxic influence on migration rate, specific proliferation inhibitors should be used in further research.
In their research, Wadhwa et al52 used CAPE compared with a complex CAPE-γ-cyclodextrin (CAPE-γCD). They studied cell viability of the MCF-7 and MDA-MB-231 breast cancer cells. It is noteworthy that in their study CAPE exhibited short-term cytotoxicity, while CAPE-γCD displayed constant growth suppression and induced apoptosis. They discovered that CAPE promoted upregulation of p53 function via interaction of mortalin and p53. Their invasion experiment on MCF-7 and MDA-MB-231 cells, as well as metastatic samples, have shown that both CAPE-γCD complex and CAPE alone manifested migration inhibition, which is in line with our research.
Wang et al53 observed that lack of FOXF2 induced EMT (epithelial-mesenchymal transition) of basal-like breast cancer cells. Moreover, they observed that TWIST1 was a target of FOXF2 transcription. Moreover, TWIST1 induced EMT by downregulating E-cadherin expression. This was possible through an indirect effect on the CDH1 promoter. In their recent study, Zhang et al54 confirmed that the miR-182/FOXF2 axis may be a new therapeutic strategy, because miR-182 significantly enhanced migration and invasion in MCF-7 cells. NF-κB is absolutely necessary for EMT and metastasis of the breast cancer,55 while on the other hand, CAPE is a specific inhibitor of NF-κB31-33; therefore, we assume that CAPE could act on FOXF2 in MCF-7 cells.
Riverso et al56 demonstrated that monocyte chemotactic protein 1 (MCP-1) induced ER-α signaling via PI3K/Akt/mTORC1. Other interesting results were from the study of Li et al,57 who found that that the MCP-1-induced EMT of MCF-7 cells and was mediated by the ERK/GSK-3β/Snail signaling pathway. Toma et al58 demonstrated that CA reduced the secretion of MCP-1 in human endothelial cells; therefore, we can also assume that CA could act on MCP-1 in MCF-7 cells.
These 2 assumptions set us in the direction of further research.
Bhat et al59 showed in their research that GROα-stimulation in MCF7 cells and GROα-knockdown in MDA-MB-231 cells induced large changes in migration and invasion abilities in vitro. At the same time, significant changes in EMT markers via the MAPK pathway were observed in breast cancer cells. In further research, we will use specific migration markers to confirm migration inhibition of breast cancer cells by CA/CAPE and also check their impact on GROα.
CA and other phenolic acids are widely present in vegetables, fruits, coffee, grains, and propolis, and simultaneously occur in ester forms.60 There is evidence that there is an association between consumption of polyphenols and prevention of some types of cancer.61 Additionally, many polyphenols are processed by gut microbiota and constitute active metabolites, which is an additional benefit of high intake of polyphenols.62 However, a main limitation of our study was to transfer the obtained data into specific recommendations of how to prepare an optimal diet containing CA and CAPE.
Presently, knowledge about cellular uptake of microbial phenolic metabolites is limited, due to the complexity of processes affecting the absorption process, including diffusion and intestinal transports, as well as the metabolism of the intestinal phase and hepatic phase II.63 Primary metabolic reactions of native polyphenols in gut and liver lead to methylation, glucuronidation, or sulfated derivatives found in urine and plasma.64,65
Omar et al66 observed in a rat model in vivo that less than 10% of the CA is excreted in urine, with a preponderance of caffeic acid 4′-O-glucuronide (42%) and sulfates (approximately 16%). On the other hand, Konishi et al67 reported that absorption of CA is significantly higher compared with gallic and p-coumaric acid.
In their research, Li et al68 studied pharmacokinetics of CAPE. The results revealed that CAPE exhibited a specific binding interaction with HSA (human serum albumin) comparing with other propolis components. The thermodynamic results indicated that the binding is mainly driven by van der Waals forces and hydrogen bonds. The docking and drugs (warfarin and ibuprofen) competitive results showed that CAPE was located in the subdomain IIA (Sudlow’s site I, FA7) of HSA, and Gln196 and Lys199 contributed to the hydrogen bonds. They also observed circular dichroism spectra, which could be interpreted as an alteration of the secondary structure of HSA due to its partial unfolding in the presence of CAPE.
These factors lead us toward further research, based on in vivo experiments that are needed to investigate liberation, absorption, distribution, metabolism, and excretion (LADME). It is also necessary to determine the concentration rate of CA and CAPE in tissues and organs to determine an optimal form of administration and recommended dosage to use these polyphenols as chemopreventive agents in an organized form.
