Abstract
Aim: This was a prospective investigation of longitudinal body composition changes in patients with nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy (CCRT) and a comparison of the Patient-Generated Subjective Global Assessment (PG-SGA) and the ESPEN (European Society for Clinical Nutrition and Metabolism) diagnostic criteria (EDC) as evaluation methods. Methods: All patients received standard CCRT according to 2 centers’ current practices. Body composition parameters were determined by bioelectrical impedance analysis and obtained weekly from baseline until the end of treatment. The nutritional status of all patients was evaluated by the PG-SGA and EDC. Results: Forty-eight patients were eligible for analysis. Most body composition parameters, including body cell mass, fat mass, fat-free mass, and skeletal mass, as well as body weight, body mass index, and PG-SGA score, significantly decreased during CCRT (P = .00). The PG-SGA was shown to have better sensitivity than the EDC; however, the 2 different evaluation methods were found to have a perfect concordance at Week 4 and Week 6 (κ = 0.91 and 0.96, P = .00 and .00, respectively). Pearson correlation analyses showed that fat-free mass index and body weight were positively correlated with global quality of life score (r = 0.81, P = .00; r = 0.78, P = .00, respectively). Conclusions: This study has shown that body composition parameters, especially fat-free mass index, are valuable for diagnosing malnutrition in patients with nasopharyngeal carcinoma receiving CCRT. We recommend that these bioelectrical impedance analysis techniques should be increasingly implemented in nutritional assessments.
Keywords: body composition, nasopharyngeal carcinoma, malnutrition, fat-free mass index
Introduction
Although concurrent chemoradiotherapy (CCRT) provides a survival benefit in locally advanced nasopharyngeal carcinoma (NPC) patients, it also increases the risk of acute toxicities.1 As a consequence of dysphagia, mucositis, nausea, and other treatment-related toxicities, malnutrition is very common in patients with NPC undergoing CCRT.2-4 Although malnutrition can be defined by both the Patient-Generated Subjective Global Assessment (PG-SGA) tool5 and the European Society for Clinical Nutrition and Metabolism (ESPEN) diagnostic criteria (EDC),6 a uniform definition of malnutrition is still lacking in oncology settings. The bioelectrical impedance analysis (BIA) technique has been validated for the assessment of body composition and nutritional status in a variety of patient populations including those with cancer.7,8 This technique is easy-to-use, noninvasive, and reproducible. It measures the impedance of the body to a small applied electric current and uses these data, together with an appropriate model, to generate body composition parameters, including body cell mass (BCM), fat mass (FM), fat-free mass (FFM), and skeletal muscle mass (SM).9 These body composition parameters have been used to allow detection, monitoring, and control of hydration and nutrition status using BIA technique for the feedback on treatment in cancer patients. It may offer objective measures to improve clinical decision-making and predict outcomes.10 This study was conducted prospectively to investigate the longitudinal body composition changes in patients with NPC undergoing CCRT and to compare the use of the PG-SGA and the EDC, in order to explore better body composition parameters that could be valuable in diagnosing malnutrition in nasopharyngeal oncology settings.
Methods
Study Design
This study was conducted and reported according to the STROBE (Strengthening The Reporting of OBservational Studies in Epidemiology) guideline. A prospective observational study was conducted in patients with histologically confirmed, stage II to IVa (the Seventh American Joint Committee on Cancer/Union for International Cancer Control staging system) NPC, eligible for CCRT, with or without induction chemotherapy from June 2014 to June 2016. All patients were treated at 2 centers. Other inclusion criteria for this study included an age of 18 to 70 years and a Karnofsky performance status score of at least 70%. Adequate hematologic, hepatic, and renal functions were also required. Exclusion criteria were as follows: (1) distant metastases; (2) other nonremission cancers except for a basocellular carcinoma of the skin; (3) prior chemoradiotherapy treatment within the last 6 months; (4) active intestinal comorbidity or a known eating disorder precluding adequate dietary intake or absorption; (5) a diagnosis of heart failure, uncontrolled diabetes, or (severe) dementia; (6) pregnancy or lactation; and (7) any known allergy to oral supplements. Informed consent was obtained from all enrolled patients. This study was approved by the institutional review boards of the 2 participating centers.
