Abstract
The prognosis of triple-negative breast cancer with metastases after chemotherapy remains dismal. We report the case of a 50-year-old female with first disease recurrence at the axillary lymph node and, later on, bilateral pulmonary metastases with severe shortness of breath. The patient received low-dose immune checkpoint blockade (concurrent nivolumab and ipilimumab) weekly over 3 weeks with regional hyperthermia 3 times a week, followed by systemic fever-range hyperthermia induced by interleukin-2 for 5 days. She went into complete remission of her pulmonary metastases with transient WHO I-II diarrhea and skin rash. The patient remained alive for 27 months after the start of treatment, with recurrence of metastases as a sternal mass, and up to 3 cm pleural metastases. This exceptional response should instigate further research efforts with this protocol, which consists only of approved drugs and treatments.
Keywords: TNBC, immunotherapy, checkpoint inhibition, hyperthermia, IL-2
Background
The prognosis of triple-negative breast cancer (TNBC) with metastases after chemotherapy remains dismal. We report the case of a 50-year-old female with first disease recurrence at the axillary lymph node and, later on, bilateral pulmonary metastases with severe shortness of breath (SOB). The patient received low-dose (LD) immune checkpoint blockade (ICB; concurrent nivolumab and ipilimumab) weekly over 3 weeks with regional hyperthermia 3 times a week, followed by systemic fever-range hyperthermia induced by interleukin-2 (IL-2) for 5 days. She went into complete remission of her pulmonary metastases with transient World Health Organization Grades I-II diarrhea and skin rash. The patient remained alive for 27 months after the start of treatment, with recurrence of metastases as a sternal mass, and up to 3 cm pleural metastases. This exceptional response should instigate further research efforts with this protocol, which consists only of approved drugs and treatments.
Introduction
The breakthrough discovery of ICB1 has revived cancer immunotherapy, but could not change the palliative management of stage IV cancer affecting millions of patients worldwide. ICB is not recommended in “individuals facing imminent death” because of potential serious immune-related adverse events (irAE).
The rationale of this combination therapy was based on the prediction that anti-CTLA-4 therapy may have a mechanism similar to that occurring in inherited human CTLA-4 haploinsufficiency.2 This prediction seems to be consistent with a study that hypothesized that ICT established by targeting CTLA-4 with ipilimumab could restore antitumor reactivity through a graft-versus-tumor effect.3 This is presumably associated with the graft-versus-host-disease against minor histocompatibility antigens expressed on hematopoietic cells.4
In an effort to synergize treatment effects, we combined 2 ICB drugs with hyperthermia treatments and IL-2.
A “Compassionate Patient Use” protocol consisted of an off-label LD ICB anti-PD-1 and anti-CTLA-4 antibody treatment (ipilimumab/nivolumab) with IL-2 under taurolidine protection and locoregional hyperthermia and whole body hyperthermia (WBH); written informed consent was obtained from the patients. WBH was induced using external heating followed by systemic fever induction using IL-2. Previously it was reported that coadministration of taurolidine with IL-2 in stage IV melanoma patients greatly enhanced the tolerability of this regime by diminishing the IL-2-induced vascular leak syndrome without diminishing its therapeutic value.5 Our concept follows in the footsteps of Coley, father of cancer immunotherapy.6 To our knowledge, this is the first report of complete clinical remission of far advanced bilateral pulmonary metastases in stage IV TNBC induced by LD ICB in combination with IL-2 and hyperthermia without inducing severe irAEs.
