Abstract
Background and Aims: Peripheral neuropathy is a common complication of chemotherapy that can induce marked disability that negatively affects the quality of life in patients with multiple myeloma (MM). The aim of this study was to prevent the onset or the worsening of peripheral neuropathy in MM patients treated with bortezomib (BTZ), using a new nutritional neuroprotective compound. We report preliminary results of 18 out of 33 patients who completed the study. Methods: We administered a tablet of Neuronorm to patients, containing docosahexaenoic acid 400 mg, α-lipoic acid 600 mg, vitamin C 60 mg, and vitamin E 10 mg bid for the whole follow-up period. Neurological visit assessment, electroneurography, and evaluation scales were performed at baseline and after 6 months. Results: At 6 months, 8 patients had no chemotherapy-induced peripheral neuropathy, while 10 patients experienced chemotherapy-induced peripheral neuropathy of grade 1 according to the Common Terminology Criteria for Adverse Events, one of them with pain. Seventeen patients did not report painful symptoms; no limitation of functional autonomy and stability in quality of life domains explored was observed. Conclusions: Our results seem to indicate that early introduction of a neuroprotective agent in our patients with MM treated with BTZ could prevent the onset or the worsening of neuropathic pain, avoiding the interruption of the therapy with BTZ, and maintaining a good functional autonomy to allow normal daily activities. Despite the limitations due to the fact that this is a preliminary study, in a small population, with short follow-up, our data seem to indicate that the nutraceutical may have some potential to be considered for a future trial.
Keywords: docosahexaenoic acid, DHA, α-lipoic acid, ALA, peripheral neuropathy, multiple myeloma, bortezomib, peripheral neuropathy assessment
Introduction
Peripheral neuropathy (PN) is a frequent and significant clinical manifestation of multiple myeloma (MM) that may be observed at onset of disease as a disease-related clinical presentation or induced during treatment as a therapy-related complication.1-3 The most common type is a mild axonal sensory-motor neuropathy,4 but it is also possible to observe a type of demyelinating PN.5 The incidence rate of PN among MM patients has not yet been clearly determined and to date contrasting data have been reported in published clinical studies: overall, almost 20% of myeloma patients suffer from disease-related PN at diagnosis and up to 75% may experience chemotherapy-induced PN (CIPN).4,6-8
The mechanism underlying CIPN in MM has been well studied: vincristine interferes with microtubules-aided postaxonal transport and cisplatin induces apoptosis of the dorsal root ganglion through DNA damage and inhibition of protein synthesis.9,10 In contrast, the novel immunomodulator agents (i.e., thalidomide and lenalidomide) and proteasome inhibitors (i.e., bortezomib [BTZ]) exert a pleiotropic action on nerve fibers; the exact mechanism causing neurologic damage, however, is still not clear.11 It has been proposed that the most important mechanisms explaining thalidomide-related neurotoxicity included capillary damage and secondary anoxemia in nerve fibers, but also an accelerated neuronal cell death by downregulation of tumor necrosis factor-α and the consequent inhibition of nuclear factor-κβ.12 On the contrary, BTZ plays a more specific neurotoxic role through the transient release of intracellular calcium stores with consequent mitochondrial calcium influx and caspase-mediated apoptosis13,14 as well as altered intracellular calcium homeostasis with consequent symptoms of depolarization causing pain and paresthesia.14
CIPN is usually dose-dependent and it may worsen any preexisting polyneuropathy and induce disability, adversely affecting the quality of life (QoL).1,2,15 CIPN is one of the most important factors that lead to dose reduction or discontinuation of chemotherapy.1,16 Pain is a very common symptom in patients with CIPN, and often it can become so unbearable that patients need to stop chemotherapy and/or cannot perform normal acts of everyday life.15 BTZ, the first-line proteasome inhibitor drug in the treatment of MM, is one of the newer chemotherapy medications for the treatment of hemato-oncological diseases.17 BTZ may induce reversible painful distal neuropathy, with neurological symptoms that improve or disappear 3 to 4 months after treatment discontinuation.1 This neuropathy can occur as grade 1 or 2, according to the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE)18 in 33% of newly diagnosed patients and as grade 3 or 4 in 18% of newly diagnosed patients.15 In recent years, the route of administration of BTZ has progressively moved from intravenous to subcutaneous. Subcutaneous administration has a better profile of toxicity, particularly with regard to the onset and severity of PN, resulting in a better treatment, tolerability, and improved compliance.19 Nevertheless, the onset of peripheral neurotoxicity remains one of the main problems associated with BTZ treatment20 and an essential dose-limiting factor during the treatment of MM.21 Guidelines issued by the American Society of Clinical Oncology reported therapies aimed at reducing CIPN, but no method of CIPN prevention was suggested.22,23 There are some evidence supporting moderate recommendation only of antidepressant duloxetine for treatment of CIPN pain.22 Smith and colleagues24 reported that duloxetine administration produced a low 1.06 point reduction in pain on a 10-point scale from baseline, but was also associated with side effects such as fatigue and nausea.
