Abstract
Background:
Microparticles (MPs) are small extracellular plasma membrane particles shed by activated and apoptotic cells, which are involved in the development of atherosclerosis. Our previous study found that microRNA (miR)-19b encapsulated within endothelial MPs (EMPs) may contribute to the upregulation of circulating miR-19b in unstable angina patients. Hypoxia is involved in atherosclerosis as a critical pathological stimulus. However, it still remains unclear whether the increase of miR-19b levels in EMPs is related to hypoxia and if the effect of miR-19b – wrapped within EMPs – stimulates hypoxia on vascular endothelial cells. This study aimed to explore the changes of miR-19b in EMPs induced by hypoxia as well as their effects on endothelial cells.
Methods:
Human umbilical vein endothelial cells (HUVECs) were cultured in vitro and arranged to harvest EMPs in two parts: the first part consisted of EMPcontrol and EMPhypoxia and the second part included EMPvehicle, EMPNC mimic, and EMPmiR-19b mimic. Cell migration was detected by scratch migration and transwell chamber migration. Angiogenesis was assessed by tube formation assays. Furthermore, we predicted the target gene of miR-19b by bioinformatics analysis, and luciferase assay was used to verify the targeted gene of miR-19b. Data were analyzed by one-way analysis of variance. Student's t-test was used when two groups were compared.
Results:
Compared with EMPcontrol- and EMPhypoxia-inhibited migration of cells by scratch migration assay (80.77 ± 1.10 vs. 28.37 ± 1.40, P < 0. 001) and transwell chamber migration assay (83.00 ± 3.46 vs. 235.00 ± 16.52, P < 0.01), the number of tube formations was markedly reduced by 70% in the EMPhypoxia group (P < 0.001) in vitro analysis of HUVECs. Meanwhile, a strong inhibition of migration and tube formation of HUVECs in the presence of miR-19b-enriched EMPmiR-19b mimic was observed. This effect might be due to the delivery of miR-19b in EMPs. Transforming growth factor-β2 (TGFβ2) was predicted to be one of the target genes of miR-19b, and we further confirmed that TGFβ2 was a direct target gene of miR-19b using the luciferase assay. The expression of TGFβ2 in HUVECs was inhibited by treatment with EMPhypoxia and EMPmiR-19b mimic.
Conclusions:
MiR-19b in EMPs induced by hypoxia could reduce endothelial cell migration and angiogenesis by downregulating TGFβ2 expression, which may have inhibited the progression of atherosclerosis.
Keywords: Endothelial Microparticle, MicroRNA-19b, Hypoxia, Cell Migration, Angiogenesis, Transforming Growth Factor-β2
摘要
背景:
微颗粒(Microparticles ,MPs)是细胞激活或者凋亡状态下,从细胞膜上脱落下来的颗粒物质。MPs已被证实与动脉粥 样硬化的发生发展有密切联系。本课题组前期研究发现,不稳定型心绞痛患者循环中miRNA-19b水平的升高,主要是由于内皮 细胞来源的微颗粒(endothelial microparticles,EMPs)中携带miRNA-19b含量增加所致。但是,这些患者血液循环中EMPs的 升高及EMPs内miR-19b水平的升高是否与缺氧状态有关,目前尚不清楚。本研究旨在探讨缺氧诱导的EMPs中miR-19b的变化 及其对内皮细胞发挥的生物学作用。
方法:
人脐静脉内皮细胞在体外培养后获得两组EMPs:第一组是在常氧和缺氧条件下分别培养获得的EMPcontrol和EMPhypoxia; 第二组是携带不同miR-19b水平的EMPvehicle、EMPNC mimic and EMPmiR-19b mimic。细胞迁移功能研究选择划痕试验和Transwell小室试 验。血管新生功能研究通过成管试验。此外,我们运用生物信息学分析预测了miR-19b的靶基因,荧光素酶报告基因实验来验 证miR-19b的靶基因。数据分析采用单因素方差分析,组间比较采用t检验。
结果:
与EMPcontrol相比,EMPhypoxia能显著抑制划痕试验(80.77±1.10 vs 28.37±1.40, P<0. 001)及Transwell小室试验(83.00±3.46 vs 235.00±16.52, P<0.01)中内皮细胞迁移。EMPhypoxia组的细胞管型数量减少约70%。与此同时,在EMPmiR-19b mimic组中也观察到内 皮细胞迁移及血管新生功能被抑制。生物信息学预测分析预测TGFβ2(transforming growth factor β2)为miRNA-19b发挥上述 作用的潜在靶基因,并且通过荧光素酶报告基因实验证实其发挥直接调控作用。TGFβ2在 HUVECs中的表达受到EMPhypoxia和 EMPmiR-19b-mimic的抑制。
结论:
缺氧诱导产生的内皮微颗粒携带高水平miRNA-19b进入内皮细胞,通过直接抑制其靶基因TGFβ2的表达,抑制内皮细 胞迁移以及血管新生,抑制早期动脉粥样硬化的进展。
INTRODUCTION
Microparticles (MPs) are small vesicles (<1 μm) that are shed from various cells under both normal and pathological conditions.[1] It has been reported that activated endothelial cells can release endothelial MPs (EMPs), especially after stimulations such as inflammation, lipoproteins, oxidative stress, or high-stress shear forces.[2] EMPs containing nuclear materials such as DNA, RNA, and microRNA contribute to cellular communication and vascular homeostasis.[3] It has been proven that EMPs contribute significantly to endothelial dysfunction, vascular inflammation, oxidative stress, and thrombosis during the development of atherosclerosis.[4] Taraboletti et al.[5] reported that EMPs isolated from human umbilical vein endothelial cells (HUVECs) promoted formation of capillary-like structures of endothelial cells in low concentrations, whereas high levels of EMP abolished angiogenesis. More recently, Jansen et al.[6] revealed that EMPs from apoptotic endothelial cells promoted endothelial cell migration and proliferation. Aberrant migration and angiogenesis of endothelial cells play a crucial role in the pathogenesis of cardiovascular diseases, especially atherosclerosis.[7] Thus, we sought to investigate the generation of EMPs and their effects on atherosclerosis.