Results
Patient Information
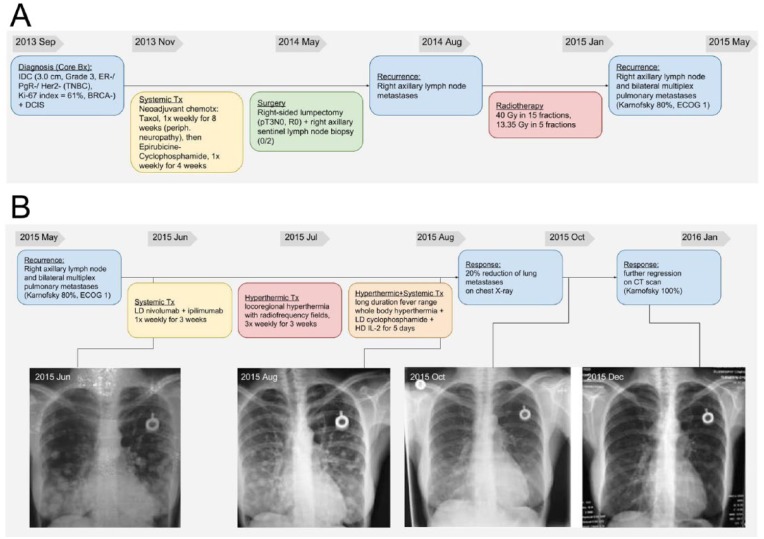
In September 2013, a 50-year-old Caucasian female was first diagnosed with breast cancer (Figure 1A). Her right breast lesion was invasive carcinoma with medullary features of 3.0 cm T2 N0 M0, Grade 3, no estrogen receptor, progesterone receptor, or Her2-neu expression (TNBC), with a 61% Ki-67 index, indicating high proliferative potential. Additional evidence of ductal carcinoma in situ and local vascular invasion was obtained. The BRCA1 mutation analysis proved wild-type.
Figure 1.
Detailed history and chest X-rays of a patient with triple-negative breast cancer from diagnosis and treatment before (A) and after attending (B) the outpatient clinic with the respective regimen. Long-term follow-up is also displayed supported by chest X-rays (C).
From November 2013, neoadjuvant paclitaxel chemotherapy (8 weekly T cycles) was administered, which due to peripheral neuropathy was changed to epirubicin and cyclophosphamide (4 weekly EC cycles). In May 2014, lumpectomy was performed (pT2 N0, R0) with sentinel lymph node biopsy (2 tumor-free nodes); postoperative histology proved viable tumor cells in the primary breast tumor. Six months later, a repeated axillary lymph node biopsy demonstrated metastatic tumor cells within these lymph nodes. In August 2014, she underwent radiotherapy of the right chest wall with 40 Gy in 15 fractions plus 13.35 Gy in 5 fractions. In February 2015, right axillary lymph node metastases of up to 1.2 cm in size and advanced disseminated pulmonary metastases up to 2 cm in size were detected. Metastasis was histologically proven with the right axillary lymph node biopsy demonstrating recurrence of the previously described TNBC; infection and Candida were excluded with sputum analysis and Candida serology, respectively.
Clinical Findings
The patient first presented at the Immunology & Integrative Oncology day-clinic in May 2015 (Figure 1B), with a Karnofsky score of 80% and mild neuropathy with distal predominance in all extremities. During inspiration she felt severe pain in the left lateral chest wall becoming extremely painful during sneezing. She complained about severe SOB on exertion, lack of appetite, insomnia, and exhaustion. She was unable to climb more than a few steps of a stairway prior to immunotherapy treatment.
Therapeutic Intervention
In June 2015, LD nivolumab (0.5 mg/kg) and LD ipilimumab (0.3 mg/kg) was administered weekly, over 3 weeks. This dosage was based on a recent article suggesting the use of LD checkpoint inhibitors in the adjuvant setting.7 Total cumulative dosage of nivolumab during 3 weeks in this patient was 82.5 mg, and the total cumulative dosage of ipilimumab during 3 weeks was 49.5 mg. This was accompanied by locoregional hyperthermia with radiofrequency fields 13.56 MHz (Syncrotherm RF1200) 3 times per week (maximum output 400 W) over the thoracic region in combination with high-dose (HD) vitamin C (0.5 g/kg) and α-lipoic acid (600 mg) 3 times per week over 3 weeks. This was followed by long-duration fever range WBH (Heckel-HT3000) in combination with LD chemotherapy using cyclophosphamide 300 mg/m2 intravenous one time to down-modulate T-reg cells.8 Then, a HD IL-2 (54 Mio/m2 as decrescendo regimen) therapy was administered under taurolidine 2% (bis(1,1dioxoperhydro-1,2,4-thiabiazin-4-yl) methane; Geistlich Pharma) protection for 5 days.5
Follow-up and Outcomes
The patient experienced a major and durable response of widespread bilateral lung metastasis following the described immunotherapy.
