Abstract
Background: Radiotherapy is one of the primary therapies for localized prostatic carcinoma. Therefore, there is an emerging need to sensitize prostatic cancer cells to chemotherapy/radiotherapy. Modified citrus pectin (MCP) is an effective inhibitor of galectin-3 (Gal-3), which is correlated with tumor progression, proliferation, angiogenesis, and apoptosis. Purpose: This study was directed to evaluate the efficacy of combining ionizing radiation (IR) with MCP on PCa cells. Study Design: Effects of treatments on PCa cells survival were evaluated using XTT assay, flow cytometry, and clonogenic survival assay. Expression of selected proteins was estimated using western blotting. Cell motility, migration, and invasion were determined. Contribution of reactive oxygen species production to treatment effects on cell viability was tested. Results: Radiotherapy combined with MCP reduced viability and enhanced radiosensitivity associated with a decrease in Gal-3, cleavage of the precursor of caspase-3, increased expression of the pro-apoptotic protein Bax, and downregulation of DNA repair pathways, poly-ADP-ribose polymerase, and proliferating cell nuclear antigen. MCP significantly reduced the invasive and migratory potential of PCa cells. Combining sodium pyruvate with MCP and IR mitigated the effect on cell viability. Conclusion: Our findings demonstrated that MCP sensitized PCa cells to IR by downregulating anti-apoptotic Gal-3, modulating DNA repair pathways, and increasing ROS production. For the first time the correlation between MCP, radiotherapy, and Gal-3 for prostatic cancer treatment was found. In addition, MCP reduced the metastatic properties of PCa cells. These findings provide MCP as a radiosensitizing agent to enhance IR cytotoxicity, overcome radioresistance, and reduce clinical IR dose.
Keywords: prostate cancer, modified citrus pectin, ionizing radiation, radiosensitivity, galectin-3
Introduction
Prostate cancer (PCa) is the most diagnosed noncutaneous malignancy and the third leading cause of cancer-related deaths in men, after lung and colorectal cancer.1 The PCa disease varies from a dormant localized state to a highly invasive tumor that metastasizes preferentially to bones like other epithelial cancers such as breast and lung cancers. Conventional treatment modalities for PCa are radiotherapy, radical prostatectomy, androgen deprivation, and chemotherapy. Ionizing radiation (IR) damages tumor cells’ DNA directly by single and double strand breaks or indirectly by reactive oxygen species (ROS), which causes injury to biomolecules, including DNA.2 A major obstacle to IR therapy is that there is a maximum amount of radiation that can be safely administered. Despite IR advances in delivery technology, the rate of biochemical relapse/clinical recurrence for a considerable number of PCa patients who have undergone IR therapy unfortunately remains high. Understanding the mechanisms of radioresistance will help overcome recurrence after IR therapy in PCa patients and prevent metastasis. Combining radiotherapy with radiosensitizing agents could offer a way to selectively enhance toxicity and overcome radioresistance.
Natural compounds from plants provide an important source of new radiosensitizing agents with limited or no toxicity. For example, curcumin, which has been reported as a radiosensitizer in PCa cells, such as PC-3, DU-145, and LNCaP partly via epigenetic activation of miR-143- and miR-143-mediated autophagy inhibition3; capsaicin, the active compound in chili peppers, has been shown to modulate the response to IR in PCa models through the inhibition of NF-κB signaling4; the polyphenol phytochemical, resveratrol, can enhance the radiosensitivity by either targeting the mitochondrial functionality, modulating the tumor necrosis factor-mediated or Fas-FasL-mediated pathways of apoptosis in different cancers.5
Esophageal squamous cell carcinoma is resistant to radiation therapy, resulting in the modulation of its invasion, infiltration, and metastasis. Astaxanthin was shown to increase radiosensitivity in esophageal squamous cell carcinoma through inducing apoptosis and G2/M arrest via inhibiting Bcl-2, Cyclin B1, Cdc2, and promoting Bax expression.6
Modified citrus pectin (MCP) is a soluble polysaccharide fiber dietary supplement produced from enzymatically hydrolyzed citrus pectin, and has GRAS (generally regarded as safe) designation from the US Food and Drug Administration (Code of Federal Regulations, Title 21, Volume 3, 21, CFR184.1588).7 MCP has shown to exhibit antineoplastic properties in various laboratory models: it inhibited proliferation and induced apoptosis of human and mouse PCa cells,8 decreased invasive behavior of human prostate and breast cancer cells,9 and inhibited liver metastasis in animal colon cancer model.10
Ramachandran et al have shown that MCP induced the activation of human blood lymphocyte subsets like T, B, and NK cells.11
Moreover, MCP was found to sensitize PCa cells to doxorubicin12 and ovarian cancer cells to paclitaxel.13
In a pilot clinical trial performed in the University of California that included 10 patients, the authors assessed the effect of MCP on PSA levels in patients with biochemical PSA failure after definitive local therapy for PCa. The authors found that 70% of the patients had a statistically significant increase in PSA doubling time after taking MCP orally for 12 months.14
It has been shown that galectin-3 (Gal-3) has an important role in tumor aggressiveness through the regulation of anchorage-independent cell growth, invasion, migration, and androgen receptor signalling.15,16 MCP acts mainly as an antagonist to extracellular Gal-3.17
To date, there has been no report about the combined effect of MCP and IR. Therefore, the aim of the present study was to investigate the effect of IR alone and in combination with MCP on survival and metastatic activity of PCa cells.
