Abstract
Purpose: Ninety percent of patients with advanced cancer have moderate to severe pain, and up to 70% of patients with cancer pain do not receive adequate pain relief. This randomized controlled clinical trial was designed to determine the feasibility and evaluate the effects and safety of intradermal acupuncture (IA) in patients who were being administered analgesics for cancer pain. Methods: Advanced cancer patients experiencing pain were randomly assigned to IA or sham IA treatment for 3 weeks (15 patients for each group), wherein the CV12, bilateral ST25, LI4, LR3, PC06, and Ashi points were selected and stimulated. Follow-up evaluations were conducted 3 weeks after the end of treatments. The grade and dosage of analgesics for cancer pain, pain intensity, quality of life, and safety were assessed. Results: Twenty-seven patients (90%) completed 6-week trial, and no serious adverse events were associated with either IA or sham IA procedures except the transient side effect such as fatigue. Nine patients in the IA group (64.3%) and 5 in the sham IA group (38.5%) responded to the 3-week intervention. These patients were mostly in the nonopioid and the weak opioid levels of the World Health Organization analgesic ladder. Self-reported pain declined by −1.54 ± 1.45 and −1.15 ± 1.57 in the IA and sham IA groups, respectively, with improved quality of life reported. Conclusions: IA treatment appears feasible and safe for advanced cancer patients. It might reduce analgesic usage in the early World Health Organization analgesic ladder stage cancer patient, though it could not show significant outcome differences due to design limitation of sham IA.
Keywords: acupuncture, intradermal, advanced cancer, analgesics, pain management
Introduction
Pain is the most common symptom in patients with cancer. A systematic review of the literature from 1966 to 2005 revealed that pain was reported in 25% for those patients newly diagnosed with cancer, 33% for those undergoing active treatment, and greater than 75% for those with advanced disease.1 Relief of pain is a priority oncology care to improve quality of life (QOL) in activities of daily living and the patient’s ability to endure treatment.2 Since 1986, the most widely accepted strategy for cancer pain management has been proposed by the World Health Organization (WHO), which suggests administering a sequential 3-step analgesic ladder from nonopioids to weak opioids to strong opioids according to pain intensity.3,4 However, nearly half of all cancer patients are not adequately treated for pain.5 Most patients are reluctant to receive opioids for fear of adverse effects,6 and a combination of pharmacologic and nonpharmacologic treatment modalities for cancer pain is the standard of care, as cancer pain is complex and multifactorial.7 Nevertheless, most health care providers overlook nonpharmacologic and complementary therapies. Therefore, a comprehensive pain management strategy is required.
Acupuncture has been suggested as a safe treatment intervention for cancer pain as well as for cases of chemotherapy-induced nausea and vomiting, neuropathy, xerostomia, and hot flashes.8-10 Although acupuncture has been clinically proven to relieve various types of pain such as chronic low back pain or tension-type headache,11,12 the recent Cochrane Reviews on acupuncture for cancer pain still demand well-designed clinical trials to provide evidence for effectiveness of acupuncture for cancer pain.13,14 However, a complete double-blinded, placebo-controlled trial is very challenging in acupuncture treatment, as in many trials of nonpharmacological pain management.15
Acupuncture is not widely used in cancer patients, which might be due to poor access to acupuncture treatment.16 Conventional acupuncture treatment is usually administered twice or thrice a week, thus lacking the benefit of continuous relief for cancer pain. Intradermal acupuncture (IA), which can be self-managed and stimulated for breakthrough pain, is thus a good candidate for investigation. Although several studies have investigated the effects of auricular IA on cancer pain, few randomized controlled trials have assessed the effects of whole body stimulation using IA on cancer pain.17,18 The aims of this pilot study were to evaluate the feasibility and safety of an IA treatment protocol designed to reduce cancer pain in advanced disease, and to explore pretreatment to posttreatment changes.