Following the first diagnosis of far advanced bilateral pulmonary metastases (Figure 1B) in June 2015, the second X-ray in August 2015 (2 months after completion of therapy) already demonstrated a 20% reduction of lung metastases. In October 2015 (4 months after completion of therapy), chest X-ray demonstrated remarkable further partial response. Six months later the X-ray demonstrated near complete response (Figure 1B). A first computed tomography scan of the thorax, abdomen, and pelvis also demonstrated major response of lung metastases and, interestingly, some progression of mediastinal and right internal mammary chain lymph nodes. Radiologically, this was first interpreted as possible pseudo-progression,9 a radiological and clinical phenomenon increasingly reported following immunotherapy with checkpoint inhibitors.10 Importantly, this was associated with an excellent clinical condition with a Karnofsky score of 100%. SOB on exertion or any other cancer-related symptoms had vanished, and her functional capacity was vastly improved; she was able to climb multiple flights of stairs without difficulty. Side effects (irAEs) consisted of transient World Health Organization Grades I-II diarrhea and skin rash.
In April 2016, the patient suffered right axillary recurrence and underwent right axillary clearance; in 1/20 resected lymph nodes from the right axilla breast cancer metastasis was detected. A gene expression analysis was performed by PANTHER-Chip test as described previously.11 About 1000 genes with high clinical relevance were present as 10-fold replicates. Applying thresholds of >31 the tumor recurrence was classified as high-risk basal-like breast cancer with high claudin expression pattern and high proliferative activity; the “invasiveness” signature was found to be unfavorably high (see Supplementary Figure 1; available in the online version of the article).
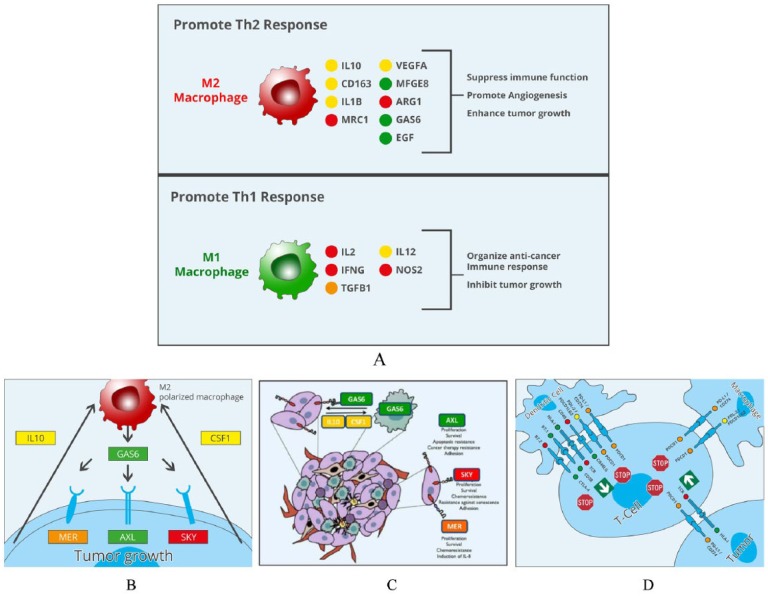
Genes promoting immune surveillance (eg, IL-2, IFN-γ, TGF-β, etc) were very active (see Figure 2A, B, and C). Not unexpectedly, high activity of beneficial macrophage M1 polarization was demonstrated (Figure 2D). A total gene expression analysis of a metastatic axillary lymph node demonstrated that several checkpoint genes were overexpressed even after 1 year past initiation of therapy. There was no immunohistochemistry available but expression of checkpoints was measured with real-time mRNA. Checkpoint receptor and ligand genes important for immune therapy like CD40, CD86, PD-L1, glucocorticoid-induced TNFR-related ligand (GITRL) and inducible costimulator ligand (ICOSL) in T cells, dendritic cells, or tumor cells were overexpressed in this tumor, whereas growth arrest-specific 6 gene was not.