To date there are some preliminary studies regarding the use of several agents for the treatment of oxaliplatin-induced neuropathy that show positive effects: calcium and magnesium infusions, glutathione, carbamazepine, gabapentin, amifostine, acetyl-L-carnitine, α-lipoic acid (ALA), Gingko biloba extract, and celecoxib, but those treatments require further investigation.25,26 To our knowledge, there are only 2 studies available regarding the treatment of BTZ-induced PN in MM patients. The first randomized trial by Zhang et al27 shows that mecobalamin administration in these patients leads to a reduction of PN in 77.7%. In contrast, in the study of Callender et al28 the addition of acetyl-carnitine for prophylactic purposes leads to a lesser appearance of grade 3 CIPN in the treated group compared with the control group.
Published data indicate that nutraceuticals with antioxidant and axonal regrowth action may be useful in reducing inflammation and favoring the formation of new axons and intersynaptic connections. Among the newly marketed nutraceuticals, those containing both docosahexaenoic acid (DHA) and ALA exert powerful neurotrophic, neuroprotective, antioxidant, and anti-inflammatory actions due to the simultaneous presence of these 2 molecules.29 These properties have been demonstrated in studies on PN related to diabetes.30-34
DHA and ALA are extremely safe35-37 and can be used in young and old patients, even when concomitant pathologies are present. DHA and ALA also demonstrated high tolerability, with no side effects to gastrointestinal and renal systems.38 They can be given in association with steroidal and nonsteroidal anti-inflammatory drugs, pain relief medications, and gabaergic drugs.29 On this basis, we conducted a Phase II prospective study to evaluate whether the use of a nutraceutical during the first 6 months of BTZ treatment in 33 patients with MM at first diagnosis was able to prevent the onset of neurotoxicity. To date, 18 patients out of 33 have completed the study (6 months), 8 patients are ongoing while 7 dropped out. We decided to report the preliminary data regarding the results of the analysis of 18 patients who completed the study.
Methods
The primary objective of the study was to assess if the nutraceutical can prevent the onset of chemo-induced neurotoxicity of grade II according to NCI-CTCAE.18 Secondary objectives were to assess if the nutraceutical can prevent the onset of painful symptoms of PN and to assess the stability of QoL and functional autonomy.
Subjects
Adult patients newly diagnosed with MM according to the International Myeloma Working Group Criteria39 admitted to the unit of Hematology and Stem Cell Transplantation of the Regina Elena National Cancer Institute and planned to receive BTZ chemotherapy for subcutaneous administration were consecutively enrolled in the study. Patients were managed according to the current standard of care and no additional diagnostic or therapeutic procedure was performed. The study was approved by the Institute’s Ethics Committee (RS661/15-RS571/15-1629), and each participant signed an informed consent form.
Inclusion criteria were as follows: patients of both genders aged between 18 and 75 years, newly diagnosed with MM, and naïve to chemotherapy; patients who gave written informed consent; a baseline neurophysiological evaluation resulting in absence of neurotoxicity (NCI-CTCAE: 0); and absence of painful symptoms (Visual Analog Scale [VAS] = 0).
Patients with any other neuropathy (e.g., diabetic neuropathy) and any other hematological disorder, patients currently or previously treated with chemotherapy, patients with preexisting neurological disorders, history of alcohol abuse, or documented severe vitamin deficiency were excluded from the study.
Study Treatment
The study treatment (Neuronorm) is composed of DHA (400 mg), ALA (600 mg), vitamin C (60 mg), and vitamin E (10 mg). It is a nutritional supplement containing DHA, ALA, vitamin C (the origin is synthetic), and vitamin E (the origin is vegetable). Thanks to these components it has trophic and antioxidant properties that act on the nervous system.30,31,33,37,40 Since DHA is the fatty acid with the highest number of double bonds, it is extremely exposed to oxidation risk; for this reason, the presence of vitamin C and E (as well as ALA) is very important for its preservation (Inpha Duemila srl, Leeco, Italy).
Vitamin C is needed as a cofactor of dopamine β-monooxygenase for the biosynthesis of noradrenaline (and adrenaline); the European Food Safety Authority Panel concludes that a cause and effect relationship has been established between the dietary intake of vitamin C and normal function of the nervous system.41,42 Vitamin E possesses neuroprotective properties; therefore, its deficiency is linked to PN.42,43
The investigational product has been freely supplied by Inpha Duemila srl unconditionally and was dispensed to the patients at the study site (Inpha Duemila srl; headquarters via Cardinale Ferrari, 6 Mariano Comense (CO) Italy; made in via Ariosto, 50/60 Trezzano sul Naviglio (MI), Italy; licensed by Ambros Pharma via Larga 2 Milano, Italy).
Pill bottles were given to patients at each visit. Patient compliance was verified by checking the number of pills returned. The study treatment was then taken orally, 1 tablet twice daily, for 6 months.
All enrolled patients received a 6-month treatment with subcutaneous BTZ at a dose of 1.3 mg/m2.
Regarding the CT schedule with BTZ, 7 patients were treated with VTD (velcade [BTZ], thalidomide, and dexamethasone), with a median of 4 cycles (range = 3-5) administered every 21 days using a twice weekly schedule of BTZ; 9 patients received 5 cycles of VMP (velcade [BTZ], melphalan, and prednisone) every 35 days with biweekly subcutaneous BTZ; while the remaining 2 patients were treated with 5 VMP cycles using a 1-week schedule of BTZ established “a priori,” considering age and comorbidity.