Materials and Methods
Reagents
PectaSol-CMCP (ecoNugenics, Santa Rosa, CA) was prepared in a stock of 25 mg/mL in H2O, stored at −20°C, thawed and diluted in a cell culture medium immediately before treatment. Antibiotics (penicillin, streptomycin, amphotericin) and a kit for XTT-based cell proliferation assay were obtained from Biological Industries (Beit-HaEmek, Israel). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Life Technologies (Rehovot, Israel). Propidium iodide (PI) was obtained from Sigma-Aldrich (St. Louis, MO). Antibodies for western blot analysis were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Lines
Human prostate carcinoma cells (PC-3, DU-145, and Cl-1) were obtained from the American Type Culture Collection and maintained in DMEM supplemented with 10% heat-inactivated FBS and 1% penicillin-streptomycin-amphotericin B solution. All cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2, harvested by trypsin/EDTA (ethylenediaminetetraacetic acid) and passaged 1 to 2 times/week. In all the experiments, 24-hour cell cultures were used.
Radiotherapy
The cells in 96-well plates were irradiated with single 2 to 4 Gy dose 30 minutes after adding MCP. Irradiation was performed using a linear accelerator operated at 6 MeV photon beam at a dose rate of 418 cGy/min.
XTT Assay for Cell Survival
The cells were seeded in 96-well plates (1.5-2 × 103 cells/well) and attached overnight before being treated with MCP and/or irradiated. After 72 hours of incubation, cell viability was evaluated using XTT cell proliferation assay (Biological Industries, Beit-HaEmek, Israel). Highly colored formazan was measured at 450 nm by absorbance plate reader (TECAN, Sunrise, Männedorf, Switzerland). Each plate included blank wells containing media but no cells and the control wells containing nontreated cells. Each variant of the experiment was performed in triplicate and repeated at least twice.
Clonogenic Assay for Cell Survival
DU-145 cells were carefully pipetted, then counted, and 300 to 500 cells/plate were seeded in 60-mm tissue culture plates (Corning, NY). After 24 hours incubation, the cells were treated with MCP and/or irradiated and then allowed to grow for 10 to 14 days until the surviving cells produced colonies consisting of 50 or more cells. The colonies were washed with phosphate-buffered saline (PBS), fixed with 70% ethanol, and stained with Giemsa stain (Beckman Coulter, Brea, CA) for 5 to 10 minutes at room temperature. The stained colonies were washed with PBS and then counted using light microscopy. The surviving fraction is the ratio of colony number to the number of cells plated with a correction for plating efficiency (PE; percent of intact cells producing colonies).
Analysis of Mode of Combined Treatment
The mode of IR and MCP interaction was analyzed using “CalcuSyn” software (Biosoft, Cambridge, UK), based on Chou and Talalay’s equation for calculation of combination index (CI).18 The dose-effect curves, CI, and normalized isobolograms were determined: CI < 1.0 indicates synergism of treatments tested, whereas CI = 1.0 indicates an additive effect of combined treatment.
