Abstract
Background:
Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). This study assessed the efficacy and safety of tofacitinib in Chinese patients with RA enrolled in Phase 3 and long-term extension (LTE) studies.
Methods:
ORAL Sync was a 1-year, randomized, placebo-controlled, Phase 3 trial. Patients received tofacitinib 5 or 10 mg twice daily (BID) or placebo advanced to tofacitinib 5 or 10 mg BID at 3 or 6 months. All patients remained on ≥1 background conventional synthetic disease-modifying antirheumatic drug. ORAL Sequel is an open-label LTE study (data-cut: March 2015; data collection and analyses were ongoing, and study database was not locked at the time of analysis; study was closed in 2017). Efficacy outcomes: American College of Rheumatology (ACR) 20/50/70 response rates and Disease Activity Score in 28 joints using erythrocyte sedimentation rate (DAS28-4 [ESR]). Patient- and physician-reported outcomes: Health Assessment Questionnaire-Disability Index (HAQ-DI), Patient and Physician Global Assessment of Arthritis, and pain (visual analog scale). Safety was assessed throughout.
Results:
ORAL Sync included 218 patients; 192 were subsequently enrolled into ORAL Sequel. In ORAL Sync, more patients achieved ACR20 (tofacitinib 5 mg BID, 67.4%; 10 mg BID, 70.6%; placebo, 34.1%) and DAS28-4 (ESR) <2.6 (tofacitinib 5 mg BID, 7.1%; 10 mg BID, 13.1%; placebo, 2.3%) with tofacitinib versus placebo at Month 6. Mean changes from baseline in HAQ-DI were greater with tofacitinib versus placebo at Month 6. In ORAL Sequel, efficacy was consistent to Month 48. Incidence rates for adverse events of special interest in tofacitinib-treated patients were similar to the global population.
Conclusions:
Tofacitinib significantly reduced signs/symptoms and improved physical function and quality of life in Chinese patients with moderate-to-severely active RA up to Month 48. The safety profile was consistent with the global population.
Clinical Trial Identifier:
NCT00856544 and NCT00413699.
Keywords: Clinical Efficacy, Patient-Reported Outcomes, Rheumatoid Arthritis, Safety, Tofacitinib
摘要
背景:
托法替布是一种治疗类风湿关节炎(RA)口服Janus 激酶(Jak)抑制剂。我们通过3期临床研究以及长期拓展(LTE)研究, 评估托法替布在中国RA患者中的疗效和安全性。
方法:
ORAL Sync研究是一项为期1年随机双盲安慰剂对照3期临床研究。药物治疗组分别为托法替布5mg 每日两次(BID) 或10mg BID;安慰剂对照组在3个月或6个月的时候随机转换成托法替布5mg BID或10mg BID。所有患者均联合使用≥合种 csDMARDs治疗。ORAL Sequel是一个开放标签的LTE研究(数据截至:2015年3月;数据收集和分析同步进行;2017年研究 结束)。疗效评价标准为美国风湿病学会(ACR)20/50/70应答率和DAS28-4[ESR]评分。患者/医生报告结果评价标准为健 康评估调查问卷功能障碍指数(HAQ-DI),患者和医生总体评价,和疼痛(视觉模拟量表)。安全性评估贯穿整个研究。
结果:
ORAL Sync研究共纳入218例RA患者,192例患者随后进入ORAL Sequel研究。ORAL Sync研究中,药物治疗组的6个月 时的疗效显著高于安慰剂组,其中ACR20(托法替布5 mg BID,67.4%;10 mg BID,70.6%;安慰剂,34.1%),DAS28 - 4 (ESR)<2.6(托法替布5 mg BID,7.1%;10mg BID,13.1%;安慰剂,2.3%)。同样托法替布治疗组6个月HAQ-DI的平均 变化显著高于安慰剂组。ORAL Sequel研究中,疗效稳定长达48个月。安全性方面,托法替布治疗中国RA患者特别关注的不 良事件发生率与全球人口相似。
结论:
托法替布显著降低中国中重度活动性RA患者的症状和体征,显著改善患者生理功能和生活质量,疗效稳定长达48个 月。安全性数据与全球人口一致。
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic and debilitating autoimmune disease characterized by synovial inflammation, leading to the destruction of articular cartilage and progressive joint destruction.[1] Patients with RA experience impaired health-related quality of life due to pain, fatigue, and loss of physical function.[1] As a result, RA imposes a significant health and economic burden, thus highlighting the importance of optimum treatment management to prevent disease progression and to improve prognosis.
The estimated prevalence of RA in China is 0.28%, although regional differences exist and prevalence estimates range from 0.20 to 0.93%.[2,3] As a developing country, China faces several challenges and barriers to the optimal management and treatment of RA. These include the high costs of treatments, limited access to medication, and difficulty accessing public healthcare systems, particularly in rural areas.[4,5,6] Patients in China often present with high levels of disability.[7] Furthermore, compared with Europe and North America, there are differences in the pattern and prevalence of infectious disease (e.g., endemic tuberculosis) in the Asia-Pacific region, which need to be taken into consideration when prescribing immunomodulatory therapies.[8]
The current goal of RA treatment is to achieve remission or low disease activity.[9,10] To meet this goal, the Chinese Rheumatology Association and the Asia Pacific League of Associations for Rheumatology have developed guidelines to reduce misdiagnoses and improve treatment quality.[3,8,11] These guidelines are based on disease characteristics in China and have been adapted from guidelines developed by the European League Against Rheumatism[12] and the American College of Rheumatology (ACR).[9] Conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate, are generally used as first-line treatment in China due to their low costs and established efficacy.[2] csDMARDs are often followed by biologic (b)DMARDs in patients with an inadequate response to csDMARDs.[2,8,13]
While bDMARDs have improved the management of RA, only approximately one-third of the established RA patients meet the criteria for clinical remission.[14] Furthermore, bDMARDs are limited by their intravenous or subcutaneous use, and orally available treatments are desirable. Many patients with RA would prefer an orally administered treatment to an injectable therapy.[15]
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The efficacy and safety of tofacitinib 5 and 10 mg twice daily (BID), as monotherapy or in combination with csDMARDs, in patients with moderate-to-severe RA have been demonstrated in global Phase 2,[16,17,18,19,20] Phase 3,[21,22,23,24,25,26] and Phase 3b/4[27] trials of up to 24 months’ duration, and in long-term extension (LTE) studies of up to 114 months’ observation.[28,29,30]
Although the efficacy and safety of tofacitinib have been demonstrated in global Phase 3 studies and improved patient-reported outcomes of tofacitinib in Chinese patients with RA have been shown in a Phase 3 study,[31] the clinical effectiveness and tolerability of tofacitinib have not been previously reported exclusively in Chinese patients enrolled in Phase 3 and LTE studies. Given that different patient populations may present different clinical characteristics, different responses, and different risks, it is of interest to investigate clinical outcomes specific to Chinese patients.[7]
ORAL Sync was a Phase 3, randomized, double-blind, placebo-controlled, 12-month, clinical trial of tofacitinib, the results of which have been previously reported.[23] Of the six global Phase 3 trials of tofacitinib, ORAL Sync is the only trial with study sites in China. Patients in ORAL Sync could enroll in the open-label LTE study, ORAL Sequel. In this study, we describe for the first time, the efficacy and safety of tofacitinib in Chinese patients enrolled in ORAL Sync[23] and ORAL Sequel.[28]
METHODS
Ethical approval
Both studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each investigational center. All patients provided written informed consent.
