Abstract
Background: Chemoradiotherapy-associated mucositis can manifest as pain, inflammation, dysphagia, diarrhea, weight loss, rectal bleeding, and infection. Mucositis is a major dose-limiting side effect of chemotherapy, affecting nutritional intake and oral and intestinal function. Despite several interventions being available, there is a need for safe and effective preventative and treatment options for treatment-induced mucositis. The goals of this review are to discuss interventions based on foods and natural products and present the research to date. Methods: A narrative literature review identified 60 clinical studies examining various nutritional compounds and 20 examining probiotics. 9 studies on probiotics for the prevention of diarrhea were also assessed on methodological quality and limitations identified. Results: Several compounds have been posited as useful adjuvants for cancer treatment–related mucositis. Probiotics demonstrate efficacy for the prevention and treatment of chemoradiotherapy-induced gastrointestinal toxicity without significant side effects. Glutamine and activated charcoal were reported to reduce chemotherapy-induced diarrhea but not radiation-induced intestinal mucositis. Honey has been reported to decrease treatment interruptions, weight loss, and delays the onset of oral mucositis. Zinc, glutamine, and topical vitamin E were demonstrated efficacy for oral mucositis. Conclusion: There is plausible clinical evidence for the administration of several adjunctive treatments for the prevention and treatment of mucositis. Probiotics were reported to reduce the burden of intestinal mucositis and treatment-induced diarrhea. Activated charcoal and glutamine are beneficial for chemotherapy-induced diarrhea, whereas the administration of honey, zinc, and glutamine reduce the risk of developing oral mucositis during chemotherapy or radiotherapy.
Keywords: chemotherapy, radiotherapy, mucositis, diarrhea, probiotics, adjunctive compounds
Introduction
Chemotherapy- and radiotherapy-induced mucositis is a significant burden for cancer patients (Table 1). Symptoms of oral mucositis become apparent 5 to 10 days after chemotherapy and may progress from erythema, cracking, and inflammation to pain, bleeding, ulceration,1,2 and pain.1,3 Pelvic radiotherapy is reported to induce changes in the bowel habits of 90% of patients, with half of all patients reporting that quality of life is significantly adversely affected,4 and that serious complications can persist decades post treatment cessation.4-6 Epidemiological studies on cancers of the head and neck report a prevalence of oral mucositis of 80% for patients undergoing radiotherapy and 40% of patients receiving chemotherapy.3 Moreover with high-dose chemotherapy, mucositis can develop in 100% of bone marrow transplant patients. Mucositis is the most frequent and serious reported side effect in the first 3 months following a transplant and is the most common condition requiring systemic analgesics during cancer therapy.3 The frequency of mucositis is also higher in patients receiving continuous infusion therapy for breast and colon cancer.7
Table 1.
Chemoradiotherapy-Induced Gastrointestinal Toxicities.
| Intervention | Pathophysiology | Possible Symptoms |
|---|---|---|
| Radiotherapy138-141 | Direct epithelial injury | Mouth ulcers |
| Mucositis | Pain | |
| Loss of mitotic activity | Anorexia | |
| Acute inflammation | Bloating dysphagia | |
| Abscess formation | Diarrhea | |
| Swelling of vascular endothelial lining | Lactose intolerance malabsorption | |
| Tissue ischemia mucosal friability | Nausea | |
| Neovascularization progressive fibrosis | Ulceration | |
| Weight loss | ||
| IBS | ||
| ISBO | ||
| Irinotecan6,142-144 | Cholinergically mediated diarrhea | Rhinitis |
| Cytokine release | Early-onset diarrhea | |
| Altered motility | Abdominal cramping | |
| Villous blunting and crypt degeneration | Malabsorption | |
| TJ dysfunction | Delayed-onset diarrhea | |
| Changes to claudin-1 and occludin | ||
| Bacterial translocation | ||
| Fluoropyrimidines including 5-FU8,145-149 | Gastrointestinal mucositis | Altered bowel movement |
| Villi shortening, increased crypt depth | Diarrhea | |
| Increased intestinal myeloperoxidase activity, reduced glutathione (GSH) concentrations, and increased levels of inflammatory mediators | Lactose intolerance | |
| Reduced expressions of occludin and claudin-1 and TJ dysfunction | Malabsorption | |
| SIBO | ||
| Paclitaxel150,151 | Increase apoptosis of intestinal villi, increased intestinal permeability, reduced white blood cell count, and induced bacterial translocation | Stomatitis |
| Vomiting | ||
| Diarrhea | ||
| Colitis | ||
| Oxaliplatin151,152 | DNA denaturation and neuronal ablation | Potentiation of 5-FU related GIT toxicities |
| Apoptosis of intestinal epithelial cells | Anorexia | |
| Inflammation | Stomatitis | |
| Bacterial translocation | Nausea | |
| Sepsis | Emesis | |
| Diarrhea/constipation | ||
| Lapatinib153 | Increased jejunal crypt length, increased mitotic rate, and goblet cell morphology | Malabsorption |
| Altered bowel function | ||
| Diarrhea | ||
| Methotrexate154 | Reduced claudin-1 and occludin expression and TJ dysfunction | Inflammation |
| Increased proinflammatory cytokine production | Sepsis | |
| Neutropenia | ||
| Taxanes151 | Ischemic colitis | Nausea |
| Neutropenia | Diarrhea | |
| Mucosal edema | Emesis | |
| Hemorrhage | Stomatitis | |
| Inflammatory infiltrates | Colitis | |
| Ulceration | Hepatitis | |
| Cisplatin, carboplatin151,155 | Decreased total surface area of villi | Anorexia |
| Reduced villus height and villus/crypt ratio | Stomatitis | |
| Decreased intestinal motility | Nausea | |
| Altered digestive and metabolic functions | Emesis | |
| Inflammatory infiltrates | Diarrhea | |
| Malabsorption | ||
| Anthracyclines151 | Inflammation | Stomatitis |
| Steatosis | Ulceration | |
| Anorexia | ||
| Diarrhea | ||
| Nausea | ||
| Emesis | ||
| Cytarabine, gemcitabine151 | Necrotizing colitis | Anorexia |
| Veno-occlusive disease | Nausea | |
| Emesis | ||
| Ulceration | ||
| Diarrhea |
Abbreviations: IBS, irritable bowel syndrome; ISBO, intermittent small bowel obstruction; TJ, tight junctions; 5-FU, 5-fluorouracil; SIBO, small intestinal overgrowth syndrome.
Unlike oral mucositis, clinical data reflecting the long-term effects of treatment-induced gastrointestinal mucositis are lacking. An important and debilitating symptom of intestinal mucositis is diarrhea. Gastrointestinal mucositis has been reported in 80% of patients treated with 5-fluorouracil (5-FU).8 The frequency of chemotherapy-induced diarrhea depends on the drug administered and the schedule implemented. The highest rate of diarrhea has been reported to occur with a weekly regimen of irinotecan and 5-FU bolus with 10% of patients going on to develop grade 3 to 4 mucositis. Late-onset diarrhea may occur within a week following higher dosages of irinotecan and after approximately 2 weeks following a weekly administration of lower doses.9 In stage III colorectal cancer (CRC), chemotherapy with FOLFOX induced diarrhea in 56% of patients, yet with FOLFIRI, the prevalence of diarrhea increased to 89%. The risk of a first episode was highest during the first cycle (35 %) and dropped to less than 10% during subsequent cycles.10
The frequency of treatment-induced gastrointestinal toxicity in CRC has been posited to likely increase with the introduction of novel drugs and the use of more intense combination regimens of polychemotherapy combined with monoclonal antibodies.6,10 Targeted therapies, including erlotinib, gefitinib, lapatinib, sorafenib, and sunitinib, have been associated with a 2- to 8-fold increased risk of all or high-grade diarrhea compared with conventional chemotherapy regimens.11
Despite several interventions being available, including cryotherapy and loperamide, for the control of oral mucositis and diarrhea, respectively, there is a need to further explore additional safe and effective preventative and treatment options for treatment-induced mucositis and related symptoms. The goal of this narrative review was to present an overview of the safety and efficacy reported for various interventions posited to reduce the adverse effects of antineoplastic agents. The majority of clinical research has focused on prevention or treatment of oral mucositis, while intestinal toxicity has been less well reported.
Methods
The inclusion criteria were any type of clinical trial examining the use of any oral or topical probiotic or nutritional intervention with both the abstract and the journal article written in English. All clinical trial designs and methodology were included, namely, prevention as well as treatment studies. The following databases were used to retrieve journal articles: PubMed, the Cochrane Library, Science Direct, Scopus, Embase, and Google Scholar, and searches were current up to November 2016.
Electronic databases were searched using the following search string:
Probiotics OR Diet OR Food OR Nutrition OR Micronutrients OR Vitamins OR Minerals OR Dietary supplements OR Functional foods OR Honey AND Chemotherapy AND Mucositis AND Chemotherapy AND Diarrhea AND Radiotherapy AND Mucositis AND Radiotherapy AND Diarrhea.
Examination of references in retrieved articles was also conducted.
Results
Fifty articles examining various nutritional compounds were identified, 49 from PubMed and 1 from Embase.12 Reports included 1 study on activated charcoal,13 1 study on β-glucan,14 2 studies on multinutrient formulations,15,16 2 studies on an amino acid–rich oral formulation,17,18 1 study with folic acid with B12,19 5 studies on vitamin E,12,20-23 17 intervention studies containing minerals,24-40 and 21 studies with glutamine (Table 2).41-61 Moreover, 25 clinical trials were identified examining honey alone or in combination as a prophylaxis/intervention for oral mucositis (Table 3).62-87
Table 2.
Clinical Trials Investigating Nutrients for Oral and Intestinal Mucositis.