Figure 2.
The upper half of the graph (A) illustrates the crosstalk of “bad” growth stimulating macrophages (type 2 tumor-associated macrophages [TAMs]). In the lower part of the graph (B) the interplay of “beneficial” type 1 TAMs with tumor cells and antigen-presenting cells is depicted. As illustrated by color code (green to red meaning low to high expression) genes promoting immune surveillance are very active. As is to be seen in the tumor tissue of the patient a high activity of the beneficial types of macrophages related to immune activity was found as compared with “bad” growth stimulating ones (C). Activity of checkpoint genes important for tumor immune therapy is demonstrated (D). Figures reproduced by permission from H. Bojar, NextGen Oncology Group, Dusseldorf, Germany.
Computed tomography of the thorax from June 2016 (London) and magnetic resonance imaging of the thorax from August 2016 (Vienna) confirmed durable response of lung metastases (Figure 1B); however, the computed tomography scan also demonstrated a 4.5 cm right parasternal mass suspicious for local recurrence and several up to 3 cm pleural deposits and mediastinal lymph adenopathy. Therefore, pseudoprogression had to be discarded as a possible diagnosis, and subsequently the new lesions had to be specified as recurrent metastatic disease, but none was found within the lung parenchyma. The patient did not undergo further biopsy for these recurrences.
Discussion
The most common irAE for both anti-CTLA-4 and anti-PDL-1/PD-1 therapy involves dermatologic toxicity, which is also typically the irAE with the earliest onset, within several weeks. Anti-PD-1 therapy induces dermatitis through a T-cell-mediated mechanism separate from those typically reported with anti-CTLA-4 treatment.12 Dermatologic toxicity was also observed in this patient, but due to the LD ICB (0.3 mg/kg and 0.5 mg/kg of ipilimumab and nivolumab, respectively), the clinically most relevant irAEs (diarrhea and colitis) occurred only mildly. These typically do not present until approximately 6 weeks into treatment after several doses of registered CTLA-4 blockade (3 mg/kg or 10 mg/kg).
We hypothesized that using LD checkpoint blockade in combination with hyperthermia and IL-2 would create synergies, which each of the modalities alone could not achieve. Therefore, the rationale for our combination was as follows:
Block the checkpoints of the T-cell response with LD checkpoint inhibitors (inhibit the inhibitors).
Combine with exogenous local regional and WBH, inducing fever-range temperatures in the tumor microenvironment, reducing interstitial pressure and facilitating immune cell extravasation.
Follow by IL-2 administered for 5 days.