Flow Cytometry for Cell Cycle and Induction of Apoptosis
The cells treated with MCP and/or irradiated were incubated 24 to 72 hours and then harvested, washed twice with PBS, and fixed in 70% ethanol for 1 hour. The cells were resuspended in PBS containing 20 µg/mL PI and 200 µg/mL of DNase-free RNase and incubated in the dark for 30 minutes. Data were acquired on FACSCalibur/Arya instrument (BD Bioscience, San Jose, CA). The distinct phases of the cell cycle were fitted using the mathematical Watson Pragmatic model with the FlowJo Analysis Software (Tree Star, Ashland, OR). Necrotic cells were counted following trypan blue staining.
Annexin V-FITC/7-AAD Assay for Cell Apoptosis
The cells were double-stained with fluorescein isothiocyanate-conjugated annexin V (Annexin V-FITC) and 7-amino-actinomycin D (7-AAD) using apoptosis kit (Life Technologies, Rehovot, Israel). The percentage of apoptotic cells was determined by FACS (fluorescence-activated cell sorting) analysis according to the manufacturer’s instructions.
Western Blot Analysis of Protein Expression
Expression of anti-apoptotic factors and other regulatory molecules was evaluated according to the standard protocols. Protein concentration was determined using a Bradford/Bio-Rad Protein Assay (Bio-Rad, Hercules, CA), based on bovine serum albumin standard curve. Blots were detected using SuperSignal West Pico Chemiluminescent Substrate (ThermoScientific, Waltham, MA), and band densities were quantified using LI-Cor Image Studio Lite software (LI-Cor Biotechnology, Lincoln, NE).
Preparation of Cytoplasmic and Nuclear Extracts
Intact or treated DU-145 cells were washed twice with ice-cold PBS, trypsinized, and centrifuged at 500 g for 5 minutes. Cell pellets were used for extraction of the nuclear and cytoplasmic fractions using a NucBuster protein extraction kit (Novagen, Madison, WI.
Wound Healing Assay for Cell Motility
DU-145 cells (15-20 × 105 cells/well) were seeded in 6-well culture plates. The confluent cell monolayer was scraped using a 200-µL micropipette tip. The cells were washed 3 times with PBS and incubated for 48 hours with MCP. Scratch area photographs were taken using a Nikon Eclipse TE2000-s microscope (Nikon GmbH, Germany). The data were described as the closed width of the wound compared with the initial width (%, mean ± standard error [SE]).
Transwell Cell Migration Assay
Cell migration was assayed using a modified Boyden Chamber (Greiner Bio-One GmbH, Germany) with 8-µm pore size membrane in a 24-well plate (NUNCLON, Sigma-Aldrich, St. Louis, MO). The lower part of the chambers was filled with DMEM containing 10% FBS. The cells in serum-free DMEM (5 × 105 cells/mL) were placed in the upper part of the chambers with and without MCP and incubated at 37°C with 5% CO2 for 24 hours. Assays were carried out in duplicate. The culture media were discarded, and the top side of the membrane was scraped with a wet cotton swab to remove nonmigrated cells. Invading cells were fixed in 70% ethanol and stained with Giemsa stain (Beckman Coulter, Brea, CA). The average number of migrated cells was counted from 6 randomly selected microscopic fields at 40× magnification. The migration index was calculated by comparing the migration of treated cells relative to control.
Transwell Cell Invasion Assay
Cell invasion was assayed as described above, but the membrane was covered with Matrigel at 300 µg/cm2.
Evaluation of ROS Production Contribution to Treatments Effects on Cell Viability
The cells plated in 96-well plates were treated with MCP and/or IR in full DMEM with or without sodium pyruvate (1 mM). Cell viability was determined using XTT assay.
Statistical Analysis
Experiments were performed in triplicate and repeated 2 to 4 times. Mean values and standard errors were calculated for each point from the pooled normalized data. The significance of the difference between the arms was analyzed using the 1-tailed Student t test with unequal variance and was considered as statistically significant if P < .05.