Study design and patients
This analysis included efficacy and safety data from Chinese patients with RA enrolled in a Phase 3 study (ORAL Sync, A3921046 [NCT00856544])[23] and an open-label LTE study (ORAL Sequel, A3921024 [NCT00413699]).[28]
Patients who participated in ORAL Sync were randomized 4:4:1:1 to receive tofacitinib 5 mg BID, tofacitinib 10 mg BID, placebo advanced to tofacitinib 5 mg BID at Month 3 or 6, or placebo advanced to tofacitinib 10 mg BID at Month 3 or 6, respectively, all in combination with csDMARDs. Patients receiving placebo who did not respond at Month 3 (i.e., those not achieving ≥20% reduction from baseline in swollen and tender joint counts) were advanced blindly to tofacitinib 5 or 10 mg BID. At Month 6, all remaining placebo patients were advanced to tofacitinib.[23]
ORAL Sequel is an open-label LTE study that enrolled patients who completed qualifying Phase 1, 2, or 3 index studies of tofacitinib. All Chinese patients included in this analysis were from ORAL Sync. For this analysis, a data cutoff date of March 31, 2015 was used (data collection and analyses were ongoing, and the study database was not yet locked). ORAL Sequel was closed in October 2017, and the last subject last visit of a Chinese patient was May 27, 2015; therefore, some values may have changed for the final, locked study database. When enrolled into ORAL Sequel, Chinese patients initially received tofacitinib 5 mg BID and were allowed to increase their dose to 10 mg BID at the discretion of the investigator. Patients were analyzed in tofacitinib 5 and 10 mg BID dose groups based on average total daily dose (TDD; sum of doses received divided by number of days a dose was received) in the LTE study. The 5 and 10 mg BID dose groups were defined as TDD <15 mg/d and TDD ≥15 mg/d, respectively. Baseline values for the LTE study were those of the index studies for patients enrolling in the LTE within 14 days of the index study; for all other patients, baseline was the start of the LTE study.
Detailed inclusion and exclusion criteria have been reported previously.[23,28] Eligible patients were aged ≥18 years and had active RA based on the ACR 1987 revised criteria,[32] despite receiving treatment with ≥1 stably dosed csDMARDs or bDMARDs. Key inclusion criteria included ≥4 tender or painful joints (68- or 66-joint count) and an erythrocyte sedimentation rate of ≥28 mm/h or a C-reactive protein level of >7 mg/L. Key exclusion criteria included serious chronic or recurrent infections, evidence of active or inadequately treated latent tuberculosis infection, history of recurrent herpes zoster, disseminated herpes zoster or herpes simplex, hepatitis B or C, HIV or other opportunistic infections, and history of lymphoproliferative disorder and malignancy (except adequately treated or excised nonmetastatic basal or squamous cell skin cancer or cervical carcinoma in situ). Concomitant RA medications were permitted at the discretion of the investigator and included: methotrexate, leflunomide, sulfasalazine, antimalarials, auranofin injectable gold preparations, nonsteroidal anti-inflammatory drugs, and/or glucocorticoids at approved doses.[28]
Efficacy endpoints
Efficacy endpoints included ACR20/50/70 response rates (defined as an improvement from baseline of at least 20%, 50%, and 70%, respectively, in the number of tenders and swollen joints and at least three of the five ACR components); mean change from baseline in Disease Activity Score in 28 joints using erythrocyte sedimentation rate (DAS28-4 [ESR]); and the proportions of patients achieving remission, defined by DAS28-4 (ESR) <2.6, and low disease activity, defined by DAS28-4 (ESR) ≤3.2. Efficacy data were reported up to Month 12 for ORAL Sync and up to Month 48 for ORAL Sequel.
Patient- and physician-reported outcomes
Outcomes included mean change from baseline in: Health Assessment Questionnaire-Disability Index (HAQ-DI); Patient Global Assessment of Arthritis (PtGA); Physician Global Assessment of Arthritis (PGA); and Patient Assessment of Arthritis Pain (pain; visual analog scale [VAS]).
Safety endpoints
All available safety data through Month 12 for ORAL Sync and Month 48 for ORAL Sequel are presented. Safety endpoints included reporting of adverse events (AEs), serious AEs (SAEs), discontinuations due to AEs, and deaths. AEs were recorded using the Medical Dictionary for Regulatory Activities (MedDRA) Preferred Terms.
Statistical analysis
In this exploratory, post hoc analysis, efficacy analyses were based on the full analysis set (FAS), which included all patients who received ≥1 dose of study drug and for whom data were available from ≥1 postbaseline assessment. All safety analyses were based on observed cases. Incidence rates (IRs; unique patients with events per 100 patient-years of observation) for AEs of special interest were based on the number of patients with an event and the total exposure time censored at the time of event, death, or discontinuation from the study and compared between treatment groups. The 95% confidence intervals for IRs were based on Exact Poisson adjusted for exposure time.
