Abstract
Background/Objectives: In the Middle East, people consume camel milk regularly as it is believed to improve immunity against diseases and decrease the risk for cancer. Recently, it was noted that most of the beneficial effects of milk come from their nanoparticles, especially exosomes. Herein, we evaluated the anticancer potential of camel milk and its exosomes on MCF7 breast cancer cells (in vitro and in vivo) and investigated the possible underlying molecular mechanism of action. Methods/Results: Administration of camel milk (orally) and its exosomes (orally and by local injection) decreased breast tumor progression as evident by (a) higher apoptosis (indicated by higher DNA fragmentation, caspase-3 activity, Bax gene expression, and lower Bcl2 gene expression), (b) remarkable inhibition of oxidative stress (decrease in MDA levels and iNOS gene expression); (c) induction of antioxidant status (increased activities of SOD, CAT, and GPX), (d) notable reduction in expression of inflammation-(IL1b, NFκB), angiogenesis-(VEGF) and metastasis-(MMP9, ICAM1) related genes; and (e) higher immune response (high number of CD+4, CD+8, NK1.1 T cells in spleen). Conclusions: Overall, administration of camel milk–derived exosomes showed better anticancer effect, but less immune response, than treatment by camel milk. Moreover, local injection of exosomes led to better improvement than oral administration. These findings suggest that camel milk and its exosomes have anticancer effect possibly through induction of apoptosis and inhibition of oxidative stress, inflammation, angiogenesis and metastasis in the tumor microenvironment. Thus, camel milk and its exosomes could be used as an anticancer agent for cancer treatment.
Keywords: camel milk, exosomes, MCF7, apoptosis, immunity
Introduction
Modern Western lifestyle along with smoking in young people, obesity, and industrial and agricultural pollution resulted in higher incidence rate of breast cancer in most Middle East countries.1 Lack of awareness and hesitancy to perform regular health checks are among the major causes for failure of breast cancer early diagnosis.2 Many treatment strategies are currently available, including surgery, radiation therapy, and chemotherapy. Specific treatment modalities depend on certain characteristics as hormone receptor status, tumor grade, metastatic potential, and molecular and patient profile. Despite notable advances in chemotherapy, the severe drawbacks and the possibility of drug resistance and relapse reduce treatment efficacy.3 This in turn increases the need for exploring novel therapeutic strategies to not only kill the tumor cells but also stop their metastasis.
There is a traditional belief in the Middle East that regular consumption of camel milk can improve patients’ immunity against diseases and decreases the risk for cancer. Consumption of camel milk can improve the immunity of patients against hepatitis C virus.4,5 Camel milk and its constituents also showed anticancer effect against a wide variety of cancer cell lines, including human breast cancer MCF7 cells.6-10
Exosomes are extracellular nanovesicles of 30 to 100 nm diameter that are produced by nearly all cell types.11 They play a significant role in cellular communication by transferring their nucleic acid (ie, mRNA, micro RNA [miR], long noncoding RNA [lncRNA] molecules, and circulating DNA) and protein cargoes between cells.12 Milk exosomes have special significance because they have the capacity of transferring genetic information from mother to infants.13,14 Human milk has been demonstrated as a potent immune stimulant and its exosomes have an anticancer impact through NF-κB pathway.15 Moreover, exosomes derived from cow milk also inhibit breast cancer cell viability in vitro.16 However, to the best of our knowledge to date no publications are available in the literature that address the in vitro or the in vivo effect of camel milk exosomes on breast cancer cells. Herein, we evaluated the potential effect of camel milk and its exosomes on MCF7 breast cancer cells, in vitro and in vivo, and the involved underlying mechanism.
Materials and Methods
Exosomes Isolation and Characterization
Fresh camel milk samples were collected from healthy lactating she-camels at mid lactation period. The exosomes were isolated using differential ultracentrifugation as previously described17 with some modifications. The milk sample (20 mL) was centrifuged at 5000 × g for 15 minutes at 4°C to remove fat globules, casein aggregates, and other debris. The supernatants containing the exosomes were centrifuged at 13,000 × g for 30 minutes at 4°C to remove debris and apoptotic bodies. Exosomes were isolated from supernatants by twice ultracentrifugation at 100,000 × g (Optima L-90K; Beckman Coulter) for 90 minutes each at 4°C, with an interval wash with phosphate buffered saline (PBS), to remove large particles and microvesicles. The exosome pellets were pooled and resuspended in PBS to give homogenous suspension. The total exosomal protein content was measured by Bradford method. The isolated exosomes were identified by transmission electron microscopy (JEM2100, Joel Inc) at 80 kV. The exosomes were pelleted, fixed in 2.5% glutaraldehyde in cacodylate buffer at 20°C for 1 hour, and stained with 2% uranyl acetate. The specific structural proteins of exosomes (CD63, CD81; Santa Cruz) were verified by Western blotting. In brief, exosomes pellets were lysed by RIPA buffer, then their protein contents in the collected supernatants were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes which were incubated with the CD63 (1:200) and CD81 (1: 200) primary antibodies. Secondary horseradish peroxidase–conjugated anti-rabbit IgG antibody detection was done with enhanced chemiluminescence reagents (Santa Cruz).
Cell Viability by MTT Assay
The cytotoxic effect of both camel milk and its exosomes on MCF7 cells was evaluated by MTT assay. The cells were cultured in a 96-well plate (1 × 104 cells/well, 100 μL/well) containing Dulbecco’s modified Eagle medium (DMEM) provided with 10% fetal bovine serum, and 1% penicillin/streptomycin (GIBCO). The cells were then incubated at 37°C for 24 hours under 5% CO2, 95% air until reaching a confluence of 70% to 80%. Two-fold dilution of fat-free camel milk (obtained by centrifugation at 1400 × g for 30 minutes at 4°C) and exosomal proteins at different concentrations (0, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL) were added and the cells were reincubated for further 24 hours. The cells were incubated with 5 mg/mL of MTT (Sigma) for 4 hours and then the medium was replaced with 100 μL dimethyl sulfoxide (DMSO; Sigma) and vortexed for 20 minutes. Absorbance was recorded at 570 nm using microplate reader. The concentration of milk and its exosomes inhibiting 50% of cells (IC50) was calculated using the sigmoidal curve using GraphPad (Prism) statistics software.
In Vitro Scratch Assay
This assay was achieved as previously described.18 A scratch in form of a straight line in the middle of each well was made by a sterile yellow tip in MCF7 cells (2.5 × 105 cells/mL in 6-well plates) seeded in DMEM at a 70% to 75% confluence. A fresh media with different concentrations of camel milk and its exosomal proteins (1/2 IC50) were added to the wells and the cells were photographed at 0 and 24 hours. The migration rate was calculated using the following formula: area of scratch at 0 hours − area of scratch at 24 hours / area of scratch at 0 hours × 100.
Animals and Experimental Design
Healthy female albino rats (n = 50) of similar age (~3 weeks) and weight (~80 g) were housed in a temperature-controlled (25°C-27°C) and light-controlled room (12-hour light/dark cycle) with free access to food (standard diet) and water. Rats were acclimatized to laboratory conditions for 2 weeks prior to experiments. All experimental procedures described herein followed the guidelines of the Institutional Animal Care and Use Committee of Kafrelsheikh and Northern Border Universities and was performed in accordance with the National Institutes of Health guidelines.
