Abstract
Objectives: Cancer-related fatigue and fear of recurrence (FOR) are the most common symptoms in cancer survivors and severely affect quality of life (QOL). This study aims to promote and evaluate the effectiveness of physical and psychological rehabilitation activities for cancer survivors. Methods: A longitudinal study with an interventional research design was conducted. A total of 80 participants were randomly assigned to experimental groups E1 (Qigong exercise [QE]) or E2 (stress management [SM]) or the control group. The E1 and E2 groups received QE and SM, respectively, as interventions once a week for 12 weeks, and effects were assessed. Cancer-related fatigue, FOR, QOL, and heart rate variability (HRV) were evaluated at baseline (T0), after 12 weeks (T1), and at the 3-month follow-up (T2). Results: QE and SM effectively strengthened the physical and psychological functions of cancer survivors at the T1 phase. Although differences in FOR and QOL were not statistically significant, the scores were decreased and increased, respectively. Although the effects during the T2 phase were not as significant as those during T1, the score progress was maintained. The effects on HRV were significantly different among the E1, E2, and control groups at T1, which shows that the performance of both experimental groups was better than that of the control group. Conclusions: Physical and psychological rehabilitation activities should be practiced periodically and should be led by professional staff. Long-term educational resources and care should also be provided. HRV can be used to efficiently monitor the status of the mind-body balance and is a more suitable index than questionnaires for physical and psychological function evaluation in cancer survivors.
Keywords: cancer survivors, cancer-related fatigue, fear of recurrence, quality of life, heart rate variability, Qigong exercise, stress management
Introduction
According to the Global Burden of Disease Report, cancer is the second leading cause of death worldwide. In the past 10 years, the incidence of cancer has increased by 33%. Thus, cancer poses a great threat to the health of humanity.1 Cancer-related fatigue (CRF) and fear of recurrence (FOR) are common seriously disturbing problems faced by cancer survivors.2-4 CRF is accompanied by lack of strength, depressed mood, and sleep disorders, which lead to reduced physical, social, cognitive, and vocational function. Moreover, CRF can easily be overlooked by medical staff, resulting in delayed treatment.5,6
Cancer recurrence is the most difficult stage during the course of the disease. FOR can lead to emotional distress and cognitive and behavioral disorders that can affect recovery. FOR does not decrease as the treatment ends or as the survival duration increases. Patients with a good prognosis or a low risk of recurrence remain fearful or worried, which severely affects their quality of life (QOL) and results in burdens to the individual, family, and society.7 Approximately one third of cancer survivors suffer from the long-term impact of CRF,8 and more than 70% are currently experiencing FOR.9,10
Cancer-related physical and physiological disorders can cause autonomic nervous system (ANS) dysfunction. Compared with healthy individuals, cancer survivors generally have lower heart rate variability (HRV).11-13 HRV can be used to monitor the regulatory functions and status of the human ANS.13 In clinical practice, HRV has not only been adopted as a diagnostic indicator of ANS dysfunction14 but has also been used as a marker for cancer-related distress and recovery status.12
Cancer survivors must tolerate pain caused by the disease and are also under greater psychological pressures than other people. The influence of the mind-body interaction leads to decreased physical functions and can even diminish the effects of medical treatments. In recent years, more than 75% of cancer survivors have undergone traditional cancer treatment in combination with complementary and alternative medicine to reduce the disturbing symptoms of cancer. This integrative treatment has yielded improved effects and increased QOL.15,16 Some studies have shown that 12 weeks of Qigong exercise (QE) intervention can reduce fatigue, anxiety, and depression and promote quality of sleep in breast and prostate cancer patients.17-19 Therefore, this study adopted “Guide health-preserving Qigong” as a QE intervention. QE, which is widely applied in complementary and alternative medicine, can increase blood circulation, improve lung and heart function, regulate homeostasis, moderate emotions, and allow self-healing.20-22
Some research has indicated that participation in stress management (SM) training courses may promote life value, self-efficacy, and QOL and may reduce the physical and mental symptoms of cancer patients.3,6,23 SM helps cancer survivors realize the value of life, seek self-development, and improve their interpersonal relationships.3,23,24 Hence, QE and SM can both promote the self-regulation functions of the body, improve HRV, and restore balance to the ANS.11,25-27 Furthermore, QE and SM can alleviate symptoms such as fatigue, anxiety, depression, and sleep disorders and can thus improve the QOL of cancer survivors.18,27-31 After regular treatments, providing cancer survivors with immediate and effective interventions during the recovery stage to assist in reducing distress due to the mental and physical symptoms of the disease is necessary and urgent. The results of this study can assist cancer survivors in maintaining recovery activities and restoring QOL and can provide a reference for care following treatment for cancer.
Methods
A longitudinal study with an interventional research design was conducted separately in 2 cancer centers in northern and southern Taiwan. The participants were randomly assigned to experimental groups E1 (QE) or E2 (SM) or to the control group. A 12-week intervention and a 3-month follow-up evaluation were employed in this study.
Participants and Study Design
Eligibility Criteria
The inclusion criteria for the participants were cancer survivors who (1) had completed regular treatment, including surgery, chemotherapy, radiation therapy, and target therapy, at least 1 month prior to the beginning of the study; (2) had experienced no cancer recurrence; and (3) had received hormone replacement therapy for breast cancer. The exclusion criteria were (1) participants with a diagnosis of stage IV cancer; and (2) participants with moderate or severe arrhythmia.