Results
MCP and IR Reduced PCa Cells Viability
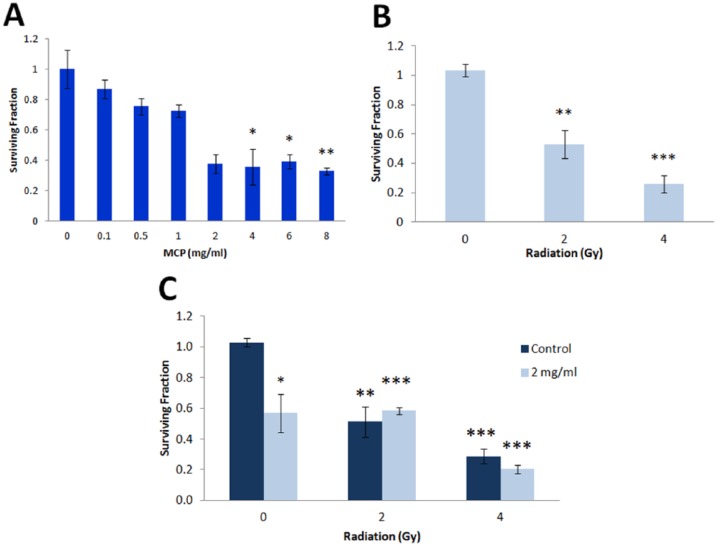
As found by XTT assay, the treatment of all 3 tested cultured prostate carcinoma cells (PC-3, Cl-1, and Du-145) with MCP for 72 hours induced a dose-dependent decrease in cell viability (Figure 1B). DU-145 cells were more sensitive to this treatment.
Figure 1.
Effect of MCP (B) and IR (A) alone on PCa cells viability. Cell viability was evaluated by XTT assay. The graphs represent mean ± SE survival values of irradiated/treated cells from 3 experiments each performed in triplicate (*P < .05; **P < .01; ***P < .001).
The irradiation of PCa cells with a single dose of IR (2-4 Gy) resulted in significant survival decrease (Figure 1A): PC-3 demonstrated the highest radiosensitivity, while DU-145 cells were the most radioresistant.
The combined effect of MCP and IR on cell survival was more significant than the effect of each treatment alone (Figure 2). CalcuSyn software used to analyze the mode of interaction between these treatments revealed that on DU-145 cells the combination of MCP and IR resulted in a synergistic effect at high and low doses, whereas the effect was additive at median doses (Figure 2). On PC-3 and Cl-1 cells, the combined treatment resulted in mostly additive effect (Figure 2).
Figure 2.
Combined effect of MCP and IR on cell viability. (A, B, and C) Survival of cells evaluated by XTT assay. (D, E, and F) Normalized isobolograms indicating mode of treatments interaction.
DU-145 cells, in which the maximal synergistic effect was observed, were chosen for further studies.
In addition, the effect of treatments on DU-145 cell survival was also evaluated by a more sensitive clonogenic assay. The inhibitory effect of each treatment alone and in combination was more significant than the effect found by XTT assay (Figure 3). The highest inhibition was found at 4 mg/mL MCP. The inhibitory effect of 2 and 4 Gy was very significant. MCP and IR in combination resulted in enhanced inhibition, thus corroborating synergistic effect observed by the XTT assay.
Figure 3.
Effect of MCP and IR on DU-145 cell survival evaluated by clonogenic assay. Cell survival after MCP (A) and IR (B) treatments alone and after combined treatment (C).
MCP Induced Apoptosis and Moderated G2/M Cell Cycle Arrest
The effect of MCP on PCa cell cycle was evaluated by flow cytometry of PI-stained Du-145 cells as more sensitive to MCP treatment and characterized by high radioresistance.
After 12 hours of MCP treatment, the cell distribution in the cell cycle revealed accumulation of cells in the G0/G1 phase (58.9% for 1 mg and 68.2% for 2 mg). Moderate G2/M phase arrest appeared after 24 hours of exposure (9.62% for 1 mg and 14.2% for 2 mg). More obvious changes in G2/M phase were observed after 72 hours of treatment (19.1% for 1 mg and 17.9% for 2 mg, compared with 12.4% in control; Figure 4A).
Figure 4.
Induction of apoptosis in DU-145 cells treated by MCP. (A) PI staining and (B) double Annexin-V-FITC/7-AAD staining. Double-negative cells are intact cells, Annexin-V-FITC positive cells indicated early apoptosis, double-positive cells indicated late apoptosis, and 7-AAD positive cells indicated necrotic cells.