Visits Schedule
At baseline and final follow-up at 6 months, the following assessments were performed: neurological examination, electroneurography, and evaluation scales: NCI-CTCAE,18 reduced version of Total Neuropathic Score (TNSr),44,45 VAS for pain,46 European Organization for Research and Treatment of Cancer Quality-of-Life-Questionnaire-Core-30 (EORTC QLQ-C30),47 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-CIPN 20-item scale (QLQ-CIPN20),48 and scale of Daily Life Activities (ADL) and Instrumental Activity Daily Life (IADL).49 At baseline, after eligibility assessment, Neuronorm was given to patients. The occurrence of any adverse events was assessed.
Assessments
Primary efficacy: neurotoxicity less than grade 2 under the NCI-CTCAE system and grade 10 of TNSr system at 6 months.
Secondary efficacy: stability at all other scales at final follow-up compared with baseline.
To assess tolerability, the incidence of adverse events during treatment with BTZ and Neuronorm was assessed using the NCI-CTCAE.18
An “adverse event” (AE) is any unfavorable and unintended sign, symptom, or disease temporally associated with the use of a medical treatment or procedure that may or may not be considered related to the medical treatment or procedure. Progression of disease was not considered an AE. All the patients who assumed at least one dose of drug were included in the analysis of toxicity. Spontaneously reported, or observed AE were recorded along with details of time of onset and resolution, intensity, need for concomitant treatment, and the investigator’s opinion of a possible relationship with study treatment.
Assessment Tools/Scales
Neurological Examination
Examinations included a medical history specifically addressing symptoms of numbness, tingling or pain, weakness in the extremities, in addition to a complete neurological examination focused on tendon reflexes, strength, and sensory modalities, assessed with Rydel Seiffer tuning fork.50
Electroneurography
Nerve conduction was examined using conventional procedures and a standard electromyography machine (Medelec Synergy). To examine motor nerves, the right median and right peroneal nerves were evaluated. To examine sensory nerves, right median and bilateral sural nerves were evaluated. Compound muscle action potentials were recorded from the abductor pollicis brevis, and sensory nerve action potentials (SNAPs) were recorded from the second digit. Skin temperature was measured near the stimulation site and maintained above 32.0°C. Median SNAPs were recorded from the index finger after antidromic stimulation at the wrist, and sural SNAPs were noted at lateral malleolus after antidromic stimulation delivered 14 cm proximally at mid-calf.3
Evaluation Scales
NCI-CTCAE is an oncology toxicity scale that grades CIPN according to symptoms and disability12; the severity of sensory and motor PN was graded from grade 1 (mild) to 4 (disabling).51
TNSr is a composite score that includes evaluation of motor, sensory, and autonomic symptoms and signs, the quantitative determination of the vibration perception threshold, and the neurophysiological examination of one motor and one sensory nerve in the leg. TNSr ranges between 0 and 32 points, and the severity of the neuropathy can be classified according to the following neuropathy scale: 1 to 10, mild; 11 to 20, moderate; and >20, severe.44,45
EORTC QLQ-C30 Scale was used to assess the functional status, symptoms, and QoL of cancer patients.47
EORTC QLQ-CIPN20 Scale was used to assess sensory symptoms, motifs, and autonomic PNs induced by chemotherapy.48
Pain was self-assessed by patients through a VAS ranging between 0 (no pain) and 10 (unbearable pain).46
Functional autonomy was assessed using ADL/IADL scale that monitors daily life activities.49 ADL ranges between 0 and 6 and IADL ranges between 0 and 8, where 0 indicates full dependency and the highest score indicates independence in all functions.
Statistical Analysis
This was an exploratory trial in order to get information on possible effects of the nutraceutical. The results obtained will be used to design a double-blinded placebo-controlled study with the appropriate sample size.
The primary endpoint was the incidence of patients with grade 2 (or higher) toxicity at follow-up. The design of the study was a single stage, as proposed by A’Hern.52 According to literature data, a mean incidence rate of 40%13,21 was taken as the null hypothesis (p0, i.e., an incidence of toxicity that, if true, will imply that the proposed treatment could not be considered adequate). As an alternate hypothesis, an incidence rate of 20% was taken (i.e., an incidence that, if true, will imply that our treatment had an acceptable toxicity control). Assuming a level of significance of 5% and a power of 80%, 33 patients were enrolled in the study. If at least 25 patients did not experience grade 2 or higher toxicity, the experimental treatment would be considered adequate to control toxicity and may be proposed for a subsequent, larger comparative study.
Primary and secondary results were reported with their 95% confidence interval as well as patient characteristics and treatments were described by mean, median, standard deviation, and range if related to quantitative variables and absolute frequencies relative to qualitative variables.
The incidence of patients with grade 2 (or higher) toxicity at follow-up was estimated as the ratio between the number of patients with grade 2 (or higher) toxicity and the number of enrolled patients.
