Abstract
Objective. This study aimed to conduct a meta-analysis to establish the effect of exercise interventions on physical symptoms, including fatigue, nausea/vomiting, pain, dyspnea, insomnia, loss of appetite, constipation, and diarrhea in cancer patients and survivors. Methods. We searched articles published before April 2017 using the following databases: Cochrane Library, PubMed/MEDLINE, CINAHL, Scopus, PEDro, Health & Medical Collection, and Psychology Database. Randomized controlled trials (RCTs) of exercise intervention in cancer patients, which evaluated cancer-related physical symptoms using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30, were included. Symptom scale data were extracted for meta-analysis. Subgroup analyses were performed for exercise types (aerobic, resistance, and mixed exercise programs). Results. Of the 659 articles, 10 RCTs were included in the meta-analysis, of which the mean PEDro score was 5.43 (SD = 1.28). Fatigue, pain, dyspnea, and insomnia were significantly lower in the intervention group than in the control group at postintervention in cancer patients. However, exercise intervention did not promote or suppress nausea/vomiting, loss of appetite, constipation, and diarrhea in cancer patients. The effect of exercise type on each symptom was not different. Conclusion. Exercise intervention was confirmed to improve fatigue, pain, and insomnia and might have reduced dyspnea in cancer patients. However, the benefits of exercise on nausea/vomiting, loss of appetite, constipation, and diarrhea were not shown in any exercise type. Further research is warranted to examine the effects of exercise interventions on physical symptoms in cancer patients.
Keywords: cancer, meta-analysis, exercise, physical symptoms, dyspnea
Introduction
An estimated 21.6 million new cancer cases are expected worldwide by 2030. This estimate is a stark 53% increase from the latest statistics reported by the World Health Organization in 2012. As screening and treatment continue to progress, the overall number of cancer patients and survivors will increase.1 Although mortality rates have reduced, many cancer patients still suffer from physical and psychological symptoms. Cancer patients have various physical symptoms. Common symptoms include fatigue, nausea/vomiting, pain, dyspnea, insomnia (sleep disturbance), loss of appetite, constipation, diarrhea, drowsiness, hair loss, sore mouth, and sweating.2 The 3 types of symptoms are acute, chronic, and late symptoms. Acute symptoms develop before or during treatment but have a short duration. Chronic symptoms may continue for months or years, and late symptoms develop months or years after treatments are completed. These 3 types of symptoms at any stage of the cancer trajectory have significant adverse effects on cancer patients.3 Symptoms also occur as side effects of opioids,4 chemotherapy,5 and radiotherapy.6 All symptoms affect the quality of life (QOL) of cancer patients.
Exercise is widely recognized as an effective nonpharmacological therapy in cancer patients.7-9 A growing body of evidence supports the idea that increasing physical activity provides important benefits to promote psychological outcomes and physical well-being in cancer patients.9-12 Exercise has been reported to relieve cancer-related physical symptoms such as fatigue,8,13-15 pain,8,16 and insomnia.8,17,18 However, the effects of exercise on other symptoms, including nausea/vomiting, dyspnea, constipation, diarrhea, and loss of appetite, have not been confirmed by meta-analysis of randomized controlled trials (RCTs). On the other hand, the effects of exercise on physical symptoms might differ by type of exercise.19 Pain and insomnia have been reported to be relieved by aerobic, but not resistance exercise.10,16,18 Fatigue is improved by both aerobic and resistance exercises.13,14,19 Thus, aerobic and resistance exercises should be distinguished when the effects of exercise on cancer-related physical symptoms are examined.
This systematic review aimed to determine the effects of aerobic and resistance exercise interventions on physical symptoms by a meta-analysis of RCTs. These symptoms include not only fatigue, pain, and insomnia, but also nausea/vomiting, dyspnea, loss of appetite, constipation, and diarrhea in cancer patients.
Methods
Protocol and Objective
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.20 It was also conducted and reported in accordance with the PRISMA statement20 (PROSPERO Register code: CRD42018091244). No funding support was received in this study.
Search Methods
We performed a literature search to identify articles published before April 2017 using the following databases: Cochrane Library, the Centre for Reviews and Dissemination, PubMed/MEDLINE, CINAHL, Scopus, PEDro, Health & Medical Collection, and Psychology Database. The search strategy was adapted to each database and based on the following MeSH terms: cancer, tumor, randomized controlled trial, training, rehabilitation, and exercise. The words disorder for cancer were also used for the search (eg, lymphoma, hematopoietic malignancy, carcinosarcoma). In addition, the words outcome on physical symptoms were added to the search terms (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 and C15-PAL [EORTC QLQ-C30, QLQ-C15-PAL]). Cancer-related symptoms are frequently examined using the EORTC QLQ-C30.21 The EORTC QLQ-C30 consists of 30 items, and raw patient responses are transformed to produce scores from 0 to 100 on 5 functional scales, 9 symptom scales, and a scale representing global QOL. Higher functional scale scores indicate better health-related QOL, whereas higher symptom scale/item scores indicate higher level of symptoms. QLQ-C30 symptom scales include fatigue, nausea/vomiting, pain, dyspnea, insomnia, loss of appetite, constipation, and diarrhea. Similarly, QLQ-C15-PAL is a questionnaire developed to assess the QOL of palliative cancer care patients and has the same symptom scale as the QLQ-C30 except diarrhea. Attempts were made to contact authors of trial reports if clarification was necessary. Reference lists of identified eligible articles were cross-referenced and hand searched to identify any additional articles.