The animals were divided into 5 groups (n = 10 per group): normal control group (G1), MCF7-injected tumor group (G2), tumor group administrated camel milk orally (G3), tumor group given exosomes orally (G4), and tumor group injected locally by exosomes (G5). Rats in G1 were administered PBS, while the remaining 40 rats were first immunosuppressed by intraperitoneal injection of cyclophosphamide (CTX) at a dose of 40 mg/kg for 4 successive days. MCF7 cells (2 × 107 in 200 µL PBS) mixed (1:1) with matrigel were injected intradermally around the right thoracic teat of the mammary gland of the immunosuppressed rats at the 5th and 12th day of the experiment. To initiate tumor formation, rats were injected intramuscularly by estrogen (Folon, 0.01 mL/kg body weight) 1 day before MCF7 injection and continued until tumor formation. At tumor volumes between 1000 and 2000 mm3 (~at the 3rd week), rats developed tumors were orally administrated either camel milk (1 mL/rat, G3)19 or exosomal protein (20 mg/kg, in 1 mL PBS, G4)16 for 4 weeks, while the tumor was locally injected with 200 µL PBS (G2), or 1.25 mg/kg exosomal protein (G5) in 200 µL PBS20 4 times with 1-week interval. At the end of the experiment (6 weeks), blood samples were collected in vacutainer tubes and were centrifuged at 3000 rpm for 5 minutes to obtain serum. After sacrificing, spleen and tumor tissue was excised. The tumor tissue was either snap-frozen in liquid nitrogen (real-time polymerase chain reaction [PCR]) or homogenized (biochemical assay), while the spleen was freshly prepared for flow cytometry.
Detection of DNA Damage by Comet Assay
This assay was carried out on tumor tissue as previously described21 using a fluorescence microscope supplied with Komet 5 image analysis software. The extent of DNA damage was assessed by the tail length, and the damage index which ranged from 0 (undamaged [tail length = 0], 100 cells × 0) to 400 (maximal damage [tail length = 4], 100 cells × 4).
Caspase-3 Activity Assay
The homogenized tumor tissues were incubated in lysis buffer and the cell lysates were centrifuged at 15,000 rpm for 10 minutes at 4°C. Protein concentration in supernatant was measured by Bradford assay before being incubated for 1 hour with caspase 3 fluorogenic substrate (Ac-DEVD-AMC, Alexis Biochemicals). Cleavage of the caspase substrates was detected using a fluorescence microtiter plate reader at excitation/emission wavelengths of 360/460 nm.
Evaluation of Lipid Peroxidation and Antioxidant Biomarkers
Tumor tissues were homogenized in cold PBS and were centrifuged at 5000 rpm for 15 minutes at 4°C. The supernatants were used to measure the concentration of the lipid peroxidation biomarker malondialdehyde (MDA) and the activity of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) using commercial kits (Bio-Diagnostics Co, Cairo, Egypt) as previously described.22
Molecular Analysis by Real-Time PCR
Total RNA was isolated from tumor tissues using RNeasy Mini kit (Qiagen, #74104) as previously described.23 RNA concentration and purity were determined by Q5000 nanodrop (Quawell) and by running in 1% gel electrophoresis. The cDNA was synthesized by reverse transcription of RNA (5 μg) using Quantiscript reverse transcriptase (Qiagen, #205310). Specific primers for Bax, Bcl2, NFKb, IL1β, iNOS, VEGF, MMP9, and ICAM1 genes were designed by the Primer 3 web-based tool based on the published rat sequences (Table 1). Quantitative real-time PCR (qPCR) was performed using QuantiTect SYBR Green qPCR Master Mix in a StepOnePlus real-time PCR system (Applied Biosystems) and reaction cycles as previously described.24 The quantities critical threshold (Ct) of target gene were normalized with quantities (Ct) of the internal control (β actin) as previously described.25 Levels were expressed relative to normal control samples.
Table 1.
Primers Used for Real-Time Polymerase Chain Reaction.a
| Gene | Forward Primer | Reverse Primer | Size (bp) |
|---|---|---|---|
| Bax | ACACCTGAGCTGACCTTG | AGCCCATGATGGTTCTGATC | 192 |
| Bcl2 | AGTACCTGAACCGGCATCTG | CATGCTGGGGCCATATAGTT | 83 |
| iNOS | GACCAGAAACTGTCTCACCTG | CGAACATCGAACGTCTCACA | 137 |
| IL1β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC | 79 |
| NFκβ | CCTAGCTTTCTCTGAACTGCAAA | GGGTCAGAGGCCAATAGAGA | 120 |
| VEGF | GATCATGCGGATCAAACCTCACC | CCTCCGGACCCAAAGTGCTC | 209 |
| MMP9 | TCGAAGGCGACCTCAAGTG | TTCGGTGTAGCTTTGGATCCA | 82 |
| ICAM1 | AGATCATACGGGTTTGGGCTTC | TATGACTCGTGAAAGAAATCAGCTC | 75 |
| β actin | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC | 149 |
Melting temperatures (Tm) for all genes are 60°C.
Immunophenotyping of Spleen NK1.1, CD4, and CD8 T Cells
The collected spleens were mashed in PBS, filtered through 80 µm wire mesh, digested with 1X RBC Lysis Buffer (eBioscience, # 00-4333-57), pelleted down at 3000 × g for 7 minutes, and resuspended in PBS. The cells were stained with CD3-FITC (pan lymphocytic marker), CD4-PE, CD8-PE, and NK1.1-PE (Abcam) antibodies and incubated for 2 hours on ice, pelleted down, resuspended in PBS, and finally analyzed using flow cytometry (Attune, Applied Biosystem).
Statistical Analysis
One-way analysis of variance using GraphPad Prism 7 (GraphPad Software, Inc, La Jolla, CA) was used to determine the difference between the groups. Comparison of means was carried out with Tukey’s honestly significant difference test. Data were presented as mean ± standard error of mean (SEM) and significance was declared at P < 0.05.
Results
Characterization and Labeling of Camel Milk–Derived Exosomes
Transmission electron microscopy (TEM) examination revealed presence of exosomes in form of nanovesicles with average diameters ranged from 30 to 100 nm in the samples isolated from camel milk by ultracentrifugation (Figure 1A). Western blot results confirmed expression of exosomal markers CD63 and CD81 in these exosomes (Figure 1B).
Figure 1.