| References | Treatment | Intervention | Cancer Type | Design (n = Subjects), Assessment | Outcome |
|---|---|---|---|---|---|
| Oral mucositis | |||||
| Karac et al14 | Chemotherapy (FOLFOX-4) | Beta-glucan 50 mg/day versus no treatment | CRC | Retrospective, controlled (n = 62), CTCAE | Decreased incidence OM and diarrhea |
| Casbarien et al15 | Chemotherapy and radiotherapy | Multinutrient formulation (Supportan) | HNC | Open-label study (n = 7), CTCAE | OM none severe |
| Machon et al16 | Chemotherapy and radiotherapy | Multinutrient formulation (Oral Impact) | HNC | Prospective noncontrolled (n = 31), CTCAE | Decreased OM severity |
| Harada et al17 | Radiotherapy ± chemotherapy | Amino acid–rich oral formulation (Elental) versus no treatment | OC | Retrospective study (n = 74), CTCAE | Decreased OM severity and increased Tx completion rates |
| Ogata et al18 | 5-FU-based chemotherapy | Amino acid–rich oral formulation (Elental) | CRC | Prospective pilot study (n = 22), CTCAE | Decreased OM severity (P = .0002) |
| Azzoli et al19 | Pralatrexate | Folic acid IM/B12 oral | NSCLC | Nonrandomized, multicenter (n = 39), CTCAE | NS decrease OM |
| Ghoreishi et al12 | Cyclophosphamide-based conditioning regimen | Vitamin E 400 mg versus placebo | ALL/AML/CML | RCT (n = 39), CTCAE | NS decrease OM |
| Ferreira et al20 | Radiotherapy | Topical vitamin E, 400 mg versus placebo | HNC | RCT (n = 54), RTOG | Decreased OM risk of 36% |
| Wadleigh et al21 | 5-FU infusion/cisplatin or doxorubicin | Topical vitamin E, 400 mg versus placebo | HNC/OeC; HCC/AML | RCT (n = 18), WHO OMAS | Decreased OM (P < .05; vitamin E 60% complete resolution) |
| El-Housseiny et al22 | Chemotherapy | Topical vitamin E 100 mg versus 40 mg/kg/daily IM | OC | Comparative randomized study (pediatric, n = 80), WHO OMAS | Decreased OM severity (P < .05) |
| Khurana et al23 | Chemotherapy | Topically vitamin E compared with pycnogenol, glycerin, water | AL/NHL | Single-blind, randomized (n = 72, pediatric), WHO OMAS | Decreased OM severity (P < .05) with vitamin E |
| Büntzel et al24 | Chemotherapy and radiotherapy | Sodium selenite oral fluid 0.5 mg versus no treatment | HNC | RCT (n = 39), RTOG | NS benefit |
| Jahangard-Rafsanjani et al25 | HDC HSCT conditioning regimen | Selenium 200 µg versus placebo | ALL/AML | RCT (n = 64), WHO OMAS | Decreased OM severity grade 3-4 (P < .05) |
| Watanabe et al26 | Chemotherapy and radiotherapy | Zinc L-carnosine solution versus azulene rinse | HNC | RCT (n = 31), CTCAE | Decreased OM severity ⩾grade 2 (P < .05) |
| Lin et al27 | Chemotherapy and radiotherapy | Zinc chelate equiv 25 mg 2-4 times daily versus placebo | HNC | RCT (n = 100), RTOG | Decreased OM severity grade 3 radiotherapy only |
| Lin et al28 | Radiotherapy | Zinc chelate equiv 25 mg 2-4 times daily versus placebo | NPC/OC | RCT (n = 100), Kaplan–Meier survival method | Delayed development of severe OM in OC only |
| Ertekin et al29 | Radiotherapy | Zinc sulfate equiv 50 mg tid versus placebo | HNC | RCT (n = 21), RTOG | Decreased OM severity (P < .05) |
| Sangthawan et al30 | Radiotherapy | Zinc sulfate equiv 50 mg oral syrup versus placebo | HNC | RCT (n = 104), WHO OMAS | NS benefit |
| Arbabi-kalati et al31 | Chemotherapy | Zinc sulfate eqiv. 50 mg tid versus placebo | HNC | RCT (n = 50), WHO OMAS | Decreased OM severity (P < .05) |
| Mehdipour et al32 | Chemotherapy | 0.2% zinc sulfate versus chlorhexidine gluconate mouthwashes | AML | Comparative randomized (n = 30), Spijkervet scale | Decreased OM severity (P < .05) |
| Mansouri et al33 | HDC HSCT conditioning regimen | Zinc sulfate equiv 50 mg bid versus placebo | HM | RCT (n = 60), WHO OMAS | NS benefit |
| Hayashi et al34 | Radiotherapy or HDC HSCT conditioning regimen | Zinc sulfate/L-carnosine suspension or lozenge | HSCT | Comparative study (n = 66), CTCAE | Decreased OM severity ⩾grade 2 (P < .05) and decreased pain (P < .01) |
| Markiewicz et al35 | Radiotherapy HDC HSCT conditioning regimen | Calcium phosphate mouth rinse versus topical mouth care with sage extract, povidone-iodine, fluconazole, vitamin A (10 g), and vitamin E (10 g) with or without benzocaine (2.5 g) twice daily | AML/ALL | NBCT (n = 40), WHO OMAS | Decreased OM severity (P < .05) and decrease in pain NS |
| Lambrecht et al36 | Chemotherapy and radiotherapy | Calcium phosphate mouth rinse (Caphosol) versus standard oral care | HNC | RCT comparative (n = 58), CTCAE | NS OM grade 3 |
| Raphael et al37 | Chemotherapy or HSCT conditioning regimen | Calcium phosphate mouth rinse (Caphosol) versus standard oral care | HM | RCT (n = 34, pediatric), CTCAE | NS benefit |
| Papas et al38 | HDC HSCT conditioning regimen | Calcium phosphate (Caphosol) versus fluoride mouth rinse | ALL/AML/CML/HL/NHL/MM/MS/BC/OvC | RCT comparative (n = 58), NIDCR | Decreased OM frequency/duration/severity (P < .05) |
| Madan et al39 | Radiotherapy | 1% povidone-iodine versus 0.12% chlorhexidine, sodium bicarbonate, plain water (control) | HNC | RCT (n = 80), WHO OMAS | Decreased OM severity scores (P < .05) |
| Vokurka et al40 | HDC before PBSCT | 1% povidone-iodine mouthwash versus saline | HSCT | RCT multicenter (n = 132), WHO OMAS | NS benefit |
| Tsujimoto et al41 | Radiotherapy | Glutamine 30 g, oral/day versus placebo | HNC | RCT (n = 40), CTCAE | Decreased OM severity (P < .05) |
| Tanaka et al42 | Radiotherapy | Glutamine 9 g with or without elemental diet versus placebo | HNC | RCT (n = 40), CTCAE | Decreased OM severity (P < .05) |
| Huang et al43 | Radiotherapy | Glutamine 30 g, oral/day versus saline | HNC | RCT (n = 17), WHO OMAS | NS benefit |
| Vidal-Casariego et al44 | Radiotherapy | Glutamine 30 g oral/day versus late or no treatment | HNC/Mel/LC/OeC/Lym | Retrospective cohort (n = 117), WHO OMAS | Decreased OM risk RR = −9.0% (95% CI = −18.0% to −1.0%) |
| Jebb et al45 | 5-FU and folinic acid | Glutamine 16 g oral/day versus placebo | mCRC | RCT (n = 28), WHO OMAS | NS benefit OM or IM |
| Skubitz and Anderson46 | Chemotherapy | Glutamine 8 g oral/day | KS | Open trial (n = 14), CALGB | Decreased OM severity (P < .05) |
| Anderson et al47 | Chemotherapy | Glutamine 4 g/m2/dose/day versus placebo | Sar/NB | RCT crossover study (pediatric n = 24), patient questionnaire | Decreased OM duration/severity (P < .05) |
| Okuno et al48 | 5-FU | Glutamine 30 g oral/day versus placebo | Not defined | RCT (n = 134), assessed by physician | NS benefit |
| Cockerham et al49 | Paclitaxel and melphalan | Glutamine 24 g oral/day | mBC | Retrospective analysis (n = 21), CTCAE | Decreased OM days/severity (P < .05) |
| Dickson et al50 | HDC | Glutamine 30 g oral/day versus placebo | ALL/AL/CML/MM/NHL | RCT (n = 58), BMT scale | NS benefit OM and diarrhea |
| Daniele et al51 | 5-FU and follinic acid | Glutamine, 18 g oral/day versus placebo | mCRC | RCT (n = 70), CTCAE | Decreased rescue meds (P < .05) |
| Cerchietti et al52 | Chemoradiotherapy | L-Alanyl-L-glutamine, IV 300/400 mg/kg bw versus placebo | HNC | RCT (n = 29), WHO OMAS | Decreased OM severity (NS) and decreased pain (P < .05) |
| Li et al53 | Chemotherapy | Glutamine 30 g oral/day versus placebo | BC | RCT (n = 60), WHO OMAS | NS benefit OM or diarrhea |
| Choi et al54 | 5-FU/leucovorin | Glutamine 10 g oral/day versus supportive care | AST | RCT (n = 51), CTCAE | Decreased OM severity (P < .05) |
| Peterson et al55 | Anthracycline chemotherapy | Glutamine, 7.5 g oral/day versus placebo | BC | RCT crossover (n = 326), WHO OMAS | Decreased OM severity grade 3 (P < .05) |
| Oesophagitis | |||||
| Topkan et al57 | Radiotherapy | Glutamine 30 g oral/day versus no treatment | LC | Retrospective (n = 63), RTOG | Decreased grade 2-3 esophagitis (27.2%) |
| Topkan et al56 | Chemotherapy and radiotherapy | Glutamine 30 g oral/day versus no treatment | NSCLC | RCT (n = 104), RTOG | Decreased grade 3 esophagitis (P < .05) |
| Tutanc et al58 | Radiotherapy | Glutamine 30 g oral/day versus no treatment | LC | RCT (n = 46), RTOG | Decreased grade 2-3 esophagitis (P < .05) |
| Chattopadhyay et al59 | Radiotherapy | Glutamine 10 g oral/day versus no treatment | HNC | Randomized case-control study (n = 70), WHO OMAS | Decreased grade 2-3 esophagitis (P < .05) |
| Gul et al60 | Radiotherapy | Glutamine 30 g oral/day versus no treatment | LC | RCT (n = 32), RTOG | Decreased esophagitis (P < .05) |
| Intestinal mucositis/diarrhea clinical studies | |||||
| Vidal-Casariego et al61 | Radiotherapy | Glutamine, 30 g, oral versus placebo | AC/PC | RCT (n = 69), RTOG | NS benefit on acute enteritis/diarrhea |
| Michael et al13 | Irinotecan | Activated charcoal, 1000 mg | CRC | Single-arm open-label (n = 24), CTCAE | Decreased grade 3/4 diarrhea (7.1% vs 25%) |
Abbreviations: CRC, colorectal cancer; CTCAE, Common Terminology Criteria for Adverse Events; OM, oral mucositis; HNC, head and neck cancer; OC, oral cancer; Tx, treatment; 5-FU, 5-fluorouracil; IM, intramuscular; NS, nonsignificant; ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; RCT, randomized controlled trial; RTOG, Radiation Therapy Oncology Group; OeC, esophageal cancer; HCC, hepatocellular carcinoma; WHO OMAS, World Health Organization Oral Mucositis Assessment Scale; im, intramuscularly; OC, oral cancer; AL, acute leukemia; NHL, non-Hodgkin’s lymphoma; HDC, high-dose chemotherapy; HSCT, hematopoietic stem cell transplantation; equiv, equivalent; NPC, nasopharyngeal carcinoma; tid, three times a day; bid, two times a day; HM, hematologic malignancies; HL, Hodgkin’s lymphoma; MM, multiple myeloma; MS, myelodysplastic syndrome; BC, breast cancer; OvC, ovarian cancer; NIDCR, National Institute of Dental and Craniofacial Research; PBSCT, peripheral blood stem cell transplantation; Mel, melanoma; LC, lung carcinoma; Lym, lymphoma; RR, risk ratio; CI, confidence interval; mCRC, metastatic colorectal cancer; KS, Kaposi sarcoma; CALGB, Cancer and Leukemia Group B scale; Sar, sarcoma; NB, neuroblastoma; mBC, metastatic breast cancer; BMT, Stanford University Bone Marrow Transplant toxicity scale; IV, intravenous; bw, body weight; AST, aspartate aminotransferase; NSCLC, non–small cell lung cancer, AC, abdominal cancer; PC, pelvic cancer.
Table 3.