Sample Size
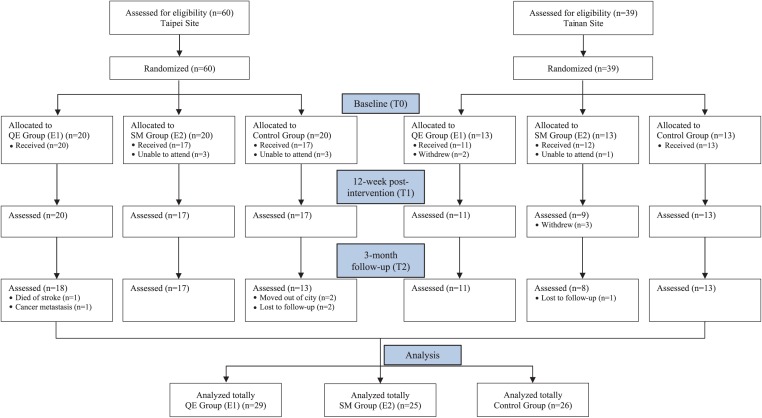
The medium effect sizes recommended in relevant studies before and after implementing intervention and during follow-up were adopted for the sample calculation.18 Repeated-measures analysis of variance (ANOVA) was performed using G*Power software. The effect size was 0.3, the α was 0.05, and the power was 0.8. The expected dropout rate was 25%. Thus, a minimum of 98 participants was required for this study. There were 99 qualified participants recruited from the 2 cancer centers. The participants were randomly assigned to the QE group (E1 = 33), the SM group (E2 = 33), or the control group (n = 33). However, only 80 participants completed the intervention. Twenty-nine of these were in the E1 group (n = 29), 25 were in the E2 group (n = 25), and 26 were in the control group (n = 26; Figure 1).
Figure 1.
CONSORT flow diagram of this study.
Study Design
Prior to the intervention, a 2-hour basic nutrition management course was provided to all participants. Subsequently, the E1 group received QE training, and the E2 group received SM training. The intervention was conducted once a week for 2 hours for a total of 12 weeks. No intervention was performed in the control group; the participants in that group maintained their daily routines. Data for overall CRF, FOR, the QOL questionnaire, and HRV were collected at 3 time points at 3-month intervals. The time points used to assess the potential improvement were baseline (T0), 12 weeks postintervention (T1), and 3-month follow-up (T2).
Outcome Measures
A pilot study was conducted by performing pretesting on 99 cancer patients in outpatient clinics to verify the reliability and validity of the CRF and FOR scales.
CRF Scale
The CRF scale was designed based on the diagnosis criteria of the ICD-10 (International Classification of Diseases, Tenth Revision) and included 15 items.32 A 5-point Likert-type scale was utilized for all items used to evaluate the degree of fatigue of cancer survivors within the past 2 weeks. Higher scores indicated severe CRF. Exploratory factor analysis was adopted. Factor loadings were evaluated using the maximum likelihood method; the Kaiser-Meyer-Olkin value was 0.839. Bartlett’s test of sphericity yielded χ2 = 541.686 (P < .001). Finally, principal component analysis with axis rotation was applied to examine the construct validity of the scale. Factors with eigenvalues >1 were extracted, and only 10 questions remained after multiple examinations. The 10 questions can be categorized into 3 domains: lifestyle function, mind-body function, and sleep condition. The accumulated variation was 74.998%. The overall Cronbach’s α of the internal consistency reliability of the scale was 0.878, suggesting very good reliability and effectiveness. Moreover, within each domain, the correlation coefficient of each item was greater than 0.500 after removing the question, indicating good reliability and effectiveness of the questionnaire as a measurement tool for evaluating CRF (Table 1).
Table 1.
A Pilot Study for Factor Analysis of Cancer-Related Fatigue and Fear of Recurrence Scales.
| Cancer-Related Fatigue (CRF) | Factor | ||
|---|---|---|---|
| 1 |
2 |
3 |
|
| Lifestyle Function | Mind-Body Function | Sleep Condition | |
| Difficulty completing daily tasks attributed to feeling fatigued | 0.855 | ||
| Significant diminished energy in daily life or social of functioning | 0.811 | ||
| Decreased motivation or interest to engage in usual activities | 0.768 | ||
| Perceived need to struggle to overcome inactivity | 0.750 | ||
| Perceived problems with short-term memory | 0.865 | ||
| Diminished concentration or attention | 0.845 | ||
| Complaints of generalized weakness or limb heaviness | 0.750 | ||
| Post-exertional malaise lasting several hours | 0.667 | ||
| Hypersomnia | 0.893 | ||
| Experience of sleep as unrefreshing or nonrestorative | 0.796 | ||
| Cronbach’s α: | |||
| Factor 1 = 0.873 | KMO = 0.839, χ2 = 541.686*** | ||
| Factor 2 = 0.864 | Total variance explained 87.8% | ||
| Factor 3 = 0.715 | |||
| Fear of Recurrence (FOR) | Factor | ||
| 1 | 2 | 3 | |
| Psychological Distress | Lifestyle Function | Triggers | |
| Sadness, discouragement, or disappointment | 0.858 | ||
| Worry, fear, or anxiety | 0.833 | ||
| Helplessness or resignation | 0.780 | ||
| Frustration, anger, or outrage | 0.779 | ||
| I am worried or anxious about the FOR | 0.774 | ||
| I am afraid of a cancer recurrence | 0.768 | ||
| When I think about FOR, other unpleasant thoughts or images come to mind (death, suffering consequences for my family) | 0.748 | ||
| My social or leisure activities (eg, outings, sports, travel) | 0.880 | ||
| My work or everyday activities | 0.866 | ||
| My relationship with my partner, my family, or those close to me | 0.836 | ||
| My quality of life in general | 0.762 | ||
| My ability to make future plans or set life goals | 0.690 | ||
| Seeing or hearing someone who is ill | 0.859 | ||
| Going to funeral or reading the obituary section of the paper | 0.768 | ||
| Television shows or newspaper articles about cancer or illness | 0.684 | ||
| Cronbach’s α: | |||
| Factor 1 = 0.956 | KMO = 0.915, χ2 = 1254.630*** | ||
| Factor 2 = 0.936 | Total variance explained 95.4% | ||
| Factor 3 = 0.794 | |||
Abbreviations: KMO, Kaiser-Meyer-Olkin.