MicroRNAs (miRNAs) are endogenously expressed small, noncoding RNAs that negatively regulate gene expression at posttranscriptional level in plants and animals.[8] Circulating miRNAs (including heart-, vascular-, and endothelium-enriched miRNAs) have been explored as novel biomarkers in various diseases, including coronary artery disease.[9] MiRNAs are partially derived from MPs and can be efficiently delivered into target cells.[10] Zhang et al.[11] identified that miR-150 was packaged into MPs by human blood cells and monocytic cells, which improved endothelial cell migration. Moreover, miR-126 was delivered into endothelial cells by EMPs to promote vascular endothelial repair.[6] In addition, miR-19b is closely associated with cardiovascular disease. It has been proven that miR-19b was able to promote cardiac fibroblast proliferation and migration.[12] Our previous study found that the elevated levels of miR-19b wrapped in EMPs might contribute to the upregulation of circulating miR-19b in unstable angina patients.[13] Therefore, we speculated that miR-19b encapsulated within EMPs has a potential role in endothelial function.
Hypoxia triggers various cellular processes in both physiological and pathological conditions and has been implicated in atherosclerosis.[14] MPs from coronary artery disease patients with obstructive sleep apnea-hypopnea syndrome increased endothelial cell dysfunction in vivo.[15] Higher levels of EMPs in the blood also reflected vascular injury under hypoxia.[16] In vitro, hypoxic conditions have been shown to trigger the release of MPs from tumor cell lines.[17] EMPs derived from hypoxia/reoxygenation (H/R) might defect the myocardium by promoting apoptosis and oxidative stress in vitro.[18] Nonetheless, it remains unclear what impact of EMPs, induced by hypoxia, have on vascular endothelial cell, in vitro.
Therefore, this study aimed to elucidate the levels of miR-19b in EMPs, stimulated by hypoxia, and the roles of miR-19b – wrapped within EMPs – on vascular endothelial cells.
METHODS
Cell culture and transfection
HUVECs were isolated from the umbilical cord according to the operational process described in the Supplementary Data (191.1KB, pdf) . The cells were cultured at 37°C in a humidified atmosphere with 5% CO2 and 95% O2. The hypoxic condition was made in a sealed chamber with 5% CO2, 92% N2, and 3% O2. EMPcontrol and EMPhypoxia were generated from HUVECs, as previously described. HUVECs were seeded on 6-well plates and transfected the following day using Lipofectamine 2000 (Invitrogen, USA) at a final concentration of 3 mg/ml according to the manufacturer's recommendation. For miR-19b overexpression, miR-19b mimics or negative control (NC) mimics (GenePharma, China) were transfected into HUVECs (70–80% confluence) at a final concentration of 30 pmol/ml for 24 h. Each group of HUVECs was cultured for another 12 h in serum-free medium to harvest EMPvehicle, EMPNC mimic, and EMPmiR-19b mimic.
Fluorescence labeling of human umbilical vein endothelial cells for confocal microscopy
After incubation for 12 h, HUVECs were labeled with calcein-AM (10 μmol/L, Sigma) for 30 min and subsequently washed, fixed, and subjected to basal media. Pictures were taken every 20 min to visualize EMP release using a confocal microscope (FV1000; Olympus, Tokyo, Japan).
Isolation and characterization of endothelial microparticles
EMPs were isolated from the cell culture medium by sequential centrifugation[19] for further identification by electron microscopy and flow cytometry [Supplementary Data (191.1KB, pdf) ].
Endothelial microparticle-treated human umbilical vein endothelial cells
EMPs were isolated from the HUVEC culture medium in advance. The pelleted EMPs were re-suspended in endothelial cell medium at a concentration of 1.0 × 107 MP/ml. HUVECs seeded on 6-well plates were treated with an equal number of EMPs (50 μl)[20] for 24 h under normoxic condition. Finally, HUVECs were used for migration and tube formation assays or were collected for real-time polymerase chain reaction (RT-PCR) and quantitative protein assay.
Migration assay and tube formation assay
HUVECs treated with EMPs for 24 h were further applied to scratch migration assays, transwell chamber migration assays, and tube formation assays [Supplementary Data (191.1KB, pdf) ].
Real-time polymerase chain reaction
Total RNAs were isolated from HUVECs or EMPs using miRNeasy Mini Kit (Qiagen, USA). RT-PCR reactions were performed on an Applied Biosystems system (ViiA7). Values are expressed as 2△△ CT. Amplification conditions and primers were shown in the Supplementary Data (191.1KB, pdf) .
Western blotting analysis
The details of Western blotting were described in the Supplementary Data (191.1KB, pdf) .
