Abstract
Purpose. This study aimed to measure symptoms of posttraumatic stress disorder (PTSD) in Chinese patients following a new diagnosis of lung cancer. Secondary aims were to explore factors at diagnosis that may predict PTSD symptoms at 6 months. Methods. This was a prospective longitudinal observational study that included 93 patients with newly diagnosed lung cancer. PTSD symptomology was assessed using the PTSD Checklist Civilian Version (PCL-C) and health-related quality of life (HRQoL) was assessed with the European Organisation for the Research and Treatment of Cancer questionnaire. Measures were completed at diagnosis and 6 months. Results. No patient had PTSD at baseline or 6 months as measured by a score of ⩾50 in the PCL-C. However, at diagnosis, 44% of patients had “mild” symptoms of PTSD. At 6 months, 64% of patients had “mild” and 8% had “moderate” PTSD symptoms. PTSD symptom scores significantly worsened over 6 months (mean difference [95% CI] = 7.2 [5.4 to 9.0]). Six months after diagnosis, higher PTSD scores were seen in people who at diagnosis were younger (P = .003), had a lower smoking pack history (P = .012), displayed less sedentary behavior (P < .005), or initially had worse cancer symptoms, including fatigue (P = .001) and poorer HRQoL (P = .004). Conclusions. Mild PTSD symptoms are common in patients with lung cancer 6 months after treatment; however, a full diagnosis of PTSD is uncommon. Screening for PTSD symptoms may be considered for at-risk patients with newly diagnosed lung cancer.
Keywords: lung cancer, posttraumatic stress disorder, survivorship, symptoms, health-related quality of life
Introduction
Lung cancer is the leading cause of cancer death worldwide. The 5-year survival across all stages of disease is only 14%.1 Lung cancer is also associated with high patient morbidity, including physical and psychological impairments, which worsen as the disease progresses.2-5 Patients with lung cancer suffer from a variety of cancer symptoms.6 Some of the most disabling symptoms are breathlessness, fatigue, and pain.7 These symptoms can occur in clusters, and their occurrence frequently results in high levels of patient distress.6,8 Distress is an important marker in lung cancer, especially because of the fact that the presence of distress at the time of lung cancer diagnosis is predictive of higher rates of mortality.9
Posttraumatic stress disorder (PTSD) is the most prevalent psychopathological consequence of trauma.10 It includes features of persistence of intense, distressing, and fearfully avoided reactions to reminders of the triggering traumatic event; alteration of mood and cognition; a pervasive sense of imminent threat; disturbed sleep; and hypervigilance.10 Factors associated with increased susceptibility to PTSD include female sex, childhood trauma, fewer years of schooling, prior mental disorders, exposure to 4 or more traumatic events, and a history of exposure to interpersonal violence.10 The lifetime prevalence of PTSD varies according to social background and country of residence, ranging from 1.3% to 12%, and the global 1-year prevalence ranges from 0.2% to 3.8%, with the lowest rates for Beijing and Shanghai and highest for Northern Ireland and the United States.11
The diagnosis of a life-threatening illness can trigger an onset of PTSD.12 Previous studies have shown that the incidence of PTSD is higher in patients with cancer than the general population,13 with the incidence of PTSD ranging from 3% to 22% in patients after they have completed cancer treatment.14 The wide variation in reported rates of PTSD are in part a result of different methods of assessment/screening used.14,15 Given that lung cancer is associated with worse prognosis, greater symptom distress, and higher disease burden than other cancer diagnoses,6,9,16 PTSD may be a common, yet underrecognized, issue in lung cancer for patients after diagnosis. However, research on PTSD in patients with lung cancer is infrequent.15 A better understanding of this issue and the potential risk factors for patients with lung cancer may assist in understanding survivorship needs in lung cancer.
Therefore, this study aimed to measure symptoms of PTSD in Chinese patients following a new diagnosis of lung cancer over the first 6 months from diagnosis. Secondary aims were to determine factors at diagnosis that predict PTSD symptoms 6 months following diagnosis and to investigate the relationship between PTSD symptoms and HRQoL. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed to report this study.17
Methods
Study Design, Setting, and Participants
This was a prospective observational longitudinal study conducted at the Affiliated Hospital of Nantong University, China, between December 2014 and July 2015. Ethics approval was obtained from the Medical Ethics Committee of the Affiliated Hospital of Nantong University, China (approval number 2014-106), and all participants or their caregivers provided informed written consent prior to participation. Participants were eligible for inclusion if they were adults, aged 18 years or older, had a new diagnosis of lung cancer, and had not started any form of cancer treatment.
Procedures
Consecutive patients were screened prospectively for inclusion into the study at the Affiliated Hospital of Nantong University. Participants were recruited any time from time of diagnosis to commencement of treatment for cancer. All patients meeting the eligibility criteria were invited to participate. Participants underwent assessment at baseline (close to diagnosis) and 6 months later. Between time points of testing, medical treatments (surgery and/or chemotherapy) were administered per usual care. According to usual care, many patients were not told of their diagnosis of lung cancer (according to the wishes of their family).