We included RCTs that evaluated the effects of exercise intervention by QLQ-C30 in cancer patients and survivors in any setting. Even if the primary outcome was not physical symptoms, studies that reported the QLQ-C30 symptom scale were included. Systematic reviews, editorials, cross-sectional studies, case reports, and case series studies were excluded. The interventions were of sufficient intensity as measured in metabolic equivalent of task, thus excluding stretching exercises, yoga, Pilates, and education. The exercise interventions for shoulder joint in breast cancer patients and pelvic floor muscle training in patients with gynecological cancer were also excluded. Comparisons were with a control group not receiving any (major) exercise intervention or other interventions (eg, cognitive behavioral therapy). Groups with only attention, relaxation, or education were considered as control groups.
Titles and subsequent abstracts of trials were retrieved and screened by 3 independent reviewers (KH, KU, and EM) to identify trials that met the inclusion criteria. A fourth independent reviewer (JN) resolved any discrepancies between the 2 reviewers. Full texts of potentially eligible trials were retrieved and assessed for eligibility by 2 independent reviewers (JN and TF). Articles deemed eligible were included after the full-text screening. To perform the meta-analysis, data details were examined. Studies that did not show numerical data of QLQ-C30 at postintervention were excluded. Final inclusion of eligible RCTs was determined in consensus meetings in which all authors participated.
Quality Assessment
The methodological quality of the studies, including their risk of bias, was assessed using the PEDro Scale, which is based on the Delphi list.22 The PEDro scale scores the methodological quality of randomized trials out of 10. A PEDro cutoff of 5 points is used widely.23 The score for each included study was determined by a trained assessor (JN). Additionally, the score, which was shown in the PEDro physiotherapy evidence database, was referred. Final scores were determined in consensus meetings in which all authors participated.
Data Extraction
Data were extracted by one of the authors (JN). When insufficient data were available in the full text, authors were contacted by email for further information. The following data were extracted from each study by 2 investigators: first author’s last name, publication year, study location and duration, sample size, type of exercise, and timing of exercise. The following data from the QLQ-C30 physical symptom scales were selected for the meta-analysis: fatigue, nausea/vomiting, pain, dyspnea, insomnia, loss of appetite, constipation, and diarrhea. Means and SDs of postintervention were extracted. It is premised that no significant difference exists between the intervention and control groups at baseline (preintervention).
Data Analysis
All statistical analyses were conducted using Review Manager (RevMan) version 5.1.24 We calculated standard mean differences (SMDs) with 95% CIs. SMDs were significant if their 95% CIs excluded zero. The random effect model was used as the pooling method to assume heterogeneity between different exercise types. We assessed statistical heterogeneity using the l2 statistic. We adopted the levels of l2 suggested by the Cochrane Handbook for Systematic Reviews of Interventions (l2 values of 0%, 25%, 50%, and 75% represented no, low, moderate, and high heterogeneity, respectively).25 The threshold for interpreting the l2 value can be misleading. Therefore, we determined the importance of the observed l2 value by looking at the magnitude and direction of the effect as well as at the strength of evidence for clinical heterogeneity. Subgroup analyses were performed for exercise types (aerobic, resistance, and mixed exercise programs).
Results
Study Selection
The database searches retrieved 743 references, which were reduced to 659 after excluding duplicate articles. The 659 studies were subjected to title and abstract screening, and 614 RCTs were excluded because of irrelevant study design, or issues with population or intervention. A full-text review was performed for 45 RCTs and, consequently, 35 RCTs were excluded. Although 2 articles were appropriate RCTs, they were not included in the meta-analysis because of differences in data form.26,27 Thus, data extraction was performed on 10 RCTs. Figure 1 shows the outcome of the search process and study selection.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) study flow diagram of the selection process.
Abbreviation: RCT, randomized controlled trial.
The included studies were conducted in various countries: 4 in Germany,28-31 2 in the United States,32,33 and 1 each in Denmark,34 South Korea,35 Australia,36 and Switzerland.37 Some of the RCTs were published in more than 1 article.
Study Characteristics
The detailed characteristics of the 10 RCTs are shown in Table 1. All interventions in the RCTs lasted for 16 weeks,32 12 weeks,30,36,37 8 weeks,35 6 weeks,33,34 3 weeks,29 or the duration of hospitalization for cancer treatment.28,31 The most prevalent cancer type was hematological malignancy,28,31,33,37 followed by breast cancer.30,32,35 In 2 RCTs,29,34 participants with various cancer types were included.
Table 1.