Methods
Study Design and Ethics
The aim of this randomized controlled trial was to investigate the feasibility, effectiveness, and safety of IA (compared with sham IA) with analgesics in the treatment of cancer pain. The changes in grade on the WHO analgesic ladder, analgesic dosage, self-reported pain scale, and QOL were observed. All adverse events were also assessed. The clinical trial was performed as a single-center, patient-assessor-blinded, randomized, sham-controlled clinical trial with 2 parallel arms. Taking into account the minimum number of subjects necessary to assess the effects of IA as a pilot study, we calculated a total sample size of 30 patients, 15 in each group.19,20
This study was reviewed and approved by the ethics committees of the Dankook University (Approval No. DKU 2016-02-001) prior to recruitment. The study was carried out in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice. This protocol was registered on the site of “Clinical Research Information Service” of the Republic of Korea, a registry in the WHO Registry Network (https://cris.nih.go.kr/; Identifier No. KCT0001897).
Study Population
Both males and females were eligible. Other inclusion criteria included age >18 years, advanced cancer stage confirmed pathologically or radiologically with only palliative chemotherapy available, clinical symptoms of cancer pain for which analgesics had been prescribed, Eastern Cooperative Oncology Group performance status 0 to 2, life expectancy >6 months, and signed written informed consent for trial participation. Exclusion criteria included hypersensitivity to acupuncture or inability to cooperate with the acupuncture procedure, lack of willingness to comply with the study protocol for reasons including problems with visually and auditory communication (eg, reading, writing, hearing, speaking, and watching), women who were pregnant, breastfeeding, or of childbearing age and not on a proper method of birth control, and patients deemed inappropriate by the investigator based on the investigator’s expectation of rapid cancer progression.
Randomization and Blinding
The eligible participants were randomized 1:1 to the IA group or sham IA group. Administration of analgesics according to the WHO analgesic ladder would probably result in a variable baseline, because each patient would probably report a different level of pain. Having patients at different medication baselines could potentially confuse the interpretation of the study results. Therefore, randomization was stratified according to the administration of nonopioids, weak opioids, or strong opioids. Random numbers were generated by a computerized random number generator using the block randomization method using R program by an independent statistician. Sequentially numbered opaque sealed envelopes containing the randomization assignments were kept in a secure place. Only the clinician administering IA therapy knew what treatment the patient had been administered, but he was prohibited from accessing any measurements for outcomes. The subjects, the outcome assessors, and the statistician performing the data analyses were blinded to treatment allocation throughout the study.
Intervention
In the experimental group, patients received IA treatment for 3 weeks on the specified acupuncture points (CV12, bilateral ST25, LI4, LR3, PC06, and additionally 0-3 Ashi points). The acupuncture points were selected by consensus of an expert committee composed of professors and researchers who specialize in traditional Korean medicine, and on the basis of literature reviews.9,21 Single-use, sterile, stainless steel IA needles measuring 0.18 × 1.3 × 1.5 mm (Dong Bang Medical Co Ltd, Boryeong, Korea) were fixed with skin tape (Supplementary Figure S1; available in the online version of the article). Each IA needle was kept attached on the skin for 48 to 72 hours, and all patients were instructed to press all the needle sites with their hands twice a day. Every week, the attached skin sites were sterilized and checked by the clinicians. In the sham IA group, all interventions were the same as those of the experimental group, including the issuance of the same instructions. However, the tip of the needle was bent so as to cause a pricking sensation mimicking real acupuncture without actually puncturing the skin.22 All IA and sham IA treatments were performed by doctors of Korean medicine who have been certified by the Korean Ministry of Health and Welfare, had at least 3 years of clinical experience, and had received more than 6 years of college education in Korean medicine.
Outcome Measures
The effect of the intervention on cancer pain was explored by measuring 3 outcomes (Table 1). The first was the primary outcome of the change in grade and dosage of analgesics for cancer pain between baseline and 1- and 3-week posttreatment assessments. Patients were considered responders if they experienced reduction in the analgesic usage. The second outcome was pain intensity assessed using a numerical rating scale (NRS), a patient-rated pain. The NRS is validated as being superior to verbal rating scales in patients with cancer pain.23 Subjects were asked to rate their average pain symptoms on an 11-point scale (0 = no symptoms; 10 = worst possible symptoms). The third outcome was QOL measured by the European Organization for Research and Treatment of Cancer QOL questionnaire (EORTC QLQ-C30), a 30-item questionnaire assessing 5 functional scales (physical, role, cognitive, emotional, and social), 3 symptom scales (fatigue, pain, nausea, and vomiting), and other symptoms and problems frequently encountered in cancer patients (dyspnea, appetite loss, insomnia, constipation, diarrhea, and financial difficulties). The validity and reliability of the scale have previously been demonstrated.24,25
Table 1.