Conclusions
We performed this limited in vitro study to show the comparison between CA and CAPE, 2 bioactive agents that occur in bee propolis. We tested the cytotoxic effect of these 2 polyphenols using an XTT-NR-SRB assay on breast cancer MCF-7 cells. The migration rate was measured by wound healing assay. Presently, there is no doubt that CA and especially CAPE can be used as a chemopreventive agent. Nonetheless, more research is needed where CA and CAPE could be used, particularly on the migration mechanism (not only for breast cancer cells). Evaluation of the cytotoxic impact on migration rate inhibition would also be a point to consider in future research, especially using migration markers. Our comparative study of the impact of CA and CAPE on MCF-7 cells clearly proved stronger effects for CAPE inducing antitumor activities using the same concentrations and experiment times. To investigate CA and CAPE as bioactive diet compounds, in vivo research is needed. We particularly believe that LADME research could be the next step of research to develop optimal diet recommendations based on CA and CAPE intake.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a research grant from the Medical University of Silesia in Katowice, Poland, No. KNW 1-167/L/N7.
ORCID iDs: Agata Kabała-Dzik  https://orcid.org/0000-0002-3575-8159
https://orcid.org/0000-0002-3575-8159
Robert Dariusz Wojtyczka  https://orcid.org/0000-0002-0334-0938
https://orcid.org/0000-0002-0334-0938
References
- 1. Castellone RD, Leffler NR, Dong L, Yang LV. Inhibition of tumor cell migration and metastasis by the proton-sensing GPR4 receptor. Cancer Lett. 2011;312:197-208. [DOI] [PubMed] [Google Scholar]
- 2. Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5-14. [DOI] [PubMed] [Google Scholar]
- 3. Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359-369. [DOI] [PubMed] [Google Scholar]
- 4. Peter C, Waibel M, Radu CG, et al. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296-5305. [DOI] [PubMed] [Google Scholar]
- 5. Radu CG, Yang LV, Riedinger M, Au M, Witte ON. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc Natl Acad Sci U S A. 2004;101:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Buul JD, Hordijk PL. Signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2004;24:824-833. [DOI] [PubMed] [Google Scholar]
- 7. Yang LV, Heng HH, Wan J, Southwood CM, Gow A, Li L. Alternative promoters and polyadenylation regulate tissue-specific expression of hemogen isoforms during hematopoiesis and spermatogenesis. Dev Dyn. 2003;228:606-616. [DOI] [PubMed] [Google Scholar]
- 8. Yoshida M, Yoshida K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol Hum Reprod. 2011;17:457-465. [DOI] [PubMed] [Google Scholar]
- 9. Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 2005;105:1127-1134. [DOI] [PubMed] [Google Scholar]
- 10. Albini A. Tumor and endothelial cell invasion of basement membranes. The matrigel chemoinvasion assay as a tool for dissecting molecular mechanisms. Pathol Oncol Res. 1998;4:230-241. [DOI] [PubMed] [Google Scholar]
- 11. Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89-96. [DOI] [PubMed] [Google Scholar]
- 12. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895-904. [DOI] [PubMed] [Google Scholar]
- 13. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329-333. [DOI] [PubMed] [Google Scholar]
- 14. Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poujade M, Grasland-Mongrain E, Hertzog A, et al. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci U S A. 2007;104:15988-15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramer N, Walzl A, Unger C, et al. In vitro cell migration and invasion assays. Mutat Res. 2013;752:10-24. [DOI] [PubMed] [Google Scholar]
- 17. Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baselga J, Norton L. Focus on breast cancer. Cancer Cell. 2002;1:319-322. [DOI] [PubMed] [Google Scholar]
- 19. Canevari RA, Marchi FA, Domingues MA, et al. Identification of novel biomarkers associated with poor patient outcomes in invasive breast carcinoma. Tumour Biol. 2016;37:13855-13870. [DOI] [PubMed] [Google Scholar]
- 20. Feldman M, Ruan W, Cunningham BC, Wells JA, Kleinberg DL. Evidence that the growth hormone receptor mediates differentiation and development of the mammary gland. Endocrinology. 1993;133:1602-1608. [DOI] [PubMed] [Google Scholar]
- 21. Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1:533-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaulsay KK, Mertani HC, Törnell J, Morel G, Lee KO, Lobie PE. Autocrine stimulation of human mammary carcinoma cell proliferation by human growth hormone. Exp Cell Res. 1999;250:35-50. [DOI] [PubMed] [Google Scholar]