Characteristics of Studies Included.
| Author, Year | Intervention | Participants (Gender, Number, Age) | Cancer Type | Intervention | Duration | Timing | Measure (Outcome) |
|---|---|---|---|---|---|---|---|
| Adamsen et al,34 2009 | EX, mixed exercise program vs CON, usual care |
Female, 73% EX, 135; 47.2 ± 10.6 years CON, 134; 47.2 ± 10.7 years |
Mixed | Resistance exercise: 3 sets of 5-8 repetitions at 70%-100%
of 1 RM, 45 minutes Aerobic exercise: 85%-95% of maximum heart rate, 15 minutes 3 times per week |
6 Weeks | During chemotherapy | EORTC QLQ-C30, SF-36, physical activity, questionnaire, muscular strength, maximum O2 |
| Baumann et al,28 2011 | EX, aerobic exercise program vs CON, usual rehabilitation |
Female, 52% EX, 17; 41.4 ± 11.8 years CON, 16; 42.8 ± 14.0 years |
Hematological | Aerobic exercise by bicycle ergometer: the intensity was 80% of achieved watt load in the WHO test. 10-20 Minutes of ADL training was also conducted during chemotherapy twice per day | During hospitalization EX: 56.1 days CON: 51.4 days |
Posttransplantation | EORTC QLQ-C30, aerobic endurance, maximal strength, lung function |
| Dimeo et al,29 2004 | EX, aerobic exercise program vs CON, relaxation control |
Female, 26% EX, 34; 55.1 ± 10 years CON, 35; 60.0 ± 9.5 years |
Mixed | Biking on a stationary bike; training intensity corresponded to a heart rate of about 80% of the maximal heart rate, 30 minutes daily, 5 days per week | 3 Weeks | Postsurgery | EORTC QLQ-C30, ergometer test |
| Do et al,35 2015 | EX, resistance exercise program vs CON, usual rehabilitation |
Female, 100% EX, 22; 49.7 ± 7.1 years CON, 22; 49.6 ± 10.4 years |
Breast | Resistance exercise using elastic tubing. 60% of 1 RM, 3 sets of 10 repetitions, rest for 2 minutes between exercise sets; 5 times per week | 8 Weeks | Postsurgery or/and radiotherapy | EORTC QLQ-C30 and BR23, DASH, volume and strength of muscle |
| Galvao et al,36 2010 | EX, mixed exercise program vs CON, usual care |
Female, 0% EX, 29; 69.5 ± 7.3 years CON, 28; 70.1 ± 7.3 years |
Prostate | Resistance exercise: 12 to 6 repetitions, maximum of 2 to 4
sets per exercise Aerobic exercise: 15 to 20 minutes of cardiovascular at 65% to 80% maximum heart rate, twice per week |
12 Weeks | During hormonal therapy | EORTC QLQ-C30, DXA, muscle strength, endurance, functional performance, balance, blood samples |
| Hacker et al,33 2011 | Resistance exercise program vs Usual care (control) |
Female, 26% EX, 9 CON, 10 46.3 ± 16.2 Years (total) |
Hematological | Resistance exercise consisted of 11 preselected exercises using elastic resistance bands. The Borg Scale (20-point scale) was used to estimate the intensity of the resistance; 6 times per week | 6 Weeks | Posttransplantation | EORTC QLQ-C30, timed stair climb, handgrip strength, 30-s chair-stand test, Fatigue Intensity Scale, quality of life index |
| Knols et al,37 2011 | EX, mixed exercise program vs Usual care (control) |
Female, 41.2% EX, 64; 46.7 ± 13.7 years CON, 67; 46.6 ± 12.0 years |
Hematological | Resistance exercise: dumbbells, squats, barbell rotations,
and step-ups Aerobic exercise by bicycle ergometer: 50%-60%, increasing up to 70%-80% of maximum heart rate, 20 minutes, twice per day |
12 Weeks | Posttransplantation | EORTC QLQ-C30, muscle strength, walk speed, 6MWT |
| Ligibel et al,32 2016 | EX, aerobic exercise program vs CON, wait-list control |
Female, 100% EX, 47; 49.3 ± 9.6 years CON, 51; 50.7 ± 9.4 years |
Breast | Moderate-intensity aerobic exercise program; the target goal was 150 minutes of moderate-intensity exercise per week | 16 Weeks | Presurgery | EORTC QLQ-C30, modified Bruce ramp, treadmill test, physical activity recall interview, FACIT |
| Schmidt et al,30 2015 | EX1, aerobic exercise program vs EX2, resistance exercise vs CON, usual care |
Female, 100% EX1, 21; 53 ± 12.6 years EX2, 22; 56 ± 10.2 years CON, 26; 54 ± 11.2 years |
Breast | EX 1: aerobic exercise by bicycle ergometer: Borg level
11-14, 25-30 minutes EX 2: resistance exercise: 20 repetitions, with 50% of the maximum weight. Any further increase in intensity was based on the Borg scale; twice per week |
12 Weeks | Postsurgery and during chemotherapy | EORTC QLQ-C30, QLQ-BR23, MFI, D2-Test; Borg Scale, muscular strength |
| Wiskemann et al,31 2011 | EX, mixed exercise program vs CON, attention control |
Female, 100% EX, 52; 47.6 (18-70) years CON, 53; 50.0 (20-71) years |
Hematological | Aerobic exercise by bicycling and treadmill walking: Borg
scale 12-14, 20 to 40 minutes, 3 times per
week Resistance exercise using stretch bands resistance. 8-20 repetitions, 2 or 3 sets. Borg scale 14-16, twice per week |
During hospitalization EX: 43 (22-120) days CON: 45 (24-92) days |
During and after hospitalization | EORTC QLQ-C30, MFI, HADS, POMS, NCCN |
Abbreviations: EX, experimental; CON, control; RM, repetition maximum; EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; SF, Short Form; WHO, World Health Organization; ADL, activities of daily living; DASH, Disability of Arm, Shoulder and Hand Score; DXA, dual energy X-ray absorptiometry; 6MWT, 6 Minutes Walking Test; FACIT, Functional Assessment of Chronic Illness Therapy; MFI, Multidimensional Fatigue Inventory; D2-Test, evaluation of cognitive function; HADS, Hospital Anxiety and Depression Scale; POMS, Profile of Mood States; NCCN, National Comprehensive Cancer Network.