To explore whether MCP can cause cell damage through the induction of apoptosis, the treated cells were tested by FACS analysis using Annexin V-FITC/7-AAD double staining (Figure 4A and B). According to the manufacturer’s instructions, cells stained with Annexin V-FITC alone demonstrated early apoptosis, cells double stained with Annexin V-FITC/7-AAD represented a late apoptotic population, while cells stained with 7-AAD alone indicated necrotic cells. Comparing with the negative control, MCP treatment of DU-145 cells for 72 hours resulted in early (4.49%) and late apoptosis (13.94%; Figure 4B), and the percentage of necrotic cells increased from 3.94% to 10.09%. This effect may indicate an apoptosis-independent cell death pathway.
The combination of MCP with IR (2 mg/mL + 4 Gy) resulted in an increase in cell death: 27.25% compared with 18.81% in cells treated with 2 mg/mL MCP alone. Like MCP treatment, the combined treatment affected mostly the necrotic population (13.94% compared with the negative control). Furthermore, while IR increased mostly the number of apoptotic cells, the combined treatment increased mostly the necrotic population.
Effect of MCP on DU-145 Cell Metastatic Activity
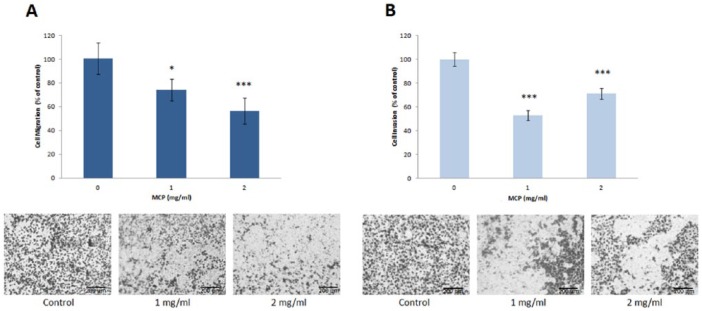
Motility as well as migration and invasion of DU-145 cells were evaluated to investigate whether MCP inhibited the metastatic activity of PCa cells. Migration and invasion were tested using corresponding Transwell assays with 8-µm pore membrane either untreated or covered with Matrigel. The treatment with 1 to 2 mg/mL MCP for 24 hours reduced significantly cell migration and invasion relative to nontreated cells (Figure 5A and B).
Figure 5.
Effect of MCP on migration (A) and invasion (B) of DU-145 cells in vitro. Migration and invasion were evaluated using Transwell assay with or without Matrigel coating of membrane. Representative images of migrated (A) or invaded (B) MCP treated cells are shown.
Motility of PCa cells treated by MCP (1 or 2 mg/mL) was evaluated by wound healing assay. The wound width was assessed by photos performed every 24 hours. Significant dose-dependent inhibition of DU-145 cell motility of 47% ± 8% and 68% ± 9% was revealed.
Role of ROS Production in Cell Death Induced by MCP and IR
The cytoprotective role of pyruvate against ROS-induced cell death is well documented. To determine whether the inhibitory effect of MCP and IR on cell viability is due to ROS production, the cells were treated for 72 hours with MCP/IR with or without 1 mM sodium pyruvate in the medium. Pyruvate co-treatment mitigated MCP effect on cell viability, and as anticipated, the effect of IR on cell viability was completely abrogated by pyruvate (Figure 6A and B).
Figure 6.
Effect of pyruvate on viability of DU-145 cells treated by MCP (A) or IR (B). Cell viability was evaluated by XTT assay. Viability of cells treated with 25 µM H2O2 was used as a positive control. The data are mean ± SE values from 3 individual experiments.
Effect of MCP and IR on PCNA, PARP, Bax, and Caspase-3 Expression
Western blot analysis revealed that treatment of DU-145 cells with MCP or IR downregulated expression of poly ADP-ribose polymerase (PARP; Figure 7A) and proliferating cell nuclear antigen (PCNA; Figure 7B). Combined treatment decreased PARP and PCNA levels more significantly compared with the effect of IR alone. These results suggested that MCP sensitized DU-145 cells to IR partly by downregulating PARP and PCNA expression. In addition, MCP alone and combined with IR induced cleavage of the zymogen precursor of caspase-3 and increased expression of Bax (Figure 7C and D). On induction of apoptosis, pro-caspase-3, the precursor form, is activated by cleavage into p20 and p12 subunits, and the p20 subunit is trimmed to yield a p17 subunit.
Figure 7.