Clinical Trials Investigating Honey for Oral Mucositis.
| References | Interventions | Cancer | Design; Assessment | Outcome |
|---|---|---|---|---|
| Radiotherapy | ||||
| Biswal et al64 | 20 mL of honey 15 minutes before and after radiation versus standard oral care | HNC | RCT (n = 40); RTOG | Decreased OM grade 3-4 (P < .0005) |
| Motallebnejad et al73 | 20 mL of honey 15 minutes before and after radiation versus saline rinse | HNC | RSB (n = 40); WHO OMAS | Decreased OM (P < .05) |
| Khanal et al70 | Swish honey for 2 minutes and expectorate, 20 mL versus lignocaine gel | OC | RSB (n = 40); RTOG | Decreased OM (P < .05) |
| Bardy et al63 | Manuka honey or placebo golden syrup 20 mL versus standard oral care | HNC | RCT (n = 131); RTOG | NS difference |
| Jayachandran and Balaji68 | Honey versus benzydamine and saline | HNC | RCT (n = 60); WHO OMAS | Decreased OM (P < .05) |
| Parsons et al85 | Manuka honey versus standard oral care | HCN | RCT (n = 28, 18 honey, 10 control); multisite mucositis scoring system | NS difference |
| Charalambous et al82 | Honey versus saline rinse | HCN | RCT (n = 30); RTOG | Grade 3 xerostomia RR = 0.13 and grade 3 oral mucositis RR = 0.26, indicating that honey is effective for both symptoms |
| Alvi et al78 | 20 mL honey versus saline rinse | HNC | RCT (n = 60); WHO OMAS | Decreased OM (P < .05) |
| Hawley et al67 | Honey versus sugar-free gel | HNC | RCT (n = 106); RTOG, WHO OMAS | NS difference |
| Samdariya et al76 | 20 mL of honey before and after radiation and salt-soda and benzydamine mouth gargles versus salt-soda and benzydamine mouth gargles alone | HNC | RCT (n = 78); Visual Analogue Pain scale | Decreased severity pain score (P < .05) |
| Jayalekshmi et al69 | 15 mL honey before and after radiation versus plain water rinse | HNC | RSB (n = 28); RTOG | Decreased OM (P < .05) |
| Rao et al86 | Honey applied before and after radiation versus povidone-iodine | HNC | RSB (n = 50); RTOG | Decreased OM (P < .002) |
| Amanat et al80 | 20 mL honey before and after radiation versus saline rinse | HNC | RCT (n = 82); RTOG | Decreased OM grade 3 (P < .016) and grade 4 (P < .032) |
| Fogh et al65 | 10 mL liquid honey versus honey lozenge versus standard supportive care | Small and non–small cell lung cancer | RCT (n = 107, 53 supportive care, 54 liquid honey honey, 56 lozenge honey); CTCAE | Honey not superior to standard care |
| Chemotherapy | ||||
| Abdulrhman et al62 | Honey versus honey, beeswax, olive oil, propolis mouthwash mixture versus standard oral care | ALL | RCT pediatric, (n = 90); CTCAE | Faster healing (P < .05) |
| Allenidekania77 | Honey versus chlorhexidine | Pediatric cancer | RCT (n = 23), WHO OMAS | Decreased OM severity (P < .001) |
| Mishra and Nayak84 | Honey ice chips versus plain ice chips | ALL | RCT (n = 40); WHO OMAS | Decreased OM occurrence (P < .001) and no difference severity |
| Kobya et al71 | Honey 1 g/kg daily versus standard oral care | HNC | Quasi-experimental study children multicenter (n = 83); WHO OMAS | Decreased OM severity (P < .05) |
| Chemoradiotherapy | ||||
| Rashad et al75 | 20 mL honey before and after radiation versus no honey | HNC | RCT (n = 40); RTOG | Decreased OM grade 3-4 (P < .05) |
| Maiti et al72 | 20 mL honey before and after radiation | HNC | RCT (n = 55); WHO OMAS | Decreased OM grade 3-4 (P < .05) |
| Berk et al81 | Manuka honey liquid/lozenges versus supportive care | LC | RCT (n = 163); CTCAE | NS difference |
| Raeessi et al74 | 300 g of honey, or ±20 g of instant coffee versus topical betamethasone | HNC | RCT (n = 75); WHO OTS | Decreased OM grade 3-4 (P < .05) |
| Francis and Williams66 | Honey mixed with turmeric powder versus standard care | Various | Nonequivalent control group, pretest posttest design (n = 60), WHO OMAS | Decreased OM (P < .05) |
| Farneti et al83 | Sodium alginate, sodium carbonate, propolis, Aloe vera, calendula, honey, and chamomile versus placebo | HNC | RCT (n = 107), CTCAE | NS difference |
| Yadav87 | Honey with glycerin versus standard care | HNC | RCT (n = 107), CTCAE | Decreased OM (P < .003) |
| Al Jaouni et al79 | Honey versus standard oral care lidocaine, mycostatin) | ALL, AML, Burkett’s lymphoma, Wilm’s tumor | Open, randomized trial (n = 40, pediatric), clinician defined OM assessment | Decreased OM grade 3-4 (P < .02) |
Abbreviations: HNC, head and neck cancer; RCT, randomized controlled trial; RTOG, Radiation Therapy Oncology Group Grading System; OM, oral mucositis; RSB, randomized single blinded; WHO OMAS, World Health Organisation Oral Mucositis Assessment Scale; OC, oral cancer; NS, nonsignificant; RR, risk ratio; CTCAE, Common Terminology Criteria for Adverse Events; ALL, acute lymphocytic leukemia; LC, lung carcinoma; WHO OTS, World Health Oraganization Oral Toxicity Scale; AML, acute myelogenous leukemia.
We also identified 19 probiotic studies (Table 4).88-105 Nine placebo-controlled clinical trials88-92,95-97,106 examined the prophylactic use of probiotic in intestinal mucositis induced by chemotherapy, radiotherapy, or a combination of both. Two trials did not include a comparator group.98,104 Four studies examined the use of a probiotic in oral mucositis,99,100,103,105 3 studies were post-chemoradiotherapy treatment trials,93,94,101 and 2 studies examined the febrile incidence during chemotherapy.102
Table 4.
Clinical Trials Investigating Probiotics for Oral and Intestinal Mucositis/Diarrhea.
| References | Treatment | Intervention | Cancer Site | Design | Outcomes |
|---|---|---|---|---|---|
| Oral mucositis clinical studies | |||||
| Sharma et al99 | Radiotherapy plus cisplatin | L brevis 2 × 109 CFU, 6 lozenges versus placebo | HNC | RCT (n = 202), efficacy analysis = 188; CTCAE | Decreased incidence mucositis grade 3-4 and decreased completion of therapy (92% vs 70%; P < .05) |
| Sharma et al105 | HSCT | L brevis 2 × 109 CFU, 3-4 lozenges, no control | MM/HL/NHL/AML/RMS | Pilot, no control (n = 18); CTCAE | 29% no mucositis, 19% grade 1 mucositis, 33% grade 2 mucositis, 9.5% grade 3-4 mucositis, and 65% <grade 2 dysphagia |
| Sharma et al100 | HSCT | L brevis 2 × 109 CFU, 4-6 lozenges | CML/MM/HL/NHL/AML/RMS | Pilot, no control (n = 31); CTCAE | 23% no mucositis, 19% grade 1 mucositis, 39% grade 2 mucositis, 13% grade 3 mucositis, and 7% grade 4 mucositis |
| Giammarco et al103 | HSCT | L brevis 2 × 109 CFU, 6 lozenges versus OM prevention including chlorhexidine, saline rinses, and nystatin | MM | RCT (n = 16); assessment method not specified | 100% mucositis; NS difference between treatments (P >.05) |
| Radiotherapy adverse events prevention studies | |||||
| Salminen et al97 | Pelvic radiotherapy, (internal and external) 80 Gy (tumor) and 50 Gy pelvis | L acidophilus NCDO 1748 + 6.5% lactulose, 2 × 1011 CFU qd versus dietary counselling | Gynecological cancer | RCT (n = 24) | Significant reduction in incidence of diarrhea (P < .01); RR = 0.3 (95% CI = 0.11-0.81); control group all with diarrhea |
| Delia et al90 | Pelvic radiotherapy (60-70 Gy) | VSL#3 1 sachet tid versus placebo | Sigmoid rectal or cervical cancers | RCT (n = 490) | Reduced incidence (124/239 [51.8%] and 77/243 [31.6%], P < .001); reduced severity 55.4% and 1.4%, P < .001); RR = 0.61 (95% CI = 0.45-0.76) |
| Castro et al88 | Radiotherapy | L casei Shirota B breve (strain and dose not provided) versus placebo | Prostate cancer | RCT (n = 40) | Reduction in proctitis; improved QoL; RR = 0.54 (95% CI = 0.27-1.06) |
| Demers et al91 | Pelvic radiotherapy (44 Gy) | L acidophilus LAC-361, B longum BB536, 1.3 × 1011 CFU bid standard dose versus high dose versus placebo | Cervical and uterine cancers | RCT (n = 229) | Reduced incidence grade 4 diarrhea; standard dose; RR = 1.09 (95% CI = 0.76-1.59) |
| Chemotherapy adverse events prevention studies | |||||
| Österlund et al96 | 5-FU and leucovorin | L rhamnosus GG, 1-2 × 1011 CFU + 11 g guar gum qd versus no prophylactic treatment | Colorectal cancer | RCT (n = 150) | Reduced grade 3 or 4 diarrhea (22% vs 37%, P = .027); reduced abdominal discomfort; reduced hospital care; fewer chemotherapy dose reductions due to bowel toxicity; RR = 0.58 (95% CI = 0.35-0.98) |
| Sharma et al106 | Irinotecan and/or fluoropyrimidines | VSL#3 1 sachet bid versus placebo | Not defined | RCT (n = 202) | No significant difference in incidence of diarrhea; RR = 2.76 (95% CI 0.89-8.51) |
| Mego et al95 | Irinotecan | Colon Dophilus 10 × 109 capsules CFU tid versus placebo | Colorectal cancer | RCT (n = 46) | Reduction grade 3 or 4 diarrhea (0% vs 17.4%, P = .11); reduced overall incidence of diarrhea (39.1% vs 60.9%, P = .24); reduced incidence of enterocolitis (0% vs 8.7%) RR = 0.1 (95% CI = 0.006-1.95) |
| Chemoradiotherapy adverse events prevention studies | |||||
| Giralt et al92 | Pelvic radiotherapy (45-50 Gy), weekly cisplatin 40 mg/m2 | 96 mL fermented yogurt with L casei DN11400, 1.1 × 1011 CFU/g yogurt tid versus placebo | Cervical squamous cell carcinoma; endometrial adenocarcionoma | RCT (n = 85) | Improved stool consistency (P = .04); no difference to presentation of end point (diarrhea) or use of loperamide; RR = 1.17 (95% CI = 0.84-1.62) |
| Chitapanarux et al89 | Radiotherapy and cisplatin | 500 mg Infloran bid versus placebo | Cervical cancer (local advanced) | RCT (n = 63) | 45% grade 2-3 diarrhea placebo group and 9% of probiotic group (P = .002); antidiarrheal medications reduced (P = .03); improved stool consistency (P < .001) respectively; RR = 0.21 (95% CI = 0.07-0.65) |
| Radiotherapy adverse effects prevention studies (no comparator groups) | |||||
| Timko104 | Radiotherapy 50-67 Gy abdomen/pelvis | Colon Dophilus 2 capsules qd | Abdominal and pelvis cancers | RNB (n = 42), stool diary | Reduction in diarrhea and antibiotic use |
| Scartoni et al98 | Radiotherapy 30-80 Gy pelvis | Dixentil 10 mL vial qd | Large bowel urological, gynecological cancers | Prevention/safety (n = 42) | Reduction in diarrhea |
| Radiotherapy treatment studies | |||||
| Henriksson et al93 | Radiotherapy 62-66 Gy pelvis | Fermented milk, L lactis, L diacetylactis, L cremoris versus nonactive fermented milk | Pelvis and urinary bladder cancers | RCT (n = 40), stool diary | Reduction in chronic bowel discomfort |
| Urbancsek et al101 | Radiotherapy to 50 Gy abdomen | L rhamnosus 1.5 × 1011 CFU 4 weeks post radiotherapy versus placebo | Pelvis and abdominal cancers | RCT (n = 206), stool diary | Reduction in self-ratings diarrhea grade and feces consistency (P < .05) |
| Lee et al94 | Radiotherapy and chemotherapy 6 weeks to 2 years prior to enrolment in study | Lacidofil with L rhamnosus R0011, L acidophilus R0052, 2 × 1011 CFU 2 capsules qd versus placebo | Colorectal cancers | RCT (n = 60); Rome III; stool diary | Decreased IBS (P < .05); increase in functional health scores (P < .05); increased FACT scores (P < .05) |
| Clinical trials investigating infectious complications | |||||
| Wada et al102 | Chemotherapy not further defined | B breve M-16-V, 1 × 1011 CFU qd | Not defined | RNB (n = 42)/pediatric | Reduction in febrile episodes, antibiotic use; no effect on diarrhea; no difference in WBC or NK cells |
Abbreviations: CFU, colony-forming unit; HNC, head and neck cancer; RCT, randomized controlled trial; CTCAE, Common Terminology Criteria for Adverse Events; HSCT, hematopoietic stem cell transplantation; MM, multiple myeloma; HL, Hodgkin’s lymphoma; NHL, non-Hodgkin’s lymphoma; AML, acute myelogenous leukemia; RMS, rhabdomyosarcoma; CML, chronic myelogenous leukemia; OM, oral mucositis; NS, nonsignificant; qd, one a day; RR, risk ratio; CI, confidence interval; NK, natural killer; VSL#3, L casei, L plantarum, L acidophilus, L delbruekii subsp bulgaricus, B longum, B breve, B infantis, Streptococcus salivarius subsp thermophiles; Colon Dophilus, B breve HA-129 (25%), B bifidum HA-132 HA (20%), B longum HA-135 (14.5%), L rhamnosus HA-111 (8%), L acidophilus HA-122 (8%), L casei HA-108 (8%), L plantarum HA-119 (8%), S thermopilus HA-110 (6%), L brevis HA-112 (2%), B infantis HA-116; Infloran, 1 billion viv Lyophilisat and 1 billion B bifidum viv Lyophilisat; Dixentil, L acidophilus and L casei (strains not provided), zinc, galacto-oligosaccharides, and vitamins B1/B2/B6 and nicotinamide; tid, three times a day; QOL, quality of life; bid, two times a day; 5-FU, 5-fluorouracil; RNB, randomized nonblinded; IBS, irritable bowel syndrome; FACT, Functional Assessment of Cancer Therapy; WBC, white blood cells.