In ORAL Sync, efficacy binary endpoints were compared between tofacitinib and placebo up to Month 6 by forming a z-score using the normal approximation to the binomial. Missing values were computed using the nonresponder imputation (NRI) method. In addition, patients at Month 3 who were advanced were treated by NRI as having nonresponse (advancement penalty). For analyses showing the patient responses by treatment sequence, the advancement penalty was not used in the NRI method (NRINAP) so that patients who advanced and remained in the study and achieved response would be counted as achieving that response. Continuous endpoints were analyzed using a linear mixed-effect model with treatment, visit, and treatment-by-visit interaction as fixed effects and patient as random effect. Estimates of mean changes from baseline for each treatment and mean differences versus placebo were derived from the model as least squares means, with corresponding standard errors.
In ORAL Sequel, efficacy analyses were based on the observed cases of its FAS population and were summarized descriptively.
RESULTS
Patients
In ORAL Sync, 218 Chinese patients were randomized to receive either tofacitinib 5 mg BID (n = 88), tofacitinib 10 mg BID (n = 86), placebo advanced to tofacitinib 5 mg BID (n = 22), or placebo advanced to tofacitinib 10 mg BID (n = 22); of these, 82 (93.2%), 78 (90.7%), 19 (86.4%), and 20 (90.9%) patients, respectively, completed the Phase 3 study. Overall, 192 Chinese patients from ORAL Sync were subsequently enrolled in ORAL Sequel and assigned to receive tofacitinib 5 mg BID (n = 153) or tofacitinib 10 mg BID (n = 39).
Patient demographics and baseline characteristics were similar among treatment groups in ORAL Sync and ORAL Sequel [Table 1]. It should be noted that the baseline disease characteristics presented for all patients in ORAL Sequel are those of the index study, ORAL Sync, for patients who enrolled in the LTE within 14 days of the index study. At baseline, all Chinese patients in ORAL Sync received ≥1 csDMARDs, and 90 patients (41.7%) had previously received glucocorticoids (primarily prednisone).
Table 1.
Baseline demographics and disease characteristics for Chinese patients enrolled in ORAL Sync and ORAL Sequel
| Characteristics | ORAL Sync | ORAL Sequel* | ||||
|---|---|---|---|---|---|---|
| Tofacitinib 5 mg BID (n = 86) | Tofacitinib 10 mg BID (n = 86) | Placebo → tofacitinib 5 mg BID (n = 22) | Placebo → tofacitinib 10 mg BID (n = 22) | Tofacitinib 5 mg BID (n = 153) | Tofacitinib 10 mg BID (n = 39) | |
| Age (years), mean (range) | 49.2 (21–70) | 47.1 (20–78) | 47.2 (22–66) | 48.7 (28–67) | 49.9 (21–72) | 48.0 (26–66) |
| Female, n (%) | 75 (87.2) | 71 (82.6) | 21 (95.5) | 17 (77.3) | 133 (86.9) | 33 (84.6) |
| Weight (Kg), mean (SD) | 57.6 (9.4) | 59.6 (9.5) | 54.2 (9.1) | 59.3 (9.5) | 58.4 (9.6) | 60.1 (9.0) |
| BMI (Kg/m2), mean (SD) | 22.3 (3.4) | 22.9 (3.1) | 20.7 (3.2) | 23.0 (3.1) | 22.5 (3.2) | 23.2 (3.6) |
| Duration of RA (years), mean (range) | 6.6 (0.3–29.2) | 7.6 (0.3–41.0) | 9.5 (0.3–39.3) | 7.1 (0.5–25.0) | 7.6 (0.3–41) | 6.4 (0.3–29.2) |
| DAS28-4 (ESR), mean (SD) | 6.2 (1.1) | 6.3 (1.0) | 6.7 (1.1) | 6.2 (0.9) | 6.1 (1.1) | 6.5 (1.0) |
| HAQ-DI, mean (SD) | 1.3 (0.7) | 1.2 (0.7) | 1.2 (0.8) | 1.1 (0.7) | 1.1 (0.7) | 1.3 (0.7) |
| Previous methotrexate use, n (%) | 72 (83.7) | 71 (82.6) | 19 (86.4) | 19 (86.4) | N/A | N/A |
*Data as of March 2015, ongoing at the time of analysis, database not locked; baseline disease characteristics presented for patients in ORAL Sequel are those of the index study, ORAL Sync, for patients who enrolled in the long-term extension within 14 days of the index study. BID: Twice daily; BMI: Body mass index; DAS28-4 (ESR): Disease Activity Score in 28 joints using erythrocyte sedimentation rate; HAQ-DI: Health Assessment Questionnaire-Disability Index; n: Number of patients; N/A: Not applicable; RA: Rheumatoid arthritis; SD: Standard deviation.
Efficacy
ORAL Sync
Chinese patients treated with tofacitinib 5 or 10 mg BID showed significantly greater ACR20/50/70 (all P < 0.05) response rates versus placebo at Month 6 [Supplementary Figure 1 (585.9KB, tif) ]. At Month 6, ACR20 response rates for patients treated with tofacitinib 5 and 10 mg BID were 67.4% and 70.6%, respectively, versus 34.1% for placebo-treated patients. Significant differences in ACR20 versus placebo were observed from Week 2 with tofacitinib 10 mg BID and from Month 1 for tofacitinib 5 mg BID. ACR50 response rates for patients who received tofacitinib 5 and 10 mg BID were 38.4% and 42.4%, respectively, versus 11.4% for patients receiving placebo at Month 6. ACR70 response rates for patients treated with tofacitinib 5 and 10 mg BID were 11.6% and 16.5%, respectively, versus 2.3% for patients receiving placebo at Month 6.
ACR20/50/70 response rates in Chinese patients up to Month 6 in ORAL Sync (FAS, NRI). *P < 0.05; †P < 0.001; ‡P < 0.0001. ACR: American College of Rheumatology; BID: Twice daily; FAS: Full analysis set; NRI: Nonresponder imputation; SE: Standard error.