The exercise carried out in the intervention group in RCTs included various exercise programs, which were mainly aerobic, resistance, stretching, and walking exercise. The intervention exercises were difficult to classify strictly. In this study, the intervention exercises in the included RCTs were classified into 3 types: aerobic, resistance, and mixed exercise programs. Aerobic exercise programs were performed in 4 RCTs,28-30,32 resistance exercise programs in 3 RCTs,30,33,35 and mixed exercise programs, including both aerobic and resistance exercise programs, in 4 RCTs.31,34,36,37 In 1 RCT,30 aerobic and resistance exercise programs were performed in 2 different groups; the data from both groups were extracted and analyzed separately. The timings of the exercise interventions performed were mainly postsurgery, posttransplantation (hematological malignancy), and during chemotherapy. The QLQ-C30 was used as outcome for physical symptoms in all RCTs but not the QLQ-C15-PAL.
Risk of Bias
Given that all included studies were RCTs, the level of evidence from all studies was II according to the National Health and Medical Research Council Hierarchy of Evidence Scale.38 The assessment of risk of bias showed a mean PEDro score of 5.43 (SD = 1.28; Table 2). Individually, 4 RCTs showed a PEDro score of 4 points,28 which were slightly lower than the cutoff for high-quality trials.23,33,35
Table 2.
Assessment of Methodological Quality and Risk of Bias With the PEDro Scale.
| Author | Scoresa |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Adamsen et al34 | Yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Baumann et al28 | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 4 |
| Dimeo et al29 | Yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Do et al35 | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 |
| Galvao et al36 | Yes | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 7 |
| Hacker et al33 | Yes | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| Knols et al37 | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ligibel et al32 | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 5 |
| Schmidt et al30 | Yes | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 5 |
| Wiskemann et al31 | No | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
The criteria addressed the following issues: 0, eligibility criteria; 1, random allocation; 2, concealed allocation; 3, groups similar at baseline; 4, participant blinding; 5, therapist blinding; 6, assessor blinding; 7, <15% dropouts; 8, intention-to-treat analysis; 9, between-group difference reported; 10, point estimate and variability reported. Each criterion was given equal weight (ie, 1 point) for a maximum sum score (criteria 1-10) of 10.
Effect of Exercise on Physical Symptoms
A total 10 RCTs were included in a random-effects meta-analysis.28-37 The efficacy of exercise on physical symptoms in cancer patients was then estimated in a forest plot. The 10 RCTs included in this review consisted of 893 participants: 434 in the exercise groups and 459 in the control group. In 1 RCT,30 both effects of aerobic and resistance exercise programs were examined. Therefore, 11 intervention groups from 10 RCTs were included in the meta-analysis.
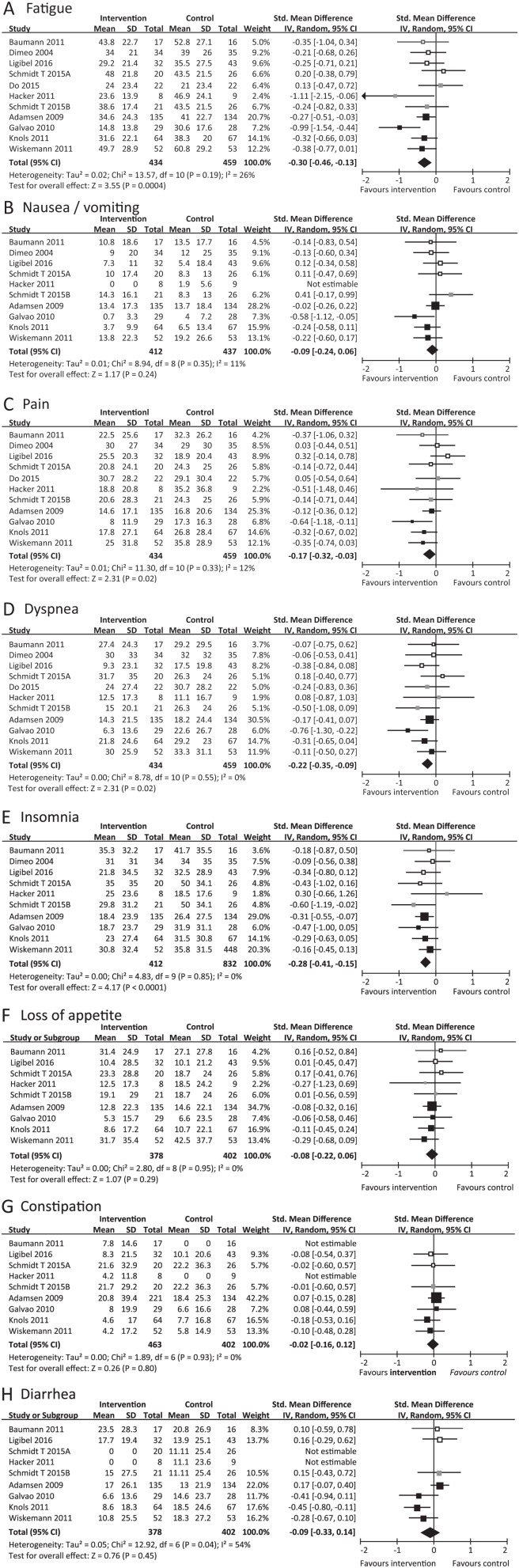
Fatigue
The meta-analysis of 11 intervention groups from 10 RCTs28-37 showed that fatigue in the intervention group was significantly lower than that in the control group (SMD = −0.30, 95% CI = −0.46 to −0.13, P = .0004; Figure 2A). The statistical heterogeneity was moderate (l2 = 26%). Subgroup analysis of exercise types demonstrated no significant difference among the 3 subgroups (P = .39; l2 = 0%). Within only the mixed exercise program subgroup,31,34,36,37 an improvement effect in favor of the intervention group was found (SMD = −0.41; 95% CI = −0.66 to −0.17; P = .0009; l2 = 46%).