Luciferase reporter assays
HUVECs plated in a 24-well plate were transfected with NC or miR-19b mimic (Applied Biosystems) and firefly luciferase reporter plasmid containing 3’UTR of transforming growth factor-β2 [TGFβ2, 200 ng/well; the details of cloning are described in the Supplementary Data (191.1KB, pdf) ] along with 5 ng/well of Renilla luciferase control plasmid (pRL-TK; Promega, Madison, WI, USA) using Lipofectamine 2000 (Invitrogen). Luciferase assays were performed using the Dual-Luciferase Assay System (Promega, Madison, WI, USA). Firefly luciferase values were normalized to Renilla, and the ratios of firefly/Renilla activity were presented.
Statistical analysis
All data are presented as mean ± standard error (SE) and were evaluated using Student's t-test or one-way analysis of variance followed by Student–Newman–Keuls test. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software, USA).
RESULTS
Characterization of endothelial microparticles
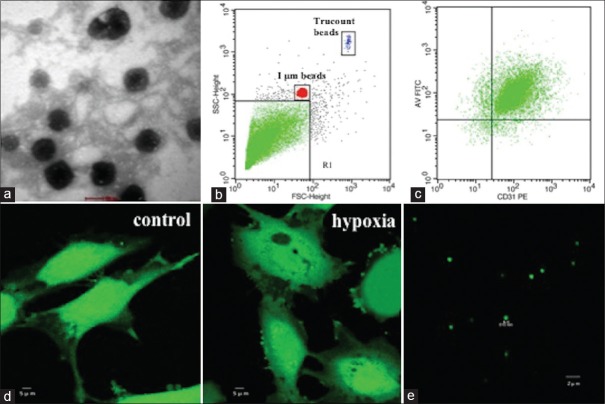
EMPs obtained from the culture medium by gradient centrifugation were fixed and observed by transmission electron microscopy (TEM). Representative TEM micrograph of EMPs is shown in Figure 1a. The characterization of isolated EMPs was confirmed by flow cytometry, which was less than 1 μm, CD31 positive, and Annexin V positive [Figure 1b and 1c]. We also performed confocal microscopy to further characterize the size of collected EMPs. The majority EMPs had a size <1 μm, suggesting an appropriate isolation of EMPs [Figure 1e]. Hypoxia of HUVECs showed obvious membrane blebbing, and vesicle release was observed by confocal microscopy [Figure 1d].
Figure 1.
EMP formation and characterization. (a) TEM micrograph of EMPs released from HUVECs. (b) Fluorescent beads of 1 μm were used to define the MP gate (gate events <1 μm; MP gate: R1). (c) Double-positive events for Annexin V-FITC and CD31-PE were used to identify EMPs and count for each sample. (d) Confocal microscopic images of calcein-AM-labeled HUVECs released membrane blebbing and vesicles after hypoxia (right) in comparison with normoxia (left). (e) Confocal microscopic images of calcein-AM-labeled EMPs. MP: Microparticle; EMP: Endothelial MP; TEM: Transmission electron microscopy; HUVECs: Human umbilical vein endothelial cells.
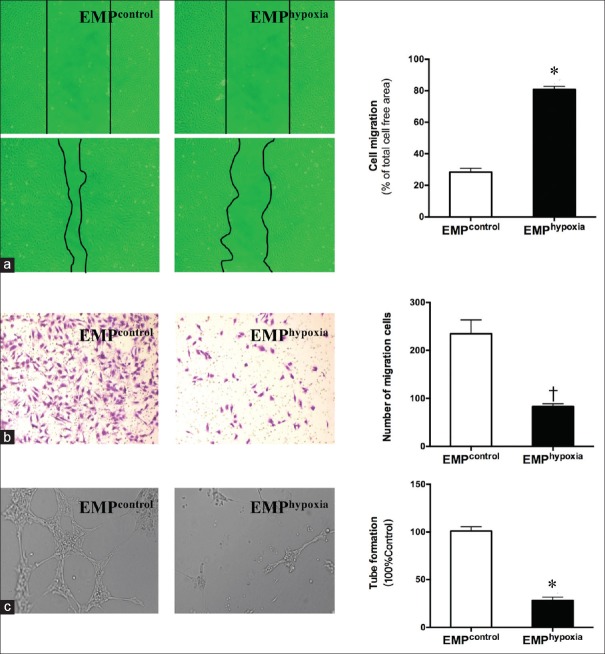
Endothelial microparticlehypoxia-inhibited migration and angiogenesis of human umbilical vein endothelial cells
Scratch migration assays and transwell chamber migration assays were performed to investigate the effects of hypoxia-induced EMPs on HUVECs. We harvested EMPcontrol and EMPhypoxia from the culture medium of HUVECs that were, respectively, exposed to in normoxic and hypoxic conditions for 12 h. After sequential centrifugation, EMPcontrol and EMPhypoxia were separately re-suspended into the basal medium of confluent HUVECs using 6-well plates. Cell migration assays were examined after 24 h of incubation. Our results demonstrated that the cell-free area of HUVEC migration was significantly increased for more than 2 times by EMPhypoxia in comparison with EMPcontrol (80.77 ± 1.10 vs. 28.37 ± 1.40, P < 0. 001) [Figure 2a]. We also examined the transwell chamber migration assay. Compared with EMPcontrol, the number of HUVEC migration cells was markedly reduced for almost 3 times by EMPhypoxia (83.00 ± 3.46 vs. 235.00 ± 16.52, P < 0.01) [Figure 2b].