All patients underwent a baseline (before treatment starting) assessment, which included demographic (age and sex), tumor-related (type, grade, stage, size, and site), and clinical (symptoms, comorbidities, and metastases) categories. The nutritional assessment, including body composition and PG-SGA, was undertaken prior to the commencement of CRT. Then, a 15- to 30-minute evaluation and nutritional counseling were performed every 2 weeks throughout the 6-week CRT period by an experienced clinical oncology nutritionist. At each evaluation, interruption of radiotherapy and/or chemotherapy as a result of toxicity was recorded.
Chemoradiotherapy
The standard oncologic therapy regimen included CCRT with cisplatin/nedaplatin according to each center’s current practices. In brief, radiotherapy consisted of a median total dose of 66 to 70.4 Gy in single fractions of 1.8 to 2.2 Gy daily. Cisplatin/nedaplatin was administered 80 mg/m2, every 3 weeks, for 2 cycles.
Measurements
Body Composition
To conduct the body composition analyses, an 8-electrode multifrequency bioelectrical impedance analyzer, the InBody S10 Biospace device (Biospace Co, Ltd, Seoul, Korea/Model JMW140), was used according to the manufacturer’s guidelines.11 The patient was checked in lying position and the electrodes were attached in both ankles for legs and thumbs and middle fingers for arms during a free intake period before fasting by the same nutritionist. Body BCM, FM, FFM, and SM were obtained weekly using the InBody software from baseline until the end of treatment. Fat-free mass index (FFMI) and fat mass index (FMI) values were calculated by dividing a patient’s FFM and FM values by the height squared (m2).
Nutritional Status
Nutritional status was evaluated by both the PG-SGA tool and the EDC for malnutrition. The scored PG-SGA was completed as described by Ottery.12 Each patient was classified by 1 of 3 categories: well-nourished (PG-SGA-A), moderately or suspected of being malnourished (PG-SGA-B), or severely malnourished (PG-SGA-C). In addition, a total PG-SGA score was calculated. For this analysis, patients were classified by PG-SGA as either well-nourished (PG-SGA-A) or malnourished (PG-SGA-B and PG-SGA-C). Using the EDC-based definition of malnutrition, diagnosis could be reached by fulfilling 1 of 2 criteria: (1) BMI <18.5 kg/m2 or (2) the combination of unintentional weight loss (>10% [indefinite of time] or >5% in the last 3 months) and either BMI (<20 kg/m2 if age <70 years or <22 kg/m2 if age >70 years) or FFMI (<15 kg/m2 for women and 17 kg/m2 for men).6 To study the additional value of FFMI in the ESPEN consensus definition, the analyses were also carried out using the EDC for malnutrition without the parameter of FFMI (ie, low BMI [<18.5 kg/m2] or the combination of weight loss and low BMI, depending on age [<20 or 22 kg/m2]).
Quality of Life
Patients completed the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 to assess health-related quality of life (QOL)13,14 at baseline and then biweekly throughout their course of CCRT. It contains 5 functional scales (physical, role, cognitive, emotional, and social), 3 symptom scales (fatigue, pain, and nausea/vomiting), a global QOL scale, and 6 single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). The results were linearly converted to a score out of 100; in addition, overall scores derived from function scales, symptom scales, and single items were calculated on the basis of the very high statistical significance of the interscale correlations, which were calculated according to the European Organization for Research and Treatment of Cancer guidelines.15
Statistical Analysis
For descriptive analysis, continuous variables are presented as the mean and SD or as the median and interquartile range, when appropriate. Categorical variables are presented as frequencies. The Shapiro-Wilk test was used to test the normality of the data distribution. For the primary outcome analysis, the repeated measures analysis of variance (repeated measures ANOVA [analysis of variance]) was used to compare outcome measures at different time points. Agreement between the PG-SGA and the EDC was analyzed by the kappa (κ) statistic. A Pearson correlation analysis was performed to determine the correlation strength between BCM, FMI, FFMI, SM, and QOL.
All statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 18.0, IBM Corp). A 2-sided P < .05 was considered statistically significant.
Results
The original study population consisted of 52 patients. Of these, 4 patients were excluded because of loss to follow-up, since they withdrew from the study during the treatment. The resulting study sample comprised 48 patients, 75.0% of whom were men, with a median age of 47 years. The patient and tumor characteristics are shown in Table 1.
Table 1.