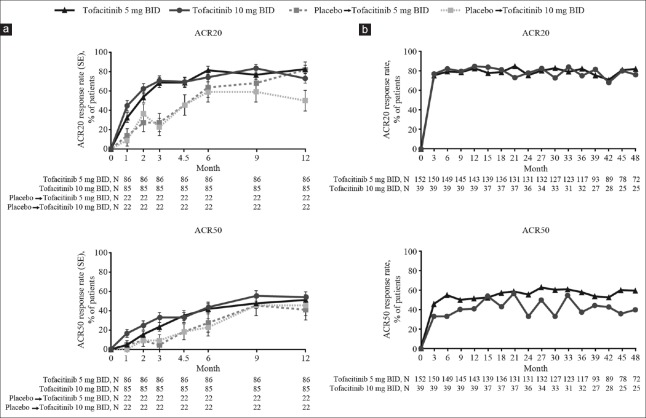
Patients randomized to placebo who were advanced to tofacitinib showed improvements in ACR20/50/70 response rates after switching at Months 3 or 6 [ACR20/50, Figure 1a; ACR70, Supplementary Figure 2 (415.8KB, tif) ]. By Month 12, ACR20 response rates for patients in the tofacitinib 5 and 10 mg BID sequences were 82.6% and 72.9%, respectively, versus 81.8% and 50.0% for patients in the placebo advanced to tofacitinib 5 mg BID and placebo advanced to tofacitinib 10 mg BID sequences, respectively. A numerically greater proportion of patients in the tofacitinib 10 mg BID sequence versus tofacitinib 5 mg BID sequence achieved ACR50 (54.1% versus 51.2%) and ACR70 (29.4% versus 25.6%) over the 12-month period in ORAL Sync. Of the patients in the placebo advanced to tofacitinib 5 mg BID and placebo advanced to tofacitinib 10 mg BID sequences, response rates were 40.9% and 45.5% for ACR50 and 31.8% and 27.3% for ACR70, respectively.
Figure 1.
ACR20/50 response rates in Chinese patients through: (a) Month 12 in ORAL Sync by treatment sequence (FAS, NRINAP); and (b) Month 48 in ORAL Sequel (OC). ACR: American College of Rheumatology; BID: Twice daily; FAS: Full analysis set; NRINAP: Nonresponder imputation, no advancement penalty; OC: Observed cases; SE: Standard error.
ACR70 response rates in Chinese patients: (a) up to Month 12 in ORAL Sync by treatment sequence (FAS, NRINAP); and (b) up to Month 48 in ORAL Sequel (OC). ACR: American College of Rheumatology; BID: Twice daily; FAS: Full analysis set; NRINAP: Nonresponder imputation, no advancement penalty; OC: Observed cases; SE: Standard error.
In ORAL Sync, mean changes from baseline in DAS28-4 (ESR) were significantly greater with tofacitinib 5 and 10 mg BID treatment versus placebo at Month 3 and Month 6 [all P < 0.05; Figure 2a]. This decrease from baseline was larger for tofacitinib 10 mg BID versus tofacitinib 5 mg BID. By Month 12, all treatment sequences showed similar changes in DAS28-4 (ESR) [Figure 2b]. A greater proportion of patients achieved DAS28-4 (ESR) <2.6 at Month 6 with tofacitinib 5 mg (7.1%) and 10 mg (13.1%) BID versus placebo (2.3%; P < 0.05). A greater number of patients achieved DAS28-4 (ESR) ≤3.2 at Month 6 with tofacitinib 5 mg (16.5%) and 10 mg (22.6%) BID versus placebo (4.6%; all P < 0.05).
Figure 2.

LS mean change from baseline in DAS28-4 (ESR) through: (a) Month 6 in ORAL Sync (FAS, longitudinal model); (b) Month 12 in ORAL Sync by treatment sequence (FAS, longitudinal model); and (c) Month 48 in ORAL Sequel (FAS, no imputation). *P < 0.05; †P < 0.001; ‡P < 0.0001. BID: Twice daily; DAS28-4 (ESR): Disease Activity Score in 28 joints using erythrocyte sedimentation rate; FAS: Full analysis set; LS: Least squares; SE: Standard error.
ORAL Sequel
ACR20/50/70 response rates were maintained from Month 1 through Month 48 [ACR20/50, Figure 1b; ACR70, Supplementary Figure 2 (415.8KB, tif) ] with both tofacitinib doses. ACR20 response rates were generally similar between the tofacitinib 5 and 10 mg BID groups whereas ACR50/70 response rates were generally higher with tofacitinib 5 versus 10 mg BID throughout the study.
Improvements with tofacitinib in mean change from baseline in DAS28-4 (ESR) were maintained through Month 48 [Figure 2c]. Reductions in DAS28-4 (ESR) score were comparable for both doses through Month 48.
Patient- and physician-reported outcomes
ORAL Sync
The change from baseline in HAQ-DI at Month 3 was one of the primary endpoints of the global ORAL Sync study. Significant improvements in HAQ-DI scores were observed at Month 3 with tofacitinib 5 and 10 mg BID versus placebo (all both P < 0.05; Figure 3a). By Month 12, all treatment groups showed similar changes in HAQ-DI scores, as previously reported.[31] Patients receiving tofacitinib 10 mg BID experienced numerically greater improvement versus tofacitinib 5 mg BID, at all time points.
Figure 3.

LS mean change from baseline in HAQ-DI through: (a) Month 6 in ORAL Sync (FAS, longitudinal model); and (b) Month 48 in ORAL Sequel (FAS, no imputation). *P < 0.05; †P < 0.001; horizontal dashed line represents MCID, 0.22. BID: Twice daily; FAS: Full analysis set; HAQ-DI: Health Assessment Questionnaire-Disability Index; LS: Least squares; MCID: Minimal clinically important difference; SE: Standard error.
Other outcomes, such as changes from baseline in PtGA, PGA, pain (VAS), and Short Form-36 scores in Chinese patients from ORAL Sync, have previously been reported.[31] Briefly, statistically significant improvements in PtGA were recorded with tofacitinib 5 and 10 mg BID versus placebo (both P < 0.05) at Month 3; the decreases continued through Month 12 for the tofacitinib 5 mg BID and placebo advanced to tofacitinib 5 mg BID groups, and Month 9 for the tofacitinib 10 mg BID and placebo advanced to tofacitinib 10 mg BID groups. Similarly, statistically significant improvements in PGA were recorded with both doses of tofacitinib at Month 3 and Month 6 (P < 0.05). Improvements were maintained until Month 12. Statistically significant changes in pain (VAS) were also observed with tofacitinib 5 and 10 mg BID versus placebo at Month 3 (P < 0.001) and Month 6 (P < 0.05), with improvements maintained up to Month 12.