Figure 2.
EMPhypoxia inhibited migration and angiogenesis of HUVECs. HUVECs treated with EMPcontrol and EMPhypoxia were subjected to scratch migration, transwell migration, and tube formation assay. (a) Scratch migration image. Migration analysis was measured as a percentage of total cell-free area. (b) Transwell migration image. Migration analysis was measured as the total migration cell number. (c) Representative images of tube formation and the relative number of tube branches measured in random 10 photographic fields were presented. *P < 0.001, †P < 0.01 compared with EMPcontrol group. EMP: Endothelial microparticle; HUVECs: Human umbilical vein endothelial cells.
The role of EMPhypoxia in angiogenesis was detected by tube formation assay. HUVECs were also treated with EMPcontrol and EMPhypoxia for 24 h and subsequently were seeded on a Matrigel substratum in a 48-well plate. Compared with EMPcontrol, the mean number of tube formation was decreased by 70% in the EMPhypoxia group [P < 0.001; Figure 2c] after 6 h. These results indicated that EMPs induced by hypoxia could inhibit migration and angiogenesis in HUVECs.
Expression level of miR-19b packed within endothelial microparticles was significantly increased under hypoxic-stimulated conditions
To determine whether miR-19b mediated the above biological effects of EMP on HUVECs, we measured the levels of miR-19b in EMPs by RT-PCR. HUVECs were separately cultured under normoxia and hypoxia for 0.5–36.0 h, and then, the EMPs in the medium were collected at each time point. RT-PCR results showed that miR-19b levels in hypoxia-induced EMPs were increased compared with controls, and the highest levels of miR-19b in EMPs were detected when HUVECs were cultured under hypoxia stimulation for 12 h [Supplementary Figure 1 (210.2KB, tif) ]. The data indicated that hypoxia could increase miR-19b in EMPs derived from HUVECs.
Hypoxia-induced changes of miR-19b in EMPs. The expression level of miR-19b in the EMPhypoxia following 0.5–36 h of hypoxia (3% O2) was measured by RT-PCR and was normalized to the spiked-in Caenorhabditis elegans miRNAs, cel-miR-39. The data were shown as mean ± SE representative of three independent experiments. *P < 0.05, †P < 0.01 compared with the normoxia group. miR-19b: MicroRNA-19b; SE: Standard error; EMPs: Endothelial microparticles; RT-PCR: Real-time polymerase chain reaction.
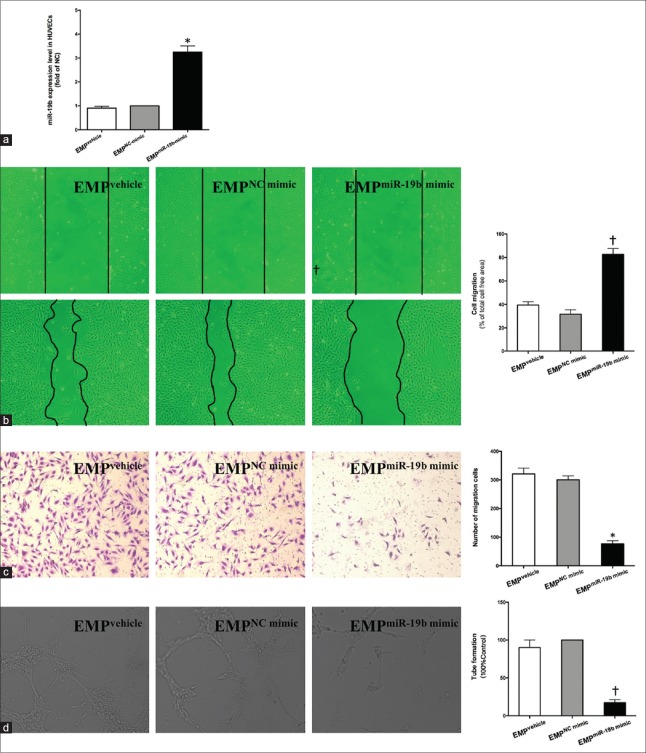
Endothelial microparticlemiR-19b mimic suppressed migration and angiogenesis of human umbilical vein endothelial cells
Next, we further investigated whether miR-19b of EMPs could affect HUVEC migration and angiogenesis. First, we detected levels of miR-19b in HUVECs transfected with miR-19b mimic or NC mimic (controls). RT-PCR results showed that, compared with controls, transfection with miR-19b mimic significantly increased the level of miR-19b in HUVECs almost 40 times [P < 0.01; Supplementary Figure 2a (315.2KB, tif) ]. Moreover, EMPs derived from HUVECs with overexpressed miR-19b also had higher levels of miR-19b compared with NC mimic group for over 10 times [P < 0.01; Supplementary Figure 2b (315.2KB, tif) ].
The levels of overexpressed miR-19b in HUVECs and EMPs. (a) The expression levels of miR-19b in HUVECs transfected with miR-19b mimic or NC mimic were measured by RT-PCR and normalized to RNU6B. (b) The expression levels of miR-19b in EMPvehicle, EMPNC mimic, and EMPmiR-19b mimic were measured by RT-PCR and normalized to the spiked-in Caenorhabditis elegans miRNAs, cel-miR-39. The data were shown as mean ± SE representative of three independent experiments. *P < 0.01 compared with the NC mimic group. miR-19b: MicroRNA-19b; NC: Negative control; Vehicle: Lipofectamine 2000; SE: Standard error; HUVECs: Human umbilical vein endothelial cells; EMPs: Endothelial microparticles; RT-PCR: Real-time polymerase chain reaction.