Characteristics of Study Sample.
| Characteristics | Number of Patients (%) |
|---|---|
| Sex | |
| Male | 36 (75.0%) |
| Female | 12 (25.0%) |
| Age (years) | |
| Median (range) | 47 (32-66) |
| Height (cm), mean ± SD | 163.23 ± 1.91 |
| Body weight (kg), mean ± SD | 62.23 ± 2.48 |
| Body mass index (kg/m2), mean ± SD | 23.34 ± 0.6 |
| TNM stage (AJCC 2007) | |
| II | 9 (18.8%) |
| III | 20 (41.7%) |
| IV A | 9 (18.8%) |
| IV B | 10 (20.8%) |
| Tumor stage | |
| T1 | 5 (10.4%) |
| T2 | 24 (50.0%) |
| T3 | 9 (18.8%) |
| T4 | 10 (20.8%) |
| Nodal stage | |
| N0 | 1 (2.1%) |
| N1 | 19 (39.6%) |
| N2 | 16 (33.3%) |
| N3 | 12 (25.0%) |
| Treatment | |
| CCRT | 3 (6.3%) |
| IC + CCRT | 45 (93.8%) |
| Radiation doses/fractions (Gy/F) | |
| 66/30 | 29 (60.4%) |
| 70.4/32 | 19 (39.6%) |
Abbreviations: TNM, tumor, node, and metastasis; AJCC, American Joint Committee on Cancer; CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy.
During CCRT, there were statistically and clinically significant changes in most body composition parameters, including BCM, FM, FFM, and SM, as well as body weight, BMI, and PG-SGA scores (Table 2). Except for BCM and SM, BMI, FMI, and FFMI decreased from Week 0 to Week 2. The PG-SGA score decreased from Week 0 to Week 2, while since Week 2 the score increased dramatically. FMI decreased approximately 13.1% during radiotherapy; FFMI, 6.8%; BCM, 6.0%; SM, 6.8%; body weight, 8.9%; and BMI, 9.4%. The influence of demographic and clinical factors on body composition showed that the differences in results were not statistically significant (Table 3).
Table 2.
Changes in Nutritional Parameters During Radiation Therapy ().
| Variable | Baseline | Week 2 | Week 4 | Week 6 | P |
|---|---|---|---|---|---|
| Weight (kg) | 64.36 ± 10.99 | 63.83 ± 11.57 | 61.69 ± 11.43 | 58.76 ± 10.98 | P = .00 |
| Weight loss (%) | 1.48 ± 1.54 | 4.42 ± 3.22 | 8.87 ± 4.04 | P = .00 | |
| BMI (kg/m2) | 23.65 ± 3.22 | 23.24 ± 3.35 | 22.46 ± 3.33 | 21.39 ± 3.26 | P = .00 |
| FMI (kg/m2) | 7.66 ± 1.99 | 7.53 ± 1.99 | 7.20 ± 1.92 | 6.61 ± 1.87 | P = .00 |
| FFMI (kg/m2) | 15.79 ± 1.82 | 15.73 ± 1.93 | 15.28 ± 1.87 | 14.79 ± 2.02 | P = .00 |
| BCM (kg) | 27.78 ± 4.60 | 27.92 ± 5.00 | 27.11 ± 4.74 | 26.35 ± 4.95 | P = .00 |
| SM (kg) | 24.47 ± 6.01 | 24.52 ± 6.15 | 23.75 ± 5.73 | 22.93 ± 5.86 | P = .00 |
| PG-SGA | 7.50 ± 5.97 | 7.50 ± 3.11 | 17.75 ± 5.56 | 20.50 ± 6.76 | P = .00 |
Abbreviations: BMI, body mass index; FMI, fat mass index; FFMI, fat-free mass index; BCM, body cell mass; SM, skeletal muscle mass; PG-SGA, Patient-Generated Subjective Global Assessment.
Table 3.
The Influence of Demographic and Clinical Factors on Body Composition.