Effect of IR and MCP on expression of selected proteins in DU-145 cells. Cell lysates were subjected to western blot analysis: (A) with mouse antihuman PARP and (B) with β-mouse antihuman PCNA; (C) with antihuman caspase-3 and (D) antihuman Bax; (E) nuclear extract with antihuman Gal-3 and (D) cytoplasmic extract with antihuman Gal-3.
Effect of MCP and IR on Intracellular Gal-3 Expression
To elucidate the mechanisms underlying MCP effects on cell survival, purified nuclear and cytoplasmic extracts from cells treated for 48 hours with MCP (1-6 mg/mL) were subjected to western blot analysis with Gal-3. Treatment with MCP decreased Gal-3 expression in the nucleus and simultaneously increased its expression in the cytoplasm (Figure 7E and F). Moreover, IR alone (4 Gy) or MCP alone (2 mg/mL) did not change significantly Gal-3 expression in whole-cell lysates. But the combined treatment reduced Gal-3 relative expression (normalized to β-actin) by 80%.
Discussion
The present study demonstrated that MCP reduced PCa cell viability in a dose-dependent manner and synergistically enhanced cell sensitivity to IR. MCP caused moderate cell arrest in G2/M phase and nonsignificant reduction of G0/G1 phase. MCP also induced cleavage of the precursor form of pro-apoptotic protein caspase-3 and increased the expression of the pro-apoptotic protein Bax. Therefore, the inhibitory effect of MCP on cell survival may be partly due to induction of apoptosis. Moreover, like MCP treatment, the combined treatment affected mostly the necrotic population.
The role of Gal-3 in malignant cell transformation, such as tumor growth, anoikis resistance, apoptosis inhibition, angiogenesis, cell adhesion, cell motility, and cell invasion is well established.19 The antimetastatic properties of MCP are attributed to critical rate limiting steps by inhibiting Gal-3 and Gal-3-mediated interactions.17 Gal-3, the only known chimeric form of vertebrate galectin, can form extracellular pentamers at its amino terminal end region, and with its carbohydrate recognition domain at its carboxyl end region forms complexes that cross-link glycosylated ligands to form a dynamic lattice on the surface of cells.20 MCP alone did not induce significant changes in intracellular Gal-3 total expression but its combination with IR did significantly decrease Gal-3 expression. Therefore, MCP plus IR induced cell death and the resultant enhanced radiosensitivity could be associated with Gal-3 downregulation and the subsequent inhibition of its anti-apoptotic activity. It may also be speculated that the MCP disruption of the Gal-3 lattice by competitive binding to the carbohydrate recognition domain in the extracellular tumor microenvironment may enhance the effect of the radiation exposure to the cells.
Since side effects of radiation can include induced inflammation and tissue damage, Gal-3 also plays a pivotal role in tissue remodeling and fibrosis. Recently, extensive data on MCP blockade of Gal-3 and reduction of fibrosis in multiple organ tissue animal models21-23 lends further support that the combination may help both ameliorate side effects of radiation therapy and enhance the benefits in a clinical situation.
Cell irradiation resulted in a transient generation of ROS or reactive nitrogen species, oxidative stress, and consequent DNA damage and activation of cellular transduction pathways leading to cell death by apoptosis.24,25 Our finding that pyruvate co-treatment with MCP mitigated the latter’s inhibitory effect on cell viability suggests that one of the mechanisms underlying MCP radiosensitizing activity is enhanced ROS generation. Other research has shown that the cholesterol-lowering statin medication, atorvastatin, enhanced the cell-killing effect of IR by reducing endogenous ROS levels and prolonging the lifespan of radiation-induced ROS via a decrease in the level of mononitrogen oxides and superoxide dismutase activity.26
