Abstract
Purpose: The aim of present study was to study the effect of osteopathic manipulation on pain relief and quality of life improvement in hospitalized oncology geriatric patients. Methods: A nonrandomized controlled clinical trial was performed in the Oncology Rehabilitation Unit, Milan, Italy, from September 2015 to March 2016. Twenty-three older cancer patients were enrolled and allocated in 2 experimental groups: the study group (OMT group, N = 12) underwent osteopathic manipulative treatment in addition to physiotherapy, and the control group (PT group, N = 12) underwent only physiotherapy. At enrollment (T0), 24 recruited oncology patients completed the sociodemographic forms and were evaluated for pain intensity and quality of life by an external examiner. All patients were revaluated every week (T1, T2, T3, and T4) for pain intensity and at the end of the study treatment (T4) for quality of life. A standard level of significance was set at α < .05. Results: The 2 groups did not significantly differ in age (P = .682), body mass index (P = .413), or gender (P = 1). The osteopathic manipulative treatment added to physiotherapy produced a significant reduction in Numeric Rating Scale (NRS) scores both at T2 (P = .004) and T4 (P = .002). The difference in quality of life improvements between T0 and T4 was not statistically significant. NRS improved in the PT group at T4. Between-group analysis of NRS and quality of life with the Mann-Whitney test did not show any significant difference between the 2 treatments. Conclusions: Our study showed a significant improvement in pain relief and a nonsignificant improvement in quality of life in hospitalized geriatric oncology patients during osteopathic manipulative treatment. Trial Registration: Protocol registered on Clinicaltrials.gov (NCT03142386).
Keywords: osteopathic manipulative treatment, malignancy, geriatrics, clinic, cancer survivors, rehabilitation
Introduction
Cancer is mainly a disease of aging and approximately 60% of all cancers and 70% of cancer mortality involves people aged 65 years and older.1 The number of geriatric cancer patients is projected to increase significantly over the next 20 years.2
Aging is an individualized process, characterized by physiologic and psychosocial changes that can affect tumor biology and decision making for cancer treatment.3 The most common complaint is chronic pain with an incidence between 83% and 93% among older persons living in institutional settings. Pain can limit participation in daily activities.4 Pain results from changes in skin, bone, nerve, and other tissues due to direct tumor involvement or metastases, treatment effects or a combination of these.5 In particular, the occurrence of these symptoms may decrease as the perception of all types of pain becomes more blunted with age.6 Furthermore, the manifestations of pain may be atypical in the elderly with cognitive impairment, another age-related problem, with delirium as one of the most common atypical manifestations.7
Considering the medical complexity of older cancer patients,3 pain management is not simple and is one of the significant aspects of oncology care. Pain has a prevalence between 40% to 90% in oncology patients.8 The treatment of cancer in elderly patients needs to be personalized given the diversity of the older population in terms of life expectancy, functional impairments, comorbidity and social, economic, and emotional support.9 In some oncology care settings, patients typically receive only pharmacological support to cope with chronic pain.10
It could be useful to introduce some alternative strategies that can relieve cancer pain11; in fact, the American Geriatrics Society has stated that pharmacologic pain management methods used in conjunction with nonpharmacologic methods can relieve persistent pain among older adults.12
Complementary and alternative medicine (CAM) therapy is emerging as an important concept for pain management in cancer patients.13 One of the effective complementary therapies for pain management is osteopathic manipulative treatment (OMT), a nonpharmacologic way of addressing chronic pain in older persons.14,15 OMT is the therapeutic application of a variety of techniques to improve physiologic functions and restore homeostasis altered by somatic dysfunction16 (ICD-101CM Diagnosis Code M99.09-09), resulting in pain and in peripheral and visceral inflammation.17,18
Several studies have demonstrated the anti-inflammatory effect of OMT, showing a reduction of several cytokines (IL-6, IL-12), substance P, and TNFα.19,20 In addition, the effect on the autonomic nervous system has been suggested as another mechanism by which OMT can act. This hypothesis is based on the increase of parasympathetic activity leading to a trophotropic effect of OMT.21 Relevant effects of OMT were found for reducing pain and improving functional status in patients with acute and chronic pain of nociceptive or neuropathic origin in different clinical conditions.22-24 On this basis, the hypothesis is that OMT could reduce the perception of pain and improve physical function in geriatric oncology patients in an institutional setting.