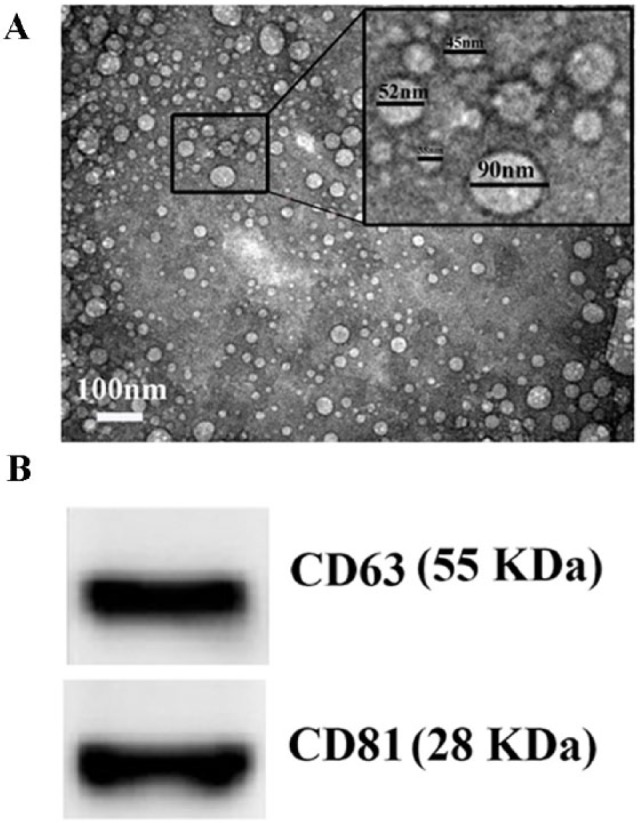
Characterization of camel milk–derived exosomes. Transmission electron microscopic examination shows small nanovesicles (30-100 nm) in the sample isolated from the camel milk by ultracentrifugation. Western blot analysis of the exosomal protein shows presence of CD63 and CD81.
Camel Milk and Its Exosomes Inhibited Proliferation of MCF7 Cells
The MTT assay was performed to detect the inhibitory effect of camel milk and its exosomes on human breast cancer cell line, MCF7. MTT assay results exhibited significant dose-dependent anti-proliferative activity for milk and its exosomes on MCF7 with IC50 values of 47.9 ± 1.02 and 27.34 ± 0.65, respectively, as compared with vehicle (DMSO)-treated MCF7 cells (Figure 2).
Figure 2.
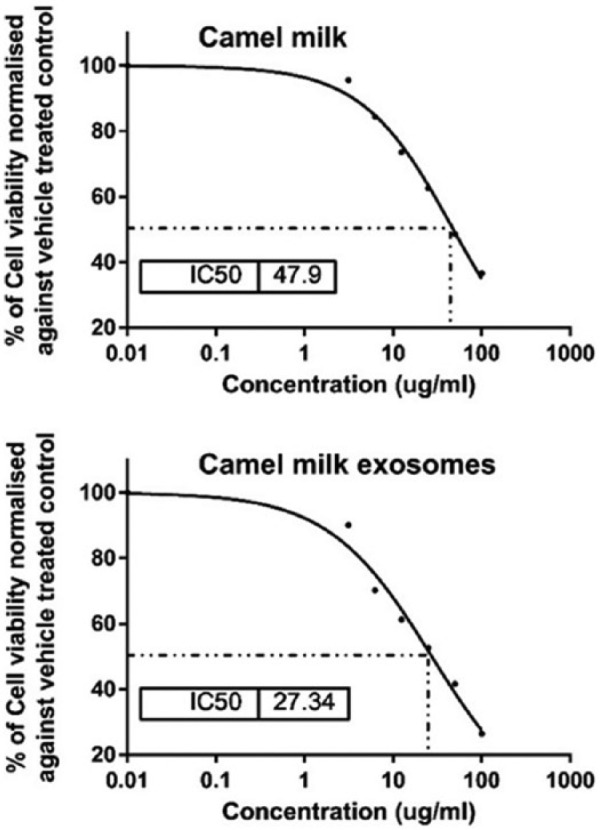
Sigmoidal curve for MTT assay showing IC50 values and the inhibition percentage (%) of camel milk and its exosomes on MCF7 cells. Each data point represents an average of three independent experiments.
Camel Milk and Its Exosomes Suppressed the Migration of MCF7 Cells
The wound-healing assay was used to monitor change in motility (migration) potential of MCF7 cells following treatment by camel milk and its exosomes. The obtained results exhibited a significant lower rate of MCF7 migration following administration of camel milk (40.25% ± 2.12%) and camel milk–derived exosomes (17.75% ± 2.86%) as compared with the DMSO-treated MCF7 cells (65.22% ± 3.74%, Figure 3).
Figure 3.
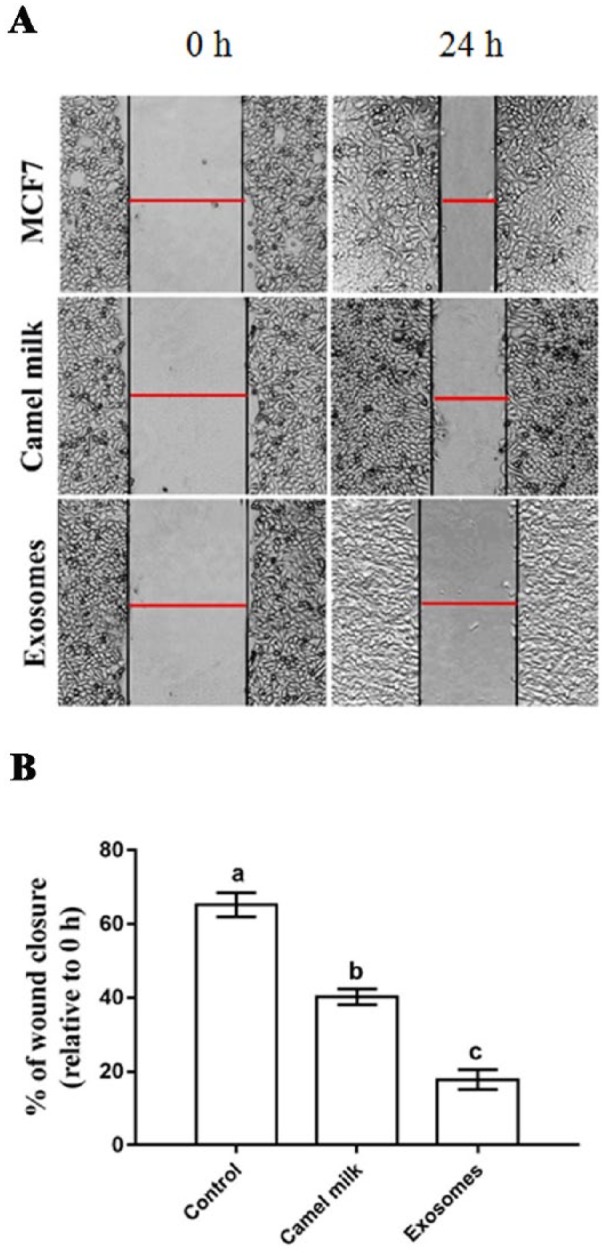
Inhibitory effect of camel milk and its exosomes on the migration of MCF7 cells. (A) Wound-healing assay of MCF7 cells treated with camel milk and its exosomes. (B) Percentage of wound closure of MCF7 cells. Images were captured at 0 and 24 hours. Red bar refers to the size of the wound. Values are expressed as mean ± SEM. Columns carrying different letters are significantly different at P < 0.0001.