Subsequently, HUVECs were treated with EMPs (EMPvehicle, EMPNC mimic, and EMPmiR-19b mimic) for 24 h. We found that compared with EMPNC mimic, EMPmiR-19b mimic could increase the levels of miR-19b in HUVECs by over 2 folds [P < 0.05; Figure 3a]. Meanwhile, a strong inhibition of migration and tube formation of HUVECs in the presence of EMPmiR-19b mimic was observed. EMPmiR-19b mimic significantly increased migration of cell-free area and reduced the number of migration cells of HUVECs in comparison with controls [Figure 3b and 3c]. Evaluation of tube formation showed that HUVECs hardly formed into branched capillary-like endothelial networks after incubation with EMPmiR-19b mimic [Figure 3d]. These results indicated that increased miR-19b, within EMPs, could suppress HUVEC migration and angiogenesis. Furthermore, the biological effect of EMPs induced by hypoxia might be due to the delivery of miR-19b.
Figure 3.
EMPmiR-19b mimic suppressed migration and angiogenesis of HUVECs. (a) The expression levels of miR-19b in HUVECs treated with EMPs and validation of miR-19b in HUVECs were determined by RT-PCR and were normalized to RNU6B. HUVECs treated with EMPs for 24 h were subjected to (b) scratch migration and (c) transwell migration test for migration capability and (d) tube formation assay for angiogenic function. EMPNC mimic was used as control in tube formation assay. The data shown are as mean ± SE representative of three independent experiments. *P < 0.05, †P < 0.01; Vehicle, Lipofectamine 2000. EMP: Endothelial microparticle; HUVECs: Human umbilical vein endothelial cells; RT-PCR: Real-time polymerase chain reaction; NC: Negative control; SE: Standard error.
Transforming growth factor-β2was a direct target gene of miR-19b
To further clarify the regulatory mechanism of EMPmiR-19b mimic in migration and tube formation of HUVECs, we predicted the target gene of miR-19b associated with migration and tube formation using miRBase (http://www.mirbase.org/), TargetScan (http://www.targetscan.org/), and miRanda (http://miracle.igib.res.in/miracle/). Results showed that TGFβ2 was a one of the potential targets commonly predicted by the three algorithms. MiR-19b-binding sites across several species including humans, mice, rats, and dogs suggested an evolutionarily conserved importance for miR-19b [Figure 4a and 4b].
Figure 4.
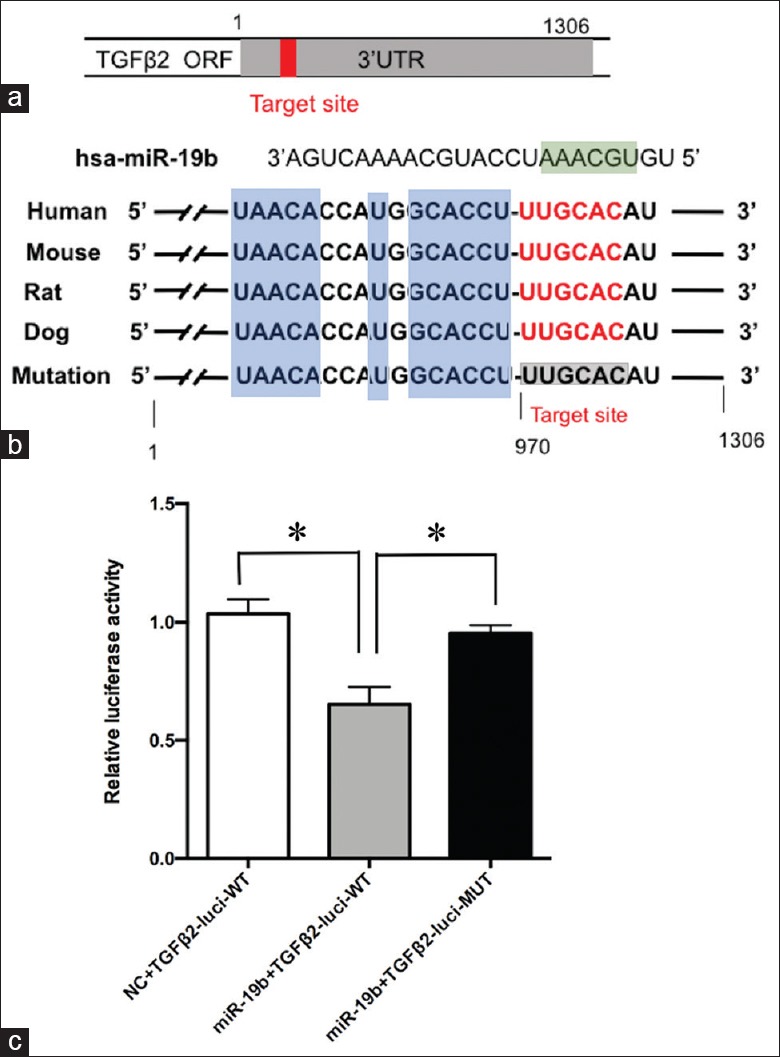
Luciferase assays of miR-19b-binding sites on TGFβ2 3’UTR. (a) 3’untranslated region of TGFβ2 mRNA with the predicted target sites for miR-19b. (b) Nucleotide resolution of the predicted target sites: seed sequence (green), target sequence (red), evolutionarily conserved regions (blue), and mutated miR-19b-binding site (gray). (c) HUVECs were transfected with either wild-type 3’UTR TGFβ2 or mutant 3’UTR TGFβ2, along with the miR-19b mimic. Luciferase activities were normalized to Renilla activities. Results shown are as mean ± SE representative of three independent experiments. *P < 0.01. TGFβ2: Transforming growth factor-β2; HUVECs: Human umbilical vein endothelial cells; SE: Standard error.