ORAL Sequel
Patients receiving tofacitinib 5 and 10 mg BID had similar magnitudes of change in HAQ-DI from baseline, which were consistent from Month 1 to Month 48 [Figure 3b].
Improvements in other patient- and physician-reported outcomes appeared to be maintained through Month 48 in ORAL Sequel. Mean changes from baseline in PtGA scores at Month 48 were −25.56 and −24.84 for tofacitinib 5 and 10 mg BID, respectively. Mean changes from baseline in PGA scores at Month 48 were −36.68 and −27.90 in the tofacitinib 5 and 10 mg BID groups, respectively.
Mean changes in pain (VAS) from baseline to Month 48 in ORAL Sequel were −27.40 and −20.04 in the tofacitinib 5 and 10 mg BID treatment groups, respectively.
Safety
ORAL Sync
The proportion of patients with AEs was generally similar across the tofacitinib treatment groups up to Month 3 and between Months 3 and 6 [Table 2]. Up to Month 3, there were more AEs with placebo than with tofacitinib, whereas between Months 3 and 6 there were fewer AEs with placebo. Common AEs for patients from any treatment group included upper respiratory tract infection (URTI) and increased alanine aminotransferase [Table 2]. After Month 6, the proportion of patients with AEs was greater with tofacitinib 10 (26.7%) versus tofacitinib 5 mg BID (10.5%). Up to Month 6, the proportions of patients who reported leukopenia and white blood cell count decreased were numerically higher with tofacitinib 5 or 10 mg BID versus placebo. Similarly, from Months 3–6, the proportion of patients reporting blood creatinine phosphokinase increased was higher in the tofacitinib 5 and 10 mg BID treatment groups (1.2% and 3.5%, respectively) compared with the placebo group (0.0%). Three patients receiving tofacitinib 10 mg BID experienced SAEs including one death due to acute heart failure, one pulmonary tuberculosis, and one tendon rupture. Eight patients discontinued due to AEs (tofacitinib 5 mg BID, n = 3; tofacitinib 10 mg BID, n = 5) [Table 2]. There were no malignancies reported.
Table 2.
Summary of AEs* per treatment period in Chinese patients enrolled in ORAL Sync and ORAL Sequel
| Items | ORAL Sync | |||||
|---|---|---|---|---|---|---|
| Up to month 3 | Month 3–6 | |||||
| Tofacitinib 5 mg BID (n = 86) | Tofacitinib 10 mg BID (n = 86) | Placebo (n = 44) | Tofacitinib 5 mg BID (n = 86) | Tofacitinib 10 mg BID (n = 86) | Placebo (n = 25) | |
| Patients with AEs, n (%) | 24 (27.9) | 28 (32.6) | 19 (43.2) | 22 (25.6) | 20 (23.3) | 3 (12.0) |
| Patients with SAEs, n (%) | 0 | 1 (1.2) | 0 | 0 | 1 (1.2) | 0 |
| Discontinuations due to AEs, n (%) | 3 (3.5) | 1 (1.2) | 1 (2.3) | 0 | 2 (2.3) | 0 |
| AE occurring in ≥5% of patients in any treatment group at any time point‡, n (%) | ||||||
| Leukopenia | 1 (1.2) | 4 (4.7) | 1 (2.3) | 2 (2.3) | 3 (3.5) | 0 |
| Toothache | – | – | – | – | – | – |
| Chest pain | – | – | – | – | – | – |
| Nasopharyngitis | 3 (3.5) | 0 | 1 (2.3) | 0 | 3 (3.5) | 0 |
| Upper respiratory tract infection | 4 (4.7) | 10 (11.6) | 4 (9.1) | 3 (3.5) | 1 (1.2) | 0 |
| Urinary tract infection | – | – | – | – | – | – |
| Herpes zoster | 1 (1.2) | 1 (1.2) | 2 (4.5) | – | – | – |
| Blood creatinine phosphokinase increased | – | – | – | 1 (1.2) | 3 (3.5) | 0 |
| Alanine aminotransferase increased | 4 (4.7) | 3 (3.5) | 3 (6.8) | 1 (1.2) | 2 (2.3) | 0 |
| Aspartate aminotransferase increased | 4 (4.7) | 2 (2.3) | 2 (4.5) | 1 (1.2) | 2 (2.3) | 0 |
| Gamma-glutamyltransferase increased | – | – | – | – | – | – |
| Lymphocyte count decreased | – | – | – | – | – | – |
| White blood cell count decreased | 2 (2.3) | 4 (4.7) | 0 (0.0) | 1 (1.2) | 2 (2.3) | 0 |
| Hyperlipidemia | – | – | – | 2 (2.3) | 0 | 0 |
| Hypertension | – | – | – | – | – | – |
| Items |
ORAL Sync
Post-Month 6 |
ORAL Sequel† | ||||
| Tofacitinib 5 mg BID (n = 86) | Tofacitinib 10 mg BID (n = 86) | Placebo → tofacitinib 5 mg BID (n = 22) | Placebo → tofacitinib 10 mg BID (n = 22) | Tofacitinib 5 mg BID (n = 153) | Tofacitinib 10 mg BID (n = 39) | |
| Patients with AEs, n (%) | 9 (10.5) | 23 (26.7) | 6 (27.3) | 4 (18.2) | 107 (69.9) | 21 (53.8) |
| Patients with SAEs, n (%) | 0 | 1 (1.2) | 0 | 0 | 14 (9.2) | 4 (10.3) |
| Discontinuations due to AEs, n (%) | 0 | 2 (2.3) | 0 | 0 | 29 (19.0) | 3 (7.7) |
| AE occurring in ≥5% of patients in any treatment group at any time point‡, n (%) | ||||||
| Leukopenia | 2 (2.3) | 1 (1.2) | 2 (9.1) | 0 | 9 (5.9) | 2 (5.1) |
| Toothache | – | – | – | – | 1 (0.7) | 3 (7.7) |
| Chest pain | – | – | – | – | 1 (0.7) | 2 (5.1) |
| Nasopharyngitis | – | – | – | – | 8 (5.2) | 3 (7.7) |
| Upper respiratory tract infection | 0 | 3 (3.5) | 0 | 1 (4.5) | 27 (17.6) | 4 (10.3) |
| Urinary tract infection | – | – | – | – | 8 (5.2) | 1 (2.6) |
| Herpes zoster | – | – | – | – | 8 (5.2) | 2 (5.1) |
| Blood creatinine phosphokinase increased | 1 (1.2) | 5 (5.8) | 0 | 1 (4.5) | 16 (10.5) | 1 (2.6) |
| Alanine aminotransferase increased | 0 | 3 (3.5) | 0 | 0 | 17 (11.1) | 2 (5.1) |
| Aspartate aminotransferase increased | 0 | 2 (2.3) | 0 | 0 | 16 (10.5) | 2 (5.1) |
| Gamma-glutamyltransferase increased | 0 | 3 (3.5) | 0 | 0 | 8 (5.2) | 0 |
| Lymphocyte count decreased | – | – | – | – | 10 (6.5) | 0 |
| White blood cell count decreased | – | – | – | – | 11 (7.2) | 1 (2.6) |
| Hyperlipidemia | – | – | – | – | 3 (2.0) | 3 (7.7) |
| Hypertension | – | – | – | – | 9 (5.9) | 0 |
*Based on MedDRA Preferred Terms; †Data as of March 2015, ongoing at time of analysis, database not locked; ‡Data are not shown when there were ≤2 patients in all treatment groups at that time point; these are indicated by "-". AE: Adverse event; BID: Twice daily; MedDRA: Medical Dictionary for Regulatory Activities; n: Number of patients; SAE: Serious adverse event.