Outcome Measurement
PTSD was measured in accordance with the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria18 using the PTSD Checklist Civilian Version (PCL-C).19,20 The PCL-C is a valid and reliable self-rated scale for the diagnosis of PTSD in cancer and for measurement of PTSD symptoms.19-21 The Chinese version of the PCL-C,22 which has established reliability and validity was used.23,24 The PCL-C comprises 17 questions corresponding to key symptoms of PTSD in the areas of re-experiencing, avoidance/numbing, and increased arousal. Questions are scored on a 5-point Likert type scale from 1 (not at all) to 5 (extremely). A total severity score is calculated by the summation of the scores for the 17 items, ranging from 17 to 85 points. The diagnosis of PTSD was determined using the cuff off method, with scores ⩾50 rendering a diagnosis of PTSD.22 Symptoms of PTSD are rated as mild (20-39 points), moderate (40-59 points), severe (60-79 points), and extreme (80-85 points).25
The Chinese version26 of the Hospital Anxiety and Depression Scale (HADS) was used to measure symptoms of anxiety and depression.27-29 The HADS comprises 14 questions, which ask the participant to recall how they have been feeling over the past 7 days. The items are rated on a 4-point Likert type scale from 0 to 3. The anxiety and depression items are summated to give an anxiety score and depression score, respectively, from 0 to 21. The cutoff values for the anxiety and depression subscales are as follows: 0 to 7 points, normal; 8 to 10 points, borderline (anxiety or depression); and 11 to 21 points, clinical (anxiety or depression).29 The Distress Thermometer was used to measure distress. Participants were asked to rate the distress they had been experiencing over the previous 7 days on an 11-point Visual Analogue Scale from 0 (no distress) to 10 (extreme distress).30 A cutoff value of 5+ is used to identify patients with distress.30,31
Health-related quality of life (HRQoL) was assessed with the European Organisation for the Research and Treatment of Cancer questionnaire (EORTC QLQ-C30,32,33 Chinese version). The EORTC QLQ-C30 is a core questionnaire designed to assess HRQoL over the full spectrum of cancer diagnoses. It is a widely used tool to assess HRQoL and has been used in China previously.34,35 The questionnaire assesses HRQoL over the previous week.32 The questionnaire provides a range of domain scores and symptom scores. Higher domain scores represent better status (such as higher scores on the global domain or mental health domain represent better global quality of life and better mental health, respectively). Lower scores on the symptom scores represent fewer symptoms. Demographic and medical data were also collected, including age, sex, type of cancer, type of cancer treatment, smoking history, social situation, premorbid mobility, and whether or not the patient was informed of their lung cancer diagnosis. Patients were asked to recall their sedentary time (hours spent watching television per day) and their physical activity levels (time spent in moderate or vigorous physical activities per day) from the past week.
Study Size and Statistical Analyses
A convenience sample of 93 patients, recruited over the 9-month study period, was included in this study. All data were analyzed using SPSS Windows, version 22.0 (SPSS, Chicago, IL). Data were assessed for normality using the Kolmogorov-Smirnov statistic. Parametric data are presented as means and SDs, and nonparametric data are presented as medians and interquartile ranges. Descriptive statistics were used to summarize baseline characteristics and outcome data. Change over time in outcomes were analyzed with paired t tests (as data were parametric) using available data from patients who had repeat measures (no data imputation was performed).
The Pearson correlation coefficient was used to assess the bivariate relationships between PTSD total score (at baseline and 6 months) and other variables. Coefficients were interpreted as follows: little (0.00-0.25), fair (0.25-0.50), moderate (0.50-0.75), and large (0.75-1.0) association.36 Linear regression analyses were used to investigate which factors when measured at baseline predict future PTSD scores 6 months from diagnosis. Baseline test scores and demographics were the variables of interest and were included in all regression models. The outcome of interest was PTSD at the 6-month assessment. Potential covariates included age, cancer stage, smoking pack-year history, baseline distress, baseline symptoms, and baseline physical function including physical activity levels and sedentary time. Potential covariates with significant univariate correlation with the outcome of interest were included in the model if collinearity was not identified. The α value was set at .05 for all analyses.
Subgroup Analyses
Baseline characteristics and outcome data were compared between the subgroup of patients who were aware that they had lung cancer versus those who were not (ie, their family elected not to tell them). Change over time in outcomes and linear regression analyses to predict future PTSD were repeated (as described above) for each of these subgroups separately. In addition, PTSD levels, anxiety, depression, distress, and global HRQoL of patients with early-stage disease (Ia-IIIa) were compared to those with late-stage disease (IIIb-IV) at diagnosis and 6 months.