The changes between baseline and follow-up in TNSr, mean VAS score for pain, EORTC QLQ-C30, and EORTC QLQ-CIPN20 were analyzed using repeated measure t test.
Results
To date, of 33 patients (Figure 1), 8 are ongoing and 7 dropped out for progression of oncological disease (MM). Eighteen patients have completed the study (6 months): 7 women and 11 men, with a median age of 69 years. Concerning the evaluation of the response of hematological disease to chemotherapy during the 6 months of observation, 16 patients were evaluable. Among these, 13 (81%) experienced at least partial remission, while the remaining 3 (19%) had stable disease or disease progression (Table 1).53
Figure 1.
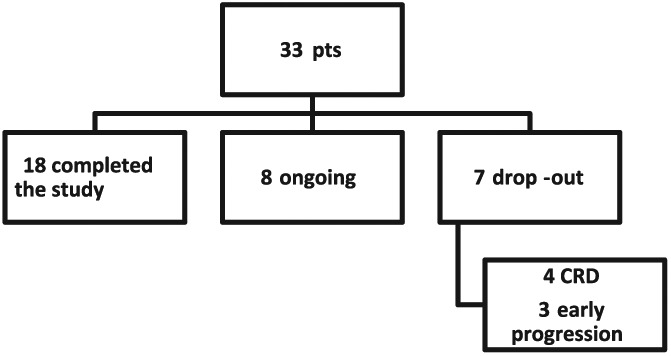
Flowchart of all 33 enrolled patients (CRD = carfilzomab, lenalidomide, and dexamethasone).
Table 1.
Baseline Clinical and Biologic Features of the First 18 Patients Enrolled in the Study.
| Parameter | n (%) |
|---|---|
| Sex, male | 11 (61) |
| Median age, years (range) | 69 (57-76) |
| Diagnosis | |
| IgG kappa | 10 (57) |
| IgG lambda | 2 (11) |
| IgA kappa | 4 (22) |
| Light chain | 1 (5) |
| IgD kalla | 1 (5) |
| ISS stage | |
| 1 | 9 (50) |
| 2 | 6 (33) |
| 3 | 3 (17) |
| Durie and Salmon stage | |
| IA | 5 (28) |
| IIA | 9 (50) |
| IIIA | 2 (11) |
| II-IIIB | 2 (11) |
| Cytogenetic analysisa | |
| Intermediate-high risk | 2 (11) |
| Standard risk | 14 (78) |
| NA | 2 (11) |
| Treatment | |
| VTDb | 7 (39) |
| VMP with biweekly BTZ administration | 9 (50) |
| VMP with weekly BTZ administration | 2 (11) |
| Response assessment28 | |
| CR | 9 (50) |
| VGPR | 3 (17) |
| PR | 2 (11) |
| SD/PD | 3 (17) |
| NE | 1 (5) |
Abbreviations: Ig, immunoglobulin; ISS, International Staging System; NA, not available; VTD, velcade, thalidomide, and dexamethasone; VMP, velcade, melphalan, and prednisone; BTZ, bortezomib; CR, complete response; VGPR, very good partial response; PR, partial response; SD/PD, stable disease/progressive disease; NE, not evaluable.
Method of Fonseca et al.53
In all patients treated with VTD, BTZ was administered twice weekly.
At baseline, all patients had no PN, according to NCI-CTCAE. At the final follow-up, 8 patients had no toxicity (NCI-CTCAE = 0), while 9 patients progressed to NCI-CTCAE grade 1, and 1 patient had NCI-CTCAE grade 1 with pain, but no patient experienced a grade 2 or higher toxicity.
TNSr increased from an average value of 3 at baseline to 4.5 at final follow-up. Although the increase was statistically significant (P = .008), the value observed at final follow-up is still within the range indicating mild neurotoxicity (1-10). No patient had moderate or severe neurotoxicity, according to TNSr.
Mean VAS score for pain increased from 0 to 0.4 between baseline and final follow-up and the increase was not statistically significant (P = .33). Only one patient reported a VAS score of 7 at the last observation, without any pain relief medications being given.
The scores of the functional scales remained stable within normal limits indicating full functional autonomy throughout the follow-up period (ADL = 6/6; IADL = 8/8).
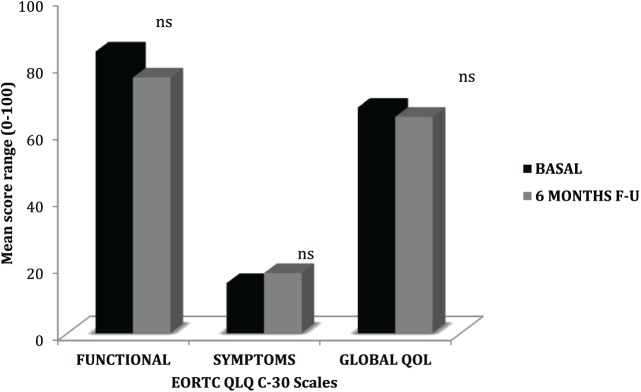
QoL Scores (EORTC QLQ-C30) remained stable and within normal limits between baseline and final follow-up, indicating good functional autonomy (mean = 84.2 vs 76.5; P = .16), good control of tumor-related symptoms (mean = 15 vs 18, P = .37), and a good QoL (mean 67.5 vs 64.7; P = .54; Figure 2).