ORAL Sequel
AEs occurred in 69.9% and 53.8% of patients receiving tofacitinib 5 and 10 mg BID, respectively [Table 2]. The most common AEs in either dose group (AEs occurring in ≥5% of patients in any treatment group) are presented in Table 2. These included URTI, nasopharyngitis, and herpes zoster. SAEs were experienced by 9.2% and 10.3% of patients treated with tofacitinib 5 and 10 mg BID, respectively. One patient treated with tofacitinib 10 mg BID died due to bacterial meningoencephalitis.
IRs for safety events of special interest were generally similar between tofacitinib doses, although the IR for discontinuations due to AEs was numerically greater with tofacitinib 5 versus 10 mg BID [Table 3]. No cases of malignancies or lymphoma were reported with tofacitinib. IRs for all-cause mortality were 0 and 0.74 with tofacitinib 5 and 10 mg BID, respectively.
Table 3.
Incidence rates for safety events of special interest (ORAL Sequel)
| Items | ORAL Sequel* | |
|---|---|---|
| Tofacitinib 5 mg BID (n = 153) | Tofacitinib 10 mg BID (n = 39) | |
| Exposure, patient-years | 488.14 | 135.09 |
| IR/100 patient-years (95% CI) | ||
| SAEs | 2.75 (1.46–4.70) | 3.08 (0.84–7.87) |
| Discontinuations due to AEs | 6.06 (4.06–8.70) | 2.23 (0.46–6.51) |
| Serious infections | 1.23 (0.45–2.68) | 1.48 (0.18–5.35) |
| Opportunistic infections (excluding tuberculosis) | 0 (0–0.76) | 0 (0–2.73) |
| Tuberculosis | 0.41 (0.05–1.48) | 0 (0–2.73) |
| All herpes zoster (serious and nonserious) | 1.72 (0.74–3.39) | 1.51 (0.18–5.44) |
| Malignancies (excluding NMSC) | 0.00 (0.00–0.76) | 0 (0–2.73) |
| NMSC | 0 (0–0.76) | 0 (0–2.73) |
| Lymphoma/lymphoproliferative disorders | 0 (0–0.76) | 0 (0–2.73) |
| MACE | 0.21 (0.01–1.14) | 0 (0–2.73) |
| All-cause mortality | 0 (0–0.76) | 0.74 (0.02–4.12) |
*Data as of March 2015, ongoing at the time of analysis, database not locked. AE: Adverse event; BID: Twice daily; CI: Confidence interval; IR: Incidence rate; MACE: Major adverse cardiovascular event; NMSC: Nonmelanoma skin cancer; SAE: Serious adverse event.
DISCUSSION
In this paper, we report the efficacy and safety of tofacitinib in Chinese patients enrolled in Phase 3 study, ORAL Sync, and the LTE study, ORAL Sequel.[23,28] In China, there is an unmet need for new RA therapies that can improve treatment outcomes for patients with an inadequate response to csDMARDs and bDMARDs. Here, we show that tofacitinib is effective in reducing the signs and symptoms of RA in Chinese patients; these findings are consistent with results reported for the global population in ORAL Sync and ORAL Sequel.
ACR20/50/70 response rates, change from baseline in DAS28-4 (ESR), and proportion of patients achieving DAS28-4 (ESR) remission (<2.6) or low disease activity (c3.2), were generally similar to those observed globally in ORAL Sync; however, a greater proportion of Chinese patients achieved an ACR20 response at Month 6 (tofacitinib 5 mg BID: 67.4%; tofacitinib 10 mg BID: 70.6%; placebo: 34.1%) versus the global population (tofacitinib 5 mg BID: 52.7%; tofacitinib 10 mg BID: 58.3%; placebo: 31.2%). For Chinese patients, the ACR20 response rate at Month 12 was 72.9% with tofacitinib 10 mg BID versus 50.0% for patients who advanced from placebo to tofacitinib 10 mg BID. This difference in ACR20 response rates could be due to the small number of patients randomized to the placebo treatment arm. In the global population, significant differences in ACR20 with tofacitinib 5 mg BID versus placebo were first reported at Week 2[23] whereas for the Chinese population, significant differences in ACR20 were observed from Month 1. This late response versus the global population may be due to the low numbers of Chinese patients in this analysis, particularly in the placebo group (44 patients). In ORAL Sequel, ACR20/50/70 response rates were generally higher in the tofacitinib 5 mg BID group, which is likely due to the low numbers of patients receiving tofacitinib 10 mg BID (per protocol, all patients were initiated on tofacitinib 5 mg BID; dose increases were based on the investigator's discretion).