Results
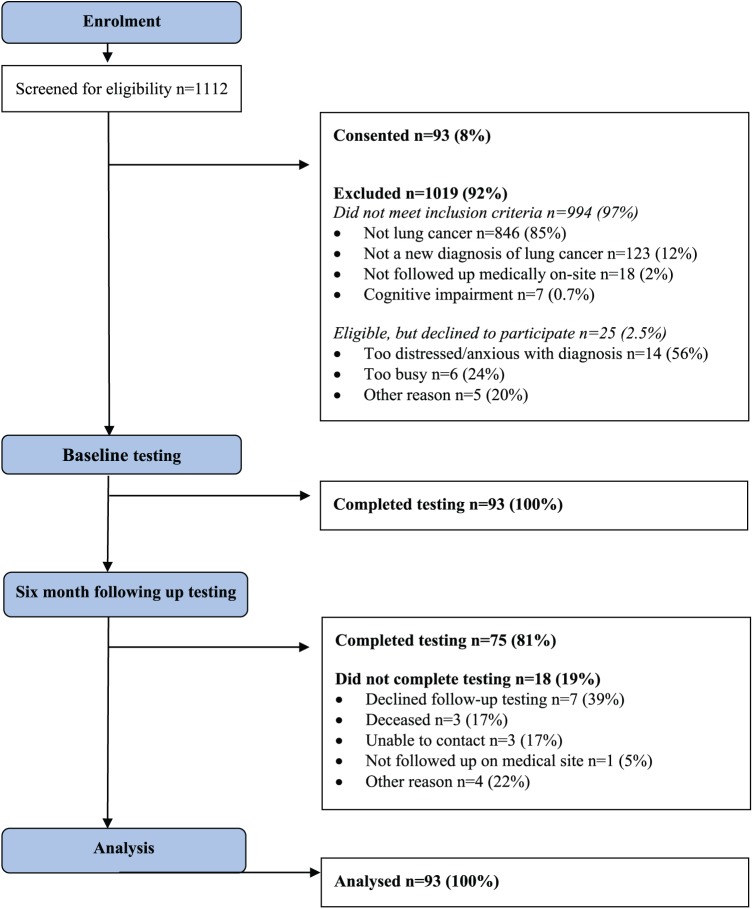
Between December 2014 and July 2015, 1112 patients were screened for inclusion into this study; 118 patients were eligible and approached for inclusion (Figure 1). Among them, 93 participants consented (79% consent rate) and were included in the study. The main reason for nonconsent was that patients were too distressed/anxious with the diagnosis (n = 14). The flow of participants through the study is presented in Figure 1. All participants (n = 93, 100%) completed the assessments at baseline, and 81% (n = 75) completed assessments at 6 months. The main reason for missing data at 6 months was that the participant declined the follow-up testing (n = 7, 39% of missing data; Figure 1).
Figure 1.
Flow through the study.
The medical and social demographics are shown in Table 1. The mean (SD) age was 62 ± 10 years, and 72% were male. Of the cohort, 76% were ECOG 0 or 1 at diagnosis, and a little more than half of the patients (58%) had stage IV disease.
Table 1.
Medical and Social Demographic Characteristics of the Cohort.
| Variable | n = 93 |
|---|---|
| Age at baseline, years, mean (SD) | 62.4 (10.3) |
| Sex, male (%) | 67 (72%) |
| Social situation, n (%) | |
| Home alone independent | 1 (1.1%) |
| Home with family | 85 (91%) |
| Home with supports | 3 (3.2%) |
| Retirement village | 0 |
| Other | 4 (4.3%) |
| Employment status, n (%) | |
| Working | 0 |
| Sick leave | 20 (21%) |
| Home duties | 13 (14%) |
| Not employed | 24 (26%) |
| Retired | 34 (37%) |
| Other | 2 (2.2%) |
| Smoking status, n (%) | |
| Never smoker | 35 (38%) |
| Ex-smoker | 26 (28%) |
| Current smoker | 32 (34%) |
| Smoking history pack years, median [IQR] | 25 [0-40] |
| Use of walking aid at baseline, n (%) | 0 |
| Self-reported limitations to walking, n (%) | 26 (28%) |
| ECOG-PS, n (%) | |
| 0 | 6 (6.4%) |
| 1 | 71 (76%) |
| 2 | 13 (14%) |
| 3 | 2 (2.2%) |
| 4 | 1 (1.1%) |
| Meeting PA guidelines,a n (%) | 64 (69%) |
| Television viewing time per day, hours, median [IQR] | 2 [0-2] |
| Weight loss at diagnosis, n (%) | 32 (34%) |
| Histological type, n (%) | |
| Squamous | 33 (33%) |
| Adenocarcinoma | 49 (53%) |
| Large cell | 0 |
| Other | 11 (12%) |
| Cancer stage, n (%) | |
| Stage IA | 1 (1.1%) |
| Stage IB | 12 (13%) |
| Stage IIA | 13 (14%) |
| Stage IIB | 2 (2.2%) |
| Stage IIIA | 10 (11%) |
| Stage IIIB | 1 (1.1%) |
| Stage IV | 54 (58%) |
| Medical treatment, n (%) | |
| Chemotherapy only | 42 (45%) |
| Surgery and chemotherapy | 36 (39%) |
| Surgery only | 2 (2.1%) |
| No surgery, chemotherapy or RT | 13 (14%) |
| Type of surgery, n (%) | |
| Lobectomy | 15 (16%) |
| Wedge resection | 5 (5.4%) |
| Lobectomy and wedge resection | 0 |
| Sleeve resection | 1 (1.1%) |
| Segmentectomy | 2 (2.2%) |
| Resection from different lobes | 3 (3.2%) |
| Aware of cancer diagnosis, yes, n (%) | 32 (34%) |
Abbreviations: ECOG, Eastern Cooperate Oncology Group–Performance Status; IQR, interquartile range; PA, physical activity; RT, radiotherapy.