P < .001
FOR Scale
The FOR scale was designed based on the Chinese edition of the Fear of Cancer Recurrence Inventory (FCRI) and included 42 items. A 5-point Likert-type scale was utilized for all items used to evaluate the degree of fear of cancer recurrence among the cancer survivors during the past month; higher scores indicated more severe FOR.33 Exploratory factor analysis and verification were performed. The Kaiser-Meyer-Olkin value was 0.915, and Bartlett’s test of sphericity yielded χ2 = 1254.630 (P < .001). Only 15 questions were retained, and they were categorized into 3 domains: psychological distress, lifestyle function, and triggers. The accumulated variation was 79.073%. The overall Cronbach’s α of the internal consistency reliability of the scale was 0.954, suggesting very good reliability and effectiveness. Moreover, within each area, the correlation coefficient of each item was greater than 0.500 after removing the question. The results indicate that the questionnaire was reliable and effective as a measurement tool for evaluating FOR (Table 1).
QOL Scale
The QOL scale was designed based on the fourth Chinese edition of the Functional Assessment of Cancer Therapy-General (FACT-G) and included 27 items. The original English version contained 28 questions. To improve the internal consistency and stability of the theme, the construct validity factor analysis and verification were performed again. A total of 4 component themes were selected, and the scale was finally reduced to 27 questions, becoming the fourth edition of the Chinese version of the FACT-G scale. A 5-point Likert-type scale was utilized for all items used to examine the level of QOL within the past 7 days. Four domains were included in the scale. The questions of physical and emotional well-being were reverse-scored. Higher scores indicated poorer QOL. For social/family and functional well-being, higher scores indicated higher life functions and better QOL.34
HRV Indicators
The CheckMyHeart Handel HRV, which has received safety certification from the Food and Drug Administration of the United States and the CE marking of the European Economic Area, was adapted for HRV examination. Participants were asked to avoid foods with intense flavors, smoking, and caffeinated and alcoholic beverages the day before the test.35 The room temperature was maintained at 23°C to 25°C. The participants were seated or lying prone during a 5-minute Lead II electrocardiography recording. The recorded data were converted to HRV indicators using time domain and frequency domain analysis methods as described below:
Standard deviation of normal R-R intervals (SDNN) represents the overall activity of HRV in the time domain; a higher score indicates higher HRV overall activity.
Total power (TP): the clinical meaning of TP in the frequency domain is similar to that of SDNN in the time domain; a lower score indicates a higher level of fatigue, a lack of energy, or a lack of strength.
High frequency (HF) represents the parasympathetic nervous system activity in the frequency domain; a lower score indicates that the subject is experiencing anxiety or stress, whereas a higher score indicates relaxation.
Interventions
Mind-body support and social support are indispensable components of education and care for cancer patients. The Memorial Sloan-Kettering Cancer Center and the MD Anderson Cancer Center in the United States actively promote mind-body support treatment, and such treatment has become part of the traditional cancer treatment method.36,37 Moreover, provision of balanced nutrition and supplements is considered critical in the care of cancer survivors. Therefore, CRF, FOR, QOL, and HRV were monitored to evaluate the effects of physical and psychological activities as interventions.
Nutrition Management
Two hours of nutrition management education were provided to the 3 groups of participants by a certified dietitian. Accurate dietary principles were introduced, and dietary habits were adjusted to achieve a balanced diet and to supply adequate nutrition.
Qigong Exercise
A certified martial arts specialist of the International Wushu Federation taught the method of 12-style QE, including standing position, meditation, and leg massage. The exercises allow enhancement of heart and lung function, facilitate blood and energy circulation, regulate physical functions, allow deep relaxation of the body, and promote self-healing capabilities. Handouts and demonstration DVDs were provided to encourage the participants to practice at home and to improve their skills.
Stress management
A rehabilitation and mental health counseling professor led the patients in the SM group. The program involved conversation and sharing of cognitive behavior modification and mindfulness decompression for a total of 90 minutes. During the remaining 20 minutes, body relaxation and meditation techniques that patients could use to relieve mental and emotional stress were demonstrated. The cognitive behavior modification process was divided into 3 phases as follows38:
The first phase was “self-observation.” During this phase, the patients learned how to perceive their behavior, listen to their inner voice, and develop a new viewpoint on their problems.
The second phase was “starting a new internal dialogue.” In this phase, the patients learned to change their inappropriate inner dialogue and were helped restructure their cognition.
The third phase was “learning new skills.” In this phase, the patients were taught effective response skills, how to use these skills to deal with pressure in life situations, and how to maintain these skills.
Data Analyses
The data were analyzed using SPSS 22.0 for Windows (SPSS Inc, Chicago, IL). Chi-square tests were used for comparisons of baseline categorical data among E1, E2, and the control group. The paired sample t test was used to compare the improvement effects of single interventions, and 1-way ANOVA was used to analyze the differences in effects among groups. The AR (1) model in the generalized estimating equation was determined to be the best model by QIC (quasi-information criterion) value and was implemented to evaluate correlations among the 3 time points as a basis for assessing improvement over time.
Results
Demographic Characteristics
Among the 80 study participants, there were more females (n = 70) than males (n = 10); the age of the participants ranged from 33 to 75 years, and 47.5% were between 50 and 59 years of age. The cancer survival period ranged from 1.1 to 14.2 years, and 30.1% of the participants had survived for more than 5 years. The most frequent type of cancer among the participants was breast cancer (65%), and most cancers were at stage I or II (72.6%) at diagnosis. A total of 56.3% of the participants felt fatigue, and approximately 25% experienced anxiety or depression. According to baseline (T0) data, almost all participants suffered from CRF (100%) and FOR (98.7%). In total, 77.5% had moderate or severe levels of fatigue, 47.5% suffered from moderate to severe emotional distress, and 7.5% experienced severe fatigue along with a tendency toward emotional distress. With the exception of the survival period, no significant differences in demographic characteristics were found among the 3 groups (Table 2).
Table 2.