Schedule of Enrollment, Interventions, and Assessments.
| Period | Baseline | Treatment Phase | Follow-up Phase | |||
|---|---|---|---|---|---|---|
| Week | 0 | First | Second | Third | Fourth | Sixth |
| Informed consent | ✓ | |||||
| Demographic characteristics | ✓ | |||||
| IA/sham IA treatment | ✓ | ✓ | ✓ | |||
| Administered analgesics | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| NRS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| EORTC QLQ-C30 | ✓ | ✓ | ✓ | ✓ | ||
| Blood tests | ✓ | ✓ | ||||
| Safety assessment | ✓ | ✓ | ✓ | ✓ | ✓ | |
Abbreviations: IA, intradermal acupuncture; NRS, numerical rating scale; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life questionnaire.
Feasibility, Adherence, Safety, and Credibility
Feasibility of recruitment to the study was assessed as proportion of eligible and interested persons, and feasibility of the protocol was assessed as the proportion of individuals completing the treatment against those who originally started the treatment regimen. An adherence standard of 80% of IA or sham IA attended as scheduled, without no-shows or cancellations, was set as acceptable feasibility. Any expected or unexpected adverse events related to this study were recorded and monitored until their resolution. Safety was also be assessed by performing blood tests, including a complete blood count, and renal and liver function tests at the screening visit and after the end of treatment. Vital signs were measured, and adverse events were recorded at each visit. The patients were asked at the end of follow-up to guess which treatment they were receiving to determine the credibility of the IA control condition.
Statistical Analyses
Baseline demographic and clinical characteristics of patients are reported as mean ± standard deviation for continuous variables and as frequencies and percentages for categorical variables. Continuous variables were analyzed by independent sample t tests or Wilcoxon rank sum tests, and categorical variables were analyzed using the χ2 or Fisher’s exact test, according to whether or not the data were normally distributed. Cochran-Mantel-Haenszel test stratified by the WHO analgesic ladder was used for the differences in primary outcome between the 2 groups as well as independent t tests between the 2 groups and a repeated-measures analysis of variance in secondary outcomes. The factors were modes of treatment (4 levels: preacupuncture, postacupuncture, 1-week follow-up, and 3-week follow-up), groups (2 levels: IA treatment and sham IA), and their interaction. The significance level was set at P < .05, and post hoc analyses were performed where appropriate using the SPSS for Windows, Version 22.0 (IBM SPSS Statistics, Armonk, NY).
Results
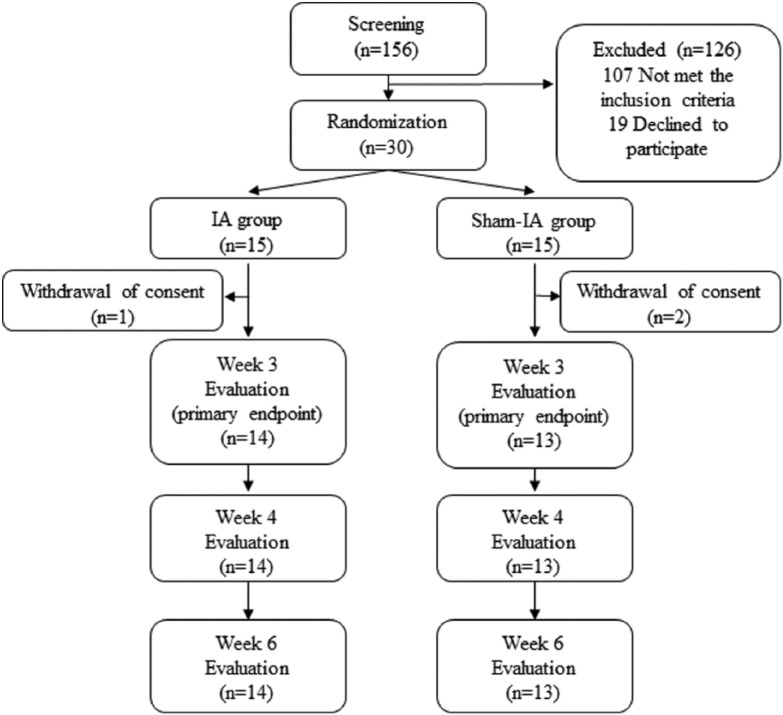