As a result of the impaired self-tolerance, irAEs may present with a broad clinical spectrum that mainly involve the gut, skin, endocrine glands, liver, and lung, but can potentially affect any tissue, and their incidence may reach up to 90% of patients.13,14 Meta-analysis indicates an overall incidence of <75% with anti-CTLA-4 monotherapy (ipilimumab) and ⩽30% in phase 3 trials of anti-PD-1/PD-L1 agents. IrAEs of ⩾grade 3 severity occur in up to 43% of patients taking ipilimumab and ⩽20% taking PD-1/PD-L1 agents. The incidence of irAEs with ipilimumab and pembrolizumab is dose-dependent, with greater toxicity at higher dose levels; toxicity also varies between the adjuvant and metastatic disease settings. Death due to irAEs occurred in up to 2% of patients.13
These immune-mediated adverse reactions can be interpreted to mean that both ipilimumab and nivolumab blockades break down immune tolerance, though via different checkpoints. This is consistent with the suggestion that the mechanisms of action of the checkpoint-modulatory antibodies are independent of tumor origin or specific mutations and, therefore, they demonstrate clinical activity across a broad range of different types of cancer.15
Inducible co-stimulator (ICOS) molecule is T-cell-specific and belongs to the CD28/CTLA-4/B7 immunoglobulin superfamily, which is expressed only after activation. In a presurgical clinical trial, a marked increase in the frequency of T-cells expressing ICOS in both tumor tissues and blood of anti-CTLA-4 treated patients was observed. In fact, a sustained increase in ICOS+ T-cells correlated with increased survival.16-18 In the absence of ICOS, antitumor T-cell responses elicited by anti-CTLA-4 are significantly diminished, thereby impairing tumor rejection. The findings that ICOSL is overexpressed in dendritic cells is consistent with the finding that the ICOS/ICOSL pathway is necessary for the optimal therapeutic effect of anti-CTLA-4, implicating this pathway as a target for future combinatorial strategies to improve the efficacy of anti-CTLA-4 therapy.19
Glucocorticoid-induced TNFR-related (GITR) gene codes for a member of the TNF receptor superfamily. GITR activation by its ligand (GITRL) influences the activity of effector and regulatory T-cells, thus participating in the development of immune response against tumors and infectious agents.20 Overexpression of GITRL in dendritic cells of the resected lymph node of this patient even 1 year after completion of combination therapy seems to confirm this finding.
Nivolumab efficacy seems to be positively correlated with the expression of PD-L1. It was assumed that significant overexpression of the PD-L1 ligand in the tumor cells, dendritic cells, and tumor-associated macrophages increased overexpression of PD-L in T-cells, which in turn contributed to the therapeutic efficacy of the combination protocol.
Sequence-specific synergy between IL-2 and ipilimumab resulted in durable complete response in 3 patients with BRAF-mutated metastatic melanoma.21 This is consistent with this report because sequential administration of LD ipilimumab/nivolumab and IL-2 therapy also resulted in durable complete response of lung metastases in this patient.
Both preclinical and clinical data have demonstrated improved antitumor immune responses with the addition of mild fever-range hyperthermia. The molecular mechanisms include the generation of heat shock proteins, the activation of antigen-presenting cells, and changes in lymphocyte trafficking. In addition, mild, fever-range (40°C) prolonged WBH may help in reducing interstitial pressure in the tumor microenvironment.22,23 Hyperthermia has been shown to modulate directly or indirectly the innate and adaptive immune system response.24
The potential of immune checkpoint inhibition in advanced TNBC currently is being studied in keynote trials.25 These studies indicate some efficacy but also raise concern for the safety of immune checkpoint inhibitions alone in advanced TNBC. Interestingly, TNBC cells stimulate the natural killer–cell immune response significantly more strongly than estrogen receptor–positive breast cancer cells.26 PD-L1 is expressed in 20% of TNBCs, suggesting PD-L1 as a therapeutic target in TNBCs.27
In this case report, we demonstrate complete remission of disseminated lung metastasis in TNBC followed 1 year later by parasternal recurrence and new pleural deposits. The progression that occurred in her pleura and in the parasternal mass 1 year following the therapy finally classifies this patient having a mixed overall response. Evidently, this patient with such far advanced lung metastasis would have had an extremely limited expected survival. Therefore, we wanted to report this case as an exceptional response to the LD ICB therapy, which resulted in 27 months’ overall survival from the beginning of our treatment. The patient eventually died in August 2017 in her home in London from unknown cause, most probably due to the progression of her disease. The last time she was in our office was 13 months earlier, in July 2016. She had not had any treatment afterward in our clinic.