IR-induced DNA damage resulted in 4 main response reactions: DNA repair, transcriptional response, DNA damage checkpoints, and apoptosis. Our data indicated that MCP downregulated the expression of 2 key components of DNA repair machinery: PARP and PCNA. PARP is required for efficient base-excision repair of apurinic sites and evidence indicates that inhibitors of DNA repair pathways can work as targeted treatment of DNA repair-defective cancers.27 DNA repair pathways can enable tumor cells to survive DNA damage that is induced by treatments; therefore, suppression of specific DNA repair pathways used in combination with DNA-damaging IR could prove to be clinically significant. In addition, alterations in DNA repair pathways that arise during tumor development can make some cancer cells dependent on a reduced set of DNA repair pathways for their survival. Metastatic PCa genomics reveals that up to 90% of patients harbor actionable mutations and >20% have somatic DNA repair gene defects.28 Clinical trials with PARP inhibitors have shown significant response rates of up to 88% for PCa patients having repair gene deficits like BRCA1/2 or ATM mutations rendering these cancers vulnerable to PARP suppression.29 The proliferation marker, PCNA, a cofactor of DNA polymerase, is important in DNA synthesis and repair. A systematic review on PCNA in clinical cancer progression concludes its expression is significantly associated with poor 5-year survival, advanced stage, or higher World Health Organization grade and suggests that it is an effective therapy target in many types of cancers.30
This report of MCP downregulation of repair processes and increased sensitivity to IR is very encouraging. Pharmaceutical inhibitors of PARP are clinically used as single-agent therapy for tumors with BRCA1 or BRCA2 mutations, and research is expanding their use to a wider range of tumors, combining them with cytotoxic chemotherapy or radiotherapy. These PARP inhibitors have shown activity in potentiating the effects of radiotherapy in several tumor types, namely, lung, colorectal, head and neck, glioma, cervix, and prostate cancers.31 Studies of cell behavior after these combined treatments show that radiosensitization is manifested predominantly in an increase in prolonged growth arrest and senescence. However, the possible recovery of senescent cells and reentry into cell cycle leading to tumor recurrence after prolonged arrest also needs to be considered outside of short-term cell assays.32 Further studies including clinical trials to assess potential benefit of combining radiotherapy with MCP are warranted.
In our study, in PCa cell lines, the combined treatment of MCP with radiation decreased Gal-3 expression by 80% in the nucleus and simultaneously increased its expression in the cytoplasm. A recent study33 showed that MCP also inhibited bladder tumor growth through downregulation of Gal-3. A possible explanation why MCP alone did not change the expression of Gal-3 in PCa cells may be related to the high variability between various cell types. It would be interesting to evaluate the effect of MCP on other human cell lines.
Conclusions
In conclusion, our study demonstrated that MCP could sensitize PCa cells to IR by (a) downregulating anti-apoptotic Gal-3, (b) modulating DNA repair machinery, and (c) increasing ROS production. In addition, MCP antagonized the metastatic phenotype of PCa cells by targeting Gal-3. Collectively, these preliminary findings provide a rationale for further studies in animals and humans on using MCP as a radiosensitizing agent in cancer therapy to enhance IR cytotoxicity, overcome radioresistance, and reduce clinical IR dose.
Acknowledgments
The authors would like to thank Barry Wilk and Esther Eshkol for their contributions to this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author Isaac Eliaz acknowledges that he is the owner of a medical clinic and dietary supplement company. The other authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided by ecoNugenics, Inc.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [DOI] [PubMed] [Google Scholar]
- 2. Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311-322. [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Li M, Wang Y, Luo J. Curcumin sensitizes prostate cancer cells to radiation partly via epigenetic activation of miR-143 and miR-143 mediated autophagy inhibition. J Drug Target. 2017;25:645-652. [DOI] [PubMed] [Google Scholar]
- 4. Venier NA, Yamamoto T, Sugar LM, et al. Capsaicin reduces the metastatic burden in the transgenic adenocarcinoma of the mouse prostate model. Prostate. 2015;75:1300-1311. [DOI] [PubMed] [Google Scholar]
- 5. Jiang Z, Chen K, Cheng L, et al. Resveratrol and cancer treatment: updates. Ann N Y Acad Sci. 2017;1403:59-69. [DOI] [PubMed] [Google Scholar]
- 6. Qian X, Tan C, Yang B, et al. Astaxanthin increases radiosensitivity in esophageal squamous cell carcinoma through inducing apoptosis and G2/M arrest. Dis Esophagus. 2017;30:1-7. [DOI] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration. CFR—Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1588. Accessed May 30, 2018.