PA guidelines = 150 minutes of moderate-intensity PA per week.
Posttraumatic Stress Disorder
No patient had PTSD diagnosed with the DSM-IV criteria22 at baseline or at 6 months (ie, no participant scored ⩾50 on the PCL-C). At diagnosis, 44% of patients had mild symptoms of PTSD, but no patient had moderate, severe, or extreme PTSD symptoms. At the 6-month follow-up, 64% of patients had mild PTSD symptoms, 8% had moderate PTSD symptoms, and no patient had severe or extreme PTSD symptoms. Over 6 months, PTSD scores significantly worsened (mean difference [95% CI] = 7.2 [5.4-9.0]), and by the 6-month follow-up, the group was classified as having mild PTSD symptoms overall (Table 2).
Table 2.
PTSD Symptoms, Mood, and HRQoL in Patients at Baseline and 6-Month Follow-up.a
| Measure | Baseline, Mean (SD) | Six Months |
P Value | |
|---|---|---|---|---|
| Mean (SD) | Mean Difference (95% CI) | |||
| PCL-C PTSD total score | 19.5 (1.7) | 26.7 (8.3) | −7.2 (−9.0, −5.4) | <.0005 |
| HADS Depression score | 5.8 (1.04) | 5.9 (1.4) | −0.12 (−0.38, 0.13) | .349 |
| HADS Anxiety score | 6.4 (1.8) | 7.9 (4.1) | −1.7 (−2.6, −0.81) | <.0005 |
| Distress Thermometer | 2.8 (0.8) | 3.4 (1.2) | −0.64 (−0.91, −0.37) | <.0005 |
| Global quality of life | 70.2 (16.9) | 67.4 (15.8) | 4.2 (0.96, 7.5) | .012 |
| Physical function | 92.5 (11.5) | 86.8 (14.0) | 5.9 (2.5, 9.4) | .001 |
| Role function | 94.6 (11.3) | 86.4 (14.9) | 9.1 (6.1, 12.1) | <.0005 |
| Emotional function | 92.4 (10.1) | 89.3 (10.9) | 3.6 (1.4, 5.7) | .002 |
| Cognitive function | 98.6 (4.7) | 96.9 (6.5) | 2.0 (0.59, 3.4) | .006 |
| Social function | 70.6 (10.5) | 72 (10.3) | 0.22 (−1.4, 1.8) | .784 |
| Fatigue | 29.3 (13.6) | 32.4 (14.8) | −2.2 (−4.9, 0.50) | .108 |
| Nausea or vomiting | 5.4 (11.6) | 12.4 (15.3) | −6.0 (−9.2, −2.8) | <.0005 |
| Pain | 18.8 (18.8) | 19.3 (20.0) | −1.1 (−4.8, 2.6) | .551 |
| Dyspnea | 12.5 (18.3) | 14.2 (19.9) | −2.2 (−7.5, 3.0) | .402 |
| Insomnia | 12.9 (18.4) | 18.2 (21.4) | −6.2 (−10.7, −1.7) | .007 |
| Appetite loss | 26.5 (15.2) | 23.1 (15.5) | 2.2 (−4.0, 4.8) | .096 |
| Constipation | 26.9 (29.1) | 36 (31.8) | −7.5 (−12.2, −2.9) | .002 |
| Diarrhea | 0.35 (3.45) | 0 (0) | 0.44 (−0.44, 1.3) | .321 |
| Financial problems | 32.3 (7.69) | 32.4 (10.9) | −0.44 (−2.4, 1.5) | .658 |
Abbreviations: PTSD, posttraumatic stress disorder; HRQoL, health-related quality of life; PCL-C, PTSD Checklist Civilian Version (scored out of 85 points); HADS, Hospital Anxiety and Depression Scale.
Higher scores on the PCL-C PTSD, HADS Depression, HADS Anxiety, Distress Thermometer, and symptom domains represent worse status. Lower scores on the global quality of life and function domains represent worse status. Change over time data are shown for n =75, with repeated measures.
Mood and Health-Related Quality of Life
There was a significant increase in anxiety and distress scores over 6 months; however, there was no change in depression (Table 2). Over the 6 months, there was significant reduction in global quality of life, physical function, role function, emotional function, cognitive function, nausea/vomiting, insomnia, and constipation (P < .05; Table 2). The domains of social function, pain, dyspnea, diarrhea, and financial problems did not change over time (Table 2).
Relationship Between PTSD and Other Factors
Six months after diagnosis, higher PTSD scores were seen in people who were younger (r = 0.341; P = .003), who had a lower smoking pack history (r = 0.288; P = .012), who displayed less sedentary time (r = 0.395; P < .005), and who initially had worse HRQoL and symptoms at diagnosis: global HRQoL (r = 0.331; P = .004), fatigue (r = 0.367; P = .001), nausea/vomiting (r = 0.392; P = .001), pain (r = 0.356; P = .002), appetite loss (r = 0.555; P < .005), and constipation (r = 0.233; P = .044). Linear regression analyses were performed to assess the impact of a number of factors at diagnosis on PTSD scores at 6 months. The final model contained sedentary time, smoking status, and appetite loss (all measured at diagnosis) as the 3 independent variables, and this model explained 69.7% of the variance in PTSD at 6 months [R2 = 0.49; F(3, 71) = 22.346; P < .0005]. Smoking (β = −2.62; P = .003), sedentary time (β = −2.35; P < .0005), and appetite loss (β = 0.22; P < .0005) were significant predictors of PTSD symptoms.