The aim of this study was to evaluate effects of OMT versus physiotherapy in chronic pain relief and quality of life of hospitalized oncology geriatric patients.
Methods
Study Design
The present study was a nonrandomized pilot controlled clinical trial with allocation of participants into the 2 experimental groups using osteopathic manipulative treatment and physiotherapy. The protocol was specifically approved by the local ethical committee and registered in Clinicaltrials.gov (NCT03142386). An ethical obligation with the Oncology Rehabilitation Unit was kept providing osteopathic treatment to all patients willing to participate to the study. As consequence, after the study, all control patients have received the OMT.
Population
Twenty-four older oncology patients were recruited in the Oncology Rehabilitation Unit in Milan, Italy, from September 2015 to March 2016. This study was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all subjects. Inclusion criteria were postsurgical cancer patients, male and female, age ⩾65 years, with an oncology prognosis of 6 to 24 months and chronic pain for at least 3 months with an intensity score higher than 3, measured with the Numeric Rating Scale (NRS). Exclusion criteria were patients receiving chemotherapy or radiotherapy treatment at the time of the study, with mental disorders (Mini-Mental State Examination [MMSE] = 10-20), with infection, anticoagulation therapy, cardiopulmonary disease, or clinical instability postsurgery. Oncology patients were admitted for rehabilitation after cancer surgery. The main cancers were colorectal cancer, osteosarcoma, spinal metastasis from breast and prostatic cancer, and kidney cancer. Based on selection criteria and oncology prognosis, patients were selected and allocated into 2 groups by the physician geriatrist of the department. The study group (OMT group, N = 12) underwent osteopathic manipulative treatment in addition to physiotherapy and control group (PT group, N = 12) underwent only physiotherapy. All the selected patients were allowed to use the nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Outcome Measurements
The NRS is a validated scale to measure pain intensity, with numbers between 0 and 10, with 0 representing absence of pain and 10 the worst possible pain experienced by the patient.25
The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQC30) is a validated multidimensional health-related quality of life (HRQOL) questionnaire composed of 6 functional scales, 3 symptom scales, and several additional single item scales. We used only 3 scales for quality of life: the Global Health Status scale (GHS), Financial Difficulties scale (FD), and Summary Score (SS) because these are more specific for oncology patients.26,27
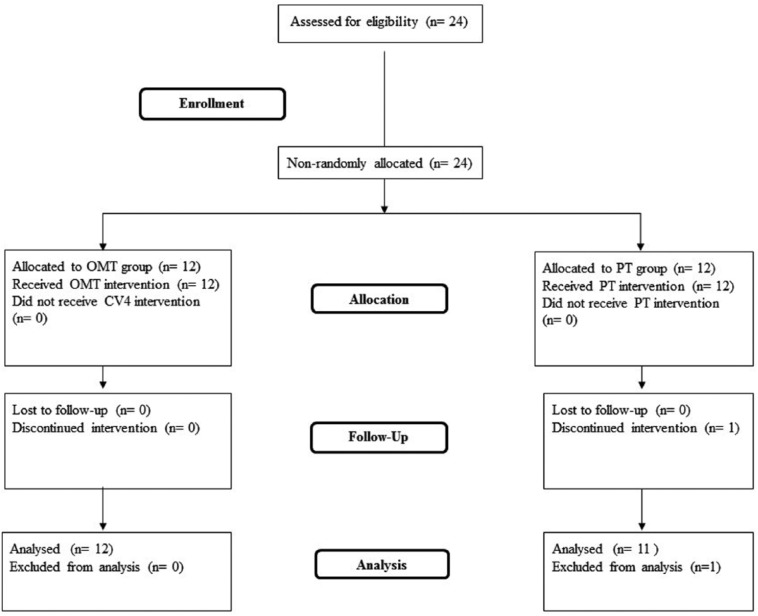
At enrollment (T0), the 23 recruited cancer patients completed the sociodemographic forms and were evaluated for pain intensity and quality of life by an external examiner blinded to the group assignment of the patients. Patients’ quality of life was reevaluated at the end of treatment (T4) while pain intensity was analyzed every week (T1, T2, T3, and T4), over a total of 4 weeks of study. Only T0, T2, and T4 scores were considered in the analyses (Figure 1).