Baseline demographics were generally similar in the Chinese subpopulation and in the global Phase 3 study; however, the mean age for patients receiving tofacitinib in the Chinese subpopulation of ORAL Sync (47.1–49.2 years; Table 1) was lower than in the global population (51.9–52.7 years). Although no substantial differences were seen in the clinical response of patients in the Chinese and global populations, the observed disparity in age may contribute to small differences in response in the Chinese population. Efficacy was generally similar in Chinese patients versus the global population in ORAL Sequel, although it should be noted that 79.7% (153/192) of Chinese patients received tofacitinib 5 mg BID versus only 24.2% (1,059/4381) of the global population in ORAL Sequel.
In line with the global Phase 3 studies[33,34,35] and as previously published,[31] significant improvements in patient-and physician-reported outcomes, including HAQ-DI, PtGA, PGA, and pain, were reported for tofacitinib-treated patients versus placebo-treated patients in the Chinese subpopulation of ORAL Sync.
The safety profile in Chinese patients was generally consistent with the safety profiles observed in the global Phase 3 and LTE analyses[23,28] including the proportion of patients with AEs. The most frequently reported AEs in Phase 3 and LTE studies were URTIs and nasopharyngitis. Rates of leukopenia and white blood cell count decreased were also reported to be numerically higher with tofacitinib versus placebo in ORAL Sync. Treatment with tofacitinib is associated with decreases in absolute lymphocyte and absolute neutrophil counts, and relevant dosage recommendations in the event of these AEs are included in the prescribing information for tofacitinib in China.[36] Blood creatinine phosphokinase increased was reported in tofacitinib-treated Chinese patients in both ORAL Sync and ORAL Sequel; this was also observed in the global Phase 3 and LTE tofacitinib studies. The mechanism for this increase is not known, however, and there was no temporal association between elevated creatinine phosphokinase levels (≥5x the upper limit of normal) and reports of myopathies in the global studies (data on file). In the LTE study, the proportions of patients with AEs and SAEs were generally lower in the Chinese population versus the global population, irrespective of dose.[28]
Patients with RA are at a greater risk of serious infections including tuberculosis, which increases with the use of immunosuppressive agents such as bDMARDs. This risk differs depending on the background rate of tuberculosis within individual countries.[37] Despite rates of tuberculosis in China falling in recent years, the background risk of tuberculosis remains high and China remains one of the top three countries for tuberculosis prevalence worldwide.[38] Only one case of tuberculosis was reported up to Month 12; this was in a patient receiving tofacitinib 10 mg BID. However, it should be noted that patient numbers were low, and patients participating in Phase 3 studies were screened for active or latent tuberculosis. In the LTE study, two cases of tuberculosis were reported in patients receiving tofacitinib 5 mg BID.
The interpretation of Phase 3 data presented in this analysis was limited by low patient numbers and low tofacitinib exposure. Fewer patients were enrolled in the placebo treatment arms compared with the tofacitinib treatment arms, thus limiting conclusions based on comparisons between tofacitinib- and placebo-treated patients.
Interpretation of data from the LTE study was limited due to the lack of a comparator arm. Furthermore, patients enrolled in the LTE study had already demonstrated a response and good tolerability to tofacitinib through their participation in the index study. As dose adjustments were permitted in the LTE study, limited comparisons could be made between tofacitinib doses. Despite this, due to scarce long-term real-world data, LTE data are important in evaluating tofacitinib in the Chinese subpopulation.
In this analysis of Chinese patients from a Phase 3 and an LTE study, tofacitinib 5 and 10 mg BID demonstrated quick onset and sustained efficacy up to Month 48, and reduced signs and symptoms in patients with moderately to severely active RA. The safety profile of tofacitinib was consistent with findings from global studies. This analysis, therefore, supports the use of tofacitinib as an oral alternative to bDMARDs for the treatment of Chinese patients with RA.
Data sharing
On request and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was sponsored by Pfizer Inc.
Conflicts of interest
An Y, Bao CD, Chen ZW, Gu JR, Li ZG, Liu Y, Xu HJ, and Zhao DB have nothing to disclose. Kremer J has served as a consultant for, and has received research support from, AbbVie, Amgen, BMS, Genentech, Pfizer Inc, and UCB; he has also received payment for lectures, including service on speakers’ bureaus, from AbbVie. Hwang LJ was an employee and shareholder of Pfizer Inc at the time of analysis. Wang L and Wu QZ are employees and shareholders of Pfizer Inc.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Strand V, Khanna D. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol. 2010;28:S32–40. [PubMed] [Google Scholar]
- 2.Wang G, Mu R, Xu H. Management of rheumatoid arthritis in People's Republic of China – Focus on tocilizumab and patient considerations. Int J Gen Med. 2015;8:187–94. doi: 10.2147/IJGM.S81633. doi: 10.2147/IJGM.S81633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Yang Y. Rheumatology in China: Challenges and development. Rheumatology (Oxford) 2012;51:1733–4. doi: 10.1093/rheumatology/kes166. doi: 10.1093/rheumatology/kes166. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Mu R, Wang X, Xu C, Duan T, An Y, et al. The impact of rheumatoid arthritis on work capacity in Chinese patients: A cross-sectional study. Rheumatology (Oxford) 2015;54:1478–87. doi: 10.1093/rheumatology/kev014. doi: 10.1093/rheumatology/kev014. [DOI] [PubMed] [Google Scholar]
- 5.Xu C, Wang X, Mu R, Yang L, Zhang Y, Han S, et al. Societal costs of rheumatoid arthritis in China: A hospital-based cross-sectional study. Arthritis Care Res (Hoboken) 2014;66:523–31. doi: 10.1002/acr.22160. doi: 10.1002/acr.22160. [DOI] [PubMed] [Google Scholar]
- 6.Langley PC, Mu R, Wu M, Dong P, Tang B. The impact of rheumatoid arthritis on the burden of disease in urban China. J Med Econ. 2011;14:709–19. doi: 10.3111/13696998.2011.611201. doi: 10.3111/13696998.2011.611201. [DOI] [PubMed] [Google Scholar]