Oral mucositis was most commonly assessed according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer objective grading system (RTOG/EORTC), the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), or the World Health Organization Oral Mucositis Toxicity Scale. Intestinal mucositis was assessed by changes in stool consistency, incidence and severity of diarrhea, and the use of antidiarrheal medication. The most frequently used assessment scales are the CTCAE diarrhea grade and the Bristol stool charting system.
Bias and quality analysis were not performed as part of this review, as several reviews have already reported on bias and quality of studies for minerals, glutamine, honey, and probiotics, the 4 types of interventions with sufficient number of trials to attempt conducting a systematic review or meta-analysis. Although some studies had low bias, the overall assessment is that many studies have high bias and most studies suffered from some methodological weaknesses.107-112 The systematic review and meta-analysis of 9 probiotic trials for the prevention of chemoradiotherapy-induced diarrhea found that while the trials were of generally good methodological quality, there were significant blinding issues, and 1 study was published as a poster abstract only. Ambiguous handling of incomplete outcome data and lack of intention-to-treat analysis were noted as further bias risks.112
Reviewed Interventions
Prophylactic activated charcoal has been shown to reduce dose-limiting chemotherapy-induced diarrhea, thereby optimizing irinotecan therapy, while reducing antidiarrheal medication in an open-label, single-arm study. The study involved 28 patients with advanced CRC receiving irinotecan 125 mg/m2 intravenously once a week for 4 weeks every 6 weeks. In cycle 1, patients received irinotecan plus activated charcoal (5 mL aqueous solution containing 1000 mg activated charcoal in 25 mL of water), given the evening before the irinotecan dose and then 3 times daily for 48 hours after the dose. The use of activated charcoal in the first cycle was associated with a significant reduction in the incidence and severity of diarrhea, reduced the use of loperamide as a rescue medication, and was well tolerated with excellent compliance13 (Table 2).
Beta-glucan is an immune-modulating polysaccharide that was shown in a retrospective, controlled trial of 62 patients with CRC to prevent significant reductions in leucocyte and neutrophil counts compared with chemotherapy alone with a FOLFOX-4 regimen. The addition of β-glucan was also associated with a lower incidence of oral mucositis and diarrhea (Table 2).14
Concurrent administration of folate and cobalamin failed to reduce mucositis in a pilot study of 39 patients with non–small cell lung cancer treated with pralatrexate. Mucositis remained the dose-limiting toxicity of pralatrexate treatment19 (Table 2).
A meta-analysis that assessed the effectiveness of oral glutamine in radiotherapy-induced mucositis in head and neck cancers reported that in 5 clinical studies (234 patients total),41,43,44,52,59 glutamine was shown to reduce the risk and severity of radiotherapy-induced oral mucositis compared with either placebo or no treatment (risk ratio [RR] = 0.17, 95% confidence interval [CI] = 0.06-0.47).107 Oral glutamine was also shown to be beneficial in 1141,44,46,49,54-60 of 15 studies investigated in a systematic review investigating the effects of glutamine for chemotherapy- or radiotherapy-induced oral mucositis. Glutamine significantly reduced the incidence of grade 2, 3, or 4 mucositis and/or reduced weight loss as well as the duration, time of onset, and/or maximum grade of mucositis.108 Four studies showed no effect45,48,50,53 (Table 2).
A recent study found that 9 g glutamine in combination with an elemental diet was associated with a significant reduction in chemotherapy-induced oral mucositis in esophageal cancer compared with no treatment or glutamine alone. The incidence of grade 2 or higher of oral mucositis was 60% in the control group, 70% in the glutamine group, and 10% in the glutamine plus elemental diet group.42 A further review of 9 randomized, controlled studies concluded that glutamine may reduce gastrointestinal mucositis and diarrhea and improve nitrogen balance, immune imbalance, and wound healing in chemotherapy-induced toxicity113 (Table 2).
Glutamine has been shown to be a principal nutrient with glucose supporting survival of mammalian cells and, unfavorably, cancer cells. However, oral glutamine has been reported to be unlikely to contribute significantly to tumor growth, local invasion, and metastatic dissemination.114 High baseline consumption of dietary glutamate has been shown in humans to be associated with a lower risk for CRC,115 and oral glutamine 2 days before tumor implantation has been shown in rodents to increase natural killer cell activity, upregulate intestinal glutathione metabolism, and decrease tumor growth by 40% to 50%.116,117 Oral glutamine administered during chemoradiotherapy did not negatively affect tumor control and survival in patients with stage IIIB non–small cell lung cancer.56 As cancer cells can manipulate host metabolism favoring tumor growth, deprivation of dietary glutamine or the use of oral supplementation for mucositis was reported unlikely to adversely affect tumor growth during chemotherapy treatments. Glutamine, however, has been shown to be ineffective in controlling acute radiation-induced intestinal mucositis.61,118
A Cochrane review of 3 studies64,73,75 investigating honey concluded that it was associated with a weak to moderate benefit in the prevention of radiotherapy-induced oral mucositis.119 Three subsequent meta-analyses concluded that oral administration of honey could prevent the incidence of radiotherapy-induced oral mucositis in head and neck cancers.109,110,120 Cho et al concluded that oral administration of honey after radiotherapy could prevent the development of moderate to severe mucositis and associated weight loss.109 Xu et al concluded that, compared with no treatment, honey could reduce the incidence of oral mucositis after chemoradiotherapy (RR = 0.35, 95% CI = 0.18-0.70, P = .003).110 Honey was also found to decrease treatment interruptions, weight loss, and delay the onset of oral mucositis. Honey, however, was inefficacious in decreasing the peak mucositis score. Co et al reported statistical pooling showing that the risk ratio of having a treatment interruption was significantly lower with the use of honey versus control 0.11 (95% CI = 0.02-0.58) with a risk ratio of developing severe mucositis when honey was administered as 0.45 with a CI of 0.09 to 2.21.120
Friend et al specifically examined pediatric trials and identified 4 trials62,71,77,79 with grade C evidence that honey was effective as a preventative and adjunctive therapy for chemotherapy-induced oral mucositis in children.121 Honey was found to reduce the frequency, duration, and stage of chemotherapy-induced mucositis.
Seven trials have been published since the meta-analyses report.109,110,120 Four trials examined honey in radiotherapy-induced mucositis in head/neck and lung cancers,65,69,80,86 1 trial in chemotherapy-induced mucositis in acute lymphocytic leukemia,84 and 2 trials in chemoradiotherapy-induced mucositis in children with various cancers.79,87 Only one trial65 reported no effect. Medical manuka honey administered as a liquid or as a lozenge was not superior to best supportive care in preventing radiation esophagitis.65
A single-blinded randomized controlled trial (n = 28) found that 15 mL of natural honey was associated with a statistically significant difference in degree of oral mucositis between the experimental and control groups in weeks 4, 5, and 6 (P < .01).69 Compared with the active comparator, povidone-iodine, honey significantly reduced radiation-induced oral mucositis, decreased the incidence of intolerable mucositis, treatment breaks, loss of treatment days (P < .0001 and < .0003), and did not affect the radiation-induced tumor response.86 Honey significantly reduced the severity of mucositis (grades 3 and 4) compared with control group at the end of 6 weeks of radiation treatment.80 Honey ice cubes were shown to significantly reduce the occurrence of chemotherapy-induced oral mucositis in pediatric cancer patients compared with plain ice cubes on the 5th (P = .001) and 15th (P = .001) days of assessment.84 A significantly higher number of patients developed grade 2 or above chemoradiotherapy-induced mucositis in the control arm compared with the experimental arm (P = .003).87 Absolute risk reduction between honey and control for developing grades III and IV oral mucositis was found to be significant in a study of pediatric cancer patients receiving chemoradiotherapy (P < .0579; Table 3).
A meta-analysis of randomized controlled trials (RCTs) examining oral mucositis induced by chemoradiotherapy, or hematopoietic stem cell transplantation (HSCT), found that patients pretreated with mineral supplementation delayed the onset of mucositis and that fewer patients experienced less peak oral mucositis compared with controls.111 The analysis examined 7 studies with zinc,26-31,33 3 with calcium,36-38 2 with selenium,24,25 and 2 with iodine.39,40 Significant study bias was observed though and study heterogeneity, making it difficult to make specific clinical recommendations. Mineral formulations did not overall significantly reduce mean duration of mucositis, pain duration, or use of analgesics.111 Of the 14 studies included in the meta-analysis, the 3 excluded26,32,122 and 2 recent studies34,98 are presented in Table 2. Zinc is essential for proper immune function and for the integrity of connective tissue and cell membranes, and 50 to 150 mg elemental zinc daily was reported to reduce oral mucositis27-29,31 (Table 2).
Four studies examining the effects of multinutrient formulations that consisted of a mixture of open-label, retrospective, and prospective studies were identified. In a small, open-label study, it was reported that oral or parenteral administration of a multinutrient formulation was well tolerated in patients with head and neck cancers treated with chemoradiotherapy without developing severe mucositis.15 In another, multinutrient formulation composed of amino acids, omega-3 fatty acids, ribonucleic acids, vitamins, and antioxidants was shown to be associated with less severe mucositis in patients with head and neck cancers treated with chemoradiotherapy.16 In 2 other studies that examined the same amino acid–rich oral formulation, the first study found that the formulation was associated with reduced severity of mucositis in squamous cell carcinoma treated with radiotherapy with or without chemotherapy when compared with no formulation administration. The nutrient formulation was also associated with improved completion rates of chemoradiotherapy.17 The second study was a prospective pilot study in CRC treated with 5-FU-based chemotherapy. This study reported that the multinutrient formulation was associated with a decrease in the severity of oral mucositis in approximately 90% of the patients during the first course of treatment (P = .0002) and maintained in the second course of treatment (P <.000118; Table 2).
Vitamin E may reduce mucositis by regulating nrf2 activation. Gamma-tocotrienol has been shown to prevent 5-FU-induced redox signaling by regulating nrf2 activation and cell survival in human oral keratinocytes.123 Applying vitamin E directly to the mucous membranes may be more effective than orally administered. Oral vitamin E (400 mg twice daily) was shown to have no effect on the incidence or severity of mucositis in an RCT of 60 patients with leukemia receiving allogenic bone marrow transplantation.12 Despite this, topical vitamin E was shown to be beneficial in the treatment of oral lesions associated with mucositis in an RCT of patients with solid tumors (n = 17) or leukemia (n = 1). Six of 9 patients receiving vitamin E had complete resolution of oral lesions compared with 1 out of 9 in the control group (P = .025).21 In another study with 80 patients who developed oral mucositis, 100 mg of a topical, but not oral, application of vitamin E was shown to improve mucositis.22 In a further study with 54 patients with head and neck cancers it was found that vitamin E before, and for the duration of radiotherapy, decreased the incidence of mucositis.20 Topical vitamin E reduced the risk of mucositis by 36%.20 Both topically applied vitamin E and the oligomeric procyanidin known as pycnogenol were shown in an RCT to reduce mucositis in 72 children although pycnogenol was not effective for severe, grade 4 mucositis23 (Table 2).