Figure 1.
Flowchart of the study. OMT, osteopathic manipulative treatment; PT, physiotherapy.
Osteopathic Manipulative Treatment
The OMT, based on osteopathic principles of body unit, structure-function relationship, and homeostasis, was designed for each patient on the basis of the results of the osteopathic examination. Diagnosis and treatment were founded on 5 models: biomechanics, neurologic, metabolic, respiratory-circulatory, and behavior.16,28 The OMT protocol was administered by an osteopath with clinical experience of 10 years in one-on-one individual sessions. The techniques used were: dorsal and lumbar soft tissue, rib raising, back and abdominal myofascial release, cervical spine soft tissue, suboccipital decompression, and sacroiliac myofascial release. Back and abdominal myofascial release techniques are used to improve back movement and internal abdominal pressure. Suboccipital decompression involves traction at the base of the skull, which is considered to release restrictions around the vagus nerve, theoretically improving nerve function. Sacroiliac myofascial release is used to improve sacroiliac joint movement and to reduce ligament tension. Strain-counterstrain and muscle energy technique are used to diminish the presence of trigger points and their pain intensity. OMT was repeated once every week during 4 weeks for each group, for a total of 4 treatments. Each treatment lasted 45 minutes.
Physiotherapy Protocol
The PT protocol is based on passive mobilization, active-assisted or active-resisted exercises, walking and local- and global-resisted exercises, as well as on proprioceptive neuromuscular facilitation applied over joints and tight/painful muscles. The use of physical therapy was not expected to result in pain relief. The planning of exercise depended on patient response to the intervention. The PT protocol was performed every day for 4 weeks for each group in one-on-one individual sessions. Each treatment was administered by a physiotherapist of the unit and lasted 30 minutes.
Statistical Analysis
One patient of the PT group dropped out after T1 and a last-observation-carried-forward approach was applied, substituting the NRS missing score at T2 and T4 with the NRS T1 score and the QLQC30 scales missing scores at T4 with the T0 scores.
Preliminary analyses evidenced significant violations of parametric test assumptions (eg, normality distribution when tested with the Schapiro-Wilk test). Considering the limited sample size of the 2 groups and that in such cases the violations of parametric test assumption might lead to relevant distortions in the results, we decided to use nonparametric tests with exact P value calculation in our analyses. The Mann-Whitney test, a nonparametric test equivalent to the independent-sample t test, was used to investigate potential between-group differences in age, body mass index, and outcome scores at T0, while the Fisher’s exact test was used on gender. The former test was also performed to investigate whether the 2 treatments were differently efficacious in improving the levels of the outcome measurements throughout the treatment period. The 2 groups were compared on the T2-T0 and T4-T0 change scores of NRS, and on the T4-T0 change scores of the SS, GHS, and FD scores of the QLQC30. Changes occurring within each group were investigated using the Wilcoxon signed-rank test, a nonparametric test equivalent to the paired-sample t test.
A standard level of significance of α < .05 was maintained in the analyses. Considering that this is a pilot study aiming to explore novel hypotheses, the decision was to give more importance in having a reduced probability of false negative (type II error) at the expense of a higher probability of false positive (type I error). Additional studies will be necessary to provide further evidence for any significant result found by this study. All data analyses were performed using R (R Core Team, R Project for Statistical Computing, 2017, https://www.r-project.org/).
Results
Twenty-three elderly cancer patients were analyzed and the 2 treatment groups did not significantly differ on age (OMT group: median = 76.5 years, interquartile range [IQR] = 8.75; PT group: median = 77 years, IQR = 14; P = .682), body mass index (OMT group: median = 27.02 kg/m2, IQR = 3.775; PT group: median = 25.71 kg/m2, IQR = 7.225; P = .413), and gender (OMT group: females = 7, males = 5; PT group: females = 7, males = 4; P = 1). Data are reported in Table 1. The 2 groups also did not significantly differ on the QLQC30-SS (P = .260), QLQC30-GHS (P = .748), and QLQC30-FD (P = .147) scores at T0, while the NRS scores at T0 were significantly higher in the OMT group than in the PT group (P = .048) (Table 2).
Table 1.