To examine whether TGFβ2 is a direct target of miR-19b in HUVECs, we cloned the putative miR-19b-binding site on TGFβ2 3’UTR into the luciferase reporter plasmid and co-transfected it with the miR-19b mimic into HUVECs. Results showed that overexpression of miR-19b could decrease the expression of the luciferase reporter gene to 50%, which contained the wild-type-binding site of miR-19b. However, when the putative binding site of miR-19 was mutated, miR-19b-mediated inhibition of luciferase gene expression was rescued in HUVECs [Figure 4c]. These results demonstrated that TGFβ2 was a direct target of miR-19b in HUVECs.
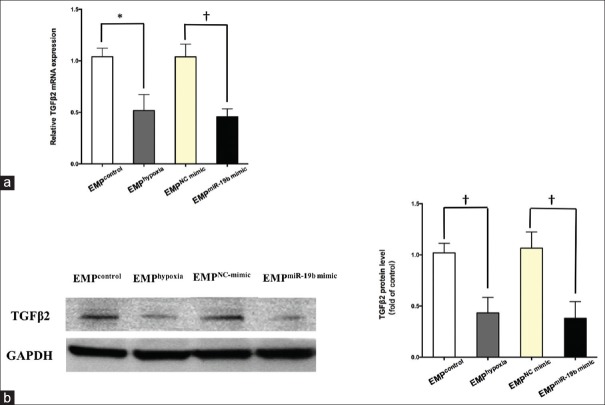
Transforming growth factor-β2 was downregulated in human umbilical vein endothelial cells treated with endothelial microparticlehypoxia or endothelial microparticlemiR-19b mimic
To investigate if the EMP-derived miR-19b suppression function was mediated by targeting TGFβ2, confluent HUVECs were respectively incubated with EMPcontrol, EMPhypoxia, EMPNC mimic, and EMPmiR-19b mimic. Then, mRNA and protein were collected from HUVECs after 24 h. RT-quantitative PCR and Western blotting results indicated that EMPhypoxia could decrease TGFβ2 mRNA levels by 50% [P < 0.05; Figure 5a] and protein levels by 60% [P < 0.01; Figure 5b] in HUVECs. Similarly, EMPmiR-19b mimic could decrease TGFβ2 mRNA levels by 60% [P < 0.01; Figure 5a] and protein levels by 65% [P < 0.01; Figure 5b] in HUVECs.
Figure 5.
The effect of EMPhypoxia and EMPmiR-19b mimic on TGFβ2 expression in HUVECs. (b) TGFβ2 mRNA was measured by RT-PCR and normalized to GAPDH mRNA. The data were expressed as changes relative to the data of the cells treated by EMPNC mimic. (b) TGFβ2 expression was measured by Western blotting with GAPDH used as a loading control. Densitometry was performed and normalized to GAPDH expression level. The data shown are as mean ± SE of three independent experiments. *P < 0.05, †P < 0.01. EMP: Endothelial microparticle; TGFβ2: Transforming growth factor-β2; HUVECs: Human umbilical vein endothelial cells; RT-PCR: Real-time polymerase chain reaction; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; NC: Negative control; SE: Standard error.
DISCUSSION
The present study showed that hypoxia-induced EMPs inhibited HUVEC migration and angiogenesis, which might be partly mediated by an increase of miR-19b in EMPs. In the previous study, we showed that upregulation of plasma miR-19b in patients suffering from unstable angina was partially due to the advanced release of miRNAs wrapped in EMPs.[13] These findings indicated that miR-19b in EMPs may be an indicator of an abnormal endothelial function caused by ischemic hypoxia within circulation. This study also demonstrated that miR-19b – encapsulated within EMPs – induced by hypoxia is associated with TGFβ2 gene transcription and that miR-19b can inhibit TGFβ2 expression in HUVECs. Taken together, miR-19b encapsulated within EMPs could play a role in cell-to-cell signaling in atherosclerosis, and EMPhypoxia enriched with miR-19b might protect against atherosclerosis at an early stage through negatively modulating target gene TGFβ2, which restrains the migration and angiogenesis of HUVECs.
Our study found that hypoxia could induce the release of EMPs, which consequently inhibit HUVEC migration and angiogenesis. Previous studies also revealed that EMP-mediated inhibition of LDL receptor-related protein (LRP6) abrogated vascular smooth muscle cell migration and proliferation in vitro.[21] In contrast, MPs released from adipose-derived stem cells were able to increase the migration and tube formation of HUVECs through the intercellular delivery of miR-31.[22] The effects of MPs may rely on their sources and intracellular molecular substances. All results showed that EMPhypoxia could significantly decrease the HUVEC migration and angiogenesis.