- 23. Borgés S, Moudilou E, Vouyovitch C, et al. Involvement of a JAK/STAT pathway inhibitor: cytokine inducible SH2 containing protein in breast cancer. Adv Exp Med Biol. 2008;617:321-329. [DOI] [PubMed] [Google Scholar]
- 24. Chiesa J, Ferrer C, Arnould C, et al. Autocrine proliferative effects of hGH are maintained in primary cultures of human mammary carcinoma cells. J Clin Endocrinol Metab. 2011;96:E1418-E1426. [DOI] [PubMed] [Google Scholar]
- 25. Gebre-Medhin M, Kindblom LG, Wennbo H, Törnell J, Meis-Kindblom JM. Growth hormone receptor is expressed in human breast cancer. Am J Pathol. 2001;158:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhat-Nakshatri P, Sweeney CJ, Nakshatri H. Identification of signal transduction pathways involved in constitutive NF-kappaB activation in breast cancer cells. Oncogene. 2002;21:2066-2078. [DOI] [PubMed] [Google Scholar]
- 27. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scimeca M, Antonacci C, Colombo D, Bonfiglio R, Buonomo OC, Bonanno E. Emerging prognostic markers related to mesenchymal characteristics of poorly differentiated breast cancers. Tumour Biol. 2016;37:5427-5435. [DOI] [PubMed] [Google Scholar]
- 29. Biswas DK, Iglehart JD. Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J Cell Physiol. 2006;209:645-652. [DOI] [PubMed] [Google Scholar]
- 30. Frasor J, Weaver A, Pradhan M, et al. Positive cross-talk between estrogen receptor and NF-kappaB in breast cancer. Cancer Res. 2009;69:8918-8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017-6026. [DOI] [PubMed] [Google Scholar]
- 32. Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappaB. Proc Natl Acad Sci U S A. 1996;93:9090-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Onori P, DeMorrow S, Gaudio E, et al. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int J Cancer. 2009;125:565-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yildirim O, Yilmaz A, Oz O, et al. Effect of caffeic acid phenethyl ester on treatment of experimentally induced methicillin-resistant Staphylococcus epidermidis endophthalmitis in a rabbit model. Cell Biochem Funct. 2007;25:693-700. [DOI] [PubMed] [Google Scholar]
- 35. Lee DH, Kim HH, Cho HJ, Bae JS, Yu YB, Park HJ. Antiplatelet effects of caffeic acid due to Ca(2) mobilization inhibition via cAMP-dependent inositol-1, 4, 5-trisphosphate receptor phosphorylation. J Atheroscler Thromb. 2014;21:23-37. [DOI] [PubMed] [Google Scholar]
- 36. Omene CO, Wu J, Frenkel K. Caffeic acid phenethyl ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Invest New Drugs. 2012;30:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu J, Omene C, Karkoszka J, et al. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011;308:43-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akyol S, Ozturk G, Ginis Z, Armutcu F, Yigitoglu MR, Akyol O. In vivo and in vitro antineoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr Cancer. 2013;65:515-526. [DOI] [PubMed] [Google Scholar]
- 39. Morin P, St-Coeur PD, Doiron JA, et al. Substituted caffeic and ferulic acid phenethyl esters: synthesis, leukotrienes biosynthesis inhibition, and cytotoxic activity. Molecules. 2017;22:E1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rzepecka-Stojko A, Kabala-Dzik A, Moździerz A, et al. Caffeic acid phenethyl ester and ethanol extract of propolis induce the complementary cytotoxic effect on triple-negative breast cancer cell lines. Molecules. 2015;20:9242-9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grosso G, Godos J, Lamuela-Raventos R, et al. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: level of evidence and limitations. Mol Nutr Food Res. 2017;61(4). doi: 10.1002/mnfr.201600930 [DOI] [PubMed] [Google Scholar]
- 42. Kabala-Dzik A, Rzepecka-Stojko A, Kubina R, et al. Comparison of two components of propolis: caffeic acid (CA) and caffeic acid phenethyl ester (CAPE) induce apoptosis and cell cycle arrest of breast cancer cells MDA-MB-231. Molecules. 2017;22:E1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dziedzic A, Kubina R, Kabala-Dzik A, Wojtyczka RD, Morawiec T, Buldak RJ. Caffeic acid reduces the viability and migration rate of oral carcinoma cells (SCC-25) exposed to low concentrations of ethanol. Int J Mol Sci. 2014;15:18725-18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kabala-Dzik A, Rzepecka-Stojko A, Kubina R, et al. Migration rate inhibition of breast cancer cells treated by caffeic acid and caffeic acid phenethyl ester: an in vitro comparison study. Nutrients. 2017;9:E1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011;728:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Omene C, Kalac M, Wu J, Marchi E, Frenkel K, O’Connor OA. Propolis and its active component, caffeic acid phenethyl ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. J Cancer Sci Ther. 2013;5:334-342. [PMC free article] [PubMed] [Google Scholar]