General Characteristics of the Study Participants.
| OMT Group (N = 12) | PT Group (N = 11) | P | |
|---|---|---|---|
| Demographics | |||
| Age (y), mean ± SD | 76.50 ± 8.34 | 76.50 ± 8.34 | .68a |
| Gender (female), % | 27 | 31 | 1.00b |
| BMI (kg/m2), mean ± SD | 25.10 ± 4.53 | 24.16 ± 4.73 | .41a |
| Type of cancer, % | |||
| Colorectal cancer | 46 | 43 | .88b |
| Breast cancer | 23 | 25 | .78b |
| Prostatic cancer | 21 | 20 | .85b |
| Osteosarcoma | 10 | 12 | .56b |
| Duration (mo), mean ± SD | 32.12 ± 3.52 | 30.56 ± 4.01 | .58a |
Abbreviations: BMI, body mass index; OMT, osteopathic manipulative treatment; PT, physiotherapy.
Obtained from nonparametric test, Mann-Whitney test.
Obtained from Fisher’s exact test.
Table 2.
Outcome Scores at T0, T2, and T4.a
| OMT Group (N = 12) |
PT Group (N = 11) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T2 | T4 | Pc (T0-T2) | Pc (T0-T4) | T0 | T2 | T4 | Pc (T0-T2) | Pc (T0-T4) | P b | |
| NRS | 6.08 ± 3.40 | 3.25 ± 2.89 | 2.67 ± 2.67 | .004 | .002 | 3.36 ± 2.20 | 2.00 ± 2.05 | 1.64 ± 1.63 | 0.158 | .047 | .150 |
| QLQC30-SS | 59.79 ± 19.59 | — | 65.10 ± 15.10 | .058 | 70.28 ± 8.78 | 76.67 ± 7.29 | .005 | .650 | |||
| QLQC30-GHS | 39.58 ± 28.68 | — | 54.86 ± 19.93 | .074 | 42.42 ± 19.88 | 53.03 ± 17.98 | .031 | .700 | |||
| QLQC30-FD | 30.55 ± 41.34 | — | 22.22 ± 41.03 | .500 | 6.06 ± 13.48 | 6.06 ± 13.48 | 1.000 | .490 | |||
Abbreviations: FD, Financial Difficulties scale; GHS, Global Health Status scale; NRS, Numeric Rating Scale; OMT, osteopathic manipulative treatment; PT, physiotherapy; QLQC30, Quality of Life Questionnaire Core 30; SS, summary score.
Values in table are mean ± SD. Alpha level is set at .05. Boldfaced P values indicate statistical significance.
Between-group differences.
Within-group differences.
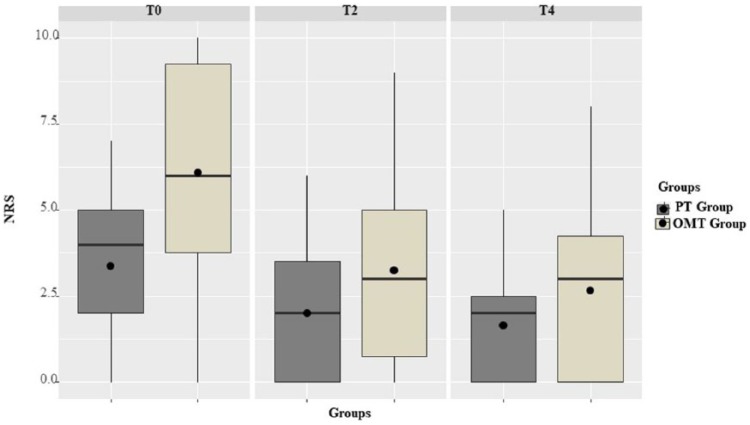
OMT added to PT produced a significant reduction in subjects’ NRS scores both at T2 (P = .004) and T4 (P = .002) (Figure 2). Differences in quality of life improvement between T0 and T4 for the OMT group were not statistically significant (QLQC30-SS: P = .058; QLQC30-GHS: P = .074; QLQC30-FD: P = .500). The NRS score reduction produced by PT alone showed a significant difference only at T4 (P = .047) and not at T2 (P = .158). Quality of life in the PT group was partially improved at T4, with a significant increase in QLQC30-SS (P = .005) and QLQC30-GHS (P = 0.031) while the QLQC30-FD scores did not improve (P = 1) (Table 2).
Figure 2.