MiR-19b belongs to the miR-17-92 gene cluster (encode miR-17, miR-18a, miR-19a/b, and miR-92a), which was highly expressed in human endothelial cells.[23] Members of miR-17-92 cluster have been directly implicated in tumor angiogenesis.[24] In vivo studies determined that miR-19b was one of the promoting factors of tumor growth and metastasis.[25] Another study also indicated that miR-19b was oncogenic in the formation of a glioma by negatively regulating phosphatase and tensin (PTEN).[26] The miR-17-92 cluster was also involved in many cardiovascular diseases. A recent study pointed out that increased miR-19b expression might delay unstable plaque progression in unstable angina patients by inhibiting endothelial cell migration and angiogenesis.[27] Furthermore, the pro-angiogenesis protein fibroblast growth factor receptor-2 was inhibited by miR-19b which could reduce the intrinsic angiogenic properties of endothelial cells in vitro.[28] Upregulation of miR-19b was associated with the early phase of acute myocardial infarction.[29] We found that the upregulation of miR-19b in EMPs was induced by hypoxia. Maximum amounts of EMP-encapsulated miR-19b were released by HUVECs when under hypoxia for 12 h. The increased miR-19b in EMPhypoxia might be the main cause of its effect on HUVECs, so we further confirmed that miR-19b in EMPs generated from HUVECs transfected with miR-19b mimic was also involved in migration and angiogenesis of HUVECs. The same effects of EMPmiR-19b mimic and EMPhypoxia illustrated that miR-19b in EMPs was a negative regulator of endothelial cell migration and angiogenesis.
TGFβ2 has been proved to play a critical role in the development of cardiovascular diseases such as atherosclerosis and restenosis.[30] In our study, database-based target gene prediction software predicted that TGFβ2 was the most probable target gene of miR-19b. The inhibitory effects of miR-19b on TGFβ2 transcriptional activity were further confirmed by TGFβ2-driven promoter luciferase assay. We demonstrated that mRNA and protein levels of TGFβ2, in HUVECs, could be inhibited by EMPhypoxia and EMPmiR-19b mimic. Other than this, TGFβ2 targeted by miR-599 could repress vascular smooth muscle cell migration.[31] It has been observed that TGFβ2 regulated by miR-30 could suppress capillary morphogenesis in HUVECs.[32] In addition, miR-342-5p was proven to reduce angiogenesis by negatively regulating TGFβ signaling both in vitro and in vivo.[33] Taken together, these results highlight TGFβ2 as an inhibitor to cell migration and over-vascularization, which was responsible for miR-19b in EMP function of controlling endothelial cell migration and angiogenesis. This biological effect might protect against atherosclerosis at the early stage.
In conclusion, our study proved that miR-19b in EMPs induced by hypoxia could be transferred from EMPs into HUVECs and reduce HUVEC migration and angiogenesis by downregulating TGFβ2 expression. This research suggested that miR-19b within EMPs may be a novel target for further study of atherosclerosis.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81770356, 81470473, and 81600340) and the Capital Health Research and Development of Special (No. 2016-2-4083).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79. doi: 10.1038/nri855. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Lovren F, Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin Chem. 2013;59:1166–74. doi: 10.1373/clinchem.2012.199711. doi: 10.1373/clinchem.2012.199711. [DOI] [PubMed] [Google Scholar]
- 3.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 4.Badimon L, Suades R, Arderiu G, Peña E, Chiva-Blanch G, Padró T, et al. Microvesicles in atherosclerosis and angiogenesis: From bench to bedside and reverse. Front Cardiovasc Med. 2017;4:77. doi: 10.3389/fcvm.2017.00077. doi: 10.3389/fcvm.2017.000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V, et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–80. doi: 10.1016/S0002-9440(10)64887-0. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, et al. Endothelial microparticle-mediated transfer of microRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–38. doi: 10.1161/CIRCULATIONAHA.113.001720. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 7.Shoeibi S, Mozdziak P, Mohammadi S. Important signals regulating coronary artery angiogenesis. Microvasc Res. 2018;117:1–9. doi: 10.1016/j.mvr.2017.12.002. doi: 10.1016/j.mvr.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J, et al. Circulating microRNAs: Novel biomarkers for cardiovascular diseases. J Mol Med (Berl) 2012;90:865–75. doi: 10.1007/s00109-011-0840-5. doi: 10.1007/s00109-011-0840-5. [DOI] [PubMed] [Google Scholar]
- 10.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–95. doi: 10.1161/CIRCRESAHA.111.247452. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Zhong C, Wang K, Liu Y, Lv D, Zheng B, Zhou Q, et al. MiR-19b controls cardiac fibroblast proliferation and migration. J Cell Mol Med. 2016;20:1191–7. doi: 10.1111/jcmm.12858. doi: 10.1111/jcmm.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Ren J, Xu N, Zhang J, Geng Q, Cao C, et al. MicroRNA-19b functions as potential anti-thrombotic protector in patients with unstable angina by targeting tissue factor. J Mol Cell Cardiol. 2014;75:49–57. doi: 10.1016/j.yjmcc.2014.06.017. doi: 10.1016/j.yjmcc.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Jain T, Nikolopoulou EA, Xu Q, Qu A. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol Ther. 2018;183:22–33. doi: 10.1016/j.pharmthera.2017.09.003. doi: 10.1016/j.pharmthera.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Jia L, Fan J, Cui W, Liu S, Li N, Lau WB, et al. Endothelial cell-derived microparticles from patients with obstructive sleep apnea hypoxia syndrome and coronary artery disease increase aortic endothelial cell dysfunction. Cell Physiol Biochem. 2017;43:2562–70. doi: 10.1159/000484508. doi: 10.1159/000484508. [DOI] [PubMed] [Google Scholar]