- 8. Yan J, Katz A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and- independent prostate cancer cells. Integr Cancer Ther. 2010;9:197-203. [DOI] [PubMed] [Google Scholar]
- 9. Jiang J, Eliaz I, Sliva D. Synergistic and additive effects of modified citrus pectin with two polybotanical compounds, in the suppression of invasive behavior of human breast and prostate cancer cells. Integr Cancer Ther. 2013;12:145-152. [DOI] [PubMed] [Google Scholar]
- 10. Liu HY, Huang ZL, Yang GH, Lu WQ, Yu NR. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J Gastroenterol. 2008;14:7386-7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramachandran C, Wilk BJ, Hotchkiss A, Chau H, Eliaz I, Melnick SJ. Activation of human T-helper/inducer cell, T-cytotoxic cell, B-cell, and natural killer (NK)-cells and induction of natural killer cell activity against K562 chronic myeloid leukemia cells with modified citrus pectin. BMC Complement Altern Med. 2011;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tehranian N, Sepehri H, Mehdipour P, et al. Combination effect of PectaSol and doxorubicin on viability, cell cycle arrest and apoptosis in DU-145 and LNCaP prostate cancer cell lines. Cell Biol Int. 2012;36:601-610. [DOI] [PubMed] [Google Scholar]
- 13. Hossein G, Keshavarz M, Ahmadi S, Naderi N. Synergistic effects of PectaSol-C modified citrus pectin an inhibitor of galectin-3 and paclitaxel on apoptosis of human SKOV-3 ovarian cancer cells. Asian Pac J Cancer Prev. 2013;14:7561-7568. [DOI] [PubMed] [Google Scholar]
- 14. Guess BW, Scholz MC, Strum SB, Lam RY, Johnson HJ, Jennrich RI. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6:301-304. [DOI] [PubMed] [Google Scholar]
- 15. Li L, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther. 2014;351:336-343. [DOI] [PubMed] [Google Scholar]
- 16. Fukumori T, Dondoo TO, Daizumoto K, et al. Galectin-3 is involved in the progression of castration-resistant prostate cancer through the regulation of tumor invasion, angiogenesis and androgen receptor signaling. Eur Urol Suppl. 2017;16:e257-e258. [Google Scholar]
- 17. Glinsky VV, Raz A. Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydr Res. 2009;344:1788-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55. [DOI] [PubMed] [Google Scholar]
- 19. Fortuna-Costa A, Gomes AM, Kozlowski EO, Stelling MP, Pavão MSG. Extracellular galectin-3 in tumor progression and metastasis. Front Oncol. 2014;4:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. J Cell Sci. 2015;128:2213-2219. [DOI] [PubMed] [Google Scholar]
- 21. Martínez-Martínez E, Calvier L, Rossignol P, et al. Galectin-3 inhibition prevents adipose tissue remodelling in obesity. Int J Obes. 2016;40:1034-1038. [DOI] [PubMed] [Google Scholar]
- 22. Abu-Elsaad NM, Elkashef WF. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can J Physiol Pharmacol. 2016;94:554-562. [DOI] [PubMed] [Google Scholar]
- 23. Kolatsi-Joannou M, Price KL, Winyard PJ, Long DA. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6:e18683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894-3901. [PubMed] [Google Scholar]
- 25. Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734-5754. [DOI] [PubMed] [Google Scholar]
- 26. Yu Y, Zhu C, Liu C, Gao Y. Effect of prior atorvastatin treatment on the frequency of hospital acquired pneumonia and evolution of biomarkers in patients with acute ischemic stroke: a multicenter prospective study. Biomed Res Int. 2017;2017:5642704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193-204. [DOI] [PubMed] [Google Scholar]
- 28. Geethakumari PR, Schiewer MJ, Knudsen KE, Kelly WK. PARP inhibitors in prostate cancer. Curr Treat Options Oncol. 2017;18:37. [DOI] [PubMed] [Google Scholar]
- 29. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lv Q, Zhang J, Yi Y, et al. Proliferating cell nuclear antigen has an association with prognosis and risks factors of cancer patients: a systematic review. Mol Neurobiol. 2016;53:6209-6217. [DOI] [PubMed] [Google Scholar]
- 31. Powell C, Mikropoulos C, Kaye SB, et al. Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers. Cancer Treat Rev. 2010;36:566-575. [DOI] [PubMed] [Google Scholar]
- 32. Gewirtz DA, Alotaibi M, Yakovlev VA, Povirk LF. Tumor cell recovery from senescence induced by radiation with PARP inhibition. Radiat Res. 2016;186:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fang T, Liu D, Ning H, et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3 [published online May 16, 2018]. Acta Pharmacol Sin. doi: 10.1038/s41401-018-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]