Among the 156 patients screened between February and June 2016 in our institution, 49 met the inclusion criteria. The recruitment goal of 30 participants (61.2%) was met and enrolled in the trial after 19 patients refused to participate (Figure 1). Randomization resulted in comparable groups: 15 randomly assigned to receive the IA treatment and 15 to receive the sham IA treatment. One participant in the IA group and 2 in the sham IA group withdrew consent after enrollment. Any evaluation was not available for them, because they withdrew from the study before week 1. Therefore, they are excluded from the analysis. There was no significant difference among groups in baseline demographic and clinical characteristics (Table 2). The remaining 27 subjects constituted the IA group (n = 14) and the sham IA group (n = 13). The subjects had mean age of 56 years (range = 42-73), mean NRS of 5.70 ± 1.76 points, and mean EORTC QLQ-C30 score of 71.18 ± 13.01 points.
Figure 1.
Consort diagram.
Table 2.
Patient Demographics and Baseline Measurements.
| IA (n = 14) | Sham IA (n = 13) | P | |
|---|---|---|---|
| Female, n (%) | 9 (64.3) | 7 (53.8) | .581 |
| Age (year) | 54.0 ± 9.1 | 58.2 ± 11.4 | .304 |
| Primary cancer, n (%) | .709 | ||
| GI tract | 5 (35.7) | 4 (30.8) | |
| Lung | 5 (35.7) | 4 (30.8) | |
| Breast | 3 (21.4) | 2 (15.4) | |
| Others | 1 (7.1) | 3 (23.1) | |
| Concurrent chemotherapy, n (%) | 7 (50.0) | 8 (61.5) | .547 |
| WHO analgesic ladder, n (%) | .964 | ||
| Level I | 4 (28.6) | 4 (30.8) | |
| Level II | 5 (35.7) | 4 (30.8) | |
| Level III | 5 (35.7) | 5 (38.5) | |
| Symptom score | |||
| ECOG PS grade, n (%) | .581 | ||
| Level I | 9 (64.3) | 7 (53.8) | |
| Level II | 5 (35.7) | 6 (46.2) | |
| NRS | 5.79 ± 1.88 | 5.62 ± 1.66 | .806 |
| EORTC QLQ-C30 | 71.00 ± 14.61 | 71.38 ± 11.77 | .941 |
Abbreviations: IA, intradermal acupuncture; GI, gastrointestinal; WHO, World Health Organization; ECOG PS, the Eastern Cooperative Oncology Group performance status; NRS, numerical rating scale; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life questionnaire.
Twenty-seven of the 30 enrolled patients (90.0%) completed 3 weeks of IA or sham IA treatment, and they completed all 6 weeks of the trial. The 3-week and 6-week completion rates met the feasibility criteria (80%). Adverse events related to IA treatment occurred in only one IA participant. The patient experienced fatigue, which resolved quickly. No other adverse events including any changes in blood tests were observed in both groups.
In the primary outcome, 64.3% of the patients (9/14) in the IA treatment group reported a reduction in the consumption of analgesics, with regard to the baseline level, in comparison with 38.5% (5/13) of the sham IA group (P = .180; Figure 2). Stratification analysis by the WHO analgesic ladder also failed to demonstrate statistical significance between the groups (P = .111). However, 12 patients in the WHO analgesic ladder levels I and II in both groups had reduced analgesic usage compared with only 2 subjects in level III (P = .017). The self-reported level of pain using NRS had significantly decreased by −1.54 ± 1.45 and −1.15 ± 1.57 after treatment, by −1.57 ± 2.06 and −1.54 ± 1.20 at the first follow-up, and by −1.00 ± 2.22 and −1.08 ± 1.38 at the end of follow-up in the IA group and the sham IA group, respectively (P < .001; Figure 3A). The QOL score using EORTC QLQ-C30 had significantly improved by −7.29 ± 10.58 and −8.77 ± 11.14 after treatment, by −7.86 ± 10.45 and −5.38 ± 10.70 at the first follow-up, and by −11.43 ± 6.77 and −7.69 ± 15.36 at the end of follow-up in the IA group and the sham IA group, respectively (P = .003; Figure 3B).