Why does our concept yield such good results, specifically in the lung parenchyma? One can only speculate: the excellent perfusion of lung tissue could be one reason for this exceptional response in the lungs, which we have also seen in other patients with lung metastasis. Moreover, the use of taurolidine has been previously reported to reduce the incidence of infections in patients with long-term central venous lines indwelling catheters.28 This indicates the additional potential benefit of using taurolidine as coadministration to IL-2 by preventing IL-2-associated endothelial cell dysfunction.29
Despite the fact that the TNBC patient eventually died from recurrent disease, the safety and exceptional response to the LD ICB was demonstrated. “Rare cancer successes should instigate ‘exceptional’ research efforts.”30 In many clinical trials that failed to help enough patients, there were exceptions, rare patients with advanced cancer whose tumors shrank or even disappeared for many months or years. Previous National Cancer Institute director and Nobel Laureate Harold Varmus stated that we can really learn from such “exceptional responders” since they may explain why a drug sometimes has dramatic beneficial effects in certain patients, which in turn could allow more people to benefit from it.
Conclusion
Immunotherapy is used to encompass both nonspecific immune stimulation, such a Bacillus Calmette-Guérin and cytokines, as well as immune modulators, which include checkpoint inhibitor antibodies and drugs.31 The concept of such combination treatment approach has been described and is consistent with the generation 3 immunotherapeutics.
In previous years we have treated a variety of stage IV cancer patients with IL-2 and hyperthermia alone; only when we had started integrating LD checkpoint inhibitors did we start seeing responses such as the one described in this case report. Based on the response of this TNBC patient and several other stages III and IV patients with a variety of cancer types who have been successfully treated with the LD ICB (unpublished results), it seems that we might only be “at the tip of the iceberg” concerning the oncologic treatment potential of ICB therapy.32
Since this protocol consists only of approved drugs and treatments, our prediction that this protocol (LD ICB induced autoimmune T-cells further stimulated by IL-2 and hyperthermia) represents a potentially powerful therapeutic strategy would seem deserving of evaluation in controlled clinical trials.
Supplemental Material
Supplemental material, Figure_S1_TNBC_JICT for Complete Clinical Remission of Stage IV Triple-Negative Breast Cancer Lung Metastasis Administering Low-Dose Immune Checkpoint Blockade in Combination With Hyperthermia and Interleukin-2 by Ralf Kleef, Ralph Moss, A. Marcell Szasz, Arthur Bohdjalian, Hans Bojar and Tibor Bakacs in Integrative Cancer Therapies
Acknowledgments
The authors thank Angus Dalgleish, St George’s University of London, and Dwight McKee for comments and suggestions for this article.
Footnotes
Authors’ Note: Part of this article was presented at ITOC3 Munich, 2016; Abstract Book, Eur J Cancer. 55(suppl 1), March 2016 (see poster 085 at: http://www.ecco-org.eu/Events/ITOC3/Searchable-Programme#anchorScpr). An earlier version of the abstract was selected for publication by ASCO 2016; J Clin Oncol. 2016;34(suppl):Abstract e23111 (see http://abstracts.asco.org/176/AbstView_176_166013.html).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ralf Kleef and Tibor Bakacs declared potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakacs T, Mehrishi JN. Anti-CTLA-4 therapy may have mechanisms similar to those occurring in inherited human CTLA4 haploinsufficiency. Immunobiology. 2015;220:624-625. [DOI] [PubMed] [Google Scholar]
- 3. Davids MS, Kim HT, Bachireddy P, et al. Leukemia and Lymphoma Society Blood Cancer Research Partnership. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Brien GC, Cahill RA, Bouchier-Hayes DJ, Redmond HP. Co-immunotherapy with interleukin-2 and taurolidine for progressive metastatic melanoma. Ir J Med Sci. 2006;175:10-14. [DOI] [PubMed] [Google Scholar]