- 7.Wang GY, Zhang SL, Wang XR, Feng M, Li C, An Y, et al. Remission of rheumatoid arthritis and potential determinants: A national multi-center cross-sectional survey. Clin Rheumatol. 2015;34:221–30. doi: 10.1007/s10067-014-2828-3. doi: 10.1007/s10067-014-2828-3. [DOI] [PubMed] [Google Scholar]
- 8.Lau CS, Chia F, Harrison A, Hsieh TY, Jain R, Jung SM, et al. APLAR rheumatoid arthritis treatment recommendations. Int J Rheum Dis. 2015;18:685–713. doi: 10.1111/1756-185X.12754. doi: 10.1111/1756-185X.12754. [DOI] [PubMed] [Google Scholar]
- 9.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li ZG. A new look at rheumatology in China – Opportunities and challenges. Nat Rev Rheumatol. 2015;11:313–7. doi: 10.1038/nrrheum.2014.218. doi: 10.1038/nrrheum.2014.218. [DOI] [PubMed] [Google Scholar]
- 12.Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R, Liu X, Ye H, Yao HH, Guo JL, Li GT, et al. Magnetic resonance imaging in early rheumatoid arthritis: A multicenter, prospective study. Clin Rheumatol. 2016;35:303–8. doi: 10.1007/s10067-016-3180-6. doi: 10.1007/s10067-016-3180-6. [DOI] [PubMed] [Google Scholar]
- 14.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: Current and emerging paradigms of care. Clin Ther. 2011;33:679–707. doi: 10.1016/j.clinthera.2011.05.044. doi: 10.1016/j.clinthera.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augustovski F, Beratarrechea A, Irazola V, Rubinstein F, Tesolin P, Gonzalez J, et al. Patient preferences for biologic agents in rheumatoid arthritis: A discrete-choice experiment. Value Health. 2013;16:385–93. doi: 10.1016/j.jval.2012.11.007. doi: 10.1016/j.jval.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–29. doi: 10.1002/art.33383. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 17.Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–905. doi: 10.1002/art.24567. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 18.Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–81. doi: 10.1002/art.33419. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH Tofacitinib Study Investigators. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–8. doi: 10.1002/acr.20494. doi: 10.1002/acr.20494. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S, et al. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: A 12-week, randomized, phase 2 study. Mod Rheumatol. 2015;25:514–21. doi: 10.3109/14397595.2014.995875. doi: 10.3109/14397595.2014.995875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: A randomised phase 3 trial. Lancet. 2013;381:451–60. doi: 10.1016/S0140-6736(12)61424-X. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 22.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 23.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: A randomized trial. Ann Intern Med. 2013;159:253–61. doi: 10.7326/0003-4819-159-4-201308200-00006. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–86. doi: 10.1056/NEJMoa1310476. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 25.van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: Twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–70. doi: 10.1002/art.37816. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 26.van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–19. doi: 10.1056/NEJMoa1112072. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL strategy): A phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457–68. doi: 10.1016/S0140-6736(17)31618-5. doi: 10.1016/S0140-6736(17)31618-5. [DOI] [PubMed] [Google Scholar]
- 28.Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41:837–52. doi: 10.3899/jrheum.130683. doi: 10.3899/jrheum.130683. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: An open-label, long-term extension study. Arthritis Res Ther. 2016;18:34. doi: 10.1186/s13075-016-0932-2. doi: 10.1186/s13075-016-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wollenhaupt J, Silverfield J, Lee EB, Terry K, Kwok K, Strengholt S, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open-label, long-term extension studies over 9 years. Arthritis Rheumatol. 2017;69(suppl 10):683–4. [Google Scholar]
- 31.Li Z, An Y, Su H, Li X, Xu J, Zheng Y, et al. Tofacitinib with conventional synthetic disease-modifying antirheumatic drugs in Chinese patients with rheumatoid arthritis: Patient-reported outcomes from a phase 3 randomized controlled trial. Int J Rheum Dis. 2018;21:402–14. doi: 10.1111/1756-185X.13244. doi: 10.1111/1756-185x.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 33.Strand V, Burmester GR, Zerbini CA, Mebus CA, Zwillich SH, Gruben D, et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: Patient-reported outcomes from a phase III trial. Arthritis Care Res (Hoboken) 2015;67:475–83. doi: 10.1002/acr.22453. doi: 10.1002/acr.22453. [DOI] [PubMed] [Google Scholar]
- 34.Strand V, Kremer J, Wallenstein G, Kanik KS, Connell C, Gruben D, et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther. 2015;17:307. doi: 10.1186/s13075-015-0825-9. doi: 10.1186/s13075-015-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strand V, van Vollenhoven RF, Lee EB, Fleischmann R, Zwillich SH, Gruben D, et al. Tofacitinib or adalimumab versus placebo: Patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology (Oxford) 2016;55:1031–41. doi: 10.1093/rheumatology/kev442. doi: 10.1093/rheumatology/kev442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfizer Inc. Tofacitinib Citrate Tablets Labeling Document. China: Pfizer Inc; 2017. [Google Scholar]
- 37.Carmona L, Hernández-García C, Vadillo C, Pato E, Balsa A, González-Alvaro I, et al. Increased risk of tuberculosis in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1436–9. [PubMed] [Google Scholar]
- 38.World Health Organization. Global Tuberculosis Report 2015. [Last accessed on 2016 Oct 19]. Available from: http://www.who.int/tb/publications/global_report/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ACR20/50/70 response rates in Chinese patients up to Month 6 in ORAL Sync (FAS, NRI). *P < 0.05; †P < 0.001; ‡P < 0.0001. ACR: American College of Rheumatology; BID: Twice daily; FAS: Full analysis set; NRI: Nonresponder imputation; SE: Standard error.
ACR70 response rates in Chinese patients: (a) up to Month 12 in ORAL Sync by treatment sequence (FAS, NRINAP); and (b) up to Month 48 in ORAL Sequel (OC). ACR: American College of Rheumatology; BID: Twice daily; FAS: Full analysis set; NRINAP: Nonresponder imputation, no advancement penalty; OC: Observed cases; SE: Standard error.