Figure 2.
Quality of life in cancer assessed by EORTC QLQ-C30: comparison of mean scores of all evaluated patients between baseline and final follow-up (6 months).
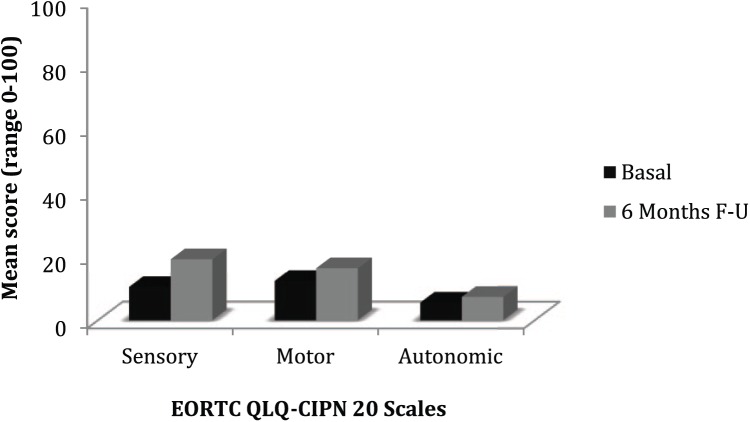
The EORTC QLQ-CIPN20 scores remained stable from baseline until final follow-up (sensitivity: mean = 10.4 vs 19.1, P = .09; motor: mean = 12.3 vs 16.3, P = .28; and autonomic: mean = 5.5 vs 7.3; P = .16; Figure 3).
Figure 3.
Patients’ perspective of chemotherapy-induced peripheral neuropathy assessed by EORTC QLQ-CIPN20: comparison of mean scores of all evaluated patients between baseline and final follow-up (6 months).
During the study period, no patient reported side effects or suspended Neuronorm treatment. Furthermore, no patients had to stop treatment with BTZ. Only one patient needed a 50% reduction in BTZ dosage due to hematological toxicity, not to PN.
Discussion
PN may be a relevant issue in myeloma patients due to its significant impact on the QoL and on pain intensity, which can lead to dose reduction or treatment discontinuation of the therapy.3,11,22
Considering our preliminary results, which highlight a possible positive role of a neuroprotective agent on the appearance of CIPN, we decided to submit the preliminary data of 18 (out of 33) newly diagnosed patients with MM, undergoing to first-line treatment with BTZ in association with Neuronorm bid. for 6 months.
At baseline, the neurological examination gave normal results for all patients and no neurological symptoms or pain was reported. ADL and IADL confirmed the absence of any toxicity or limitation in daily life activities. TNSr scores were indicative of mild neuropathy in 88.9% of patients and EORTC QLQ-CIPN20 scores indicated the presence of subtle symptoms (motor, sensory, and autonomic) in all patients.
After 6 months of chemotherapy with BTZ in combination with Neuronorm, no patient stopped chemotherapy and only one patient had the dose reduced by 50% due to hematological toxicity, not to neurotoxicity.
Eight patients did not experience any neurotoxicity during the study, while 10 patients out of 18 (55.5%) had NCI-CTCAE scores for mild sensory PN (grade 1), but no patient reached grade 2 (moderate PN), against the 40% expected according to the null hypothesis formulated on the bases of literature data.
All TNSr scores remained stable, within the range of mild neurotoxicity, and no patient reached the score of 10, indicating moderate neurotoxicity.
Regarding painful symptomatology, only one patient experienced pain during the study, but no specific therapy was required. VAS scores confirm the absence of pain during the study and remained stable (mean = 0.4 cm).
The scores for ADL, IADL, and EORTC QLQ-C30 remained stable, indicating preserved and complete functional autonomy and persistence of a good QoL: the mean ADL/IADL scores were 6/6 and 8/8 and mean global QoL score was 64.7. EORTC QLQ-CIPN20 scores remained stable, even if within a range indicative of a subtle polyneuropathy.
This is the first study evaluating the protective action of a nutraceutical compound in naive patients during treatment with BTZ, using a multilevel neurological assessment.