Box plots of the Numeric Rating Scale (NRS) scores at the T0, T2, and T4. OMT, osteopathic manipulative treatment; PT, physiotherapy.
Whiskers of the plot extends to the minimum and maximum scores. The lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles). Black line inside the hinges corresponds to the median. Black-filled circles correspond to the mean.
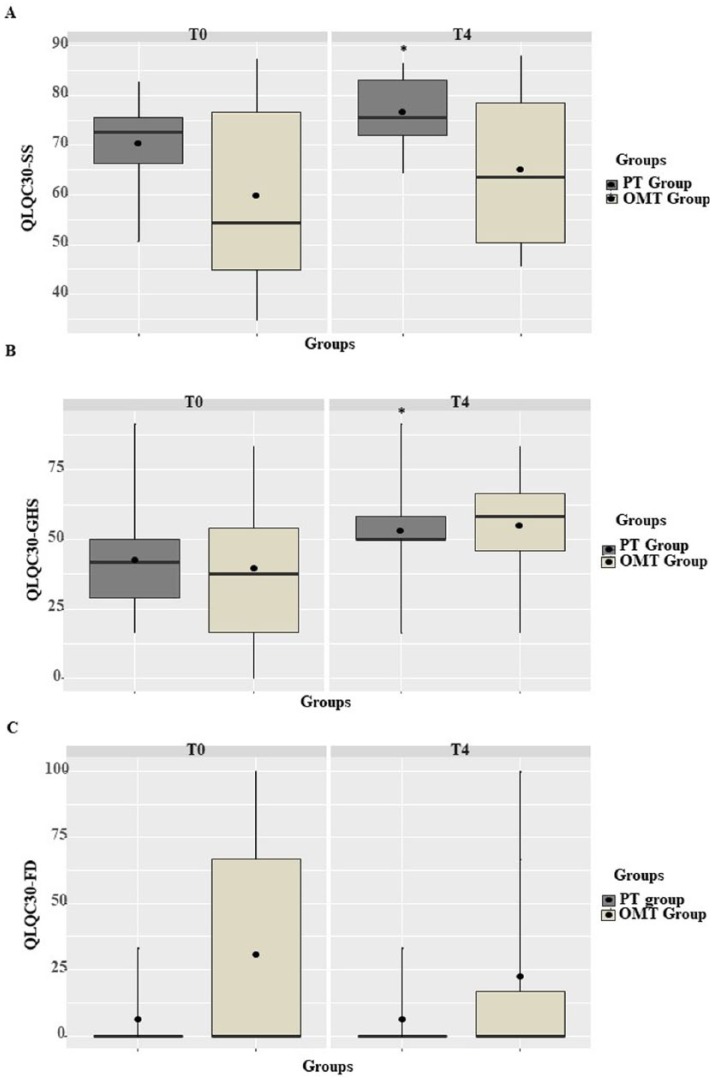
However, between-group analysis with the Mann-Whitney test did not show any significant difference between the two treatments (NRS change score between T2-T0: p = 0.266; NRS change score between T4-T0: p = 0.149; QLQC30-SS change score between T4-T0: p = 0.651; QLQC30-GHS change score between T4-T0: p = 0.699; QLQC30-FD change score between T4-T0: p = 0.491) (Figure 3).
Figure 3.
Box plots of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQC30) scores at the T0 and T4. OMT, osteopathic manipulative treatment; PT, physiotherapy.
Whiskers of the plot extends to the minimum and maximum scores. The lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles). Black line inside the hinges corresponds to the median. Black-filled circles correspond to the mean.
Adverse Events
No adverse events occurred during the study.