Demographic Characteristics of Participants at Baseline.
| Frequency Distribution (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | E1 (n = 29) | E2 (n = 25) | Control (n = 26) | Total (N = 80) | χ2 Test | P |
| Gender | 0.705 | .703 | ||||
| Male | 4 (13.8%) | 2 (8.0%) | 4 (15.4%) | 10 (12.5%) | ||
| Female | 25 (86.2%) | 23 (92.0%) | 22 (84.6%) | 70 (87.5%) | ||
| Age (years) | 3.848 | .871 | ||||
| 30-39 | 3 (10.3%) | 1 (4.0%) | 1 (3.8%) | 5 (6.3%) | ||
| 40-49 | 7 (24.1%) | 6 (24.0%) | 5 (19.2%) | 18 (22.5%) | ||
| 50-59 | 11 (37.9%) | 12 (48.0%) | 15 (57.7%) | 38 (47.5%) | ||
| 60-69 | 5 (17.2%) | 5 (20.0%) | 3 (11.5%) | 13 (16.3%) | ||
| 70-75 | 3 (10.3%) | 1 (4.0%) | 2 (7.7%) | 6 (7.5%) | ||
| Marital status | 7.013 | .535 | ||||
| Single | 4 (13.8%) | 3 (12.0%) | 5 (19.2%) | 12 (15.0%) | ||
| Married | 23 (79.3%) | 19 (76.0%) | 15 (57.7%) | 57 (71.3%) | ||
| Separation | 1 (3.4%) | 1 (4.0%) | 1 (3.8%) | 3 (3.8%) | ||
| Divorced | 0 (0.0%) | 1 (4.0%) | 4 (15.4%) | 5 (6.3%) | ||
| Widowed | 1 (3.4%) | 1 (4.0%) | 1 (3.8%) | 3 (3.8%) | ||
| Education | 11.041 | .199 | ||||
| Below high school | 3 (10.3%) | 1 (4.0%) | 6 (23.0%) | 10 (12.5%) | ||
| High school | 6 (20.7%) | 9 (36.0%) | 7 (26.9%) | 22 (27.5%) | ||
| College and higher | 20 (68.9%) | 15 (60.0%) | 13 (50.0%) | 48 (60.0%) | ||
| Survival period | 12.402 | .015 | ||||
| Within 5 years | 25 (86.2%) | 14 (56.0%) | 17 (65.4%) | 56 (70.0%) | ||
| 5-10 years | 2 (6.9%) | 11 (44.0%) | 6 (23.1%) | 19 (23.8%) | ||
| >10 years | 2 (6.9%) | 0 (0.0%) | 3 (11.5%) | 5 (6.3%) | ||
| Cancer type | 4.149 | .940 | ||||
| Colorectal cancer | 2 (6.9%) | 2 (8.0%) | 3 (11.5%) | 7 (8.8%) | ||
| Lung cancer | 1 (3.4%) | 1 (4.0%) | 1 (3.8%) | 3 (3.8%) | ||
| Malignant lymphoma | 1 (3.4%) | 3 (8.0%) | 0 (0.0%) | 3 (3.8%) | ||
| Breast cancer | 18 (62.1%) | 16 (64.0%) | 18 (69.2%) | 52 (65.0%) | ||
| Gynecological cancer | 2 (6.9%) | 2 (8.0%) | 2 (7.7%) | 6 (7.5%) | ||
| Other types of cancera | 5 (17.2%) | 2 (8.0%) | 2 (7.7%) | 9 (11.3%) | ||
| Cancer staging | 5.097 | 0.531 | ||||
| None stageb | 1 (3.4%) | 1 (4.0%) | 1 (3.8%) | 3 (3.7%) | ||
| I | 7 (24.1%) | 11 (44.0%) | 11 (42.3%) | 29 (36.3%) | ||
| II | 15 (51.7%) | 7 (28.0%) | 7 (26.9%) | 29 (36.3%) | ||
| III | 6 (20.7%) | 6 (24.0%) | 7 (26.9%) | 19 (23.8%) | ||
| Sense of fatigue | 0.622 | .733 | ||||
| No | 12 (41.4%) | 10 (40.0%) | 13 (50.0%) | 35 (43.8%) | ||
| Yes | 17 (58.6%) | 15 (60.0%) | 13 (50.0%) | 45 (56.3%) | ||
| Sense of anxiety | 0.010 | .995 | ||||
| No | 22 (75.9%) | 19 (76.0%) | 20 (76.9%) | 61 (76.3%) | ||
| Yes | 7 (24.1%) | 6 (24.0%) | 6 (23.1%) | 19 (23.8%) | ||
| Sense of depression | 1.126 | .569 | ||||
| No | 22 (75.9%) | 17 (68.0%) | 21 (80.8%) | 60 (75.0%) | ||
| Yes | 7 (24.1%) | 8 (32.0%) | 5 (19.2%) | 20 (25.0%) | ||
| Level of CRF at baseline | 4.188 | .381 | ||||
| None (score of 1-6) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Mild (score of 7-20) | 5 (17.2%) | 4 (16.0%) | 9 (34.6%) | 18 (22.5%) | ||
| Moderate (score of 21-35) | 21 (72.4%) | 20 (80.0%) | 15 (57.7%) | 56 (70.0%) | ||
| Severe (score of 36-50) | 3 (10.3%) | 1 (4.0%) | 2 (7.7%) | 6 (7.5%) | ||
| Level of FOR at baseline | 4.259 | .642 | ||||
| None (score of 1-15) | 1 (3.4%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) | ||
| Mild (score of 16-35) | 15 (51.7%) | 11 (44.0%) | 15 (57.7%) | 41 (51.2%) | ||
| Moderate (score of 36-55) | 10 (34.5%) | 13 (52.0%) | 9 (34.6%) | 32 (40.0%) | ||
| Severe (score of 56-75) | 3 (10.3%) | 1 (4.0%) | 2 (7.7%) | 6 (7.5%) | ||
Abbreviations: E1, Qigong exercise group; E2, stress management group; CRF, cancer-related fatigue; FOR, fear of recurrence.
Other types of cancer including gastric cancer, liver cancer, leukemia, nasopharyngeal carcinoma, and peritoneal mesothelioma.
None stage including leukemia and peritoneal mesothelioma.