- 48. Mates JM, Segura JA, Alonso FJ, Márquez J. Anticancer antioxidant regulatory functions of phytochemicals. Curr Med Chem. 2011;18:2315-2338. [DOI] [PubMed] [Google Scholar]
- 49. Khoram NM, Bigdeli B, Nikoofar A, Goliaei B. Caffeic acid phenethyl ester increases radiosensitivity of estrogen receptor-positive and -negative breast cancer cells by prolonging radiation-induced DNA damage. J Breast Cancer. 2016;19:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jung BI, Kim MS, Kim HA, et al. Caffeic acid phenethyl ester, a component of beehive propolis, is a novel selective estrogen receptor modulator. Phytother Res. 2010;24:295-300. [DOI] [PubMed] [Google Scholar]
- 51. Rosendahl AH, Perks CM, Zeng L, et al. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clin Cancer Res. 2015;21:1877-1887. [DOI] [PubMed] [Google Scholar]
- 52. Wadhwa R, Nigam N, Bhargava P, et al. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ cyclodextrin. J Cancer. 2016;7:1755-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang QS, Kong PZ, Li XQ, Yang F, Feng YM. FOXF2 deficiency promotes epithelial-mesenchymal transition and metastasis of basal-like breast cancer. Breast Cancer Res. 2015;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang X, Ma G, Liu J, Zhang Y. MicroRNA-182 promotes proliferation and metastasis by targeting FOXF2 in triple-negative breast cancer. Oncol Lett. 2017;14:4805-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huber MA, Azoitei N, Baumann B, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Riverso M, Kortenkamp A, Silva E. Non-tumorigenic epithelial cells secrete MCP-1 and other cytokines that promote cell division in breast cancer cells by activating ERα via PI3K/Akt/mTOR signaling. Int J Biochem Cell Biol. 2014;53:281-294. [DOI] [PubMed] [Google Scholar]
- 57. Li S, Lu J, Chen Y, et al. MCP-1-induced ERK/GSK-3β/snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol Immunol. 2017;14:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Toma L, Sanda GM, Niculescu LS, Deleanu M, Stancu CS, Sima AV. Caffeic acid attenuates the inflammatory stress induced by glycated LDL in human endothelial cells by mechanisms involving inhibition of AGE-receptor, oxidative, and endoplasmic reticulum stress. Biofactors. 2017;43:685-697. [DOI] [PubMed] [Google Scholar]
- 59. Bhat K, Sarkissyan M, Wu Y, Vadgama JV. GROα overexpression drives cell migration and invasion in triple negative breast cancer cells. Oncol Rep. 2017;38:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manach C, Scalbert A, Morand C, Rémésy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727-747. [DOI] [PubMed] [Google Scholar]
- 61. Bohn T, McDougall GJ, Alegría A, et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—a position paper focusing on carotenoids and polyphenols. Mol Nutr Food Res. 2015;59:1307-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williamson G, Clifford MN. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr. 2010;104(suppl 3):S48-S66. [DOI] [PubMed] [Google Scholar]
- 63. Gonthier MP, Remesy C, Scalbert A, et al. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed Pharmacother. 2006;60:536-540. [DOI] [PubMed] [Google Scholar]
- 64. Stalmach A, Mullen W, Barron D, et al. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab Dispos. 2009;37:1749-1758. [DOI] [PubMed] [Google Scholar]
- 65. Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33:220-235. [DOI] [PubMed] [Google Scholar]
- 66. Omar MH, Mullen W, Stalmach A, et al. Absorption, disposition, metabolism, and excretion of [3-(14)C]caffeic acid in rats. J Agric Food Chem. 2012;60:5205-5214. [DOI] [PubMed] [Google Scholar]
- 67. Konishi Y, Hitomi Y, Yoshioka E. Intestinal absorption of p-coumaric and gallic acids in rats after oral administration. J Agric Food Chem. 2004;52:2527-2532. [DOI] [PubMed] [Google Scholar]
- 68. Li H, Wu F, Tan J, et al. Caffeic acid phenethyl ester exhibiting distinctive binding interaction with human serum albumin implies the pharmacokinetic basis of propolis bioactive components. J Pharm Biomed Anal. 2016;122:21-28. [DOI] [PubMed] [Google Scholar]