Figure 2.
Meta-analysis for the effect estimate of exercise on physical symptoms in cancer patients.
Standardized mean difference (SMD) was calculated for the Random effects model of meta-analysis. IV, inverse of variance; CI, confidence interval. Subgroups were indicated by color in forest plot: aerobic exercise (white), resistance exercise (gray) and mixed exercise program (black). The pooled effects in each subgroups were not shown (see the main text for more details).
Nausea/Vomiting
A meta-analysis of 10 intervention groups from 9 RCTs28-34,36,37 was performed. However, in 1 RCT,33 the SMD was not calculated because the values of the mean and SD were zero. As a result, no significant difference in nausea/vomiting was found between the intervention and control groups (SMD = −0.09, 95% CI = −0.24 to 0.06, P = .24; Figure 2B). Subgroup analysis of exercise types also demonstrated no significant differences among the 3 subgroups (P = .13; l2 = 51.6%).
Pain
The meta-analysis of 11 intervention groups from 10 RCTs28-37 showed that pain in the intervention group was significantly lower than that in the control group (SMD = −0.17, 95% CI = −0.32 to −0.03, P = .02; Figure 2C). The statistical heterogeneity was low (l2 = 9%). A subgroup analysis of exercise types demonstrated no significant difference among the 3 subgroups (P = .18; l2 = 41.1%). Within only the mixed exercise program subgroup,31,34,36,37 an improvement effect in favor of the intervention group was found (SMD = −0.28; 95% CI = −0.47 to −0.09; P = .005; l2 = 20%).
Dyspnea
The meta-analysis of 11 intervention groups from 10 RCTs28-37 showed that dyspnea in the intervention group was significantly lower than that in the control group (SMD = −0.22, 95% CI = −0.35 to −0.09, P = .001; Figure 2D). The statistical heterogeneity was low (l2 = 0%). Subgroup analysis of exercise types demonstrated no significant differences among the 3 subgroups (P = .62; l2 = 0%). Within only the mixed exercise program subgroup,31,34,36,37 an improvement effect in favor of the intervention group was found (SMD = −0.27; 95% CI = −0.49 to −0.06; P = .01; l2 = 33%).
Insomnia
The meta-analysis of 10 intervention groups from 9 RCTs28-34,36,37 showed that insomnia in the intervention group was significantly lower than that in the control group (SMD = −0.28, 95% CI = −0.41 to −0.15, P < .0001; Figure 2E). The statistical heterogeneity was low (l2 = 0%). Subgroup analysis of exercise types demonstrated no significant differences among the 3 subgroups (P = .99; l2 = 0%). Within only the mixed exercise program subgroup,31,34,36,37 an improvement effect in favor of the intervention group was found (SMD = −0.28; 95% CI = −0.41 to −0.15; P = .0005; l2 = 0%).
Loss of Appetite
The meta-analysis of 9 intervention groups from 8 RCTs28,30-34,36,37 also showed no significant difference in loss of appetite between the intervention and control groups (SMD = −0.08, 95% CI = −0.22 to 0.06, P = .29; Figure 2F). No significant difference was found among the 3 subgroups (P = .50; l2 = 0%).
Constipation
Nine intervention groups from 8 RCTs28,30-34,36,37 were included in the meta-analysis. However, the SMD was not calculated in 2 RCTs28,33 because the values of the mean and SD were zero in the constipation symptom scale. Therefore, the analysis was performed for 7 groups from 6 RCTs.30-32,34,36,37 No significant difference in constipation was found between the intervention and control groups (SMD = −0.02, 95% CI = −0.16 to 0.12, P = .80, l2 = 0%; Figure 2G). No significant difference was found among the 3 subgroups (P = .97; l2 = 0%).
Diarrhea
In 2 RCTs,30,33 the SMD was not calculated because the values of the mean and SD were zero on the Diarrhea Symptom Scale. Seven intervention groups from 7 RCTs were included in the meta-analysis.30-32,34,36 No significant difference was noted in diarrhea between the intervention and control groups (SMD = −0.09, 95% CI = −0.33 to 0.14, P = .45, l2 = 54%; Figure 2H). No significant difference was found among the 3 subgroups (P = .32, l2 = 12.7%).