Figure 2.

Analgesic usage after the intradermal acupuncture (IA) or the sham IA treatment according to the World Health Organization (WHO) analgesic pain ladder.
Figure 3.

Pain intensity (A) and quality of life measured by the European Organization for Research and Treatment of Cancer questionnaire (EORTC QLQ-C30) and (B) assessed by a numerical rating scale according to the intradermal acupuncture (IA) or the sham IA treatment.
Discussion
Pain is common and devastating, frequently cited by cancer patients as having a substantial impact on activities of daily living and QOL. Previous data have shown that a higher QOL is strongly associated with longer survival among patients with advanced cancer.26,27 Therefore, active and early integration of palliative care for patients with advanced cancer is a clinically important strategy with positive effects on survival and QOL.28
IA treatment is usually used on the ear, known as auricular therapy.17 It can also be applied on acupuncture points or pain sites so that the pain relief can be self-managed and continuously stimulated by subjects. In this trial, IA treatment on meridian acupoints was demonstrated to be very feasible and safe for advanced cancer patients with pain. The effect of IA with analgesics on cancer pain was evaluated against the primary outcome measure—changes in dosage of analgesics for cancer pain. Fourteen participants (51.9%) reduced their analgesics for cancer pain during the trial, which was not statistically significant between the groups. NRS scores also confirmed the effect of IA or sham IA treatment on pain, which was maintained at the first follow-up (at 1 week) and was slightly decreased at the end of the follow-up (at 3 weeks). Similar alteration was observed for QOL as a secondary outcome, allowing the assessment of how much pain relief due to acupuncture treatment improved the QOL of cancer patients.
As the design of a credible sham acupuncture is always an issue, we should be cautious as to whether the sham intervention acted as a plausible control.15 Sham methods in IA were categorized into several types: (1) same treatment on nonacupuncture points or acupuncture points that are not theoretically effective for the disease and (2) sham needles or adhesive patches without pellet/seed on the same acupuncture points as experimental group. The second type was used in this study. For the blinding of the treatments, all interventions were the same as those of the IA group except that bent stainless steel needles were used, not intended for insertion into the skin. In the credibility evaluation, only 4 patients in the sham IA group identified the procedure as sham acupuncture, compared with 2 patients in the IA group when they were asked about the treatment allocation at the end of the study. Acupressure, acupoint stimulation with fingers or hands has been shown to be effective for relieving a variety of pains.29 It is additionally suspected that the bent needle was providing stimulation via the skin due to design limitation of sham needle.
Despite the lack of difference between groups, the responders to IA or sham IA treatment mostly belonged to level I or II categories in the WHO analgesic ladder, depicting nonopioid and weak opioid levels. The analgesics that were reduced included acetaminophen (n = 4), acetaminophen/tramadol hydrochloride (n = 4), ibuprofen (n = 3), and acetaminophen/ibuprofen/codeine phosphate (n = 1). Therefore, an IA treatment could be recommended for an early WHO analgesic ladder stage rather than one depending on strong opioids. The low dropout rate, with only 3 subjects withdrawing consent before week 1, reflected the patients’ satisfaction with IA treatment. Considering the adverse effects from strong opioids, it would be helpful to delay the stronger morphine administration with IA treatment.