Camel Milk and Its Exosomes Limited Growth of Breast Tumor Through Induction of Apoptosis
The final weight of breast tumor was significantly decreased in all treated groups (G3-G5) with lowest weight in G5 (0.50 ± 0.02 g), followed by G4 (0.67 ± 0.02 g) and then G3 (0.84 ± 0.04 g) as compared with that of the tumor group (G2, 1.13 ± 0.06 g) (Figure 4A). Comet assay was applied to assess the DNA integrity in breast tumor tissues of rats. Groups treated by camel milk (G3) and its exosomes (G4 and G5) showed a significant increased level of DNA damage, as revealed by a high damage index as compared with that in the tumor (G2, 65.31 ± 5.09 µm) and normal (G1, 77.36 ± 4.49 µm) groups. The highest DNA damage was noticed in exosome injected group (G5, 310.0 ± 17.32 µm), followed by G4 (240.58 ± 12.53 µm) and G3 (184.05 ± 9.02 µm, Figure 4B and C).
Figure 4.
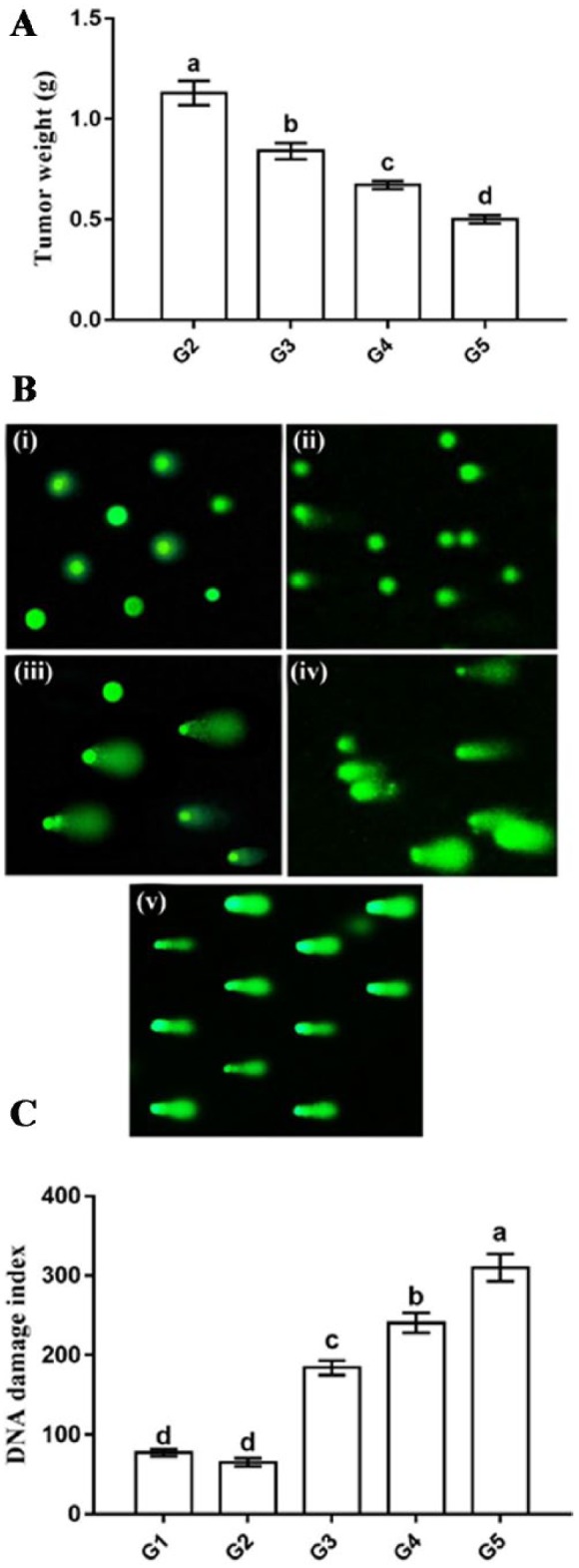
Effect of camel milk and its exosomes on tumor weight (A) and DNA damage of normal mammary gland and tumor tissue detected by comet assay (B, C). In (B), G1 (i); G2 (ii); G3 (iii); G4 (iv); G5 (v). Values are expressed as mean ± SEM. Columns carrying different letters are significantly different at P < 0.05.
Further analysis was conducted by measuring caspase-3 activity, as a final marker of apoptosis, to assess the possible apoptotic role of camel milk and its exosomes in breast tumor. As expected, breast tumor tissues from rats treated by camel milk (G3) and its exosomes (G4 and G5) exhibited higher caspase-3 activity as compared with that in the tumor (G2) and normal (G1) groups (Figure 5). Again, the highest activity was observed in G5 (342.79% ± 17.52%), followed by G4 (273.02% ± 13.44%) and G3 (215.8% ± 12.86%). Moreover, G2 (35.62% ± 2.41%) showed a significant lower caspase-3 activity than G1 (100% ± 6.03%).
Figure 5.
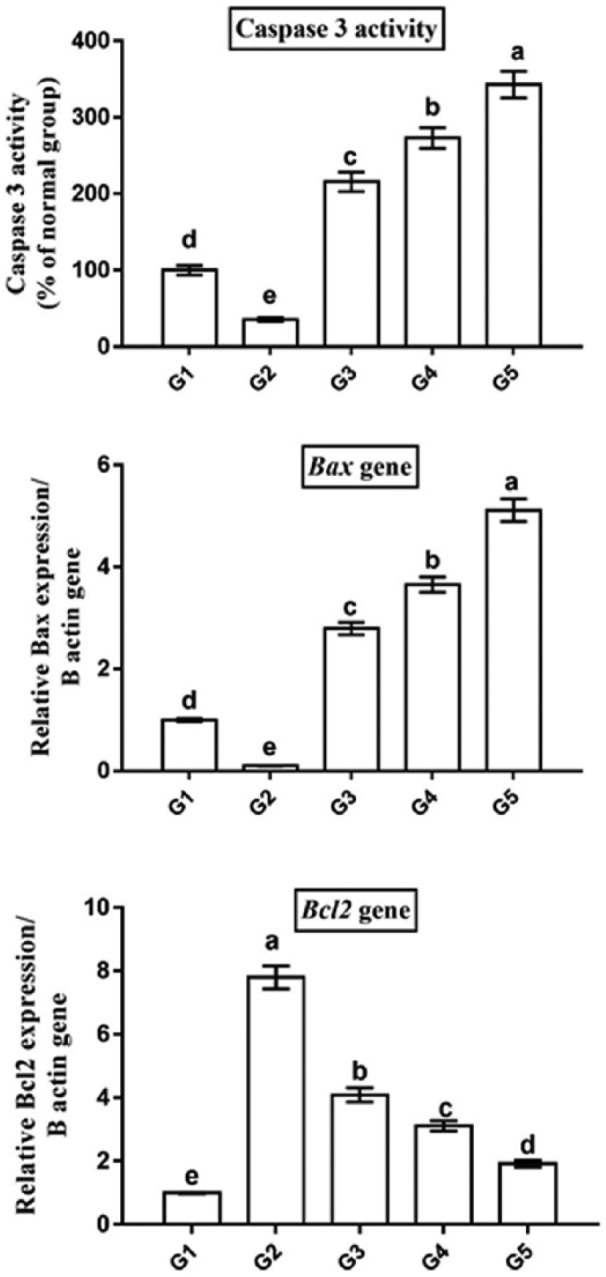
Camel milk and its exosomes induced cancer cell death via apoptosis as revealed by higher caspase-3 activity, upregulated Bax and downregulated Bcl2 gene expression. Data presented as fold change (mean) ± SEM from the normal group. Columns carrying different letters are significantly different at P < 0.05.