Discussion
This systematic review aimed to examine the current body of evidence on the benefits of an exercise intervention for cancer-related physical symptoms. The physical symptoms included fatigue, nausea/vomiting, pain, dyspnea, insomnia, loss of appetite, constipation, and diarrhea, which were evaluated using the EORTC QLQ-C30. The effects of exercise on cancer-related fatigue, pain, and insomnia were examined in some systematic reviews with meta-analysis.8,39-41 However, to the best of our knowledge, this systematic review with meta-analyses is the first to focus on nausea/vomiting, dyspnea, loss of appetite, constipation, and diarrhea in cancer patients.
The benefits of exercise on fatigue,8,15 pain,8 and insomnia8,18 in cancer patients were previously confirmed statistically by meta-analysis. In particular, a large number of RCTs and systematic reviews on fatigue exist. Most studies showed the benefit of exercise on fatigue in cancer patients, and the result of our analysis show similar evidence. In contrast, only a few studies investigated the effect of exercise on pain and insomnia. Several meta-analyses showed the benefit of exercise on pain and insomnia8,18 but had insufficient reliable evidence. Our meta-analysis including 10 RCTs showed the pooled effect of exercise on pain significantly, which establishes the evidence for an effect of exercise on pain and insomnia in cancer patients.
The important result is that exercise intervention leads to mild subjective improvements in dyspnea in cancer patients. Dyspnea is a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity.42 Dyspnea is also a relatively common and highly debilitating symptom in cancer patients. It often leads to anxiety, depression, and exercise avoidance, thereby worsening deconditioning and reducing health-related QOL.43 Additionally, cancer treatments are a major cause of dyspnea. Specifically, radiation and chemotherapy can cause pneumonitis, pulmonary fibrosis, pulmonary and cardiac toxicity, anemia, pulmonary emboli, and cachexia in a significant proportion of patients, all of which can initiate or worsen the direct cancer-related sensations of dyspnea.44 Comorbid disease can be a significant contributor, particularly if cardiac or pulmonary diseases are involved in cancer patients.45 The main treatment for dyspnea in cancer patients is pharmacotherapy, such as opioids.46 Exercise may be an effective care for dyspnea in cancer patients. The effect of exercise on dyspnea in cancer patients was examined. Although the benefit of exercise on respiratory function was shown clearly,47 evidence on the effect of exercise on dyspnea in cancer patients from a meta-analysis was not indicated because of insufficient RCTs.48 Our analysis showed evidence of this effect through a meta-analysis of 10 RCTs. Dyspnea is known to occur frequently in patients with lung cancer, such as non–small-cell lung cancer.48 However, the number of patients with lung cancer was small in this study; at most 46 out of 780 patients with lung cancer participated in RCTs, which included cancer patients with mixed cancer types.29,34 Therefore, the influence of cancer type was not strong enough. The results of dyspnea were not different when subgroup analysis by cancer type was performed (data not shown). Although this study has several limitations, the possibility of the effect of exercise on dyspnea in cancer patients was indicated.
Meta-analyses of the effects of exercise on nausea/vomiting, loss of appetite, constipation, and diarrhea in cancer patients have not been performed. Generally, regular exercise and physical activity are speculated to be related to constipation, but only limited evidence is available.49 According to our meta-analysis, exercise of any type might not be effective on constipation in cancer patients, which might be induced by impact of tumor, opioid as side effect, or physical inactivity.50,51 This result also supports the negative opinion on the effect of exercise on constipation.52 With regard to nausea/vomiting and loss of appetite, it could not be concluded whether exercise suppressed or promoted these symptoms. Exercise has been reported to promote nausea/vomiting53 and could promote loss of appetite54 in healthy people. In contrast, exercise could also suppress these symptoms in noncancer patients.55,56 No information is available on the effect of exercise on diarrhea. Our meta-analysis showed that an effect of exercise on these symptoms was not found. It was also considered that exercise at least does not promote these symptoms in cancer patients.
In this study, subgroup analyses were performed for exercise types (aerobic, resistance, and mixed exercise programs). Although a statistically significant difference of effect among exercise types was not detected in all physical symptoms, the pooled effect was different for each subgroup of exercise type. Improvement was observed in fatigue, insomnia, pain, and dyspnea only within the mixed exercise program subgroup. Further RCTs are required to examine the different effects of exercise type on each physical symptom.
This review has several important limitations that should be considered. First, the number of trials was small. The number of RCTs was reduced because the outcome of physical symptom was limited to QLQ-C30 and C15-PAL, which was intended by the authors. In this review and meta-analysis, we found a new possibility of exercise as supportive care to common cancer-related physical symptoms. Detailed meta-analyses with various outcomes and assessment tools should be consequently performed, especially on dyspnea. Second, the number of RCTs that showed the significant effect of exercise on physical symptoms was small, but the result of overall effect (SMD) was significant. Only the RCT by Galvao et al36 reported a significant effect on all of fatigue, nausea/vomiting, pain, and dyspnea in 28 patients. However, the weight in meta-analysis of the RCT was not very high (7.2% in fatigue; 7.3% in nausea/vomiting; 6.8% in pain; 6.0% in dyspnea), but the risk of bias was low (PEDro score = 7). However, the heterogeneity (l2) in meta-analysis was low to moderate (26% in fatigue, 11% in nausea/vomiting, 12% in pain, 0% in dyspnea).25 Additionally, when meta-analysis was performed without RCTs, which had high risk of bias (PEDro score < 5) per the sensitivity analysis, the result of overall effect (SMD) was not changed for all physical symptoms. Therefore, we believe that the results of meta-analyses in this study are acceptable statistically. Third, the cancer type and treatment were not limited in this meta-analysis. Physical symptoms may differ by cancer type. When treatment differs by cancer type, physical symptoms as a side effect of treatments are changed. Fourth, RCTs included in the review had different time frames. Some were performed postsurgery, and others were performed posttransplantation, during chemotherapy, and at other time points. The RCTs that were performed during chemotherapy recorded high values of physical symptoms comparatively.30,34 Finally, this review included only studies published in the English language as a result of selection; there is low possibility that selection was limited by language.