| Variable | Age | Sex | Radiation Dose | ||||||
|---|---|---|---|---|---|---|---|---|---|
| >45 Years | ⩽45 Years | P | Male | Female | P | >68 Gy | ⩽68 Gy | P | |
| ΔWeight | 4.90 ± 2.44 | 6.09 ± 3.44 | .057 | 5.75 ± 3.24 | 6.08 ± 2.35 | .816 | 5.31 ± 2.80 | 6.57 ± 3.39 | .382 |
| ΔBMI | 1.79 ± 0.89 | 2.89 ± 1.92 | .072 | 2.22 ± 1.62 | 2.40 ± 0.93 | .792 | 1.90 ± 0.91 | 2.42 ± 1.23 | .306 |
| ΔFMI | 1.22 ± 1.42 | 1.05 ± 0.64 | .638 | 1.14 ± 1.27 | 1.19 ± 0.40 | .927 | 1.34 ± 1.44 | 1.15 ± 1.04 | .746 |
| ΔFFMI | 0.84 ± 0.63 | 1.16 ± 0.83 | .075 | 1.08 ± 0.81 | 1.21 ± 0.63 | .706 | 0.76 ± 0.74 | 1.41 ± 0.79 | .078 |
| ΔBCM | 1.33 ± 0.97 | 2.38 ± 1.62 | .082 | 1.74 ± 1.43 | 1.90 ± 1.15 | .797 | 1.29 ± 1.23 | 2.34 ± 1.49 | .103 |
| ΔSM | 1.27 ± 1.09 | 2.32 ± 1.63 | .057 | 1.69 ± 1.51 | 1.77 ± 1.01 | .91 | 1.26 ± 1.48 | 2.28 ± 1.49 | .138 |
| ΔPG-SGA | 14.33 ± 7.66 | 13.40 ± 4.84 | .752 | 13.75 ± 6.38 | 14.33 ± 6.03 | .885 | 18.80 ± 6.14 | 14.57 ± 5.68 | .247 |
Abbreviation: Δ, change in body composition from baseline to Week 6; BMI, body mass index; FMI, fat mass index; FFMI, fat-free mass index; BCM, body cell mass; SM, skeletal muscle mass; PG-SGA, Patient-Generated Subjective Global Assessment.
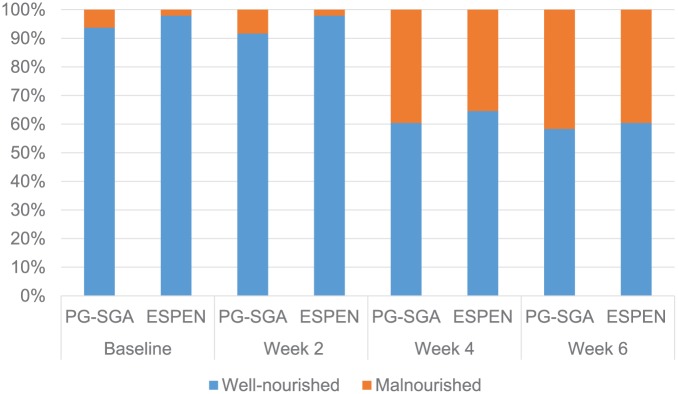
The malnutrition rate of the patient cohort evaluated by both the PG-SGA and the EDC increased from baseline to Week 6, as shown in Table 4. The PG-SGA showed better sensitivity than the EDC, especially for the EDC without FFMI at baseline and at Week 2. The proportion of malnourished patients diagnosed by the EDC without FFMI at Week 4 was only 20.8%, the same as Week 6. However, these proportions increased to 35.4% and 39.6% in Week 4 and Week 6, respectively, when FFMI data were added to the EDC, which were almost equal to those found for the PG-SGA (39.6% and 41.7%; Figure 1). Moreover, the 2 different evaluation methods were found to show perfect concordant agreement at Week 4 and Week 6 (κ = 0.911 and 0.957, P = .00 and .00, respectively; Table 4). Values of 90% sensitivity, 100% specificity, 100% positive predictive value (PPV), and 93.5% negative predictive value (NPV) were found at Week 4. Values of 95% sensitivity, 100% specificity, 100% PPV, and 97% NPV were found at Week 6.
Table 4.
The Prevalence Rate of Malnutrition According to the EDC With/Without FFMI and PG-SGA.
| EDC for Malnutrition Without FFMI |
EDC for Malnutrition With FFMI |
PG-SGA Score |
EDC Versus PG-SGA, κ Coefficient (P) | |
|---|---|---|---|---|
| BMI <18.5 kg/m2 Unintentional WL + Low BMI | Unintentional WL + Low FFMI | B+C (Malnutrition) | ||
| Baseline, n (%) | 1 (2.1%) | 0 (0.0%) | 6 (12.5%) | 0.48 (P = .00) |
| Week 2, n (%) | 1 (2.1%) | 0 (0.0%) | 7 (14.6%) | 0.38 (P = .00) |
| Week 4, n (%) | 10 (20.8%) | 17 (35.4%) | 19 (39.6%) | 0.91 (P = .00) |
| Week 6, n (%) | 10 (20.8%) | 19 (39.6%) | 20 (41.7%) | 0.96 (P = .00) |
Abbreviations: EDC, ESPEN diagnostic criteria; FFMI, fat-free mass index; PG-SGA, Patient-Generated Subjective Global Assessment; BMI, body mass index; WL, weight loss.