Outcome Evaluations
Improvement Effects of Single Interventions
The results of paired sample t tests for single interventions are shown in Table 3. After 12 weeks of both QE and SM training (T1 vs T0), CRF was significantly reduced, whereas significant increases were observed for SDNN, TP, and HF. After a 3-month follow-up (T2 vs T0), continuous improvement was observed for CRF (P < .05), but significant decreases (T2 vs T1) were observed for SDNN and TP. Nevertheless, nonsignificant trends toward improvement were observed in FOR and QOL. Although FOR and QOL were not significantly decreased (P > .05), the FOR score was decreased, and the QOL score gradually increased.
Table 3.
The Paired Sample t Test and 1-Way ANOVA of 3 Groups (N = 80).
| Variables | Improvement Effects of Single Interventionsa | Between-Group Effectsb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 vs T0 |
T2 vs T0 |
T2 vsT1 |
T1-T0 |
T2-T0 |
|||||||||
| Mean (SD) | t | P | Mean (SD) | t | P | Mean (SD) | t | P | F | P | F | P | |
| CRF | 1.850 | .164 | 1.416 | .249 | |||||||||
| E1 | −0.213 (0.499) | −2.307** | .029 | −0.213 (0.550) | −2.093** | .046 | 0.000 (0.376) | 0.000 | 1.000 | ||||
| E2 | −0.228 (0.514) | −2.216** | .036 | −0.212 (0.506) | −2.095** | .047 | 0.016 (0.484) | 0.165 | .870 | ||||
| Control | 0.026 (0.602) | 0.228 | .822 | 0.015 (0.640) | 0.122 | .903 | −0.011 (0.554) | −0.106 | .916 | ||||
| FOR | 0.957 | .389 | 0.750 | .476 | |||||||||
| E1 | −0.147 (0.687) | −1.153 | .259 | −0.211 (0.751) | −1.516 | .141 | −0.064 (0.437) | −0.793 | .453 | ||||
| E2 | −0.173 (0.495) | −1.750 | .093 | −0.090 (0.619) | −0.732 | .471 | 0.082 (0.422) | 0.979 | .337 | ||||
| Control | 0.028 (0.493) | 0.292 | .773 | −0.007 (0.436) | −0.090 | .929 | −0.035 (0.439) | −0.417 | .680 | ||||
| QOL | 1.092 | .341 | 0.116 | .890 | |||||||||
| E1 | 0.168 (0.587) | 1.545 | .134 | 0.026 (0.674) | 0.212 | .834 | −0.141 (0.476) | −1.603 | .120 | ||||
| E2 | 0.090 (0.386) | 1.173 | .252 | 0.078 (0.370) | 1.057 | .301 | −0.012 (0.337) | −0.182 | .857 | ||||
| Control | −0.017 (0.377) | −0.240 | .813 | 0.011 (0.431) | 0.140 | .890 | 0.029 (0.284) | 0.529 | .601 | ||||
| SDNN | 9.332*** | <.001 | 0.272 | .763 | |||||||||
| E1 | 5.267 (5.657) | 5.014*** | <.001 | 1.178 (8.217) | 0.772 | .446 | −4.088 (10.036) | −2.194** | .037 | ||||
| E2 | 8.390 (5.274) | 7.954*** | <.001 | 2.369 (11.085) | 1.069 | .296 | −6.021 (10.922) | −2.756** | .011 | ||||
| Control | −0.881 (11.230) | −0.400 | .692 | 0.140 (12.851) | 0.056 | .956 | 1.021 (6.737) | 0.773 | .447 | ||||
| TP | 7.307** | .001 | 2.129 | .126 | |||||||||
| E1 | 203.008 (317.483) | 3.443** | .002 | 47.782 (252.675) | 1.018 | .317 | −155.225 (403.743) | −2.070** | .048 | ||||
| E2 | 255.459 (259.103) | 4.930*** | <.001 | 55.154 (369.998) | 0.745 | .463 | −200.304 (380.110) | −2.635** | .015 | ||||
| Control | −72.903 (404.670) | −0.919 | .367 | −9.673 (479.527) | −0.103 | .919 | 63.230 (237.924) | 1.355 | .188 | ||||
| HF | 0.943 | .394 | 0.280 | .756 | |||||||||
| E1 | 35.358 (59.786) | 3.185** | .004 | 2.733 (77.064) | 0.191 | .850 | −32.625 (89.502) | −1.963 | .060 | ||||
| E2 | 34.447 (77.034) | 2.236** | .035 | 18.478 (144.497) | 0.639 | .529 | −15.969 (119.591) | −0.668 | .511 | ||||
| Control | 5.365 (124.007) | 0.221 | .827 | 29.711 (170.228) | 0.890 | .382 | 24.346 (106.030) | 1.171 | .253 | ||||
Abbreviations: ANOVA, analysis of variance; T0, baseline; T1, 12-weeks postintervention; T2, 3-month follow-up; CRF, cancer-related fatigue; E1, Qigong exercise group; E2, stress management group; FOR, fear of recurrence; QOL, quality of life; SDNN, standard deviation of normal R-R intervals, the index of overall autonomic nervous system activity; TP, total power, the index of overall activity heart rate variability; HF, high frequency, the index of parasympathetic nerve activity.
Paired sample t test.
One-way ANOVA.
P < .01, ***P < .001 (2-tailed test).
The control group received only nutrition guidance. After 12 weeks (T1 vs T0), the control group participants had increased CRF, FOR and decreased QOL, SDNN, and TP. Only HF showed a slight increase (t = 0.221, P = .827). After 3 months of follow-up (T2 vs T0), FOR, QOL, SDNN, and HF showed gradual improvement; however, the changes were not significant (P > .05), and the participants remained affected by CRF (t = 0.122, P = .903) and TP (t = −0.103, P = .919). This result indicates that an appropriate intervention was crucial to managing participants’ fatigue, emotional distress, and functional decline in ANS.