To investigate the underlying molecular mechanism of apoptosis in breast cancer induced by camel milk and its exosomes, qPCR was used to detect change in mRNA levels of the apoptotic marker, Bax, and the anti-apoptotic marker, Bcl2 (Figure 5). Breast tumor tissues of rats treated with camel milk (G3) and its exosomes (G4 and G5) showed a significant upregulation in Bax (2.79 ± 0.12, 3.65 ± 0.15, 5.11 ± 0.22 fold change, respectively) and a significant downregulation in Bcl2 (4.09 ± 0.22, 3.11 ± 0.16, 1.92 ± 0.11 fold change, respectively) mRNA levels compared with the tumor group (G2). Again, G5 and G4 showed higher Bax and lower Bcl2 mRNA levels than G3. Furthermore, G2 exhibited significant lower Bax (0.1 ± 0.005 fold change) and higher Bcl2 (7.8 ± 0.36 fold change) mRNA levels than G1 (1 ± 0.03 for Bax and 1 ± 0.02 for Bcl2).
Camel Milk and Its Exosomes Inhibited Oxidative Stress in Breast Tumor Tissues
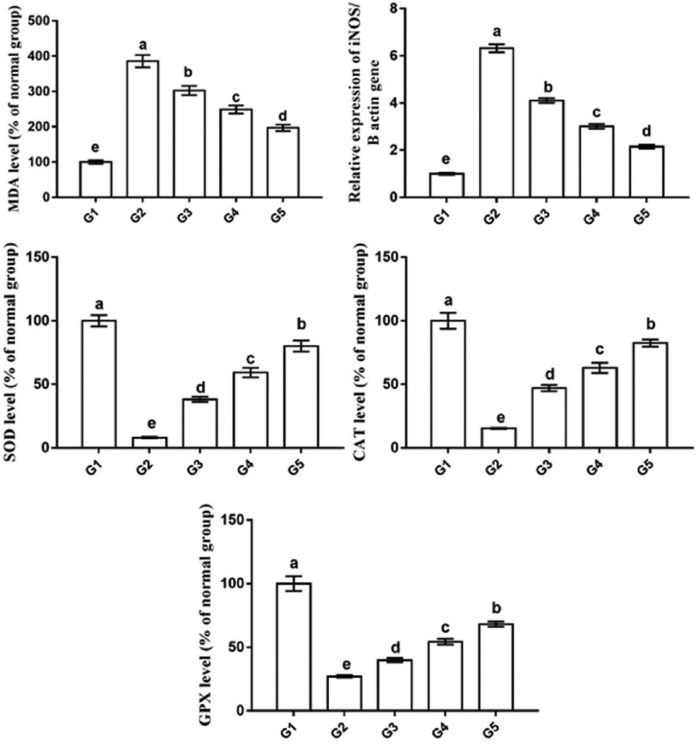
Figure 6 shows the levels of the lipid peroxidation marker (MDA), the mRNA levels of oxidative stress–related gene (iNOS), and the activities of the antioxidant enzymes (SOD, CAT, and GPX) in rat mammary tissues from all the groups. The tumor group (G2) exhibited higher MDA levels (386% ± 10.74%) and iNOS mRNA levels (6.32 ± 0.17 fold change) and lower activities of SOD (8.11% ± 0.52%), CAT (15.26% ± 0.45%), and GPX (27.12% ± 0.9%) as compared with the normal control group (G1, 100% ± 5.11%, 1 ± 0.04 fold change, 100% ± 4.47%, 100.26% ± 6.33%, 100% ± 5.77%, respectively). In contrast, rats treated with camel milk and its exosomes exhibited a significant decrease in the MDA levels and iNOS mRNA levels and a significant increase in SOD, GPX, and CAT activities, with the best results in G5 (204% ± 6.05%, 2.25 ± 0.08 fold change, 80.10% ± 4.38%, 82.52% ± 2.79%, 68.19% ± 2.01%, respectively), followed by G4 (282% ± 6.62%, 3.01 ± 0.12 fold change, 59.25% ± 3.74%, 62.9% ± 4.01%, 54.36% ± 2.21%, respectively) and then G3 (301% ± 9.49%, 4.11±0.1 fold change, 38.24% ± 2.01%, 47.11% ± 2.39%, 39.86% ± 1.7%, respectively) as compared with G2.
Figure 6.
Oxidative stress/antioxidant status of breast tumor after treatment by camel milk and its exosomes shows higher MDA level and iNOS mRNA level and lower levels of SOD, CAT, and GPX in tumor tissue. Values are expressed as mean ± SEM. Columns carrying different letters are significantly different at P < 0.05.
Camel Milk and Its Exosomes Reduced Inflammation, Angiogenesis, and Metastasis in Tumor Tissues
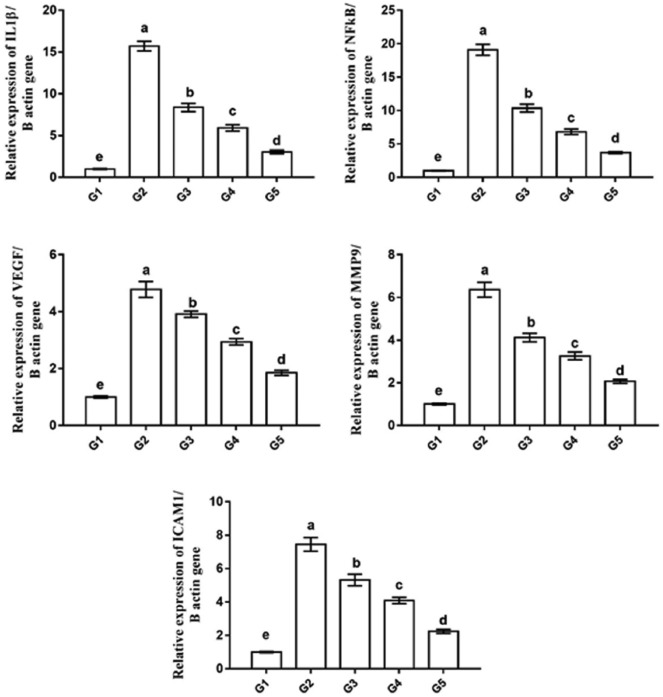
The qPCR used to monitor the changes in the expression levels of inflammation-related genes (IL1β and NFκB), angiogenesis-related gene (VEGF), and metastasis-related genes (MMP9 and ICAM1) in rat mammary tissues from all groups (Figure 7). The tumor group (G2) exhibited the most significantly increased expression levels of IL1β, NFκB, VEGF, MMP9, and ICAM1 genes (15.72 ± 0.58, 19.11 ± 0.82, 4.78 ± 0.27, 6.36 ± 0.35, and 7.45 ± 0.41 fold change, respectively) as compared with the normal control group (G1, 1 ± 0.05, 1 ± 0.05, 1 ± 0.04, 1 ± 0.04, and 1 ± 0.04 fold change, respectively). This elevated expression levels was significantly decreased in tumor tissues of rats treated with camel milk (G3) and its exosomes (G4 and G5), with the best improvement in the G5 (3.04 ± 0.22, 3.72 ± 0.15, 1.85 ± 0.09, 2.07 ± 0.09, and 2.24 ± 0.12 fold change, respectively), followed by G4 (5.92 ± 0.38, 6.83 ± 0.41, 2.94 ± 0.12, 3.26 ± 0.18, and 4.09 ± 0.18 fold change, respectively), then G3 (8.37 ± 0.49, 10.35 ± 0.58, 3.91 ± 0.11, 4.12 ± 0.20, and 5.32 ± 0.34 fold change, respectively) compared with the G2 group.