Subgroup Analyses of Patients Who Knew They Had Lung Cancer Versus Those Who Did Not Know of Their Cancer Diagnosis
In all, 34% (n = 32) of the cohort were aware that they had lung cancer, whereas 66% (n = 61) were not aware of their diagnosis (ie, their family elected not to tell them). There were significantly more male (n = 37/67, 55%) than female patients (n = 24/26, 92%) who were aware that they had lung cancer (P = .001). There were no other statistically significant differences in the medical or social demographics between these 2 subgroups at baseline (P > .05).
There were no statistically significant differences in PTSD scores between patients who knew of their diagnosis versus those who did not at baseline (P = .251) or at 6 months (P = .497). Both groups had a statistically significant increase (worsening) of PTSD scores over time (P < .05). However, at baseline, the subgroup who did not know of their diagnosis had higher depression scores (mean difference = 0.47; 95% CI = 0.02-0.91, P = .039), worse global quality of life (mean difference = 10.8; 95% CI = 3.8-17.8; P = .003), and worse social function (mean difference = 5.1; 95% CI = 0.63-9.6; P = .026) than the subgroup who knew. The subgroup of patients who knew of their diagnosis experienced worsening of PTSD scores, distress, role function, emotional function, and nausea over time (P < .05). The subgroup of patients who did not know of their diagnosis had worsening of PTSD scores, anxiety, distress, global HRQoL, physical function, role function, emotional function, cognitive function, fatigue, nausea, insomnia, and constipation over time (P < .05).
The linear regression model to predict PTSD at 6 months (sedentary time, smoking status, and appetite loss measured at diagnosis) remained true for the subgroup of patients who knew of their diagnosis, explaining 71.4% of the variance in PTSD at 6 months, whereas for the subgroup of patients who were not aware of their diagnosis, the final model contained sedentary time, smoking status, and age (all measured at diagnosis) as the 3 independent variables, and this model explained 73.1% of the variance in PTSD at 6 months [R2 = 0.53; F(3, 42) = 16.06; P < .0005]. Smoking (β = −3.80; P = .001), sedentary time (β = −3.24; P < .0005), and age (β = −0.32; P = .001) were significant predictors of PTSD symptoms for this subgroup.
Early- Versus Late-Stage Disease
Compared to patients with early-stage disease Ia to IIIa (n = 38), patients with late-stage disease (IIIb-IV, n = 55) had worse distress at baseline (mean difference = 0.4 points; 95% CI = 0.03-0.69; P = .032) and worse global HRQoL at 6 months (mean difference = 7.8 points; 95% CI = 0.70-14.90; P = .032). There was no difference in PTSD, depression, or anxiety between these groups.
Discussion
To our knowledge, this is 1 of only 2 studies to measure symptoms of PTSD in people within 6 months of a new diagnosis of lung cancer. We found that it is common for people with lung cancer to experience mild symptoms of PTSD close to diagnosis, and this rate increases over 6 months; however, no one in our study had a full diagnosis of PTSD based on the PCL-C cutoff method22 at any time point. The nonoccurrence of a PTSD diagnosis contrasts previous literature, which shows that 5% to 12% patients with cancer have PTSD based on the PCL-C criteria.14,37 However, the majority of the past literature is from the United States, with only a few studies from Asia.14,37
There are a number of differences between our study and prior work,14 which may explain the differences. First, we studied people with newly diagnosed lung cancer, whereas most of the previous work has focused on women with early- to midstage breast cancer.14,37 Lung cancer is associated with significant symptom burden and physical impairment38,39 as well as greater disease burden than most other cancer types.6,9 In the breast cancer literature, it has been shown that advanced disease stage and more intrusive medical treatment are risk factors for PTSD40; it is also possible that rates of PTSD may be specific to the cancer type as well.14 We hypothesized that rates of PTSD in our sample of Chinese people with lung cancer would be greater than the published rates in breast cancer, but this was not found. The only other similar study in lung cancer is that recently published by Dougall et al.41 They included 115 patients undergoing treatment for lung or pleural cancer and found that PTSD rates were between 5% and 16% when measured with the PTSD Checklist-Specific version PCL-S (a slightly different version of the PCL than the one we used).41 However, when PTSD was measured with the PCL-S cutoff method, rates were only 3% to 8%.41 It is important to note that this study had a significant number of dropouts (n = 93 baseline; n = 57 by the 4-month follow-up), so results should be interpreted with caution. Further studies specific to lung cancer are required to investigate PTSD rates.