Between-Group Effects
One-way ANOVA between T1 versus T0 and T2 versus T0 showed that there were significant differences between E1, E2, and the control group at T1 in SDNN (T1-T0: F = 9.332, P < .001) and TP (T1-T0: F = 7.307, P = .001; Table 3).
Improvement Over Time
For generalized estimating equation, T0 was specified as the baseline category, and the control group was considered the reference group. At T1, CRF (T1: χ2 = 5.344, P = .021), SDNN (T1: χ2 = 19.682, P < .001), TP (T1: χ2 = 10.616, P = .001), and HF (T1: χ2 = 6.391, P = .011) all showed significant improvement (P < .05). CRF (T2: χ2 = 4.777, P = .029) still showed significant improvement at T2 (P < .05; Table 4).
Table 4.
Time Effects of 3 Groups From AR1 Model of GEE Analysis (N = 80).
| DV | IV | Estimate β | SE | Wald χ2 | P |
|---|---|---|---|---|---|
| CRF | Intercept | 2.402 | 0.138 | 303.075*** | <.001 |
| E1 vs control group | 0.173 | 0.182 | 0.903 | .342 | |
| E2 vs control group | 0.259 | 0.161 | 2.575 | .109 | |
| T1-T0 | −0.140 | 0.060 | 5.344* | .021 | |
| T2-T0 | −0.139 | 0.635 | 4.777* | .029 | |
| FOR | Intercept | 2.422 | 0.143 | 286.048*** | <.001 |
| E1 vs control group | −0.094 | 0.185 | 0.258 | .611 | |
| E2 vs control group | 0.119 | 0.196 | 0.364 | .546 | |
| T1-T0 | −0.098 | 0.063 | 2.393 | .122 | |
| T2-T0 | −0.108 | 0.068 | 2.439 | .118 | |
| QOL | Intercept | 3.953 | 0.125 | 997.134*** | <.001 |
| E1 vs control group | −0.014 | 0.153 | 0.008 | .928 | |
| E2 vs control group | −0.062 | 0.156 | 0.158 | .691 | |
| T1-T0 | 0.084 | 0.052 | 2.595 | .107 | |
| T2-T0 | 0.038 | 0.056 | 0.448 | .503 | |
| SDNN | Intercept | 29.883 | 2.104 | 201.597*** | <.001 |
| E1 vs control group | −4.446 | 2.765 | 2.585 | .108 | |
| E2 vs control group | −3.897 | 2.393 | 2.651 | .103 | |
| T1-T0 | 4.245 | 0.956 | 19.682*** | <.001 | |
| T2-T0 | 1.213 | 1.188 | 1.043 | .307 | |
| TP | Intercept | 492.471 | 72.249 | 46.462*** | <.001 |
| E1 vs control group | −105.858 | 91.606 | 1.335 | .248 | |
| E2 vs control group | −98.154 | 78.120 | 1.579 | .209 | |
| T1-T0 | 129.728 | 39.815 | 10.616** | .001 | |
| T2-T0 | 31.413 | 41.245 | 0.580 | .446 | |
| HF | Intercept | 104.976 | 20.797 | 25.479*** | <.001 |
| E1 vs control group | −37.619 | 26.734 | 1.980 | .159 | |
| E2 vs control group | −19.075 | 27.803 | 0.471 | .493 | |
| T1-T0 | 25.326 | 10.018 | 6.391* | .011 | |
| T2-T0 | 16.421 | 14.800 | 1.231 | .267 |
Abbreviations: AR, autoregressive; GEE, generalized estimating equation; DV, dependent variable; IV, independent variable; SE, standard error; CRF, cancer-related fatigue; E1, Qigong exercise group; E2, stress management group; T0, baseline; T1, 12-week postintervention; T2, 3-month follow-up; FOR, fear of recurrence; QOL, quality of life; SDNN, standard deviation of normal R-R intervals, the index of overall autonomic nervous system activity; TP, total power, the index of overall activity heart rate variability; HF, high frequency, the index of parasympathetic nerve activity.
P < .05, **P < .01, ***P < .001 (2-tailed test).
Discussion
The results of this study show that the participants suffered from CRF (100%) and that 77.5% of them experienced moderate to severe fatigue. These findings suggest that the severity of CRF has been underestimated. Cancer survivors are often unaware of this condition; thus, its severity can be overlooked, resulting in delayed treatment. Our findings are consistent with those reported in related studies.5,39
Moreover, 98.7% of the participants displayed emotional distress symptoms due to FOR. Among these participants, 47.5% experienced moderate to severe fear, and 7.5% experienced severe fear. These findings are consistent with the results of related studies in which it was reported that 49% and 7% of cancer survivors suffered from moderate to severe and severe emotional distress, respectively.4 A previous study reported that approximately 50% of cancer survivors experienced a lack of psychological support and care and urgently needed the support provided by social groups and activities.7 Therefore, active participation by the medical staff in providing care and intervention support is critical.
Regarding CRF, QE and SM yielded statistically significant improvements at T1 and T2. QE restores mind-body balance by utilizing breathing exercises, repeated physical movements, and meditation. Symptoms of fatigue can be effectively alleviated, and the improvements can be maintained for a minimum of 3 months.40 Intervention can be achieved by psychological education focused on emotional support, response strategies, and relaxation skills. Moreover, cognitive behaviors were reconstructed, which can actively relieve fatigue and emotional distress and improve problem-solving skills to promote self-efficacy and goal achievement.28 These findings are consistent with the results obtained in our study.
Regarding FOR, a nonsignificant trend toward improvement was noted, but the scores were decreased at T1 and T2, indicating that emotional distress was slightly decreased. Cancer-related emotional distress is different from the distress experienced by healthy individuals. Such distress increases as the cancer survivor ages, as the disease progresses, or as new health issues develop. Moreover, the symptoms of distress can be persistent.41 Symptoms tend to be more severe among females, individuals with lower socioeconomic status, and younger individuals.7,42 Emotional distress has not been correlated with cancer prognosis, survival time, or cancer type. Correlated factors include the level of education received, whether recurrence or metastasis is experienced, and satisfaction with social support.43 Cancer survivors realize the uncertainties in disease progress, health, and QOL. As found in our study, FOR does not decrease with longer survival time or with longer interventions. These findings are consistent with the results of related studies.7,43 Therefore, continuous guidance on physical and psychological activities, nutrition management education, and periodic care should be provided. The fact that 87.5% of the participants in our study were female, a population in which FOR is relatively more severe, might explain the diminished and insignificant intervention effects observed.