Figure 7.
Real-time PCR analysis shows changes in relative gene expression of inflammation-related genes (IL1b and NFκB), angiogenesis-related gene (VEGF), and metastasis-related genes (MMP9 and ICAM1) in mammary gland tissue of all groups. Data presented as fold change from the normal control group (G1). Values are expressed as mean ± SEM. Columns carrying different letters are significantly different at P < 0.05.
Camel Milk and Its Exosomes Increased Numbers of CD4+, CD8+, and NK1.1+ T Cells in Rat Spleen
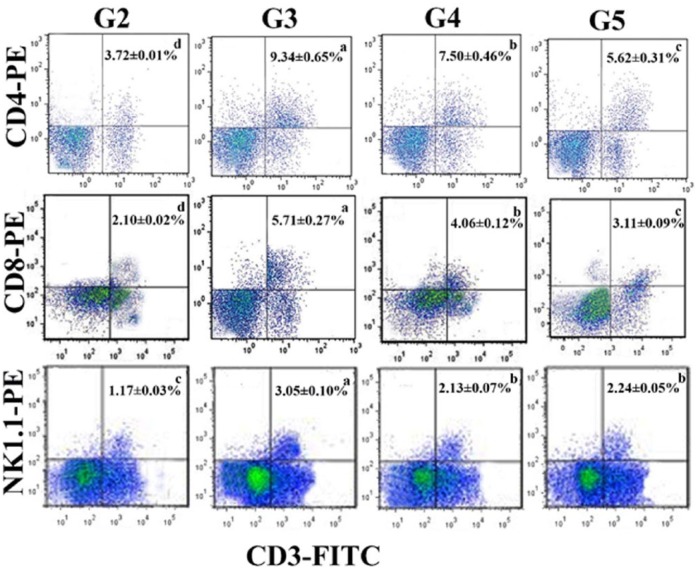
Flow cytometry data revealed a notable decrease in the number of CD3+/CD4+ (3.72% ± 0.01%), CD3+/CD8+ (2.10% ± 0.02%), and CD3+/NK1.1+ (1.17% ± 0.03%) T cells in rat spleen of tumor group (G2) (Figure 8). This number was significantly increased following treatment by camel milk (G3, 9.34% ± 0.65%, 5.71% ± 0.27%, 3.05% ± 0.10%, respectively) and its exosomes (G4: 7.50 ± 0.46, 4.06 ± 0.12, 2.13 ± 0.07 and G5: 5.62 ± 0.31, 3.11 ± 0.09, 2.24 ± 0.05). Surprisingly, rats given camel milk showed the highest number of CD3+/CD4+, CD3+/CD8+, and CD3+/NK1.1+ cells as compared with those treated by exosomes.
Figure 8.
Flow cytometry analysis of CD4, CD8, and NK1.1 on the splenocytes of G2-G5. The data are expressed as means ± SEM for triplicates. Rows containing different letters are significantly different at P < .05.
Discussion
This study was conducted to evaluate the therapeutic effect of camel milk and its exosomes on MCF7 breast cancer cells, in vitro and in vivo, and to investigate the involved underlying mechanism. Cancer cells survive through 3 mechanisms: inhibition of apoptosis, induction of inflammation, and immunosuppression.26,27 Therefore, treatment strategies targeting these 3 mechanisms can hinder cancer cell proliferation and subsequently interfere with tumor progression. In the present study, we investigated the potential role of camel milk and its exosomes on the 3 mechanisms and how this effect differs when camel milk or its exosomes were given orally or by local injection. To the best of our knowledge, this is the first study to report that administration of camel milk and its exosomes inhibited MCF7 cells proliferation and decreased breast tumor progression as revealed by (a) higher apoptosis; (b) remarkable inhibition of oxidative stress; (c) notable reduction in inflammation, angiogenesis, and metastasis; and (d) higher immune response.
It is well known that apoptosis is accompanied by higher levels of DNA fragmentation and caspase-3 activity followed by cancer cell death. In accordance, we found that administration of camel milk induced higher cytotoxic effect on MCF7 cells in vitro in a dose-dependent manner. In agreement, a previous in vitro study showed a similar inhibitory effect for camel milk on MCF7 cells.6 This inhibitory effect was also confirmed by resulted obtained from the in vivo rat model of breast cancer, which revealed a reduced size tumor, a higher level of DNA fragmentation (detected by a comet assay), and increased activity of the end result of apoptosis, caspase 3, in breast tumor tissues of rats treated with camel milk. To further confirm the induction of apoptosis, changes in the expression of the apoptotic gene, Bax, the anti-apoptotic gene, Bcl2, were investigated. As expected, rats treated by camel milk exhibited a significant upregulated Bax and downregulated Bcl2 mRNA levels in their tumor tissues as compared with the normal control and tumor groups. In agreement, previous studies also showed that camel milk induced apoptosis of MCF7 cells6 and other cell lines, including HepG2, Hela, and BT-474, mainly through a similar molecular pathway.6,10,28 This proapoptotic stimulatory effect for camel milk may be attributed to high content of lactoferrin and α-lactalbumin10; however, to date the actual mediator(s) for camel milk involved in apoptosis has not elucidated yet. Lactoferrin inhibits proliferation in colorectal cancer cells through activation of Fas-dependent apoptotic pathway29; however, in breast cancer cells, bovine milk–derived lactoferrin growth inhibitory effect was modulated by cell cycle arrest at the G1 and S phases, rather than by apoptosis.30 Concerning camel milk exosomes, to the best of our knowledge, this may be the first study to report apoptosis-mediated cytotoxic effect for camel milk exosomes when given either orally or locally in the tumor tissues. The apoptosis (indicated by higher DNA fragmentation, caspase-3 activities, Bax gene expression and lower Bcl2 gene expression) induced by exosomes was significantly higher in exosomes-treated groups than camel milk–treated groups, suggesting that camel milk induction of apoptosis, at least in part, mediated by exosomes. Taken together, the cytotoxic effect of camel milk and its exosomes on MCF7 cells and their inhibitory effect on tumor growth are probably related to apoptosis induction, but further investigations are required to unveil the actual molecular pathway regulating this effect.