- 6. Kleef R, Jonas WB, Knogler W, Stenzinger W. Fever, cancer incidence and spontaneous remissions. Neuroimmunomodulation. 2001;9:55-64. [DOI] [PubMed] [Google Scholar]
- 7. Slavin S, Moss RW, Bakacs T. Control of minimal residual cancer by low dose ipilimumab activating autologous anti-tumor immunity. Pharmacol Res. 2014;79:9-12. [DOI] [PubMed] [Google Scholar]
- 8. Ouyang Z, Wu H, Li L, Luo Y, Li X, Huang G. Regulatory T cells in the immunotherapy of melanoma. Tumour Biol. 2016;37:77-85. [DOI] [PubMed] [Google Scholar]
- 9. Michaels AY, Keraliya AR, Tirumani SH, Shinagare AB, Ramaiya NH. Systemic treatment in breast cancer: a primer for radiologists. Insights Imaging. 2016;7:131-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Velasco G, Krajewski KM, Albiges L, et al. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol Res. 2016;4:12-17. [DOI] [PubMed] [Google Scholar]
- 11. Modlich O, Prisack HB, Munnes M, Audretsch W, Bojar H. Immediate gene expression changes after the first course of neoadjuvant chemotherapy in patients with primary breast cancer disease. Clin Cancer Res. 2004;10:6418-6431. [DOI] [PubMed] [Google Scholar]
- 12. Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22. [DOI] [PubMed] [Google Scholar]
- 13. Puzanov I, Diab A, Abdallah K, et al. Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brahmer JR, Lacchetti C, Schneider BJ, et al. National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoos A. Development of immuno-oncology drugs—from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235-247. [DOI] [PubMed] [Google Scholar]
- 16. Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987-14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen H, Liakou CI, Kamat A, et al. Anti-CTLA-4 therapy results in higher CD4 + ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A. 2009;106:2729-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71:5445-5454. [DOI] [PubMed] [Google Scholar]
- 20. Nocentini G, Ronchetti S, Petrillo MG, Riccardi C. Pharmacological modulation of GITRL/GITR system: therapeutic perspectives. Br J Pharmacol. 2012;165:2089-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wyluda EJ, Cheng J, Schell TD, et al. Durable complete responses off all treatment in patients with metastatic malignant melanoma after sequential immunotherapy followed by a finite course of BRAF inhibitor therapy. Cancer Biol Ther. 2015;16:662-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skitzki JJ, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs. 2009;10:550-558. [PMC free article] [PubMed] [Google Scholar]
- 23. Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28:528-542. [DOI] [PubMed] [Google Scholar]
- 25. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engel JB, Honig A, Kapp M, et al. Mechanisms of tumor immune escape in triple-negative breast cancers (TNBC) with and without mutated BRCA 1. Arch Gynecol Obstet. 2014;289:141-147. [DOI] [PubMed] [Google Scholar]
- 27. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jurewitsch B, Jeejeebhoy KN. Taurolidine lock: the key to prevention of recurrent catheter-related bloodstream infections. Clin Nutr. 2005;24:462-465. [DOI] [PubMed] [Google Scholar]
- 29. Finnegan N, Toomey D, Condron C, Redmond HP, Da Costa M, Bouchier-Hayes DJ. Potentiation of the therapeutic index of interleukin-2 immunotherapy by combination with taurine in a syngeneic murine tumour model. Ir J Med Sci. 2002;171:85-88. [DOI] [PubMed] [Google Scholar]
- 30. Kaiser J. Biomedicine. Rare cancer successes spawn “exceptional” research efforts. Science. 2013;340:263. [DOI] [PubMed] [Google Scholar]
- 31. Dalgleish AG. Vaccines versus immunotherapy: overview of approaches in deciding between options. Hum Vaccin Immunother. 2014;10:3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med. 2017;23:540-547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Figure_S1_TNBC_JICT for Complete Clinical Remission of Stage IV Triple-Negative Breast Cancer Lung Metastasis Administering Low-Dose Immune Checkpoint Blockade in Combination With Hyperthermia and Interleukin-2 by Ralf Kleef, Ralph Moss, A. Marcell Szasz, Arthur Bohdjalian, Hans Bojar and Tibor Bakacs in Integrative Cancer Therapies