Articles published so far have used different neurological assessments, often not comparable with each other, to evaluate BTZ neurotoxicity in newly diagnosed MM patients. Considering the prominent role of BTZ for the treatment of MM54 and the paucity of data about the risk factors associated with PN due to BTZ, several authors have suggested continuous neurological monitoring of patients planned to receive BTZ.45,54,55 The dose-limiting toxicity of this drug is peripheral neurotoxicity, reported in more than one third of the patients,54-57 which necessitates a reduction or suspension of the treatment in up to about 40% of cases.54,55 Even though symptoms improve in many patients with dose modification, they can persist in some patients.58,59 BTZ can also induce painful neuropathy at a variable rate ranging from 30% to 60%,54,60 with consequent significant limitations in QoL related to neuropathic symptomatology, lasting over time.61
Several nutrient compounds have shown promise for selective neurotoxic chemotherapy agents such as vitamin E with cisplatin,62 vitamin B6 with hexamethylmelamine administration and cisplatin,63 intravenous glutathione for oxaliplatin administration,64 and omega-3 fatty acids for paclitaxel.65 Acetyl-L-carnitine has also demonstrated potential for efficacy as a treatment option for paclitaxel- and cisplatin-induced CIPN.66
However, the same authors express the need to carry out large scale randomized controlled trials.23
As far as we know, no nutraceutical compound like Neuronorm has been used in any of these studies to prevent CIPN during BTZ therapy. In the literature, only 2 small trials have studied the therapeutic effect of ALA alone in patients with CIPN due to docetaxel and oxaliplatin,26 observing an improvement of neuropathic symptoms. Another trial failed to note any protective effect of ALA against CIPN.67
The study has some limitations: first of all, the fact that these are preliminary data from a small population and, second, the absence of a parallel control group, but the results are suggestive of a preventive effect on PN by the early introduction of Neuronorm in patients with MM in the first-line treatment with BTZ. BTZ was used in 2 different CT schedules: VTD and VMP. In particular, none of the 18 patients, treated with VTD or VMP, using Neuronorm for 6 months, experienced a worsening of their mild neuropathy and they did not discontinue the treatment with BTZ, while maintaining a good functional autonomy and performing normal daily activities.
We hypothesized that this could be due to the synergy of its 4 active components: DHA contributes to the maintenance of the regular cerebral functioning. The beneficial effect is obtained by a daily intake of at least 250 mg of DHA.29 ALA is a cofactor of several enzymes that intervene in the oxidative pyruvate and other keto acids decarboxylation, and is also involved in several antioxidant mechanisms such as reduced gluthatione and ascorbic acid regeneration.29 Also, the combination of DHA and ALA represents a new approach for sustaining the subjects with neuropathy, independently of its origin, such as canicolar syndromes of the hand, lumbar and cervical syndromes, phenomena of axonal degeneration or edema, and other.29
Moreover, taking into account neurotoxic effect of thalidomide, the evaluation of the possible differences in the appearance of neurotoxicity between the 2 treatments schedule (VTD/VMP) will be analyzed through a comparison between the 2 groups, at the end of the total follow-up (33 patients).
Conclusions
Our preliminary data in a small population with short follow-up seems to indicate that early introduction of a neuroprotective agent in this patient population may prevent the onset or the worsening of PN, avoiding the interruption of the therapy with BTZ, and maintaining a good functional autonomy to allow normal daily activities. These results seem to indicate that the nutraceutical may have some potential to be considered for a future trial.
We also believe that the combined and daily follow-up of patients, being evaluated by both a hematologist and a neurologist, could provide a safer and more accurate assessment, reducing CIPN risk.54
Footnotes
Author Contributions: MM: Conception and design of the study, interpretation of data, drafting and revising the article; AZ: Acquisition of data, drafting the article; AMP: Acquisition of data, drafting the article; FM: Acquisition of data, drafting the article; DG: Analysis and interpretation of data; SG: Acquisition of data; FP: Acquisition of data; DR: Acquisition of data; EG: Acquisition of data; AM: Revising the article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Marta Maschio has received support for travel to congresses from EISAI Srl and Inpha 2000; has participated in scientific advisory boards for EISAI; has participated in pharmaceutical industry-sponsored symposia for UCB Pharma; and has received research grants from UCB Pharma. No conflicts of interest are declared for the other authors.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Marta Maschio  https://orcid.org/0000-0002-3075-4108
https://orcid.org/0000-0002-3075-4108
References
- 1. Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51-77. [DOI] [PubMed] [Google Scholar]
- 2. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419-437. [DOI] [PubMed] [Google Scholar]
- 3. Morawska M, Grzasko N, Kostyra M, Wojciechowicz J, Hus M. Therapy-related peripheral neuropathy in multiple myeloma patients. Hematol Oncol. 2015;33:113-119. [DOI] [PubMed] [Google Scholar]
- 4. Malhotra P, Choudhary PP, Lal V, Varma N, Suri V, Varma S. Prevalence of peripheral neuropathy in multiple myeloma at initial diagnosis. Leuk Lymphoma. 2011;52:2135-2138. [DOI] [PubMed] [Google Scholar]
- 5. Ramchandren S, Lewis RA. An update on monoclonal gammopathy and neuropathy. Curr Neurol Neurosci Rep. 2012;12:102-110. [DOI] [PubMed] [Google Scholar]
- 6. Koeppen S. Treatment of multiple myeloma: thalidomide-, bortezomib-, and lenalidomide-induced peripheral neuropathy. Oncol Res Treat. 2014;37:506-513. [DOI] [PubMed] [Google Scholar]
- 7. Sonneveld P, Jongen JL. Dealing with neuropathy in plasma-cell dyscrasias. Hematology Am Soc Hematol Educ Program. 2010;2010:423-430. [DOI] [PubMed] [Google Scholar]
- 8. Richardson PG, Xie W, Mitsiades C, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27:3518-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27-46. [DOI] [PubMed] [Google Scholar]
- 10. Antoine JC, Camdessanché JP. Peripheral nervous system involvement in patients with cancer. Lancet Neurol. 2007;6:75-86. [DOI] [PubMed] [Google Scholar]
- 11. Delforge M, Bladé J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol. 2010;11:1086-1095. [DOI] [PubMed] [Google Scholar]
- 12. Fernyhough P, Smith DR, Schapansky J, et al. Activation of nuclear factor-kappaB via endogenous tumor necrosis factor alpha regulates survival of axotomized adult sensory neurons. J Neurosci. 2005;25:1682-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112:1593-1599. [DOI] [PubMed] [Google Scholar]
- 14. Bennett GJ, Paice JA. Peripheral neuropathy: experimental findings, clinical approaches. J Support Oncol. 2007;5:61-63. [PubMed] [Google Scholar]
- 15. Badros A, Goloubeva O, Dalal JS, et al. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007;110:1042-1049. [DOI] [PubMed] [Google Scholar]
- 16. Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol. 2003;1:194-205. [PMC free article] [PubMed] [Google Scholar]
- 17. Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609-2617. [DOI] [PubMed] [Google Scholar]
- 18. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Published May 28, 2009. Accessed September 12, 2018.