The present study showed that camel milk and its exosomes significantly increased the activities of antioxidant enzymes (SOD, GPX, and CAT) and decreased the level of lipid peroxidation marker MDA and the expression of the oxidative stress marker iNOS in tumor tissues as compared to the tumor group. This suggests that oxidative stress/antioxidant pathway may be involved in the inhibitory effects of camel milk and its exosomes on tumor progression. Consistent with this, camel urine (which also has been known to have anticancer activity) also decreased MDA levels and iNOS mRNA expression in tumor tissues of mice31 and increased MDA levels in breast cancer patients.32 iNOS and NO accelerate tumor growth and progression through induction of the angiogenesis and metastasis.33 In contrast to our results, camel milk upregulates the expression of oxidative stress marker (HO-1) gene, and induces reactive oxygen species (ROS) overproduction in cancer cells in vitro.7 These contradictory results may be attributed to variation in experiment type (in vivo vs in vitro). Moreover, Dutta et al34 reported that breast cancer cell–derived exosomes can initiate oxidative stress damage to the recipient human primary mammary epithelial cells. However, we found that camel milk–derived exosomes can inhibit carcinogenesis, suggesting variable pathways mediated by exosomes depending on their origin from either cancer cells or normal cells (such as camel milk in this case).
Cancer cells have the ability to initiate inflammation through direct production of cytokines35 or through physical damage to normal tissue.36 Inflammation plays a crucial role in tumor progression from initiation to the metastatic process. Long exposure of breast tumor cells to cytokines causes epithelial-mesenchymal transition, which is the main initiator for metastasis and tumor invasion. Therefore, inhibition of the main inflammatory cytokine in the tumor, IL1β, can inhibit breast cancer cell migration and invasion.37 Subsequently, some anticancer drugs were designed to target inflammation-related pathways in cancer cells. We also found anti-inflammatory effect for camel milk and its exosomes, with better effect for exosomes, as revealed by downregulation of the inflammation-related genes, IL1β and NFκB in tumor tissues. A similar anti-inflammatory effect was reported for camel urine on the tumor tissues induced by MCF7 cells injection in mice.31 Comparing the constituents of camel milk and urine, it is possible that exosomes are the major players in this action as they are produced by nearly all cell subtypes and abundantly secreted in the milk and urine.
Cancer cells initiate new blood vessel formation (angiogenesis), which provides them with sufficient nutrient and oxygen to maintain their viability and to facilitate metastasis. Administration of camel milk and its exosomes led to significant downregulation in the expression of the angiogenesis-related gene, VEGF, in tumor tissues as compared to the tumor group. This suggests an inhibitory effect for camel milk and its exosomes on tumor microenvironment angiogenesis. In addition, camel milk and its exosomes also showed an antimetastatic effect on MCF7 cells as indicated by the in vitro wound healing assay, which revealed lower rate of cell migration with better effect when exosomes were used. To further verify the antimetastatic potential of camel milk and its exosomes, the metastasis-related genes, MMP9 and ICAM1 were investigated as well. As expected, administration of camel milk and its exosomes significantly downregulated the expression of MMP9 and ICAM1 in tumor tissues with best improvement by exosomes. Consistent with our data, Romli et al31 also reported lower levels of ICAM1 expression in tumor tissues of mice following administration of camel urine. Additionally, ICAM1 upregulation is correlated with metastatic and invasive ability of many cancer cells, including breast cancer.38 It is well known that chemokines in tumor microenvironment play an important role in migration and invasion of cancer cells.39 Here, we provide camel milk and its exosomes as new disruptors for chemotaxis-based migration of MCF7 cells.
The immune system plays an integral role in cancer growth and progression. This effect is mainly mediated by T cells, including CD8+ cytotoxic, CD4+ helper, and natural killer (NK) cells.40 Herein, we found that treatment by camel milk and its exosomes increased CD4+, CD8+, and NK1.1+ T cells in the spleen. Animals with higher T cells population have higher immunity and smaller tumor sizes. Similarly, cancer patients with high numbers of CD4+, CD8+, and NK+ cells have better prognosis and survival rate.41,42 CD4+ cells inhibit tumor growth through inhibition of inflammation-related cytokines. When CD8+ cells are activated, they kill cancer cells directly or indirectly through secretion of other cytotoxic cytokines.40 Surprisingly, treatment by camel milk resulted in higher numbers of CD4+, CD8+, and NK1.1+ cells than treatment by exosomes. Hence, camel milk may contain other immune-stimulant constituents than those present in their exosomes. In line with this notion, it was reported that camel milk contains abundant amount of vitamin C, vitamin E, selenium, zinc, lysozme, lactoferrin, lactoperoxidase, casein, and immunoglobulins,43 all of which have a potent immune-stimulatory effect. Additionally, camel milk contains unique nanobodies called variable heavy heavy (VHH), which have no typical light chains as those in normal antibodies. These very small nanobodies along camel milk IgGs can easily penetrate the cell membrane of any cells (including cancer cells) and localized intracellularly to perform variable immune-stimulatory functions.44 It would be worthwhile to check whether camel milk–derived exosomes carry any of these immune-stimulant constituents. Therefore, further investigations are required to check this possibility.
Several studies demonstrated that tumor exosomes play a crucial role in cancer progression by modulating local cellular microenvironments.45 Cross-talk, mediated by exosomes, between normal (healthy) cells and cancer cells in tumor microenvironment has not been fully understood yet. It is likely that transfer of exosomes among the cells in the tumor microenvironment could either induce or inhibit tumor progression depending on their source. Subsequently, we can divide exosomes according their origin into; good and bad exosomes. An example of good exosomes is the camel milk-derived exosomes which in this study showed better anticancer effect (inhibition of the growth and metastatic potential of MCF7 cells) than camel milk. The phospholipid membrane enclosing exosomes protects exosomal contents (DNA, RNA, protein) from degradation by digestive enzymes and digestive system acidic condition.16 This gastric stability may explain better anti-cancer effect for exosomes when given orally than camel milk. The absorbed exosomes will take a few hours to reach the target and may undergo dilution, by distribution all over the body, before reaching the tumor tissues. This can explain why locally injected exosomes in tumor tissues give better results than those given orally.
Conclusion
The present study demonstrated for the first time that administration of camel milk and its exosomes causes cell death of MCF7 cells through induction of apoptosis and inhibition of inflammation, oxidative stress, angiogenesis, and invasion/metastasis. Thus, camel milk and its exosomes could be useful in future as anticancer agent for cancer patients. However, additional investigations are required to confirm whether this treatment strategy is clinically relevant in human subjects.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Deanship of the Scientific Research in Northern Border University with code number (ID) MED-2017-1-7-F-7101.