A probiotic containing lozenge, specifically with Lactobacillus brevis, has been shown in an RCT to reduce radiation- and chemotherapy-induced oral mucositis in 200 patients with head and neck cancers. Use of the probiotic was associated with a reduced incidence of mucositis grades III and IV (P = .001). Supportive treatment with the probiotic was administered during treatment and for 1 week post treatment completion (radiotherapy and weekly cisplatin).99 The same probiotic lozenge formulation was also examined in 3 studies of patients undergoing HSCT for a variety of cancers including multiple myeloma. In the first pilot study, of 21 patients, only 19% developed grade III or IV mucositis compared with the expected 60%. No adverse events except occasional grade I/II nausea due to study drug were noted.105 The second pilot study by the same research group found that 20% developed grade III or IV mucositis.100 In a repeat pilot study, patients treated with HSCT were given the probiotic lozenge 4 to 7 days before initiation of chemotherapy and continuing until resolution of mucositis or till day 24. The single-arm, single-center, phase II study found that of the 31 patients enrolled, 7 (22.6%) patients did not develop any mucositis, 6 (19.4%) patients developed grade 1 mucositis, 12 (38.7%) patients developed grade 2 mucositis, and 4 (12.9%) and 2 (6.5%) patients developed grade 3 and grade 4 mucositis, respectively. Median time to onset and for resolution of mucositis was 6 days and 8 days, respectively. No adverse events were reported with usage of study drug.100 However, in the fourth pilot study by another group using the same formulation in patients undergoing HSCT, all 16 patients developed various grades of mucositis with no statistical difference between the probiotic lozenge and standard treatment103 (Table 4).
The majority of studies investigated the prophylactic use of probiotics for diarrhea, while a few investigated the efficacy of probiotics in the treatment of irritable bowel syndrome or diarrhea, weeks to years post treatment (Table 4). The studies used a variety of probiotic interventions and protocol designs and outcomes, making it difficult to identify which type of intervention and protocol was most beneficial. A systematic review and meta-analysis of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancers found that probiotics were generally beneficial in treatment-induced diarrhea, especially for grades 2 and 3.112 A recent meta-analysis that grouped 7 studies for the prophylactic use of probiotics for cancer therapy–induced diarrhea124 concluded that current evidence does not support widespread implementation of probiotics for the management of diarrhea secondary to cytotoxic therapy and the tyrosine kinase inhibitor, dacomitinib. The administration of probiotic was begun on the first day of cancer therapy initiation and as such referring to prophylactic interventions may be inaccurate. The administration of prophylactic probiotics must begin ideally 1 month prior to chemotherapy/radiotherapy initiation.
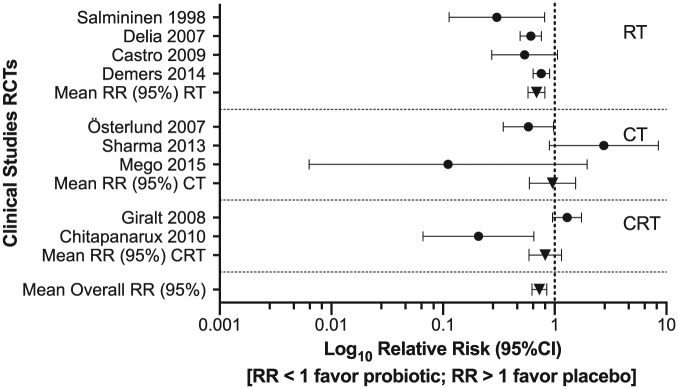
In order to investigate the probiotic effect to reduce diarrhea induced by chemotherapy and/or radiotherapy, relative risks were calculated for the 9 RCTs, and the results showed that 7 studies favored probiotics for preventing chemotherapy- and/or radiotherapy-induced grade ⩾2 diarrhea (Figure 1). The co-administration of probiotics with radiotherapy shows enhanced efficacy in preventing intestinal adverse effects induced by radiotherapy compared with chemotherapy.
Figure 1.
Forest plot of RCTs of chemotherapy-/radiotherapy-induced diarrhea.
RCT, randomized controlled trials; RT, radiotherapy; CT, chemotherapy; CRT, chemoradiotherapy; RR, relative risk, risk ratio.
Adjuvant Interventions Safety
One study implementing manuka honey mouthwash found that while it demonstrated benefit in ameliorating radiation-induced weight loss and increase quality of life in the absence of cisplatin chemotherapy, it was also reported that undiluted manuka honey caused severe nausea, vomiting, and severe stinging.85 In an additional study, Bardy et al found no difference between golden syrup and manuka honey,63 suggesting that perhaps the high sucrose content was responsible for the antibacterial effect observed. Neither study showed improvement in mucositis; however, compliance associated with the taste and texture of the interventions was an issue, which may have influenced the study outcome.
The common dose administered in clinical trials was 20 mL of honey, 3 times daily. At this dose, honey did not affect blood sugar levels when initial fasting blood sugar level was below 150 mg/dL.72 Patients undergoing radiotherapy for head and neck cancers have been reported to be prone to a range of dental complications,125 and a concern was the added risk of developing dental caries, in spite of research suggesting otherwise.126 Radiation-related caries are related to hyposalivation, shifts in the oral microbiota, and altered saliva composition. The rapid onset and progression often leads to extensive loss of dentition within short periods of time. Honey contains known cariogenic substances including glucose, fructose, sucrose, and numerous acids, including gluconic, acetic, lactic, butyric, and formic acids, that may contribute to cariogenic increased risk.127 However, honey has also been shown to prevent radiation-induced decrease enamel microhardness in xerostomic patients compared with patients with normal salvia.128 None of the trials reported that the use of honey predisposed to the development of caries.
The use of probiotics as an adjunctive medicine in oncology to enhance treatment or reduce adverse events associated with chemotherapy or radiotherapy is not part of standard practice. The principal concern being that patients treated with chemotherapy are frequently immune-compromised and, therefore, are at increased risk of sepsis from administering probiotic formulations. A systematic review of 17 studies (N = 1530) found no reports of significant side effects such as serious localized or systemic infections when administered to patients receiving cancer treatments. Five case reports showed probiotic-related bacteremia, fungemia, or positive blood cultures.129 Wang et al included 11 studies in its safety analysis. Seven studies did not report any adverse events. Four studies reported various adverse events. The reporting of adverse effects was, however, very inconsistent and poorly documented. Although some infections were reported, no probiotic bacteria growth could be found in blood cultures. Other adverse effects included mild gastrointestinal upsets, fever, and anorexia, which were also observed in the control groups.112 Okawa et al reported 1 death but no evidence of an association with the probiotic intervention was reported.130 A few probiotic trials have been performed in children.102,131 A single-blinded study found that Bifidobacterium breve reduced the frequency of fever, which was associated with a lower use of intravenous antibiotics compared with placebo in children receiving chemotherapy (1-13 years of age, n = 42). No adverse events were reported.102 A safety and feasibility study did not report Lactobacillus plantarum–associated bacteremia in children undergoing allogenic hematopoietic cell transplant;131 however, a case of septic shock caused by yogurt-derived Lactobacillus species was recently reported in a 54-year-old male patient with acute promyelocytic leukemia. The bacteremia developed a week after the patient underwent high-dose chemotherapy and autologous peripheral blood stem cell transplantation. The pathogen was identified by strain-specific polymerase chain reaction analysis to be identical to the Lactobacillus rhamnosus GG found in the yogurt.132
Discussion
Recent interventions have continued to explore and to further build the scientific and clinical understanding of oral and intestinal mucositis preventative and treatment options that may be available to clinicians and patients.13,112,121,133 The prevention and treatment of oral and intestinal mucositis that seeks to decrease the risk of formation and/or progression of these deleterious sequela of chemoradiotherapy regimens are important factors that impinge on patient quality of life and clinical decisions relevant to treatment. While it is acknowledged that oral and intestinal mucositis represent significant burdens of antineoplasitic therapies, the implementation of adjunctive treatments still remain a challenge.134
Chemotherapy and radiotherapy have significant adverse effects on the microbiota of the oral and gastrointestinal mucosa. Oral mucositis is strongly associated with bacteremia and sepsis due to Escherichia coli, Pseudomonas aeruginosa, and Candida albicans.103 How probiotics were postulated to overcome the side effects of chemotherapy and/or radiotherapy was advanced from observations that Lactobacillus brevis–containing lozenges produced anti-inflammatory metabolites.103 It was reported that L brevis produced arginine deiminase and sphingomyelinase, which hydrolyses platelet-activating factor known to be associated with oral mucositis in radiation therapy.103 Arginine deiminase then converts arginine to ammonia and citrulline, reducing the amount of available arginine to be converted to nitric oxide—a major mediator of inflammation. Furthermore, the appeal of probiotics administered for oral mucositis was enhanced when they were demonstrated to have no serious adverse effects.99,103,105 This local oral benefit did not, however, extend to the intestines, with 14 out of 16 patients developing diarrhea.103
In the intestines, cancer therapy has been found to decrease commensals such as Bifidobacteria, Clostridium cluster XIVa, and Faecalibacterium prausnitzii, combined with increases in Enterobacteriaceae and Bacteroides. These changes induce intestinal dysbiosis and contribute to the development of mucositis, particularly diarrhea and bacteremia.135 Adverse shifts in the intestinal microbiota has led to the notion that the administration of probiotics could reduce the side effects of chemotherapy and radiotherapy.
There is moderate evidence that zinc, selenium, vitamin E, glutamine, and honey may be beneficial for the prevention or treatment of oral mucositis. However, the low numbers and heterogeneity of the studies reviewed generally makes it difficult to offer specific clinical recommendations. In one review, mineral supplementation, including zinc, did not overall significantly reduce mean duration of the mucositis, pain incidences, or use of analgesics.111 The recommendation for zinc supplementation is currently restricted for patients with oral cancer having treatment with chemotherapy or radiation according to the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) guidelines. One drawback of using zinc supplementation is that it may induce nausea and even vomiting. Zinc supplementation should not be taken on an empty stomach, as it increases the adverse effects. The studies mostly used 220 mg zinc sulfate, equivalent to 50 mg elemental zinc, 2 to 3 times daily as a mouthwash or capsule/tablet.
The administration of selenium in clinical studies employed doses that ranged from 200 µg elemental selenium twice daily25 to sodium selenite oral fluid 200 to 500 µg an hour prior to radiotherapy sessions.24 Applying vitamin E directly to the oral mucosa may be more effective than orally administered.
Glutamine was found to be the most studied nutritional intervention and despite evidence suggesting that glutamine may reduce gastrointestinal mucositis and chemotherapy-induced diarrhea, the European Society for Clinical Nutrition and Metabolism (ESPEN)133 stated in its recent guidelines on nutrition in cancer patients that “there are insufficient consistent clinical data to recommend glutamine to prevent radiation-induced enteritis/diarrhea, stomatitis, esophagitis, or skin toxicity.” The MASCC/ISOO guideline has been updated from a recommendation against glutamine to “no guideline possible” for glutamine for oral or gastrointestinal mucositis.136 The safety of glutamine has also been reviewed in view of emerging evidence that malignant cells can utilize glutamine as a mitochondrial substrate.137 The glutamine doses investigated have ranged from 9 to 30 g daily in divided doses. As the lower dose has been shown to be beneficial in oral mucositis, it may be prudent to use the lower end of the dosage spectrum. Good oral hygiene is essential if honey-based interventions for mucositis are recommended. Activated charcoal may reduce symptoms associated with chemotherapy-induced mucositis including diarrhea. Clinical trials investigating the administration of probiotics to prevent treatment-induced intestinal toxicity has produced mixed results.124 Studies have used various end-point parameters including stool frequency and stool consistency (described separately or as diarrhea grade 2-4), use of rescue anti-diarrheal medications, and microbiome shifts induced by chemotherapy or radiotherapy. The use of probiotics in the prevention or treatment of chemotherapy and/or radiotherapy-induced gastrointestinal toxicity appears to be beneficial and without significant side effects. The MASCC/ISOO guideline suggests that probiotics containing Lactobacillus species be used to prevent diarrhea in patients receiving chemotherapy and/or radiation therapy for a pelvic malignancy,136 while the ESPEN guideline states that there are insufficient consistent clinical data to recommend probiotics to reduce radiation-induced diarrhea.