- 19. Mateos MV, Miguel JFS. Safety and efficacy of subcutaneous formulation of bortezomib versus the conventional intravenous formulation in multiple myeloma. Ther Adv Hematol. 2012;3:117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015;100:1254-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bilinska M, Usnarska-Zubkiewicz L, Pokryszko-Dragan A. Bortezomib-induced painful neuropathy in patients with multiple myeloma. Contemp Oncol (Pozn). 2013;17:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hershman DL, Lacchetti C, Dworkin RH, et al. American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941-1967. [DOI] [PubMed] [Google Scholar]
- 23. Schloss JM, Colosimo M, Airey C, Masci PP, Linnane AW, Vitetta L. Nutraceuticals and chemotherapy induced peripheral neuropathy (CIPN): a systematic review. Clin Nutr. 2013;32:888-893. [DOI] [PubMed] [Google Scholar]
- 24. Smith EM, Pang H, Cirrincione C, et al. Alliance for Clinical Trials in Oncology. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy; a randomized clinical trial. JAMA. 2013;309:1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90:377-387. [DOI] [PubMed] [Google Scholar]
- 26. Saif MW, Reardon J. Management of oxaliplatin-induced peripheral neuropathy. Ther Clin Risk Manag. 2005;1:249-258. [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang LL, Wang YH, Shao ZH, Ma J. Prophylaxis of bortezomib-induced peripheral neuropathy in patients with multiple myeloma by high-dose intravenous mecobalamin [in Chinese]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;25:480-484. [DOI] [PubMed] [Google Scholar]
- 28. Callender N, Markovina S, Eickhoff J, et al. Acetyl-L-carnitine (ALCAR) for the prevention of chemotherapy-induced peripheral neuropathy in patients with relapsed or refractory multiple myeloma treated with bortezomib, doxorubicin and low-dose dexamethasone: a study from the Wisconsin Oncology Network. Cancer Chemother Pharmacol. 2014;74:875-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossoni G, Stankov BM. Alpha-lipoic acid and docosahexaenoic acid. A positive interaction on the carrageenan inflammatory response in rats. Nutrafoods. 2010;9:21-25. [Google Scholar]
- 30. Papanas N, Ziegler D. Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin Pharmacother. 2014;15:2721-2731. [DOI] [PubMed] [Google Scholar]
- 31. Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomizedcontrolled trial (ALADIN study). Diabetologia. 1995;38:1425-1433. [DOI] [PubMed] [Google Scholar]
- 32. Reljanovic M, Reichel G, Rett K, et al. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha lipoic acid in diabetic neuropathy. Free Radic Res. 1999;31:171-179. [DOI] [PubMed] [Google Scholar]
- 33. Coste TC, Gerbi A, Vague P, Pieroni G, Raccah D. Neuroprotective effect of docosahexaeonic acid-enriched phospholipidis in experimental diabetic neuropathy. Diabetes. 2003;52:2578-2585. [DOI] [PubMed] [Google Scholar]
- 34. Yee P, Weymouth AE, Fletcher EL, Vingrys AJ. A role for omega-3 polyunsaturated fatty acid supplements in diabetic neuropathy. Invest Ophthalmol Vis Sci. 2010;51:1755-1764. [DOI] [PubMed] [Google Scholar]
- 35. Carlson SE, Colombo J, Gayewsky BJ, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parente E, Colannino G, Picconi O, Monastra G. Safety of oral alpha-lipoic acid treatment in pregnant women: a retrospective observational study. Eur Rev Med Pharmacol Sci. 2017;21:4219-4227. [PubMed] [Google Scholar]
- 37. Mostacci B, Liguori R, Cicero AF. Nutraceutical approach to peripheral neuropathies: evidence from clinical trials. Curr Drug Metab. 2018;19:460-468. [DOI] [PubMed] [Google Scholar]
- 38. Teichert J, Tuemmers T, Achenbach H, et al. Pharmacokinetics of alpha-lipoic acid in subjects with severe kidney damage and end-stage renal disease. J Clin Pharmacol. 2005;45:313-328. [DOI] [PubMed] [Google Scholar]
- 39. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237-259. [DOI] [PubMed] [Google Scholar]
- 41. Bresson JL, Flynn A, Heinonen M, et al. Vitamin C, collagen formation, immune function, oxidative damage, energy metabolism, non-haem iron absorption, physical exercise, health claim. EFSA J. 2009;7(9):1226. doi: 10.2903/j.efsa.2009.1226 [DOI] [Google Scholar]
- 42. Lu C, Liu Y. Interactions of lipoic acid radical cations with vitamins C and E analogue and hydroxycinnamic acid derivatives. Arch Biochem Biophys. 2002;406:78-84. [DOI] [PubMed] [Google Scholar]