ORCID iDs: Abdelnaser A. Badawy  https://orcid.org/0000-0002-4966-3839
https://orcid.org/0000-0002-4966-3839
Mohammed A. El-Magd  https://orcid.org/0000-0002-3314-9202
https://orcid.org/0000-0002-3314-9202
References
- 1. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang X, Shapiro DJ. The immune system and inflammation in breast cancer. Mol Cell Endocrinol. 2014;382:673-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deng Y, Sriwiriyajan S, Tedasen A, Hiransai P, Graidist P. Anti-cancer effects of piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J Ethnopharmacol. 2016;188:87-95. [DOI] [PubMed] [Google Scholar]
- 4. Mohamed WA, Schaalan MF, El-Abhar HS. Camel milk: potential utility as an adjunctive therapy to peg-IFN/RBV in HCV-4 infected patients in Egypt. Nutr Cancer. 2015;67:1305-1313. [DOI] [PubMed] [Google Scholar]
- 5. Abbas S, Imran R, AaliaNazir A, Qamar M, Sarfraz L. Effect of camel milk supplementation on blood parameters and liver function of hepatitis patients. Am J Ethnomed. 2014;1:129-146. [Google Scholar]
- 6. Korashy HM, Maayah ZH, Abd-Allah AR, El-Kadi AO, Alhaider AA. Camel milk triggers apoptotic signaling pathways in human hepatoma HepG2 and breast cancer MCF7 cell lines through transcriptional mechanism. J Biomed Biotechnol. 2012;2012:593195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korashy HM, El Gendy MAM, Alhaider AA, El-Kadi AO. Camel milk modulates the expression of aryl hydrocarbon receptor-regulated genes, Cyp1a1, Nqo1, and Gsta1, in murine hepatoma Hepa 1c1c7 cells. J Biomed Biotechnol. 2012;2012:782642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El Miniawy HMF, Ahmed KA, Tony MA, Mansour SA, Khattab SMM. Camel milk inhibits murine hepatic carcinogenesis, initiated by diethylnitrosamine and promoted by phenobarbitone. Int J Vet Sci Med. 2014;2:136-141. [Google Scholar]
- 9. Habib HM, Ibrahim WH, Schneider-Stock R, Hassan HM. Camel milk lactoferrin reduces the proliferation of colorectal cancer cells and exerts antioxidant and DNA damage inhibitory activities. Food Chem. 2013;141:148-152. [DOI] [PubMed] [Google Scholar]
- 10. Almahdy O, El-Fakharany EM, El-Dabaa E, Ng TB, Redwan EM. Examination of the activity of camel milk casein against hepatitis C virus (genotype-4a) and its apoptotic potential in hepatoma and hela cell lines. Hepat Mon. 2011;11:724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Bio. 2012;13:328-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Javeed N, Mukhopadhyay D. Exosomes and their role in the micro-/macro-environment: a comprehensive review. J Biomed Res. 2017;31:386-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen T, Xie MY, Sun JJ, et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep. 2016;6:33862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zempleni J, Aguilar-Lozano A, Sadri M, et al. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J Nutr. 2017;147:3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: Basic science and clinical applications. Int J Mol Sci. 2013;14:5338-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006:(chapter 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 18. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329-333. [DOI] [PubMed] [Google Scholar]
- 19. Maghraby AS, Mohamed MA, Abdel-Salam AM. Anti-schistosomal activity of colostral and mature camel milk on schistosoma mansoni infected mice. Asia Pac J Clin Nutr. 2005;14:432-438. [PubMed] [Google Scholar]
- 20. Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206-221. [DOI] [PubMed] [Google Scholar]
- 22. El-Magd MA, Kahilo KA, Nasr NE, Kamal T, Shukry M, Saleh AA. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia. 2017;49. doi: 10.1111/and.12750. [DOI] [PubMed] [Google Scholar]
- 23. Abd-Allah SH, Shalaby SM, Abd-Elbary E, Saleh AA, El-Magd MA. Human peripheral blood CD34+ cells attenuate oleic acid-induced acute lung injury in rats. Cytotherapy. 2015;17:443-453. [DOI] [PubMed] [Google Scholar]
- 24. El-Magd MA, Khamis A, Eldeen NSK, Ibrahim WM, Salama AF. Trehalose enhances the antitumor potential of methotrexate against mice bearing ehrlich ascites carcinoma. Biomed Pharmacother. 2017;92:870-878. [DOI] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCT method. Methods. 2001;25:402-408. [DOI] [PubMed] [Google Scholar]
- 26. Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [DOI] [PubMed] [Google Scholar]
- 28. Hasson SS, Al-Busaidi J, Al-Qarni A, et al. In vitro apoptosis triggering in the BT-474 human breast cancer cell line by lyophilised camel’s milk. Asian Pac J Cancer Prev. 2015;16:6651-6661. [DOI] [PubMed] [Google Scholar]
- 29. Fujita K, Matsuda E, Sekine K, Iigo M, Tsuda H. Lactoferrin enhances fas expression and apoptosis in the colon mucosa of azoxymethane-treated rats. Carcinogenesis. 2004;25:1961-1966. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Nicolau A, Lima CF, Rodrigues LR. Bovine lactoferrin induces cell cycle arrest and inhibits mTOR signaling in breast cancer cells. Nutr Cancer. 2014;66:1371-1385. [DOI] [PubMed] [Google Scholar]
- 31. Romli F, Abu N, Khorshid FA, et al. The growth inhibitory potential and antimetastatic effect of camel urine on breast cancer cells in vitro and in vivo. Integr Cancer Ther. 2017;16:540-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kilic N, Taslipinar YM, Guney Y, Tekin E, Onuk E. An investigation into the serum thioredoxin, superoxide dismutase, malondialdehyde, and advanced oxidation protein products in patients with breast cancer. Ann Surg Oncol. 2014;21:4139-4143. [DOI] [PubMed] [Google Scholar]
- 33. Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521-534. [DOI] [PubMed] [Google Scholar]
- 34. Dutta S, Warshall C, Bandyopadhyay C, Dutta D, Chandran B. Interactions between exosomes from breast cancer cells and primary mammary epithelial cells leads to generation of reactive oxygen species which induce DNA damage response, stabilization of p53 and autophagy in epithelial cells. PLoS One. 2014;9:e97580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonavita E, Galdiero MR, Jaillon S, Mantovani A. Phagocytes as corrupted policemen in cancer-related inflammation. Adv Cancer Res. 2015;128:141-171. [DOI] [PubMed] [Google Scholar]
- 36. Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han J, Bae SY, Oh SJ, et al. Zerumbone suppresses IL-1β-induced cell migration and invasion by inhibiting IL-8 and MMP-3 expression in human triple-negative breast cancer cells. Phytother Res. 2014;28:1654-1660. [DOI] [PubMed] [Google Scholar]
- 38. Roland CL, Harken AH, Sarr MG, Barnett CC., Jr. ICAM-1 expression determines malignant potential of cancer. Surgery. 2007;141:705-707. [DOI] [PubMed] [Google Scholar]
- 39. Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of cxcr4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157-167. [DOI] [PubMed] [Google Scholar]
- 40. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877-884. [DOI] [PubMed] [Google Scholar]
- 42. Malmberg KJ, Bryceson YT, Carlsten M, et al. NK cell-mediated targeting of human cancer and possibilities for new means of immunotherapy. Cancer Immunol Immunother. 2008;57:1541-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. El Agamy ESI, Ruppanner R, Ismail A, Champagne CP, Assaf R. Antibacterial and antiviral activity of camel milk protective proteins. J Dairy Res. 2009;59:169-175. [DOI] [PubMed] [Google Scholar]
- 44. Hamad EM, Abdel-Rahim EA, Romeih EA. Beneficial effect of camel milk on liver and kidneys function in diabetic sprague-dawley rats. Int J Dairy Sci. 2011;6:190-197. [Google Scholar]
- 45. Chauhan R, Lahiri N. Tissue-and serum-associated biomarkers of hepatocellular carcinoma. Biomark Cancer. 2016;8(suppl 1):37-55. [DOI] [PMC free article] [PubMed] [Google Scholar]