Conclusion
There is plausible clinical evidence for the administration of honey, zinc, selenium, topical vitamin E, and glutamine as an adjuvant treatment to reduce the risk of developing oral mucositis during chemotherapy or radiotherapy. Activated charcoal, glutamine, and probiotics may also be beneficial in chemotherapy-induced diarrhea. Considering the excellent safety profile and resulting high therapeutic index, further research examining the mechanism of action and clinical efficacy of probiotics in chemotherapy- and radiotherapy-induced intestinal mucositis is warranted. Given that adverse disturbances in the oral and intestinal microbiomes can promote immune dysregulation and increase the risk of patient mortality, there is need for further research in this area.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Luis Vitetta has received National Institute of Complementary Medicine and National Health and Medical Research Council of Australia competitive funding and industry support for research into probiotics and other compounds and is currently Director of Medical Research at Medlab Clinical and with Michael Thomsen investigating the formulation and administration of probiotics. Michael Thomsen is funded by the Francis M Hooper scholarship, Sydney Medical School Foundation.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Michael Thomsen  https://orcid.org/0000-0003-1446-8221
https://orcid.org/0000-0003-1446-8221
Luis Vitetta  https://orcid.org/0000-0002-7490-9298
https://orcid.org/0000-0002-7490-9298
References
- 1. Raber-Durlacher JE, Weijl NI, Saris MA, de Koning B, Zwinderman AH, Osanto S. Oral mucositis in patients treated with chemotherapy for solid tumors: a retrospective analysis of 150 cases. Support Care Cancer. 2000;8:366-371. [DOI] [PubMed] [Google Scholar]
- 2. Epstein JB, Schubert MM. Oral mucositis in myelosuppressive cancer therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:273-276. [DOI] [PubMed] [Google Scholar]
- 3. Harris DJ. Cancer treatment-induced mucositis pain: strategies for assessment and management. Ther Clin Risk Manag. 2006;2:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol). 2007;19:790-799. [DOI] [PubMed] [Google Scholar]
- 5. Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 suppl):1995-2025. [DOI] [PubMed] [Google Scholar]
- 6. Aprile G, Rihawi K, De Carlo E, Sonis ST. Treatment-related gastrointestinal toxicities and advanced colorectal or pancreatic cancer: a critical update. World J Gastroenterol. 2015;21:11793-11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pico JL, Avila-Garavito A, Naccache P. Mucositis: its occurrence, consequences, and treatment in the oncology setting. Oncologist. 1998;3:446-451. [PubMed] [Google Scholar]
- 8. Sonis ST. Oral mucositis in cancer therapy. J Support Oncol. 2004;2(6 suppl 3):3-8. [PubMed] [Google Scholar]
- 9. Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol. 2010;2:51-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keefe DM, Elting LS, Nguyen HT, et al. Risk and outcomes of chemotherapy-induced diarrhea (CID) among patients with colorectal cancer receiving multi-cycle chemotherapy. Cancer Chemother Pharmacol. 2014;74:675-680. [DOI] [PubMed] [Google Scholar]
- 11. Elting LS, Chang YC, Parelkar P, et al. Risk of oral and gastrointestinal mucosal injury among patients receiving selected targeted agents: a meta-analysis. Support Care Cancer. 2013;21:3243-3254. [DOI] [PubMed] [Google Scholar]
- 12. Ghoreishi Z, Shidfar F, Iravani M, Esfahani A, Ghavamzadeh A. Effect of vitamin E on chemotherapy-induced mucositis and neutropenia in leukemic patients undergoing bone marrow transplantation. Asia Pac J Clin Oncol. 2007;3:113-118. [Google Scholar]
- 13. Michael M, Brittain M, Nagai J, et al. Phase II study of activated charcoal to prevent irinotecan-induced diarrhea. J Clin Oncol. 2004;22:4410-4417. [DOI] [PubMed] [Google Scholar]
- 14. Karaca H, Bozkurt O, Ozaslan E, et al. Positive effects of oral β-glucan on mucositis and leukopenia in colorectal cancer patients receiving adjuvant FOLFOX-4 combination chemotherapy. Asian Pac J Cancer Prev. 2014;15:3641-3644. [DOI] [PubMed] [Google Scholar]
- 15. Casbarien O, Cresta P, Silva C, et al. Specific nutritional supplement (Supportan®) in the supportive care of the radio-chemotherapy treatment of head and neck cancers: biochemical parameters. Preliminary study. Endocr Metab Immune Disord Drug Targets. 2013;13:348-350. [DOI] [PubMed] [Google Scholar]
- 16. Machon C, Thezenas S, Dupuy AM, et al. Immunonutrition before and during radiochemotherapy: improvement of inflammatory parameters in head and neck cancer patients. Support Care Cancer. 2012;20:3129-3135. [DOI] [PubMed] [Google Scholar]
- 17. Harada K, Ferdous T, Horinaga D, et al. Efficacy of elemental diet on prevention for chemoradiotherapy-induced oral mucositis in patients with oral squamous cell carcinoma. Support Care Cancer. 2016;24:953-959. [DOI] [PubMed] [Google Scholar]
- 18. Ogata Y, Ishibashi N, Yamaguchi K, et al. Preventive effects of amino-acid-rich elemental diet Elental® on chemotherapy-induced oral mucositis in patients with colorectal cancer: a prospective pilot study. Support Care Cancer. 2016;24:783-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azzoli CG, Patel JD, Krug LM, et al. Pralatrexate with vitamin supplementation in patients with previously treated, advanced non-small cell lung cancer: safety and efficacy in a phase 1 trial. J Thorac Oncol. 2011;6:1915-1922. [DOI] [PubMed] [Google Scholar]
- 20. Ferreira PR, Fleck JF, Diehl A, et al. Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: a double-blind randomized trial. Head Neck. 2004;26:313-321. [DOI] [PubMed] [Google Scholar]
- 21. Wadleigh RG, Redman RS, Graham ML, Krasnow SH, Anderson A, Cohen MH. Vitamin E in the treatment of chemotherapy-induced mucositis. Am J Med. 1992;92:481-484. [DOI] [PubMed] [Google Scholar]
- 22. El-Housseiny AA, Saleh SM, El-Masry AA, Allam AA. The effectiveness of vitamin “E” in the treatment of oral mucositis in children receiving chemotherapy. J Clin Pediatr Dent. 2007;31:167-170. [PubMed] [Google Scholar]
- 23. Khurana H, Pandey RK, Saksena AK, Kumar A. An evaluation of vitamin E and pycnogenol in children suffering from oral mucositis during cancer chemotherapy. Oral Dis. 2013;19:456-464. [DOI] [PubMed] [Google Scholar]
- 24. Büntzel J, Riesenbeck D, Glatzel M, et al. Limited effects of selenium substitution in the prevention of radiation-associated toxicities. Results of a randomized study in head and neck cancer patients. Anticancer Res. 2010;30:1829-1832. [PubMed] [Google Scholar]
- 25. Jahangard-Rafsanjani Z, Gholami K, Hadjibabaie M, et al. The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: a randomized clinical trial. Bone Marrow Transplant. 2013;48:832-836. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer. 2010;127:1984-1990. [DOI] [PubMed] [Google Scholar]
- 27. Lin LC, Que J, Lin LK, Lin FC. Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2006;65:745-750. [DOI] [PubMed] [Google Scholar]
- 28. Lin YS, Lin LC, Lin SW, Chang CP. Discrepancy of the effects of zinc supplementation on the prevention of radiotherapy-induced mucositis between patients with nasopharyngeal carcinoma and those with oral cancers: subgroup analysis of a double-blind, randomized study. Nutr Cancer. 2010;62:682-691. [DOI] [PubMed] [Google Scholar]
- 29. Ertekin MV, Koc M, Karslioglu I, Sezen O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys. 2004;58:167-174. [DOI] [PubMed] [Google Scholar]
- 30. Sangthawan D, Phungrassami T, Sinkitjarurnchai W. A randomized double-blind, placebo-controlled trial of zinc sulfate supplementation for alleviation of radiation-induced oral mucositis and pharyngitis in head and neck cancer patients. J Med Assoc Thai. 2013;96:69-76. [PubMed] [Google Scholar]
- 31. Arbabi-kalati F, Arbabi-kalati F, Deghatipour M, Moghadam AA. Evaluation of the efficacy of zinc sulfate in the prevention of chemotherapy-induced mucositis: a double-blind randomized clinical trial. Arch Iran Med. 2012;15:413-417. [PubMed] [Google Scholar]
- 32. Mehdipour M, Zenoz AT, Kermani IA, Hosseinpour A. A comparison between zinc sulfate and chlorhexidine gluconate mouthwashes in the prevention of chemotherapy-induced oral mucositis. Daru. 2011;19:71-73. [PMC free article] [PubMed] [Google Scholar]
- 33. Mansouri A, Hadjibabaie M, Iravani M, et al. The effect of zinc sulfate in the prevention of high-dose chemotherapy-induced mucositis: a double-blind, randomized, placebo-controlled study. Hematol Oncol. 2012;30:22-26. [DOI] [PubMed] [Google Scholar]
- 34. Hayashi H, Kobayashi R, Suzuki A, et al. Preparation and clinical evaluation of a novel lozenge containing polaprezinc, a zinc-L-carnosine, for prevention of oral mucositis in patients with hematological cancer who received high-dose chemotherapy. Med Oncol. 2016;33:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Markiewicz M, Dzierzak-Mietla M, Frankiewicz A, et al. Treating oral mucositis with a supersaturated calcium phosphate rinse: comparison with control in patients undergoing allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2012;20:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lambrecht M, Mercier C, Geussens Y, Nuyts S. The effect of a supersaturated calcium phosphate mouth rinse on the development of oral mucositis in head and neck cancer patients treated with (chemo)radiation: a single-center, randomized, prospective study of a calcium phosphate mouth rinse + standard of care versus standard of care. Support Care Cancer. 2013;21:2663-2670. [DOI] [PubMed] [Google Scholar]
- 37. Raphael MF, den Boer AM, Kollen WJ, et al. Caphosol, a therapeutic option in case of cancer therapy-induced oral mucositis in children: results from a prospective multicenter double blind randomized controlled trial. Support Care Cancer. 2014;22:3-6. [DOI] [PubMed] [Google Scholar]
- 38. Papas AS, Clark RE, Martuscelli G, O’Loughlin KT, Johansen E, Miller KB. A prospective, randomized trial for the prevention of mucositis in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31:705-712. [DOI] [PubMed] [Google Scholar]
- 39. Madan PD, Sequeira PS, Shenoy K, Shetty J. The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: a randomized control trial. J Cancer Res Ther. 2008;4:3-8. [DOI] [PubMed] [Google Scholar]
- 40. Vokurka S, Bystrická E, Koza V, et al. The comparative effects of povidone-iodine and normal saline mouthwashes on oral mucositis in patients after high-dose chemotherapy and APBSCT—results of a randomized multicentre study. Support Care Cancer. 2005;13:554-558. [DOI] [PubMed] [Google Scholar]
- 41. Tsujimoto T, Yamamoto Y, Wasa M, et al. L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: a double-blind, randomized, placebo-controlled trial. Oncol Rep. 2015;33:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka Y, Takahashi T, Yamaguchi K, Osada S, Shimokawa T, Yoshida K. Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: a feasibility study. Support Care Cancer. 2016;24:933-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang EY, Leung SW, Wang CJ, et al. Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535-539. [DOI] [PubMed] [Google Scholar]
- 44. Vidal-Casariego A, Calleja-Fernández A, Ballesteros-Pomar MD, Cano-Rodriguez I. Efficacy of glutamine in the prevention of oral mucositis and acute radiation-induced esophagitis: a retrospective study. Nutr Cancer. 2013;65:424-429. [DOI] [PubMed] [Google Scholar]