Figure1.
Comparison between the PG-SGA score and the ESPEN diagnostic criteria for malnutrition.
Abbreviations: PG-SGA, Patient-Generated Subjective Global Assessment; ESPEN, European Society for Parenteral and Enteral Nutrition.
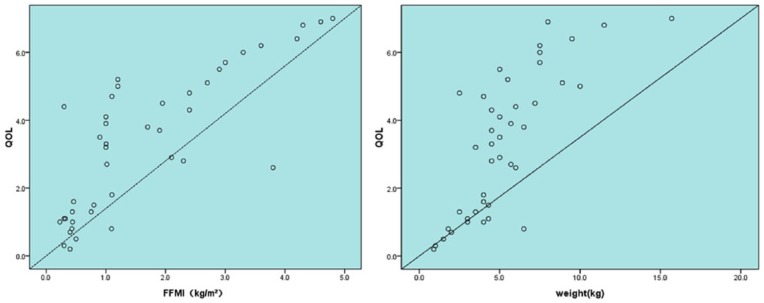
A Pearson correlation analysis showed that change in FFMI positively correlated with change in global QOL score (r = 0.805; P = .00; Figure 2). Change in body weight also positively correlated with change in global QOL score (r = 0. 777; P = .00; Figure 2). This finding suggests that during CCRT, as FFMI and body weight decreases, global QOL score significantly decreases.
Figure 2.
The relationship between change in QOL and change in FFMI and weight.
Discussion
This study, based on a cohort of patients with NPC undergoing CCRT, reports a mean body weight loss of 8.9% over 6 weeks from baseline and a decrease of 6.0% to 13.1% in body composition parameters obtained by BIA. A study conducted by de Carvalho et al16 demonstrated that patients undergoing CCRT for squamous cell carcinoma of the head and neck lost about 10% of baseline weight during treatment. Weight loss is accompanied by loss of FFM, SM, and BCM, deterioration in the QOL, more severe treatment-induced toxicity, and a shorter survival.17 Furthermore, this study also demonstrated the beneficial value of FFMI in helping diagnose malnutrition and the association of decreased FFMI with impaired QOL, which can partially support the important role of body composition parameters in nutritional assessment.
Although nutritional status has been evaluated by various objective measures historically, such as weight change, arm muscle circumference, triceps skinfold thickness, and laboratory (serum albumin, transferrin assays, and nitrogen balance studies) measurements, these measures were always challenging to put into clinical practice.18,19 Furthermore, these objective indicators are not more sensitive in assessing changes in nutritional status over a short period of time.10 The BIA technique, therefore, can overcome some of these challenges. This technique is an easy-to-use, noninvasive, and reproducible method for evaluating changes in body composition. Moreover, this study showed that body composition assessed by BIA could reflect the change of nutritional status. Compared with other methods such as the dual-energy X-ray absorptiometry, BIA has the advantages of being inexpensive, noninvasive, and it can be performed by the clinical dietitian as part of a comprehensive nutrition assessment.20
Currently, no universally accepted diagnostic criteria for malnutrition are available.21 The ESPEN has recently put forward a consensus definition for malnutrition with the aim of reaching uniformity between countries and studies, in which a low FFMI is taken into the diagnostic criteria as is the common use of BIA. While many studies showed that malnutrition was associated with lower physical functioning, lower immune status, more severe CCRT toxicities, treatment interruption, lower chemotherapy response rates, hospital readmission, impaired QOL, and increased mortality,22,23 the problem of predicting malnutrition for early intervention still remains unsolved. By using BIA to calculate body composition changes, we investigated malnutrition diagnosis in the patients with NPC undergoing CCRT “to identify valuable body composition parameters.” The result of this study adds to the growing evidence regarding the clinical applications of BIA-derived body composition measurements.