Regarding QOL, a nonsignificant trend toward improvement was noted, but the scores were increased at T1 and T2, indicating that QOL was slightly improved. Due to the severe impact of the disease on physical, emotional, and social functions, the QOL of cancer survivors decreases over time. Moreover, the decrease in QOL is more apparent among patients less than 50 years of age. Therefore, long-term care and interventions are necessary.44 Approximately one third of the participants in our study were less than 50 years of age; this could be the main factor contributing to the insignificant effect of interventions on QOL.
Qigong and SM interventions yielded significant differences in HRV at T1 versus T2. They both resulted in improvements in SDNN and TP and also induced parasympathetic nervous system activation (HF). These intervention activities allow the mind-body to enter a relaxed state and promote the regulation of ANS function, resulting in stabilized emotions. Monitoring of mental and physical states through HRV reveals the benefits and effects of Qigong and SM on physical and psychological health in real time. These results are supported by related studies.11,20,21,29,45,46
The results of our study indicated that QE and SM both enhance the physical and psychological functions of cancer survivors. Furthermore, these activities relieve fatigue and ANS functional disorders.
Thus, when facing the uncertainty of cancer progression, patients can respond with a more stable mental status, a positive attitude, and increased confidence. Adjustments in diet and nutritional supplements are also indispensable to cancer survivors. Elimination of bad dietary habits and improvement in nutrition can allow the rapid restoration of physical functions and enhance immunity. Furthermore, facing disease and life with a positive attitude can improve symptoms and physical and mental energy levels.
This study has some limitations. Regarding effects over time, the effects during the follow-up phase (T2) were not as significant as the immediate effects at T1; however, the improvements were steadily maintained. Although demonstration DVDs and CDs were provided to the participants to encourage home practice, lack of persistence can result from lack of supervision and peer encouragement. We suggest that cancer survivors actively participate in long-term physical and psychological rehabilitation activities. Continuous and periodic involvement in practices led by professional coaches at a minimum frequency of once a week is recommended. Long-term educational resources and care should be provided to maintain long-term improvements. These suggestions are provided herein as a reference for clinical practice. Moreover, significant differences between groups were not observed. Such results may be attributed to the different nature of the interventions; QE emphasizes training in physical strength, whereas SM focuses on mental support. These interventions both yielded improvements. Hence, care should be taken to cultivate both mental and physical health, as they complement each other. In future studies, a crossover design is recommended to permit accurate evaluation of the significant effects of the interventions. Moreover, such a design will allow the survivors to receive both physical and psychological support, as cancer patients can benefit from both activities.
Conclusion
In our study, questionnaires and HRV were employed to evaluate the condition of cancer survivors during recovery. The results suggest that QE and SM affect HRV in addition to CRF, which is an interesting finding on its own. Although the questionnaires employed were reliable and effective, the responses were based on subjective opinions and personal feelings and may not truly reflect the mental and physical health of cancer survivors. In contrast, HRV provides effective monitoring of heart and lung function and the status of ANS balance. Because the progress of cancer patients can be monitored easily and rapidly with HRV, this approach can serve as an indicator for evaluating the mind-body state after treatment and can assist medical providers in providing comprehensive care. Providing scientific support for the effectiveness of HRV is the focus of our future study.
Acknowledgments
We thank the Formosa Cancer Foundation and Chi Mei Hospital, Liuying, for providing intervention sites for the 12-week Qigong and stress management training courses.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Formosa Cancer Foundation (Grant No. KOAA0501C65) and Chi Mei Hospital Liuying (Grant No. CLFHR10601).
Ethical Approval: This study was reviewed by the institutional review boards (IRBs) of 2 cancer centers (IRB Nos. N201602090 and 10205-L03).
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kessels E, Husson O, van der Feltz-Cornelis CM. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018;14:479-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johns SA, Brown LF, Beck-Coon K, et al. Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Support Care Cancer. 2016;24:4085-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322. [DOI] [PubMed] [Google Scholar]
- 5. Jones JM, Olson K, Catton P, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10:51-61. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Q, Li F, Zhang H, Yu X, Cong Y. Effects of nurse-led home-based exercise and cognitive behavioral therapy on reducing cancer-related fatigue in patients with ovarian cancer during and after chemotherapy: a randomized controlled trial. Int J Nurs Stud. 2018;78:52-60. doi: 10.1016/j.ijnurstu.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 7. Butow PN, Fardell JE, Smith AB. Fear of cancer recurrence: an overview and Australian perspective. Cancer Forum. 2015;39:95-100. [Google Scholar]
- 8. Minton O, Berger A, Barsevick A, et al. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119(suppl 11):2124-2130. [DOI] [PubMed] [Google Scholar]