In conclusion, we confirmed that exercise interventions improve fatigue, pain, and insomnia in cancer patients, as observed in earlier studies.8,15,18 Additionally, the benefit of exercise on dyspnea in cancer patients was also observed, establishing the novelty of exercise as supportive care. Nausea/vomiting, loss of appetite, constipation, and diarrhea were not promoted or suppressed by any exercise type. Detailed meta-analyses with various outcomes and assessment tools should be performed, and more studies of sufficient quality are warranted.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was performed as one of the campaigns of Nagasaki Cancer Nursing a Society for the study of rehabilitation management, which is supported by Grant-in-Aid for Special Project Research in School of Health Sciences, Nagasaki University.
References
- 1. World-Health-Organization. World’s health ministers renew commitment to cancer prevention and control. http://www.who.int/cancer/media/news/cancer-prevention-resolution/en/. Accessed April 15, 2018.
- 2. Erickson JM, Macpherson CF, Ameringer S, Baggott C, Linder L, Stegenga K. Symptoms and symptom clusters in adolescents receiving cancer treatment: a review of the literature. Int J Nurs Stud. 2013;50:847-869. [DOI] [PubMed] [Google Scholar]
- 3. Mustian KM, Cole CL, Lin PJ, et al. Exercise recommendations for the management of symptoms clusters resulting from cancer and cancer treatments. Semin Oncol Nurs. 2016;32:383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khademi H, Kamangar F, Brennan P, Malekzadeh R. Opioid therapy and its side effects: a review. Arch Iran Med. 2016;19:870-876. [DOI] [PubMed] [Google Scholar]
- 5. Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155-163. [DOI] [PubMed] [Google Scholar]
- 6. Güleser GN, Taşci S, Kaplan B. The experience of symptoms and information needs of cancer patients undergoing radiotherapy. J Cancer Educ. 2011;27:46-53. [DOI] [PubMed] [Google Scholar]
- 7. Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123-133. [DOI] [PubMed] [Google Scholar]
- 8. Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;(8):CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szymlek-Gay EA, Richards R, Egan R. Physical activity among cancer survivors: a literature review. N Z Med J. 2011;124:77-89. [PubMed] [Google Scholar]
- 10. Meneses-Echavez JF, González-Jiménez E, Ramírez-Vélez R. Supervised exercise reduces cancer-related fatigue: a systematic review. J Physiother. 2015;61:3-9. [DOI] [PubMed] [Google Scholar]
- 11. Rajarajeswaran P, Vishnupriya R. Exercise in cancer. Indian J Med Paediatr Oncol. 2009;30:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carayol M, Bernard P, Boiché J, et al. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol. 2013;24:291-300. [DOI] [PubMed] [Google Scholar]
- 13. Berger AM, Mooney K, Alvarez-Perez A, et al. ; National Comprehensive Cancer Network. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13:1012-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McNeely ML, Courneya KS. Exercise programs for cancer-related fatigue: evidence and clinical guidelines. J Natl Compr Canc Netw. 2010;8:945-953. [DOI] [PubMed] [Google Scholar]
- 15. Fuller JT, Hartland MC, Maloney LT, Davison K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med. 2018;52:1311. [DOI] [PubMed] [Google Scholar]
- 16. Griffith K, Wenzel J, Shang J, Thompson C, Stewart K, Mock V. Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer. 2009;115:4874-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mansel JK, Carey EC. Nonpharmacologic approach to sleep disorders. Cancer J. 2014;20:345-351. [DOI] [PubMed] [Google Scholar]
- 18. Chiu HY, Huang HC, Chen PY, Hou WH, Tsai PS. Walking improves sleep in individuals with cancer: a meta-analysis of randomized, controlled trials. Oncol Nurs Forum. 2015;42:E54-E62. [DOI] [PubMed] [Google Scholar]
- 19. Ferioli M, Zauli G, Martelli AM, et al. Impact of physical exercise in cancer survivors during and after antineoplastic treatments. Oncotarget. 2018;9:14005-14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-e34. [DOI] [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 22. Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235-1241. [DOI] [PubMed] [Google Scholar]
- 23. Armijo-Olivo S, da Costa BR, Cummings GG, et al. PEDro or Cochrane to assess the quality of clinical trials? A meta-epidemiological study. PLoS One. 2015;10:e0132634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. RevMan [computer program]. Version 5.1. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 25. Hatala R, Keitz S, Wyer P, Guyatt G; Evidence-Based Medicine Teaching Tips Working Group. Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ. 2005;172:661-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sánchez MJY, Lacomba MT, Sánchez BS, et al. Health related quality of life improvement in breast cancer patients: secondary outcome from a simple blinded, randomised clinical trial. Breast. 2015;24:75-81. [DOI] [PubMed] [Google Scholar]