While the EDC is based on weight loss and body composition indicators (BMI and FFMI), the PG-SGA score incorporates not only recent weight loss but also assessment of nutritional status, including the patient’s symptoms (loss of appetite, nausea, swallowing difficulties, etc), dietary intake, and functional capacities, resulting in a particularly relevant multidimensional score. The PG-SGA is among the most recognizable available tools and the only tool specifically designed to assess malnutrition in oncology.24 This study showed that the sensitivity of the EDC without FFMI was relatively low. Thus, a high proportion of patients classified as undernourished according to the PG-SGA were not classified as undernourished by the EDC without FFMI. This result is in agreement with the result of a large cohort of hospitalized patients reported by Guerra et al.25 Without a BIA measurement, only less than 20% patients were identified as malnourished in Week 4 and Week 6 according to the ESPEN definition, whereas almost an additional 20% patients were identified as such based on FFMI measurements. Moreover, a recent study by Rondel et al26 showed that only the EDC with FFMI was predictive for both 3-month and 1-year survival in hospitalized patients. This finding suggests that including FFMI adds value to the EDC and highlights the importance of the BIA measurement. Since the PG-SGA sensitivity was relatively high in the first 2 weeks, this tool could be used to guide the early intervention of malnutrition in patients with NPC undergoing CCRT. The poor concordance between the PG-SGA and the EDC at Week 0 and Week 2 in our population could be explained by several factors. We found values of 33% and 25% sensitivity, 100% and 100% specificity, 100% and 100% PPV, and 95.7% NPV at Week 0 and Week 2. As the results showed that there is a minimal increase in BCM and SM from Week 0 to Week 2, as well as the PG-SGA score, it seemed that most of the patients with nasopharyngeal cancer had normal nutritional status in the initial stage. Moreover, the first sections that were completed by the patient or responsible caregiver dominated the total score of the PG-SGA at Week 0 and Week 2.
This study had some limitations. First, it was mostly restricted due to its small sample size. However, the data were collected using a prospective study design. To the best of our knowledge, this is the first prospective study to demonstrate the importance of FFMI in the EDC among patients with NPC. A more homogeneous study population would allow more precise conclusions. Second, we did not monitor nutritional status after completion of CCRT; thus, we did not know whether nutritional status affected the treatment side effect peak after CCRT. Further research with a larger sample size is needed to investigate this topic and patients with nasopharyngeal should be monitored for malnutrition not only during CCRT but also after the completion of treatment.
Conclusion
In this study, we have shown that body composition parameters, especially the FFMI, are valuable in malnutrition diagnosis. We recommend that BIA should be increasingly implemented in nutritional assessment.
Footnotes
Author Contributions: Shengjin Dou drafted and revised the manuscript. Huiping Ding analyzed and interpreted the data. Guopei Zhu and Yiqun Ling designed the study and critically reviewed the manuscript. Qiong Wang, Yan Wu, and Yong Qian collected the data. All authors approved the final version of manuscript submitted for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Methuselah Medical Technology (Shanghai) Co, Ltd.
Ethical Approval: The study was approved by the Fudan University Shanghai Cancer Center Ethics Committee (Approval Number: 1405135-4).
ORCID iDs: Yiqun Ling  https://orcid.org/0000-0001-6952-8602
https://orcid.org/0000-0001-6952-8602
Guopei Zhu  https://orcid.org/0000-0003-4770-9458
https://orcid.org/0000-0003-4770-9458
References
- 1. Du CR, Ying HM, Kong FF, Zhai RP, Hu CS. Concurrent chemoradiotherapy was associated with a higher severe late toxicity rate in nasopharyngeal carcinoma patients compared with radiotherapy alone: a meta-analysis based on randomized controlled trials. Radiat Oncol. 2015;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chasen MR, Bhargava R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 2009;17:1345-1351. [DOI] [PubMed] [Google Scholar]
- 3. Alshadwi A, Nadershah M, Carlson ER, Young LS, Burke PA, Daley BJ. Nutritional considerations for head and neck cancer patients: a review of the literature. J Oral Maxillofac Surg. 2013;71:1853-1860. [DOI] [PubMed] [Google Scholar]