The needling depth of clinical efficacy in acupuncture treatment is not standardized.30 There is lack of well-designed research to compare the therapeutic effects, thus making the proper needling depth for clinical efficacy in each acupuncture point remain obscure. In terms of safe needling depth of acupuncture points, the risk of infection may increase as the needle penetrates deep into the skin. Therefore, our study suggests that the needling depth of IA treatment for 3 weeks is safe for advanced cancer patients with or without chemotherapy. A lot of clinical and animal studies have suggested that acupuncture may be beneficial to cancer patients with pain.31 However, the selected acupoints in the clinical studies were very inconsistent.32 Therefore, the appropriate acupoints should be investigated according to the various cancer pains.
This study has several limitations. First, the analgesic usage data were based on the participants’ self-reports. Korean cancer patients have been reported to be afraid of an addiction to analgesics.6 The patients in this trial were more likely to reduce the dose of analgesics. It is thus possible that the patterns of change in medication use are attributable to the Hawthorne effect (the participants’ response to observation and assessment).33 Second, the personal manipulation of the needles in some way may affect the outcome. Third, the subjects in the trial were strictly selected from the inclusion/exclusion criteria despite having advanced cancer. Therefore, the findings should be applied to those with stable disease who have a good performance status. Finally, the follow-up observation period was only for 3 weeks, because cancer progression might have biased the outcomes.
Conclusions
The findings from this study suggested that IA treatments appear feasible and safe for advanced cancer patients. Although the study did not indicate a statistical significance between the experimental and sham groups, it provides support for expanding the usage of acupuncture with the IA method in cancer pain level I or II categories in the WHO analgesic ladder and recommends targeting the patient’s analgesic grade for IA treatment. A large-scale study with better nonpenetrating sham IA device and appropriate placebo point selection would be necessary to show the clinical benefits of IA on the body meridians.
Supplemental Material
Supplemental material, supFig_1 for Intradermal Acupuncture Along with Analgesics for Pain Control in Advanced Cancer Cases: A Pilot, Randomized, Patient-Assessor-Blinded, Controlled Trial by Kyungsuk Kim and Sanghun Lee in Integrative Cancer Therapies
Footnotes
Trial Registration: Clinical Research Information Service: KCT0001897, registered on April 25, 2016.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Bio-Synergy Research Project (NRF-2017M3A9C4065964) of the Ministry of Science, ICT and Future Planning through the National Research Foundation; and the Comprehensive and Integrative Medicine Institute (CIMI), Daegu, Republic of Korea (Grant # CIMI-15-01-07).
Supplemental Material: The online supplemental figure is available at http://journals.sagepub.com/doi/suppl/10.1177/1534735418786797.
ORCID iD: Sanghun Lee  https://orcid.org/0000-0002-0573-9555
https://orcid.org/0000-0002-0573-9555
References
- 1. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449. [DOI] [PubMed] [Google Scholar]
- 2. Paice JA, Ferrell B. The management of cancer pain. CA Cancer J Clin. 2011;61:157-182. [DOI] [PubMed] [Google Scholar]
- 3. Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management. Stepping up the quality of its evaluation. JAMA. 1995;274:1870-1873. [PubMed] [Google Scholar]
- 4. Ripamonti CI, Bandieri E, Roila F; ESMO Guidelines Working Group. Management of cancer pain: ESMO clinical practice guidelines. Ann Oncol. 2011;22(suppl 6):vi69-vi77. [DOI] [PubMed] [Google Scholar]
- 5. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19:1985-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hyun MS, Lee JL, Lee KH, et al. Pain and its treatment in patients with cancer in Korea. Oncology. 2003;64:237-244. [DOI] [PubMed] [Google Scholar]
- 7. Pujol LA, Monti DA. Managing cancer pain with nonpharmacologic and complementary therapies. J Am Osteopath Assoc. 2007;107(12 suppl 7):ES15-ES21. [PubMed] [Google Scholar]
- 8. NIH Consensus Conference. Acupuncture. JAMA. 1998;280:1518-1524. [PubMed] [Google Scholar]