- 45. Jebb SA, Osborne RJ, Maughan TS, et al. 5-fluorouracil and folinic acid-induced mucositis: no effect of oral glutamine supplementation. Br J Cancer. 1994;70:732-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Skubitz KM, Anderson PM. Oral glutamine to prevent chemotherapy induced stomatitis: a pilot study. J Lab Clin Med. 1996;127:223-228. [DOI] [PubMed] [Google Scholar]
- 47. Anderson PM, Schroeder G, Skubitz KM. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83:1433-1439. [DOI] [PubMed] [Google Scholar]
- 48. Okuno SH, Woodhouse CO, Loprinzi CL, et al. Phase III controlled evaluation of glutamine for decreasing stomatitis in patients receiving fluorouracil (5-FU)-based chemotherapy. Am J Clin Oncol. 1999;22:258-261. [DOI] [PubMed] [Google Scholar]
- 49. Cockerham MB, Weinberger BB, Lerchie SB. Oral glutamine for the prevention of oral mucositis associated with high-dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann Pharmacother. 2000;34:300-303. [DOI] [PubMed] [Google Scholar]
- 50. Dickson TMC, Wong RM, Offrin RS, et al. Effect of oral glutamine supplementation during bone marrow transplantation. JPEN J Parenter Enteral Nutr. 2000;24:61-66. [DOI] [PubMed] [Google Scholar]
- 51. Daniele B, Perrone F, Gallo C, et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut. 2001;48:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cerchietti LC, Navigante AH, Lutteral MA, et al. Double-blinded, placebo-controlled trial on intravenous L-alanyl-L-glutamine in the incidence of oral mucositis following chemoradiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;65:1330-1337. [DOI] [PubMed] [Google Scholar]
- 53. Li Y, Yu Z, Liu F, Tan L, Wu B, Li J. Oral glutamine ameliorates chemotherapy-induced changes of intestinal permeability and does not interfere with the antitumor effect of chemotherapy in patients with breast cancer: a prospective randomized trial. Tumori. 2006;92:396-401. [PubMed] [Google Scholar]
- 54. Choi K, Lee SS, Oh SJ, et al. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin Nutr. 2007;26:57-62. [DOI] [PubMed] [Google Scholar]
- 55. Peterson DE, Jones JB, Petit RG., 2nd Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer. 2007;109:322-331. [DOI] [PubMed] [Google Scholar]
- 56. Topkan E, Parlak C, Topuk S, Pehlivan B. Influence of oral glutamine supplementation on survival outcomes of patients treated with concurrent chemoradiotherapy for locally advanced non-small cell lung cancer. BMC Cancer. 2012;12:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Topkan E, Yavuz MN, Onal C, Yavuz AA. Prevention of acute radiation-induced esophagitis with glutamine in non-small cell lung cancer patients treated with radiotherapy: evaluation of clinical and dosimetric parameters. Lung Cancer. 2009;63:393-399. [DOI] [PubMed] [Google Scholar]
- 58. Tutanc OD, Aydogan A, Akkucuk S, et al. The efficacy of oral glutamine in prevention of acute radiotherapy-induced esophagitis in patients with lung cancer. Contemp Oncol (Pozn). 2013;17:520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chattopadhyay S, Saha A, Azam M, Mukherjee A, Sur PK. Role of oral glutamine in alleviation and prevention of radiation-induced oral mucositis: a prospective randomized study. South Asian J Cancer. 2014;3:8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gul K, Muge A, Taner A, Sehri E. Oral glutamine supplementation reduces radiotherapy-induced esophagitis in lung cancer patients. Asian Pac J Cancer Prev. 2015;16:53-58. [DOI] [PubMed] [Google Scholar]
- 61. Vidal-Casariego A, Calleja-Fernandez A, de Urbina-González JJ, Cano-Rodríguez I, Cordido F, Ballesteros-Pomar MD. Efficacy of glutamine in the prevention of acute radiation enteritis: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2014;38:205-213. [DOI] [PubMed] [Google Scholar]
- 62. Abdulrhman M, Elbarbary NS, Amin DA, Ebrahim RS. Honey and a mixture of honey, beeswax, and olive oil-propolis extract in treatment of chemotherapy-induced oral mucositis: a randomized controlled pilot study. Pediatr Hematol Oncol. 2012;29:285-292. [DOI] [PubMed] [Google Scholar]
- 63. Bardy J, Molassiotis A, Ryder WD, et al. A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br J Oral Maxillofac Surg. 2012;50:221-226. [DOI] [PubMed] [Google Scholar]
- 64. Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in the management of radiation mucositis: a preliminary study. Support Care Cancer. 2003;11:242-248. [DOI] [PubMed] [Google Scholar]
- 65. Fogh SE, Deshmukh S, Berk LB, et al. A randomized phase 2 trial of prophylactic manuka honey for the reduction of chemoradiation therapy-induced esophagitis during the treatment of lung cancer: results of NRG Oncology RTOG 1012. Int J Radiat Oncol Biol Phys. 2017;97:786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Francis M, Williams S. Effectiveness of Indian turmeric powder with honey as complementary therapy on oral mucositis: a nursing perspective among cancer patients in Mysore. Nurs J India. 2014;105:258-260. [PubMed] [Google Scholar]
- 67. Hawley P, Hovan A, McGahan CE, Saunders D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support Care Cancer. 2014;22:751-761. [DOI] [PubMed] [Google Scholar]
- 68. Jayachandran S, Balaji N. Evaluating the effectiveness of topical application of natural honey and benzydamine hydrochloride in the management of radiation mucositis. Indian J Palliat Care. 2012;18:190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jayalekshmi JL, Lakshmi R, Mukerji A. Honey on oral mucositis: a randomized controlled trial. Gulf J Oncolog. 2016;1:30-37. [PubMed] [Google Scholar]
- 70. Khanal B, Baliga M, Uppal N. Effect of topical honey on limitation of radiation-induced oral mucositis: an intervention study. Int J Oral Maxillofac Surg. 2010;39:1181-1185. [DOI] [PubMed] [Google Scholar]
- 71. Bulut HK, Tüfekci FG. Honey prevents oral mocositis in children undergoing chemotherapy: a quasi-experimental study with a control group. Complement Ther Med. 2016;29:132-140. [DOI] [PubMed] [Google Scholar]
- 72. Maiti PK, Ray A, Mitra TN, Jana U, Bhattacharya J, Ganguly S. The effect of honey on mucositis induced by chemoradiation in head and neck cancer. J Indian Med Assoc. 2012;110:453-456. [PubMed] [Google Scholar]
- 73. Motallebnejad M, Akram S, Moghadamnia A, Moulana Z, Omidi S. The effect of topical application of pure honey on radiation-induced mucositis: a randomized clinical trial. J Contemp Dent Pract. 2008;9:40-47. [PubMed] [Google Scholar]
- 74. Raeessi MA, Raeessi N, Panahi Y, et al. “Coffee plus honey” versus “topical steroid” in the treatment of chemotherapy-induced oral mucositis: a randomised controlled trial. BMC Complement Altern Med. 2014;14:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rashad UM, Al-Gezawy SM, El-Gezawy E, Azzaz AN. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J Laryngol Otol. 2009;123:223-228. [DOI] [PubMed] [Google Scholar]
- 76. Samdariya S, Lewis S, Kauser H, Ahmed I, Kumar D. A randomized controlled trial evaluating the role of honey in reducing pain due to radiation induced mucositis in head and neck cancer patients. Indian J Palliat Care. 2015;21:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Allenidekania A. Randomized clinical trials on effect of oral care used mead on stage of mucositis in pediatric cancer: Indonesian experience. Paper presented at: MASCC/ISOO 2015 Annual Meeting on Support Care in Cancer; June 25-27, 2015; Copenhagen, Demark. [Google Scholar]
- 78. Alvi ZA, Mahmood A, Rasool S, et al. Role of honey in prevention of radiation induced mucositis in head and neck cancer. Pak Armed Forces Med J. 2013;63:379-383. [Google Scholar]
- 79. Al Jaouni SK, Al Muhayawi MS, Hussein A, et al. Effects of honey on oral mucositis among pediatric cancer patients undergoing chemo/radiotherapy treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid Based Complement Alternat Med. 2017;2017:5861024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amanat A, Ahmed A, Kazmi A, Aziz B. The effect of honey on radiation-induced oral mucositis in head and neck cancer patients. Indian J Palliat Care. 2017;23:317-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Berk LB, Deshmukh S, Fogh SE, et al. Randomized phase 2 trial of best supportive care: manuka honey liquid and manuka honey lozenges for prevention of radiation esophagitis during chemotherapy and radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(suppl 1):S5. [Google Scholar]
- 82. Charalambous A, Charalambous M, Raftopoulos V, Lambrinou E. The effectiveness of thymus honey on radiation induced mucositis and xerostomia for head and neck cancer patients: preliminary findings of a RCT. Support Care Cancer. 2013;21(suppl 1):S125. [Google Scholar]
- 83. Farneti A, Sanguineti G, Marucci L, et al. Double-blind randomized phase 3 study comparing a mixture of natural ingredients versus placebo in the prevention of acute mucositis during chemoradiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;93(3 suppl 1):S212-S213. [Google Scholar]
- 84. Mishra L, Nayak G. Effect of flavored (honey and tulsi) ice chips on reduction of oral mucositis among children receiving chemo therapy. Int J Pharm Sci Rev Res. 2017;43:25-28. [Google Scholar]
- 85. Parsons E, Begley A, Herst P. Manuka honey mouthwash does not affect oral mucositis in head and neck cancer patients in New Zealand. J Radiother Pract. 2012;11:249-256. [Google Scholar]
- 86. Rao S, Hegde SK, Rao P, et al. Honey mitigates radiation-induced oral mucositis in head and neck cancer patients without affecting the tumor response. Foods. 2017;6:E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bansal A, Ghoshal S, Yadav BK, Bahl A. Exploring the role of “glycerine plus honey” in delaying chemoradiation induced oral mucositis in head and neck cancers. Int J Cancer Ther Oncol. 2017;5:1-7. [Google Scholar]
- 88. Castro MG, Sanchez PX, Glasberg J, Horie LM, Waitzberg DL. Effects of probiotic in prevention of radiation-induced diarrhea. Clin Nutr Supp. 2009;4:72-73. [Google Scholar]
- 89. Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 2010;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Delia P, Sansotta G, Donato V, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13:912-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33:761-767. [DOI] [PubMed] [Google Scholar]
- 92. Giralt J, Regadera JP, Verges R, et al. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: results from multicenter, randomized, placebo-controlled nutritional trial. Int J Radiat Oncol Biol Phys. 2008;71:1213-1219. [DOI] [PubMed] [Google Scholar]
- 93. Henriksson R, Franzén L, Sandström K, Nordin A, Arevärn M, Grahn E. Effects of active addition of bacterial cultures in fermented milk to patients with chronic bowel discomfort following irradiation. Support Care Cancer. 1995;3:81-83. [DOI] [PubMed] [Google Scholar]
- 94. Lee JY, Chu SH, Jeon JY, et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis. 2014;46:1126-1132. [DOI] [PubMed] [Google Scholar]
- 95. Mego M, Chovanec J, Vochyanova-Andrezalova I, et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med. 2015;23:356-362. [DOI] [PubMed] [Google Scholar]
- 96. Österlund P, Ruotsalainen T, Korpela R, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Salminen E, Elomaa I, Minkkinen J, Vapaatalo H, Salminen S. Preservation of intestinal integrity during radiotherapy using live Lactobacillus acidophilus cultures. Clin Radiol. 1988;39:435-437. [DOI] [PubMed] [Google Scholar]