- 43. Galal MK, Khalaf AAA, Ogaly HA, Ibrahim MA. Vitamin E attenuates neurotoxicity induced by deltamethrin in rats. BMC Complement Altern Med. 2014;14:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cavaletti G, Boglium G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology. 2003;61:1297-1300. [DOI] [PubMed] [Google Scholar]
- 45. Cavaletti G, Frigeni B, Lanzani F, et al. ; Italian NETox Group. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12:210-215. [DOI] [PubMed] [Google Scholar]
- 46. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175-184. [PubMed] [Google Scholar]
- 47. Apolone G, Filiberti A, Cifani S, Ruggiata R, Mosconi P. Evaluation of the EORTC QLQ-C30 questionnaire: a comparison with SF-36 Health Survey in a cohort of Italian long-survival cancer patients. Ann Oncol. 1998;9:549-557. [DOI] [PubMed] [Google Scholar]
- 48. Postma TJ, Aaronson NK, Heimans JJ, et al. ; EORTC Quality of Life Group. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41:1135-1139. [DOI] [PubMed] [Google Scholar]
- 49. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. [DOI] [PubMed] [Google Scholar]
- 50. Velasco R, Petit J, Clapés V, Verdú E, Navarro X, Bruna J. Neurological monitoring reduces the incidence of bortezomib-induced peripheral neuropathy in multiple myeloma patients. J Peripher Nerv Syst. 2010;15:17-25. [DOI] [PubMed] [Google Scholar]
- 51. Trotti A, Colevas AD, Setser A, et al. CTCAEv3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176-181. [DOI] [PubMed] [Google Scholar]
- 52. A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859-866. [DOI] [PubMed] [Google Scholar]
- 53. Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lanzani F, Mattavelli L, Frigeni B, et al. Role of a pre-existing neuropathy on the course of bortezomib-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2008;13:267-274. [DOI] [PubMed] [Google Scholar]
- 55. Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113-3120. [DOI] [PubMed] [Google Scholar]
- 56. Aghajanian C, Dizon DS, Sabbatini P, Raizer JJ, Dupont J, Spriggs DR. Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J Clin Oncol. 2005;23:5943-5949. [DOI] [PubMed] [Google Scholar]
- 57. Orlowsky RZ, Voorhees PM, Garcia RA, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058-3065. [DOI] [PubMed] [Google Scholar]
- 58. Boyette-Davis JA, Cata JP, Zhang H, et al. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011;12:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cavaletti G, Nobile-Orazio E. Bortezomib-induced peripheral neurotoxicity: still far from a painless gain. Haematologica. 2007;92:1308-1310. [DOI] [PubMed] [Google Scholar]
- 60. Lakshman A, Modi M, Prakash G, et al. Evaluation of bortezomib-induced neuropathy using Total Neuropathy Score (reduced and clinical versions) and NCI CTCAE v4.0 in newly diagnosed patients with multiple myeloma receiving bortezomib-based induction. Clin Lymphoma Myeloma Leuk. 2017;17:513-519.e1. [DOI] [PubMed] [Google Scholar]
- 61. Jordan K, Schaffrath J, Jahn F, Mueller-Tidow C, Jordan B. Neuropharmacology and management of chemotherapy-induced nausea and vomiting in patients with breast cancer. Breast Care (Basel). 2014;9:246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paksoy M, Ayduran E, Sanlı A, Enken M, Aydin S, Oktay ZA. The protective effects of intratympanic dexamethasone and vitamin E on cisplatin-induced ototoxicity are demonstrated in rats. Med Oncol. 2011;28:615-621. [DOI] [PubMed] [Google Scholar]
- 63. Wiernik PH, Yeap B, Vogl SE, et al. Hexamethylmelamine and low or moderate dose cisplatin with or without pyridoxine for treatment of advanced ovarian carcinoma: a study of the Eastern Cooperative Oncology Group. Cancer Invest. 1992;10:1-9. [DOI] [PubMed] [Google Scholar]
- 64. Cascinu S, Catalano V, Cordella L, et al. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2002;20:3478-3483. [DOI] [PubMed] [Google Scholar]
- 65. Ghoreishi Z, Esfahani A, Djazayeri A, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomised double-blind placebo controlled trial. BMC Cancer. 2012;12:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bianchi G, Vitali G, Caraceni A, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005;41:1746-1750. [DOI] [PubMed] [Google Scholar]
- 67. Guo Y, Jones D, Palmer JL, et al. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: a randomized, double-blind, placebo-controlled trial. Support Care Cancer. 2014;22:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]