- 9. Butow PN, Bell ML, Smith AB, et al. Conquer fear: protocol of a randomised controlled trial of a psychological intervention to reduce fear of cancer recurrence. BMC Cancer. 2013;13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Wal M, Servaes P, Berry R, Thewes B, Prins J. Cognitive behavior therapy for fear of cancer recurrence: a case study. J Clin Psychol Med Settings. 2018;25:390-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fong SS, Wong JY, Chung LM, et al. Changes in heart-rate variability of survivors of nasopharyngeal cancer during Tai Chi Qigong practice. J Phys Ther Sci. 2015;27:1577-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park H, Oh S, Noh Y, Kim JY, Kim JH. Heart rate variability as a marker of distress and recovery: the effect of brief supportive expressive group therapy with mindfulness in cancer patients. Integr Cancer Ther. 2018;17:825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu S, Lou J, Zhang Y, Chen P. Low heart rate variability relates to the progression of gastric cancer. World J Surg Oncol. 2018;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Y, Palmer JL, Strasser F, Yusuf SW, Bruera E. Heart rate variability as a measure of autonomic dysfunction in men with advanced cancer. Eur J Cancer Care (Engl). 2013;22:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bar-Sela G, Danos S, Visel B, Mashiach T, Mitnik I. The effect of complementary and alternative medicine on quality of life, depression, anxiety, and fatigue levels among cancer patients during active oncology treatment: phase II study. Support Care Cancer. 2015;23:1979-1985. [DOI] [PubMed] [Google Scholar]
- 16. Qureshi M, Zelinski E, Carlson LE. Cancer and complementary therapies: current trends in survivors’ interest and use. Integr Cancer Ther. 2018;17:844-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campo RA, Agarwal N, LaStayo PC, et al. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J Cancer Surviv. 2014;8:60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med. 2015;49:165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh B, Butow P, Mullan B, et al. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann Oncol. 2010;21:608-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Overcash J, Will KM, Lipetz DW. The benefits of medical Qigong in patients with cancer: a descriptive pilot study. Clin J Oncol Nurs. 2013;17:654-658. [DOI] [PubMed] [Google Scholar]
- 21. Wang F, Man JK, Lee EKO, et al. The effects of Qigong on anxiety, depression, and psychological well-being: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:152738. doi: 10.1155/2013/152738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan CL, Wang CW, Ho RT, et al. A systematic review of the effectiveness of Qigong exercise in supportive cancer care. Support Care Cancer. 2012;20:1121-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Penedo FJ, Molton I, Dahn JR, et al. Randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31:261-270. [DOI] [PubMed] [Google Scholar]
- 24. Traeger L, Penedo FJ, Benedict C, et al. Identifying how and for whom cognitive-behavioral stress management improves emotional well-being among recent prostate cancer survivors. Psychooncology. 2013;22:250-259. [DOI] [PubMed] [Google Scholar]
- 25. Burg JM, Wolf OT, Michalak J. Mindfulness as self-regulated attention. Associations with heart rate variability. Swiss J Psychol. 2012;71:135-139. [Google Scholar]
- 26. LaVoy ECP, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exerc Immunol Rev. 2016;22:82-93. [PMC free article] [PubMed] [Google Scholar]
- 27. Chuang TY, Yeh ML, Chung YC. A nurse facilitated mind-body interactive exercise (Chan-Chuang Qigong) improves the health status of non-Hodgkin lymphoma patients receiving chemotherapy: randomised controlled trial. Int J Nurs Stud. 2017;69:25-33. [DOI] [PubMed] [Google Scholar]
- 28. Corbett T, Devane D, Walsh JC, Groarke A, McGuire BE. Protocol for a systematic review of psychological interventions for cancer-related fatigue in post-treatment cancer survivors. Syst Rev. 2015;4:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koornstra RH, Peters M, Donofrio S, van den Borne B, de Jong FA. Management of fatigue in patients with cancer—a practical overview. Cancer Treat Rev. 2014;40:791-799. [DOI] [PubMed] [Google Scholar]
- 30. Castellar JI, Fernandes CA, Tosta CE. Beneficial effects of pranic meditation on the mental health and quality of life of breast cancer survivors. Integr Cancer Ther. 2014;13:341-350. [DOI] [PubMed] [Google Scholar]
- 31. Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther. 2013;12:276-290. [DOI] [PubMed] [Google Scholar]
- 32. Cella D, Davis K, Breitbart W, Curt G; Fatigue Coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385-3391. [DOI] [PubMed] [Google Scholar]
- 33. Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer. 2009;17:241-251. [DOI] [PubMed] [Google Scholar]
- 34. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579. [DOI] [PubMed] [Google Scholar]
- 35. Krygier JR, Heathers JA, Shahrestani S, Abbott M, Gross JJ, Kemp AH. Mindfulness meditation, well-being, and heart rate variability: a preliminary investigation into the impact of intensive Vipassana meditation. Int J Psychophysiol. 2013;89:305-313. [DOI] [PubMed] [Google Scholar]
- 36. Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clin North Am. 2017;101:1085-1097. [DOI] [PubMed] [Google Scholar]
- 37. Klein P. Qigong in cancer care: theory, evidence-base, and practice. Medicines (Basel). 2017;4:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Corey G. Theory and Practice of Counselling and Psychotherapy. 8th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009:296-297. [Google Scholar]
- 39. Stone PC. Methylphenidate in the management of cancer-related fatigue. J Clin Oncol. 2013;31:2372-2373. [DOI] [PubMed] [Google Scholar]
- 40. Narayanan S, Escalante CP. Clinical assessment and management of cancer-related fatigue. J Clin Outcomes Manage. 2017;24:217-228. [Google Scholar]
- 41. Deimling GT, Brown SP, Albitz C, Burant CJ, Mallick N. The relative importance of cancer-related and general health worries and distress among older adult, long-term cancer survivors. Psychooncology. 2017;26:182-190. [DOI] [PubMed] [Google Scholar]
- 42. Wu HS, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38:E29-E54. [DOI] [PubMed] [Google Scholar]
- 43. Kate J. Fear of recurrence persists for many cancers survivors. Paper presented at: European Society for Radiotherapy and Oncology (ESTRO) 3rd Forum; April 29, 2015; Barcelona, Spain. Abstract No. SP-0317. [Google Scholar]
- 44. Arndt V, Koch-Gallenkamp L, Jansen L, et al. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol. 2017;56:190-197. [DOI] [PubMed] [Google Scholar]
- 45. Antoni MH. Psychosocial intervention effects on adaptation, disease course and biobehavioral processes in cancer. Brain Behav Immun. 2013;30(suppl):S88-S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stanton AL, Luecken LJ, MacKinnon DP, Thompson EH. Mechanisms in psychosocial interventions for adults living with cancer: opportunity for integration of theory, research, and practice. J Consult Clin Psychol. 2013;81:318-335. [DOI] [PubMed] [Google Scholar]