Second, we included a longitudinal study design, whereas most prior work is cross-sectional in nature,14 and we measured outcomes in the short term (up to 6 months following diagnosis) rather than in the longer-term survivorship phase.14 Our results showed a worsening of PTSD symptoms over this time by a mean difference of 7 points. This change is greater than the minimum threshold (difference of 5 points) considered to represent a reliable change (not resulting from chance)20; however, it is below the 10-point threshold for minimal clinically meaningful change.20 Our findings of worsening symptoms contradict the prior longitudinal studies in other cancer types, which generally show a considerable decline (improvement) in PTSD symptoms over the first 3 months after diagnosis and after treatment cessation.14 Given that people with lung cancer experience significant decline in functional ability over the first 6 months from diagnosis,39 it would not be surprising if PTSD symptoms also worsened. Our results similarly showed that people had a reduction in global quality of life, physical function, role function, emotional function, cognitive function, nausea/vomiting, insomnia, constipation, anxiety, and distress also over this time. Because poorer health outcomes are seen in people who have PTSD, including poorer adherence to cancer treatments,21 early detection and treatment is vital in the context of cancer.
Third, in our cohort, we had a high proportion of patients who were not made aware of their cancer diagnosis. These patients were more commonly female and had worse HRQoL and higher depression levels at the time of diagnosis. This is an interesting topic in the context of health care in China. All patients in our study were of Chinese nationality, and 66% of these patients at initial diagnosis were unaware that they had lung cancer. The concept of death in Chinese culture is strongly influenced by Confucianism,42 and in Chinese culture, cancer is strongly related to death.43 According to Confucianism, nondisclosure in Chinese health care is common and presents a barrier for medical professionals to discuss death. Therefore, at the instruction of patients’ families, often, patients are not informed of their cancer diagnosis by the doctor (or others).44,45 This is especially the case if the family believes that the patient does not have the ability to cope with this information. Our data support this, in that these patients had worse HRQoL and higher depression levels at the time of diagnosis, suggesting that potentially, their families did not tell them of their diagnosis because they were worried about how the patient may cope with the knowledge. It is customary in conventional Chinese culture for medical professionals to respect this decision.46 It is also common for families to hide factors from the patient that could identify their diagnosis to them, such as asking for the patient to be treated away from oncology settings—for example, respiratory rather than oncology wards. Therefore, in this scenario, without the psychological reaction to being diagnosed with lung cancer,14 we hypothesize that these patients may be less likely to develop PTSD, depending on what type of illness they believe they are suffering from (whether or not this is a traumatic stressor). Andrykowski et al47 demonstrated that 37% (n = 70/189) of their patients with lung cancer (mean ± SD of 16 ± 2 months postdiagnosis) recognized that a lung cancer diagnosis and treatment was a traumatic stressor for them (based on meeting the DSM-IV stressor criteria).47 Unfortunately, we did not collect details of the diagnosis that the patient was given—that is, if they were told that they had a respiratory illness or another condition for which they were undergoing treatment—and although the traumatic event does not need to involve death to satisfy PTSD criteria (ie, it can be a threat to physical integrity),14 we do not know how this theoretical threat may be perceived by the patient. We did find that these patients experienced a similar increase in PTSD symptoms over 6 months, and there was no difference in PTSD symptoms at 6 months between patients who knew versus did not know of their diagnosis. Further investigation of the cultural factors associated with the development of PTSD symptoms is required to understand how this information can be generalized for survivorship care across countries and cultures.
Although we had a low PTSD rate, we did find that at 6 months 64% of patients had mild PTSD symptoms and 8% had moderate PTSD symptoms. Similarly, Jeantieu et al48 reported a 51% incidence of PTSD symptoms (measured with the Impact of Events Scale–Revised) in their cohort of 48 patients with lung cancer in France 3 months after surgery. They found preoperative anxiety and postoperative pain to be predictive of PTSD symptoms at 3 months after surgery.48 We found that predictors of higher PTSD scores 6 months after diagnosis included people diagnosed at a younger age, who had a lower smoking history and/or were more active, as well as those who had worse HRQoL and symptoms at diagnosis. These are 2 distinctly different groups, and as such, the mechanisms behind their PTSD symptoms may vary. The first is a young and generally healthy cohort at the time of diagnosis who are starting off at a higher health level; one could speculate that these individuals have the potential to experience greater magnitude of reduction in physical and psychological health from cancer causing a more significant impact on their daily life. Prior studies have also found younger age to be a predictor of PTSD in lung and breast cancer.14,37,41 The second group are in stark contrast. They already had poor HRQoL and high symptom burden at the time of diagnosis. It is well established that both poor HRQoL and high symptom burden at diagnosis are predictors of mortality in lung cancer.8,49 Our results suggest that these factors may also predict people who are at risk for experiencing symptoms of PTSD as well. It was beyond the scope of our study to investigate the mechanisms behind the occurrence of symptoms of PTSD. However, this is an important question for further investigation. Also, comparisons of incidence of PTSD between patients in western countries and China would be interesting.
The strengths of this study include that it was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines17 and conducted prospectively. It is limited by the inclusion of a convenience sample and a 19% rate of missing follow-up data, although a number of our missing cases were a result of death. Additionally, we did not measure prior negative life stressors, and this is a known risk factor for PTSD in the cancer population.14 Further measures at 12 months may also be warranted in future research. We did not ask participants about passive smoking, and this may explain the relatively low smoking rates in the study.