- 9. Chen ZJ, Guo YP, Wu ZC. Advances of clinical study on acupuncture and moxibustion for treatment of cancer pain [in Chinese]. Zhongguo Zhen Jiu. 2008;28:392-394. [PubMed] [Google Scholar]
- 10. Yoon SW, Jeong JS, Kim JH, Aggarwal BB. Cancer prevention and therapy: integrating traditional Korean medicine into modern cancer care. Integr Cancer Ther. 2014;13:310-331. [DOI] [PubMed] [Google Scholar]
- 11. Berman BM, Langevin HM, Witt CM, Dubner R. Acupuncture for chronic low back pain. N Engl J Med. 2010;363:454-461. [DOI] [PubMed] [Google Scholar]
- 12. Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR. Acupuncture for tension-type headache. Cochrane Database Syst Rev. 2009;(1):CD007587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paley CA, Johnson MI, Tashani OA, Bagnall AM. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev. 2015;(10):CD007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paley CA, Tashani OA, Bagnall AM, Johnson MI. A Cochrane systematic review of acupuncture for cancer pain in adults. BMJ Support Palliat Care. 2011;1:51-55. [DOI] [PubMed] [Google Scholar]
- 15. Vase L, Baram S, Takakura N, et al. Can acupuncture treatment be double-blinded? An evaluation of double-blind acupuncture treatment of postoperative pain. PLoS One. 2015;10:e0119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui Y, Shu XO, Gao YT, et al. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Res Treat. 2004;85:263-270. [DOI] [PubMed] [Google Scholar]
- 17. Alimi D, Rubino C, Leandri EP, Brulé SF. Analgesic effects of auricular acupuncture for cancer pain. J Pain Symptom Manage. 2000;19:81-82. [DOI] [PubMed] [Google Scholar]
- 18. Alimi D, Rubino C, Pichard-Léandri E, Fermand-Brulé S, Dubreuil-Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol. 2003;21:4120-4126. [DOI] [PubMed] [Google Scholar]
- 19. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180-191. [DOI] [PubMed] [Google Scholar]
- 20. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Stat. 2005;4:287-291. [Google Scholar]
- 21. Garcia MK, Driver L, Haddad R, et al. Acupuncture for treatment of uncontrolled pain in cancer patients: a pragmatic pilot study. Integr Cancer Ther. 2014;13:133-140. [DOI] [PubMed] [Google Scholar]
- 22. Karst M, Winterhalter M, Münte S, et al. Auricular acupuncture for dental anxiety: a randomized controlled trial. Anesth Analg. 2007;104:295-300. [DOI] [PubMed] [Google Scholar]
- 23. Brunelli C, Zecca E, Martini C, et al. Comparison of numerical and verbal rating scales to measure pain exacerbations in patients with chronic cancer pain. Health Qual Life Outcomes. 2010;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 25. Park KU. Assessment of change of quality of life in terminally ill patients under cancer pain management using the EORTC Core Quality of Life Questionnaire (QLQ-C30) in a Korean sample. Oncology. 2008;74(suppl 1):7-12. [DOI] [PubMed] [Google Scholar]
- 26. Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the Multicenter Italian Lung Cancer in the Elderly Study. J Clin Oncol. 2005;23:6865-6872. [DOI] [PubMed] [Google Scholar]
- 27. Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. 2009;27:5816-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733-742. [DOI] [PubMed] [Google Scholar]
- 29. Chen YW, Wang HH. The effectiveness of acupressure on relieving pain: a systematic review. Pain Manag Nurs. 2014;15:539-550. [DOI] [PubMed] [Google Scholar]
- 30. Lin JG, Chou PC, Chu HY. An exploration of the needling depth in acupuncture: the safe needling depth and the needling depth of clinical efficacy. Evid Based Complement Alternat Med. 2013;2013:740508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu W, Rosenthal DS. Acupuncture for cancer pain and related symptoms. Curr Pain Headache Rep. 2013;17:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi TY, Lee MS, Kim TH, Zaslawski C, Ernst E. Acupuncture for the treatment of cancer pain: a systematic review of randomised clinical trials. Support Care Cancer. 2012;20:1147-1158. [DOI] [PubMed] [Google Scholar]
- 33. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supFig_1 for Intradermal Acupuncture Along with Analgesics for Pain Control in Advanced Cancer Cases: A Pilot, Randomized, Patient-Assessor-Blinded, Controlled Trial by Kyungsuk Kim and Sanghun Lee in Integrative Cancer Therapies