- 98. Scartoni D, Desideri I, Giacomelli I, et al. Nutritional supplement based on zinc, prebiotics, probiotics and vitamins to prevent radiation-related gastrointestinal disorders. Anticancer Res. 2015;35:5687-5692. [PubMed] [Google Scholar]
- 99. Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer. 2012;48:875-881. [DOI] [PubMed] [Google Scholar]
- 100. Sharma A, Tilak T, Bakhshi S, et al. Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem cell transplantation. ESMO Open. 2016;1:e000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Urbancsek H, Kazar T, Mezes I, Neumann K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. Eur J Gastroenterol Hepatol. 2001;13:391-396. [DOI] [PubMed] [Google Scholar]
- 102. Wada M, Nagata S, Saito M, et al. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support Care Cancer. 2010;18:751-759. [DOI] [PubMed] [Google Scholar]
- 103. Giammarco S, Chiusolo P, Di Giovanni A, et al. A pilot study on the efficacy of Lactobacillus brevis CD2 lozenges in preventing oral mucositis by high-dose chemotherapy with autologous hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk. 2016;16(suppl 2):S79. [Google Scholar]
- 104. Timko J. Probiotics as prevention of radiation-induced diarrhoea. J Radiother Pract. 2010;9:201-208. [Google Scholar]
- 105. Sharma A, Tvsvgk T, Raina V, et al. A pilot study of efficacy of Lactobacillus cd2 lozenges in preventing high-dose chemotherapy induced oral mucositis in patients undergoing haematopoietic stem cell transplantation. Blood. 2012;120:4500. [Google Scholar]
- 106. Sharma A, Chaudhary SP, Sreenivas V, Prakash S, Raina V, Shukla NK. A phase II/III, randomized, double-blind, placebo controlled study to investigate the efficacy of a probiotic VSL#3, on chemotherapyinduced diarrhoea in cancer patients receiving fluoropyrimidines and or irinotecan (interim analysis). Eur J Cancer. 2013;49(suppl 2):S273. [Google Scholar]
- 107. Leung HW, Chan AL. Glutamine in alleviation of radiation-induced severe oral mucositis: a meta-analysis. Nutr Cancer. 2016;68:734-742. [DOI] [PubMed] [Google Scholar]
- 108. Sayles C, Hickerson SC, Bhat RR, Hall J, Garey KW, Trivedi MV. Oral glutamine in preventing treatment-related mucositis in adult patients with cancer: a systematic review. Nutr Clin Pract. 2016;31:171-179. [DOI] [PubMed] [Google Scholar]
- 109. Cho HK, Jeong YM, Lee HS, Lee YJ, Hwang SH. Effects of honey on oral mucositis in patients with head and neck cancer: a meta-analysis. Laryngoscope. 2015;125:2085-2092. [DOI] [PubMed] [Google Scholar]
- 110. Xu JL, Xia R, Sun ZH, et al. Effects of honey use on the management of radio/chemotherapy-induced mucositis: a meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2016;45:1618-1625. [DOI] [PubMed] [Google Scholar]
- 111. Lee S. Mineral derivatives in alleviating oral mucositis during cancer therapy: a systematic review. PeerJ. 2015;3:e765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang YH, Yao N, Wei KK, et al. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta-analysis. Eur J Clin Nutr. 2016;70:1246-1253. [DOI] [PubMed] [Google Scholar]
- 113. Jolfaie NR, Mirzaie S, Ghiasvand R, Askari G, Miraghajani M. The effect of glutamine intake on complications of colorectal and colon cancer treatment: a systematic review. J Res Med Sci. 2015;20:910-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. van der Hulst RR, von Meyenfeldt MF, Deutz NE, Soeters PB. Glutamine extraction by the gut is reduced in depleted [corrected] patients with gastrointestinal cancer. Ann Surg. 1997;225:112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Veloso GGV, Franco OH, Ruiter R, et al. Baseline dietary glutamic acid intake and the risk of colorectal cancer: the Rotterdam study. Cancer. 2016;122:899-907. [DOI] [PubMed] [Google Scholar]
- 116. Fahr MJ, Kornbluth J, Blossom S, Schaeffer R, Klimberg VS, Harry M. Vars Research Award. Glutamine enhances immunoregulation of tumor growth. JPEN J Parenter Enteral Nutr. 1994;18:471-476. [DOI] [PubMed] [Google Scholar]
- 117. Kaufmann Y, Spring P, Klimberg VS. Oral glutamine prevents DMBA-induced mammary carcinogenesis via upregulation of glutathione production. Nutrition. 2008;24:462-469. [DOI] [PubMed] [Google Scholar]
- 118. Kozelsky TF, Meyers GE, Sloan JA, et al. ; North Central Cancer Treatment Group. Phase III double-blind study of glutamine versus placebo for the prevention of acute diarrhea in patients receiving pelvic radiation therapy. J Clin Oncol. 2003;21:1669-1674. [DOI] [PubMed] [Google Scholar]
- 119. Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010;(12):CD000978. [DOI] [PubMed] [Google Scholar]
- 120. Co JL, Mejia MB, Que JC, Dizon JM. Effectiveness of honey on radiation-induced oral mucositis, time to mucositis, weight loss, and treatment interruptions among patients with head and neck malignancies: a meta-analysis and systematic review of literature. Head Neck. 2016;38:1119-1128. [DOI] [PubMed] [Google Scholar]
- 121. Friend A, Rubagumya F, Cartledge P. Global Health Journal Club: is honey effective as a treatment for chemotherapy-induced mucositis in paediatric oncology patients? J Trop Pediatr. 2018;64:162-168. [DOI] [PubMed] [Google Scholar]
- 122. Sharquie KE, Najim RA, Al-Hayani RK, Al-Nuaimy AA, Maroof DM. The therapeutic and prophylactic role of oral zinc sulfate in management of recurrent aphthous stomatitis (ras) in comparison with dapsone. Saudi Med J. 2008;29:734-738. [PubMed] [Google Scholar]
- 123. Takano H, Momota Y, Kani K, et al. γ-Tocotrienol prevents 5-FU-induced reactive oxygen species production in human oral keratinocytes through the stabilization of 5-FU-induced activation of Nrf2. Int J Oncol. 2015;46:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wardill HR, Van Sebille YZA, Ciorba MA, Bowen JM. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Curr Opin Support Palliat Care. 2018;12:187-197. [DOI] [PubMed] [Google Scholar]
- 125. Beech N, Robinson S, Porceddu S, Batstone M. Dental management of patients irradiated for head and neck cancer. Aust Dent J. 2014;59:20-28. [DOI] [PubMed] [Google Scholar]
- 126. Atwa AD, AbuShahba RY, Mostafa M, Hashem MI. Effect of honey in preventing gingivitis and dental caries in patients undergoing orthodontic treatment. Saudi Dent J. 2014;26:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Santos-Silva AR, Rosa GB, Eduardo CP, Dias RB, Brandao TB. Increased risk for radiation-related caries in cancer patients using topical honey for the prevention of oral mucositis. Int J Oral Maxillofac Surg. 2011;40:1335-1336. [DOI] [PubMed] [Google Scholar]
- 128. Sela MO, Shapira L, Grizim I, et al. Effects of honey consumption on enamel microhardness in normal versus xerostomic patients. J Oral Rehabil. 1998;25:630-634. [DOI] [PubMed] [Google Scholar]
- 129. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929. [DOI] [PubMed] [Google Scholar]
- 130. Okawa T, Niibe H, Arai T, et al. Effect of LC9018 combined with radiation therapy on carcinoma of the uterine cervix. A phase III, multicenter, randomized, controlled study. Cancer. 1993;72:1949-1954. [DOI] [PubMed] [Google Scholar]
- 131. Ladas EJ, Bhatia M, Chen L, et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51:262-266. [DOI] [PubMed] [Google Scholar]
- 132. Koyama S, Fujita H, Shimosato T, et al. ; Yokohama Cooperative Study Group for Hematology (YACHT). Septicemia from Lactobacillus rhamnosus GG, from a probiotic enriched yogurt, in a patient with autologous stem cell transplantation [published online February 17, 2018]. Probiotics Antimicrob Proteins. doi: 10.1007/s12602-018-9399-6. [DOI] [PubMed] [Google Scholar]
- 133. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11-48. [DOI] [PubMed] [Google Scholar]
- 134. Shankar A, Roy S, Bhandari M, et al. Current trends in management of oral mucositis in cancer treatment. Asian Pac J Cancer Prev. 2017;18:2019-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Touchefeu Y, Montassier E, Nieman K, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409-421. [DOI] [PubMed] [Google Scholar]
- 136. Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Theis VS, Sripadam R, Ramani V, Lal S. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83. [DOI] [PubMed] [Google Scholar]
- 139. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014;5:15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Buglione M, Cavagnini R, Di Rosario F, et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: dental pathologies and osteoradionecrosis: Part 1: literature review and consensus statement. Crit Rev Oncol Hematol. 2016;97:131-142. [DOI] [PubMed] [Google Scholar]
- 141. Kountouras J, Zavos C. Recent advances in the management of radiation colitis. World J Gastroenterol. 2008;14:7289-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wardill HR, Bowen JM, Al-Dasooqi N, et al. Irinotecan disrupts tight junction proteins within the gut: implications for chemotherapy-induced gut toxicity. Cancer Biol Ther. 2014;15:236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nakao T, Kurita N, Komatsu M, et al. Irinotecan injures tight junction and causes bacterial translocation in rat. J Surg Res. 2012;173:341-347. [DOI] [PubMed] [Google Scholar]
- 144. Sandmeier D, Chaubert P, Bouzourene H. Irinotecan-induced colitis. Int J Surg Pathol. 2005;13:215-218. [DOI] [PubMed] [Google Scholar]
- 145. Soares PM, Mota JM, Gomes AS, et al. Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother Pharmacol. 2008;63:91-98. [DOI] [PubMed] [Google Scholar]
- 146. Song MK, Park MY, Sung MK. 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J Cancer Prev. 2013;18:322-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wei Z, Tan B, Cao S, et al. The influence of neoadjuvant chemotherapy on gastric cancer patients’ postoperative infectious complications: what is the negative role played by the intestinal barrier dysfunction? Oncotarget. 2017;8:43376-43388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Andreyev J, Ross P, Donnellan C, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 2014;15:e447-e460. [DOI] [PubMed] [Google Scholar]
- 149. Fan X, Sellin JH. Review article: small intestinal bacterial overgrowth, bile acid malabsorption and gluten intolerance as possible causes of chronic watery diarrhoea. Aliment Pharmacol Ther. 2009;29:1069-1077. [DOI] [PubMed] [Google Scholar]
- 150. Zhang C, Xu YG, Duan XN, et al. Role of granulocyte colony-stimulating factor in paclitaxel-induced intestinal barrier breakdown and bacterial translocation in rats. Chin Med J (Engl). 2011;124:1870-1875. [PubMed] [Google Scholar]
- 151. Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol. 2012;25:106-118. [PMC free article] [PubMed] [Google Scholar]
- 152. Nukatsuka M, Saito H, Sakamoto K, et al. Efficacy of combination chemotherapy using oral fluoropyrimidine S-1 with oxaliplatin (SOX) against colorectal cancer in vivo. Anticancer Res. 2012;32:2807-2812. [PubMed] [Google Scholar]
- 153. Bowen JM, Mayo BJ, Plews E, et al. Determining the mechanisms of lapatinib-induced diarrhoea using a rat model. Cancer Chemother Pharmacol. 2014;74:617-627. [DOI] [PubMed] [Google Scholar]
- 154. Youmba SB, Belmonte L, Galas L, et al. Methotrexate modulates tight junctions through NF-κB, MEK, and JNK pathways. J Pediatr Gastroenterol Nutr. 2012;54:463-470. [DOI] [PubMed] [Google Scholar]
- 155. Shahid F, Farooqui Z, Khan F. Cisplatin-induced gastrointestinal toxicity: an update on possible mechanisms and on available gastroprotective strategies. Eur J Pharmacol. 2018;827:49-57. [DOI] [PubMed] [Google Scholar]