Conclusions
In our cohort of patients with newly diagnosed lung cancer in China, mild PTSD symptoms were common 6 months after diagnosis; however, no participant had a full diagnosis of PTSD. Over 6 months from diagnosis, participants experienced worsening of PTSD symptoms, anxiety and distress levels, HRQoL, and symptoms. Six months after diagnosis, higher PTSD symptom scores were seen in participants who at diagnosis were younger, had a lower smoking pack history, were more sedentary, or initially had worse symptoms, including fatigue and poorer HRQoL.
Footnotes
Authors’ Note: Jun Ni and Jian Feng contributed equally to the article. The Medical Ethics Committee of the Affiliated Hospital of Nantong University, China approved this study. All procedures performed were in accordance with the ethical standards of the institutional and research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study or their next of kin.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by grants from the Jiangsu Education Department, China (Overseas Research and Training Program) and the Jiangsu Key and Development Research (BE2018670), China. Dr Catherine Granger has fellowship funding from the Victorian Cancer Agency (clinical research fellow).
ORCID iD: Catherine L Granger,  https://orcid.org/0000-0001-6169-370X.
https://orcid.org/0000-0001-6169-370X.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 2. Sung MR, Patel MV, Djalalov S, et al. Evolution of symptom burden of advanced lung cancer over a decade. Clin Lung Cancer. 2017;18:274-280.e6. [DOI] [PubMed] [Google Scholar]
- 3. Granger CL, Parry SM, Edbrooke L, Denehy L. Deterioration in physical activity and function differs according to treatment type in non-small cell lung cancer—future directions for physiotherapy management. Physiotherapy. 2016;102:256-263. [DOI] [PubMed] [Google Scholar]
- 4. Tishelman C, Petersson LM, Degner LF, Sprangers MA. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381-5389. [DOI] [PubMed] [Google Scholar]
- 5. Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12:694-708. [DOI] [PubMed] [Google Scholar]
- 6. Cooley ME. Symptoms in adults with lung cancer: a systematic research review. J Pain Symptom Manage. 2000;19:137-153. [DOI] [PubMed] [Google Scholar]
- 7. Hung R, Krebs P, Coups E, et al. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J Pain Symptom Manage. 2011;41:426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheville AL, Novotny PJ, Sloan JA, et al. The value of a symptom cluster of fatigue, dyspnea, and cough in predicting clinical outcomes in lung cancer survivors. J Pain Symptom Manage. 2011;42:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10:423-431. [DOI] [PubMed] [Google Scholar]
- 10. Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N Engl J Med. 2017;376:2459-2469. [DOI] [PubMed] [Google Scholar]
- 11. Karam EG, Friedman MJ, Hill ED, et al. Cumulative traumas and risk thresholds: 12-month PTSD in the World Mental Health (WMH) surveys. Depress Anxiety. 2014;31:130-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. French-Rosas LN, Moye J, Naik AD. Improving the recognition and treatment of cancer-related posttraumatic stress disorder. J Psychiatr Pract. 2011;17:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rustad JK, David D, Currier MB. Cancer and post-traumatic stress disorder: diagnosis, pathogenesis and treatment considerations. Palliat Support Care. 2012;10:213-223. [DOI] [PubMed] [Google Scholar]
- 14. Kangas M, Henry JL, Bryant RA. Posttraumatic stress disorder following cancer: a conceptual and empirical review. Clin Psychol Rev. 2002;22:499-524. [DOI] [PubMed] [Google Scholar]
- 15. Cordova MJ, Riba MB, Spiegel D. Post-traumatic stress disorder and cancer. Lancet Psychiatry. 2017;4:330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Australian Institute of Health and Welfare. Cancer in Australia: An Overview, 2014. Canberra, Australia: AIHW; 2014. Cancer Series No. 78. Cat. No. CAN 75. [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP;, STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19. Conybeare D, Behar E, Solomon A, Newman MG, Borkovec TD. The PTSD Checklist-Civilian Version: reliability, validity, and factor structure in a nonclinical sample. J Clin Psychol. 2012;68:699-713. [DOI] [PubMed] [Google Scholar]
- 20. Weathers F, Litz B, Keane T, Palmieri P, Marx B, Schnurr P. The PTSD Checklist for DSM-5 (PCL-5). https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp. Accessed October 3, 2018.