Discussion
The novel contribution of this study is to evaluate the effectiveness of OMT on pain relief and improvement of quality of life in geriatric oncology patients. Our results showed that OMT significantly reduced pain intensity in a short period, after only 2 treatments (2 weeks) and the improvement continued until the end of treatment (after 4 weeks). Similar results were obtained with PT, but NRS differed only after 4 weeks of treatments and the NRS score difference of the PT group was minor compared with OMT (−1.73 vs −3.42, respectively). These data agree with results of previous studies, which confirmed the effectiveness of OMT on management of chronic pain in short and medium period compared with rehabilitation therapy.29 In particular, Kuchera17 showed an algorithm for integration of osteopathic principles and osteopathic treatment in diagnosis and management of chronic pain. This algorithm suggests the rationale for applying osteopathic principles and treatment to patients with chronic pain. It is structured to identify underlying etiologies, using an osteopathic differential diagnosis, and the holistic impact of pain on the body unit. There are 2 main factors that guide the planning of osteopathic treatment strategies: the patient’s capability to improve his or her own homeostatic response and the patient’s underlying pathophysiologic status as effect of palpated somatic dysfunction. Osteopathic treatment protocols formulated from this algorithm should include the interdependence of osteopathic principles of body unit, homeostasis and the relationship between structure and function for the management of chronic pain. The recent osteopathic treatment guidelines for low back pain, published in 2016,16 confirmed the effectiveness of osteopathic treatment on chronic pain and the importance of setting up the treatment on the osteopathic algorithm for chronic pain. In literature, there are many studies that show the effectiveness of this approach in various clinical conditions associated with chronic pain, such as musculoskeletal disorders, spinal cord injury,18 geriatric disorders,14 cardiological disorders,30 gynecological disorders,31 pregnancy,32 and gastrointestinal disorders.33
We obtained an improvement of quality of life with OMT, but it was not statistically significant. Physiotherapy obtained significant differences in 2 quality of life variables, but these did not differ statistically from the results of OMT. This may be due to difficulties in selecting a proper quality of life assessment tool for older cancer patients. In fact, the traditional cancer measures of performance status are not adequate in older patients, in particular in geriatric-specific measures, such as activities of daily living. The QLQC30 scale is a validated quality of life instrument for cancer patients in the clinical research setting, but not for heterogeneous hospitalized geriatric cancer patients,27 who sometimes feel too ill to fill out the questionnaire. This could be a bias in terms of reporting of results and may explain the lack of statistically significant effects of OMT on quality of life.
The present study has some limitations. The small sample size, the absence of randomization and the lack of Comprehensive Geriatric Assessment, a multidisciplinary evaluation of older patients, in addition to the issue of bias of the QLQC30 scale, do not allow us to verify the stability of the significant results.
In summary, our study showed a significant improvement in pain relief and a nonsignificant improvement in quality of life in hospitalized geriatric oncology patients during osteopathic manipulative treatment. This is the first osteopathic study in the cancer context and future studies are needed to confirm these encouraging results.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Chiara Arienti  https://orcid.org/0000-0003-0787-6075
https://orcid.org/0000-0003-0787-6075
Stefano Negrini  https://orcid.org/0000-0002-1878-2747
https://orcid.org/0000-0002-1878-2747
References
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [DOI] [PubMed] [Google Scholar]
- 2. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758-2765. [DOI] [PubMed] [Google Scholar]
- 3. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(suppl 1):i1-i57. [DOI] [PubMed] [Google Scholar]
- 5. Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236-2247. [DOI] [PubMed] [Google Scholar]
- 6. Farrell MJ. Age-related changes in the structure and function of brain regions involved in pain processing. Pain Med. 2012;13(suppl 2):S37-S43. [DOI] [PubMed] [Google Scholar]
- 7. Balducci L, Dolan D. Palliative care of cancer in the older patient. Curr Oncol Rep. 2016;18:70. [DOI] [PubMed] [Google Scholar]
- 8. van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449. [DOI] [PubMed] [Google Scholar]