- 27. Jensen BT, Jensen JB, Laustsen S, Petersen AK, Sondergaard I, Borre M. Multidisciplinary rehabilitation can impact on health-related quality of life outcome in radical cystectomy: secondary reported outcome of a randomized controlled trial. J Multidiscip Healthc. 2014;7:301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumann FT, Zopf EM, Nykamp E, et al. Physical activity for patients undergoing an allogeneic hematopoietic stem cell transplantation: benefits of a moderate exercise intervention. Eur J Haematol. 2011;87:148-156. [DOI] [PubMed] [Google Scholar]
- 29. Dimeo FC, Thomas F, Raabe-Menssen C, Pröpper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery: a randomised controlled trial. Support Care Cancer. 2004;12:774-779. [DOI] [PubMed] [Google Scholar]
- 30. Schmidt T, Weisser B, Dürkop J, et al. Comparing endurance and resistance training with standard care during chemotherapy for patients with primary breast cancer. Anticancer Res. 2015;35:5623-5629. [PubMed] [Google Scholar]
- 31. Wiskemann J, Dreger P, Schwerdtfeger R, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011;117:2604-2613. [DOI] [PubMed] [Google Scholar]
- 32. Ligibel JA, Giobbie-Hurder A, Shockro L, et al. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016;122:1169-1177. [DOI] [PubMed] [Google Scholar]
- 33. Hacker ED, Larson J, Kujath A, Peace D, Rondelli D, Gaston L. Strength training following hematopoietic stem cell transplantation. Cancer Nurs. 2011;34:238-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Do JH, Kim W, Cho YK, et al. Effects of resistance exercises and complex decongestive therapy on arm function and muscular strength in breast cancer related lymphedema. Lymphology. 2015;48:184-196. [PubMed] [Google Scholar]
- 36. Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340-347. [DOI] [PubMed] [Google Scholar]
- 37. Knols RH, de Bruin ED, Uebelhart D, et al. Effects of an outpatient physical exercise program on hematopoietic stem-cell transplantation recipients: a randomized clinical trial. Bone Marrow Transplant. 2011;46:1245-1255. [DOI] [PubMed] [Google Scholar]
- 38. National Health Medical Research Council. NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. https://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/developers/nhmrc_levels_grades_evidence_120423.pdf. Accessed October 1, 2018.
- 39. Meneses-Echávez JF, González-Jiménez E, Ramírez-Vélez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Juvet LK, Thune I, Elvsaas IKØ, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166-177. [DOI] [PubMed] [Google Scholar]
- 41. Kelley GA, Kelley KS. Exercise and cancer-related fatigue in adults: a systematic review of previous systematic reviews with meta-analyses. BMC Cancer. 2017;17:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159:321-340. [DOI] [PubMed] [Google Scholar]
- 43. O’Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol. 2009;167:116-132. [DOI] [PubMed] [Google Scholar]
- 44. Ripamonti C, Bruera E. Dyspnea: pathophysiology and assessment. J Pain Symptom Manage. 1997;13:220-232. [DOI] [PubMed] [Google Scholar]
- 45. Ripamonti C. Management of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7:233-243. [DOI] [PubMed] [Google Scholar]
- 46. Vargas-Bermúdez A, Cardenal F, Porta-Sales J. Opioids for the management of dyspnea in cancer patients: evidence of the last 15 years—a systematic review. J Pain Palliat Care Pharmacother. 2015;29:341-352. [DOI] [PubMed] [Google Scholar]
- 47. Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koelwyn GJ, Jones LW, Hornsby W, Eves ND. Exercise therapy in the management of dyspnea in patients with cancer. Curr Opin Support Palliat Care. 2012;6:129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leung L, Riutta T, Kotecha J, Rosser W. Chronic constipation: an evidence-based review. J Am Board Fam Med. 2011;24:436-451. [DOI] [PubMed] [Google Scholar]
- 50. Holzer P, Ahmedzai SH, Niederle N, et al. Opioid-induced bowel dysfunction in cancer-related pain: causes, consequences, and a novel approach for its management. J Opioid Manag. 2009;5:145-151. [DOI] [PubMed] [Google Scholar]
- 51. Iovino P, Chiarioni G, Bilancio G, et al. New onset of constipation during long-term physical inactivity: a proof-of-concept study on the immobility-induced bowel changes. PLoS One. 2013;8:e72608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robertson G, Meshkinpour H, Vandenberg K, James N, Cohen A, Wilson A. Effects of exercise on total and segmental colon transit. J Clin Gastroenterol. 1993;16:300-303. [DOI] [PubMed] [Google Scholar]
- 53. Lee J, Dodd MJ, Dibble SL, Abrams DI. Nausea at the end of adjuvant cancer treatment in relation to exercise during treatment in patients with breast cancer. Oncol Nurs Forum. 2008;35:830-835. [DOI] [PubMed] [Google Scholar]
- 54. Kolnes LJ. Exercise and physical therapy help restore body and self in clients with severe anorexia nervosa. J Bodyw Mov Ther. 2017;21:481-494. [DOI] [PubMed] [Google Scholar]
- 55. King KS, Darmani NA, Hughes MS, Adams KT, Pacak K. Exercise-induced nausea and vomiting: another sign and symptom of pheochromocytoma and paraganglioma. Endocrine. 2010;37:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Douglas JA, King JA, Clayton DJ, et al. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int J Obes (Lond). 2017;41:1737-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]