- 21. Rustad JK, David D, Currier MB. Cancer and post-traumatic stress disorder: diagnosis, pathogenesis and treatment considerations. Palliat Support Care. 2012;10:213-223. [DOI] [PubMed] [Google Scholar]
- 22. Weathers FW, Litz BT, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Paper presented at: Annual Convention of the International Society for Traumatic Stress Studies; October 24-27, 1993; San Antonio, TX. [Google Scholar]
- 23. Wang XM, Liu J, Wang Q, Yang YP. PTSD Checklist-Civilian (PCL-C) Chinese reversion in cancer inpatients (in Chinese). Med Inf. 2013;26:62-63. [Google Scholar]
- 24. Chen LL, Peng L, Tang T, Li M. Correlation between mental health and coping style in gynecological cancer patients (in Chinese). J Third Mil Med Univ. 2012;34:1097-1099. [Google Scholar]
- 25. Grubaugh AL, Elhai JD, Cusack KJ, Wells C, Frueh BC. Screening for PTSD in public-sector mental health settings: the diagnostic utility of the PTSD checklist. Depress Anxiety. 2007;24:124-129. [DOI] [PubMed] [Google Scholar]
- 26. Yang Y, Ding R, Hu D, Zhang F, Sheng L. Reliability and validity of a Chinese version of the HADS for screening depression and anxiety in psycho-cardiological outpatients. Compr Psychiatry. 2014;55:215-220. [DOI] [PubMed] [Google Scholar]
- 27. Luckett T, Butow PN, King MT, et al. A review and recommendations for optimal outcome measures of anxiety, depression and general distress in studies evaluating psychosocial interventions for English-speaking adults with heterogeneous cancer diagnoses. Support Care Cancer. 2010;18:1241-1262. [DOI] [PubMed] [Google Scholar]
- 28. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69-77. [DOI] [PubMed] [Google Scholar]
- 29. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370. [DOI] [PubMed] [Google Scholar]
- 30. Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82:1904-1908. [DOI] [PubMed] [Google Scholar]
- 31. Lynch J, Goodhart F, Saunders Y, O’Connor SJ. Screening for psychological distress in patients with lung cancer: results of a clinical audit evaluating the use of the patient Distress Thermometer. Support Care Cancer. 2010;19:193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 33. Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A:635-642. [DOI] [PubMed] [Google Scholar]
- 34. Xue D, Han S, Jiang S, et al. Comprehensive geriatric assessment and traditional Chinese medicine intervention benefit symptom control in elderly patients with advanced non-small cell lung cancer. Med Oncol. 2015;32:114. [DOI] [PubMed] [Google Scholar]
- 35. Ni J, Denehy L, Feng J, Xu Y, Wu Y, Granger C. Physical activity and health related quality of life of individuals with lung cancer in China and Australia: an observational cohort study. Integr Cancer Ther. 2017. DOI: 10.1177/1534735417699513. [Google Scholar]
- 36. Portney L, Watkins M. Foundations of Clinical Research: Applications to Practice. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 37. Wu X, Wang J, Cofie R, Kaminga AC, Liu A. Prevalence of posttraumatic stress disorder among breast cancer patients: a meta-analysis. Iran J Public Health. 2016;45:1533-1544. [PMC free article] [PubMed] [Google Scholar]
- 38. Granger CL, McDonald CF, Irving L, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer. 2014;83:292-299. [DOI] [PubMed] [Google Scholar]
- 39. Granger CL. Physiotherapy management of lung cancer. J Physiother. 2016;62:60-67. [DOI] [PubMed] [Google Scholar]
- 40. Jacobsen PB, Widows MR, Hann DM, Andrykowski MA, Kronish LE, Fields KK. Posttraumatic stress disorder symptoms after bone marrow transplantation for breast cancer. Psychosom Med. 1998;60:366-371. [DOI] [PubMed] [Google Scholar]
- 41. Dougall AL, Swanson J, Kyutoku Y, Belani C, Baum A. Posttraumatic symptoms, quality of life, and survival among lung cancer patients. J Appl Biobehav Res. 2017;22:e12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsu CY, O’Connor M, Lee S. Understandings of death and dying for people of Chinese origin. Death Stud. 2009;33:153-174. [DOI] [PubMed] [Google Scholar]
- 43. Cai C, Zhou Z, Yu L, Wan Y. Predictors of the health-related quality of life of patients who are newly diagnosed with lung cancer in China. Nurs Health Sci. 2011;13:262-268. [DOI] [PubMed] [Google Scholar]
- 44. Wang XS, Di LJ, Reyes-Gibby CC, Guo H, Liu SJ, Cleeland CS. End-of-life care in urban areas of China: a survey of 60 oncology clinicians. J Pain Symptom Manage. 2004;27:125-132. [DOI] [PubMed] [Google Scholar]
- 45. Xu W, Towers AD, Li P, Collet JP. Traditional Chinese medicine in cancer care: perspectives and experiences of patients and professionals in China. Eur J Cancer Care (Engl). 2006;15:397-403. [DOI] [PubMed] [Google Scholar]
- 46. Qian H, Hou L. Psychological impact of revealing a diagnosis of lung cancer to patients in China. J Thorac Dis. 2016;8:2879-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Andrykowski MA, Steffens RF, Bush HM, Tucker TC. Lung cancer diagnosis and treatment as a traumatic stressor in DSM-IV and DSM-5: prevalence and relationship to mental health outcomes. J Trauma Stress. 2015;28:206-213. [DOI] [PubMed] [Google Scholar]
- 48. Jeantieu M, Gaillat F, Antonini F, et al. Postoperative pain and subsequent PTSD-related symptoms in patients undergoing lung resection for suspected cancer. J Thorac Oncol. 2014;9:362-369. [DOI] [PubMed] [Google Scholar]
- 49. Tishelman C, Petersson LM, Degner LF, Sprangers MA. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381-5389. [DOI] [PubMed] [Google Scholar]