- 9. Kogan AC, Wilber K, Mosqueda L. Person-centered care for older adults with chronic conditions and functional impairment: a systematic literature review. J Am Geriatr Soc. 2016;64:e1-e7. [DOI] [PubMed] [Google Scholar]
- 10. Robb WJ. Self-healing: a concept analysis. Nurs Forum. 2006;41:60-77. [DOI] [PubMed] [Google Scholar]
- 11. Lee SH, Kim JY, Yeo S, Kim SH, Lim S. Meta-analysis of massage therapy on cancer pain. Integr Cancer Ther. 2015;14:297-304. [DOI] [PubMed] [Google Scholar]
- 12. American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain Med. 2009;10:1062-1083. [DOI] [PubMed] [Google Scholar]
- 13. Deng G, Cassileth B. Integrative oncology: an overview. Am Soc Clin Oncol Educ Book. 2014:233-242. doi: 10.14694/EdBook_AM.2014.34.233 [DOI] [PubMed] [Google Scholar]
- 14. Pannunzio A, Salemi F, Dacco S, Arienti C. Osteopathic manipulative treatment results in sustained relief from spinal pain in older patients: a pilot crossover study. Int J Med Res Health Sci. 2016;5:128-135. [Google Scholar]
- 15. Channell MK, Wang Y, McLaughlin MH, Ciesielski J, Pomerantz SC. Osteopathic manipulative treatment for older patients: a National Survey of Osteopathic Physicians. J Am Osteopath Assoc. 2016;116:136-143. [DOI] [PubMed] [Google Scholar]
- 16. Task Force on the Low Back Pain Clinical Practice Guidelines. American Osteopathic Association guidelines for osteopathic manipulative treatment (OMT) for patients with low back pain. J Am Osteopath Assoc. 2016;116:536-549. [DOI] [PubMed] [Google Scholar]
- 17. Kuchera ML. Applying osteopathic principles to formulate treatment for patients with chronic pain. J Am Osteopath Assoc. 2007;107(10 suppl 6):ES28-ES38. [PubMed] [Google Scholar]
- 18. Arienti C, Daccò S, Piccolo I, Redaelli T. Osteopathic manipulative treatment is effective on pain control associated to spinal cord injury. Spinal Cord. 2011;49:515-519. [DOI] [PubMed] [Google Scholar]
- 19. Licciardone JC, Kearns CM, Hodge LM, Bergamini MV. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: results from the OSTEOPATHIC trial. J Am Osteopath Assoc. 2012;112:596-605. [DOI] [PubMed] [Google Scholar]
- 20. Cicchitti L, Martelli M, Cerritelli F. Chronic inflammatory disease and osteopathy: a systematic review. PLoS One. 2015;10:e0121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruffini N, D’Alessandro G, Mariani N, Pollastrelli A, Cardinali L, Cerritelli F. Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: randomized controlled trial. Front Neurosci. 2015;9:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vismara L, Cimolin V, Menegoni F, et al. Osteopathic manipulative treatment in obese patients with chronic low back pain: a pilot study. Man Ther. 2012;17:451-455. [DOI] [PubMed] [Google Scholar]
- 23. Gamber RG, Shores JH, Russo DP, Jimenez C, Rubin BR. Osteopathic manipulative treatment in conjunction with medication relieves pain associated with fibromyalgia syndrome: results of a randomized clinical pilot project. J Am Osteopath Assoc. 2002;102:321-325. [PubMed] [Google Scholar]
- 24. Schwerla F, Kaiser AK, Gietz R, Kastner R. Osteopathic treatment of patients with long-term sequelae of whiplash injury: effect on neck pain disability and quality of life. J Altern Complement Med. 2013;19:543-549. [DOI] [PubMed] [Google Scholar]
- 25. Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20:88-93. [DOI] [PubMed] [Google Scholar]
- 26. Giesinger JM, Kieffer JM, Fayers PM, et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79-88. [DOI] [PubMed] [Google Scholar]
- 27. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 28. Sciomachen P, Arienti C, Bergna A, et al. Core competencies in osteopathy: Italian register of osteopaths proposal. Int J Osteopath Med. 2018;27:1-5. [Google Scholar]
- 29. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cerritelli F, Carinci F, Pizzolorusso G, et al. Osteopathic manipulation as a complementary treatment for the prevention of cardiac complications: 12-months follow-up of intima media and blood pressure on a cohort affected by hypertension. J Bodyw Mov Ther. 2011;15:68-74. [DOI] [PubMed] [Google Scholar]
- 31. Ruffini N, D’Alessandro G, Cardinali L, Frondaroli F, Cerritelli F. Osteopathic manipulative treatment in gynecology and obstetrics: a systematic review. Complement Ther Med. 2016;26:72-78. [DOI] [PubMed] [Google Scholar]
- 32. Hensel KL, Buchanan S, Brown SK, Rodriguez M, Cruser dA. Pregnancy research on osteopathic manipulation optimizing treatment effects: the PROMOTE study. Am J Obstet Gynecol. 2015;212:108.e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Müller A, Franke H, Resch KL, Fryer G. Effectiveness of osteopathic manipulative therapy for managing symptoms of irritable bowel syndrome: a systematic review. J Am Osteopath Assoc. 2014;114:470-479. [DOI] [PubMed] [Google Scholar]