- 4. Hong JS, Wu LH, Su L, et al. Effect of chemoradiotherapy on the nutrition status of patients with nasopharyngeal cancer. Nutr Cancer. 2016;68:63-69. [DOI] [PubMed] [Google Scholar]
- 5. Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779-785. [DOI] [PubMed] [Google Scholar]
- 6. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition—an ESPEN consensus statement. Clin Nutr. 2015;34:335-340. [DOI] [PubMed] [Google Scholar]
- 7. Mulasi U, Vock DM, Kuchnia AJ, et al. Malnutrition identified by the Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition Consensus Criteria and other bedside tools is highly prevalent in a sample of individuals undergoing treatment for head and neck cancer [published online October 7, 2016]. JPEN J Parenter Enteral Nutr. doi: 10.1177/0148607116672264 [DOI] [PubMed] [Google Scholar]
- 8. Wladysiuk MS, Mlak R, Morshed K, Surtel W, Brzozowska A, Malecka-Massalska T. Bioelectrical impedance phase angle as a prognostic indicator of survival in head-and-neck cancer. Curr Oncol. 2016;23:e481-e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kyle UG, Bosaeus I, De Lorenzo AD, et al. ; Composition of the ESPEN Working Group. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23:1226-1243. [DOI] [PubMed] [Google Scholar]
- 10. Malecka-Massalska T, Smolen A, Morshed K. Body composition analysis in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2014;271:2775-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang EM, Park E, Ahn YH, et al. Measurement of fluid status using bioimpedance methods in Korean pediatric patients on hemodialysis. J Korean Med Sci. 2017;32:1828-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12(1 suppl):S15-S19. [DOI] [PubMed] [Google Scholar]
- 13. Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer. 1997;33:1025-1030. [DOI] [PubMed] [Google Scholar]
- 14. Dancey J, Zee B, Osoba D, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Qual Life Res. 1997;6:151-158. [DOI] [PubMed] [Google Scholar]
- 15. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 16. de Carvalho TM, Marin DM, da Silva CA, et al. Evaluation of patients with head and neck cancer performing standard treatment in relation to body composition, resting metabolic rate, and inflammatory cytokines. Head Neck. 2015;37:97-102. [DOI] [PubMed] [Google Scholar]
- 17. Bicakli DH, Akagunduz OO, Dalak RM, Esassolak M, Uslu R, Uyar M. The effects of compliance with nutritional counselling on body composition parameters in head and neck cancer patients under radiotherapy. J Nutr Metab. 2017;2017:8631945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carney DE, Meguid MM. Current concepts in nutritional assessment. Arch Surg. 2002;137:42-45. [DOI] [PubMed] [Google Scholar]
- 19. Waitzberg DL, Correia MI. Nutritional assessment in the hospitalized patient. Curr Opin Clin Nutr Metab Care. 2003;6:531-538. [DOI] [PubMed] [Google Scholar]
- 20. Jager-Wittenaar H, Dijkstra PU, Earthman CP, et al. Validity of bioelectrical impedance analysis to assess fat-free mass in patients with head and neck cancer: an exploratory study. Head Neck. 2014;36:585-591. [DOI] [PubMed] [Google Scholar]
- 21. Dehesa-López E, Martínez-Felix JI, Ruiz-Ramos A, Atilano-Carsi X. Discordance between bioelectrical impedance vector analysis and the new ESPEN definition of malnutrition for the diagnosis of hospital malnutrition. Clin Nutr ESPEN. 2017;18:44-48. [DOI] [PubMed] [Google Scholar]
- 22. Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013;32:671-678. [DOI] [PubMed] [Google Scholar]
- 23. Li G, Gao J, Liu ZG, et al. Influence of pretreatment ideal body weight percentile and albumin on prognosis of nasopharyngeal carcinoma: long-term outcomes of 512 patients from a single institution. Head Neck. 2014;36:660-666. [DOI] [PubMed] [Google Scholar]
- 24. Prevost V, Joubert C, Heutte N, Babin E. Assessment of nutritional status and quality of life in patients treated for head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131:113-120. [DOI] [PubMed] [Google Scholar]
- 25. Guerra RS, Fonseca I, Sousa AS, Jesus A, Pichel F, Amaral TF. ESPEN diagnostic criteria for a malnutrition—a validation study in hospitalized patients. Clin Nutr. 2017;36:1326-1332. [DOI] [PubMed] [Google Scholar]
- 26. Rondel AL, Langius JA, de van der Schueren MA, Kruizenga HM. The new ESPEN diagnostic criteria for malnutrition predict overall survival in hospitalized patients. Clin Nutr. 2018;37:163-168. doi: 10.1016/j.clnu.2016.11.018 [DOI] [PubMed] [Google Scholar]