- 16.Tuleta I, França CN, Wenzel D, Fleischmann B, Nickenig G, Werner N, et al. Intermittent hypoxia impairs endothelial function in early preatherosclerosis. Adv Exp Med Biol. 2015;858:1–7. doi: 10.1007/5584_2015_114. doi: 10.1007/5584_2015_114. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111:E3234–42. doi: 10.1073/pnas.1410041111. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Shang M, Zhang M, Wang Y, Chen Y, Wu Y, et al. Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells promote apoptosis and oxidative stress in H9c2 cardiomyocytes. BMC Cell Biol. 2016;17:25. doi: 10.1186/s12860-016-0100-1. doi: 10.1186/s12860-016-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroyer AS, Isobe H, Lesèche G, Castier Y, Wassef M, Mallat Z, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–7. doi: 10.1016/j.jacc.2006.10.053. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Bammert TD, Hijmans JG, Reiakvam WR, Levy MV, Brewster LM, Goldthwaite ZA, et al. High glucose derived endothelial microparticles increase active caspase-3 and reduce microRNA-let-7a expression in endothelial cells. Biochem Biophys Res Commun. 2017;493:1026–9. doi: 10.1016/j.bbrc.2017.09.098. doi: 10.1016/j.bbrc.2017.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen F, Zietzer A, Stumpf T, Flender A, Schmitz T, Nickenig G, et al. Endothelial microparticle-promoted inhibition of vascular remodeling is abrogated under hyperglycaemic conditions. J Mol Cell Cardiol. 2017;112:91–4. doi: 10.1016/j.yjmcc.2017.09.004. doi: 10.1016/j.yjmcc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, et al. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med. 2016;5:440–50. doi: 10.5966/sctm.2015-0177. doi: 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Zhang YC, Chen Y, Xiang Y, Shen CX, Li YG, et al. The role of miR-19b in the inhibition of endothelial cell apoptosis and its relationship with coronary artery disease. Sci Rep. 2015;5:15132. doi: 10.1038/srep15132. doi: 10.1038/srep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–5. doi: 10.1038/ng1855. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Y, Yin S, Hao Y, Yang J, Zhang H, Sun C, et al. MiR-19b promotes tumor growth and metastasis via targeting TP53. RNA. 2014;20:765–72. doi: 10.1261/rna.043026.113. doi: 10.1261/rna.043026.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Z, Wang K, Zhang A, Wang G, Kang C, Han L, et al. MiR-19a and miR-19b overexpression in gliomas. Pathol Oncol Res. 2013;19:847–53. doi: 10.1007/s12253-013-9653-x. doi: 10.1007/s12253-013-9653-x. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Geng Q, Chen H, Zhang J, Cao C, Zhang F, et al. The potential inhibitory effects of miR-19b on vulnerable plaque formation via the suppression of STAT3 transcriptional activity. Int J Mol Med. 2018;41:859–67. doi: 10.3892/ijmm.2017.3263. doi: 10.3892/ijmm.2017.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin R, Bao W, Xing Y, Xi T, Gou S. MiR-19b-1 inhibits angiogenesis by blocking cell cycle progression of endothelial cells. Biochem Biophys Res Commun. 2012;417:771–6. doi: 10.1016/j.bbrc.2011.12.032. doi: 10.1016/j.bbrc.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Wang KJ, Zhao X, Liu YZ, Zeng QT, Mao XB, Li SN, et al. Circulating miR-19b-3p, miR-134-5p and miR-186-5p are promising novel biomarkers for early diagnosis of acute myocardial infarction. Cell Physiol Biochem. 2016;38:1015–29. doi: 10.1159/000443053. doi: 10.1159/000443053. [DOI] [PubMed] [Google Scholar]
- 30.Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev. 2006;17:487–99. doi: 10.1016/j.cytogfr.2006.09.002. doi: 10.1016/j.cytogfr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Xie B, Zhang C, Kang K, Jiang S. MiR-599 inhibits vascular smooth muscle cells proliferation and migration by targeting TGFB2. PLoS One. 2015;10:e0141512. doi: 10.1371/journal.pone.0141512. doi: 10.1371/journal.pone.0141512. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Howe GA, Kazda K, Addison CL. MicroRNA-30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2. PLoS One. 2017;12:e0185619. doi: 10.1371/journal.pone.0185619. doi: 10.1371/journal.pone.0185619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan XC, Cao J, Liang L, Wang L, Gao F, Yang ZY, et al. MiR-342-5p is a notch downstream molecule and regulates multiple angiogenic pathways including notch, vascular endothelial growth factor and transforming growth factor β signaling. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003042. pii: e003042. doi: 10.1161/JAHA.115.003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hypoxia-induced changes of miR-19b in EMPs. The expression level of miR-19b in the EMPhypoxia following 0.5–36 h of hypoxia (3% O2) was measured by RT-PCR and was normalized to the spiked-in Caenorhabditis elegans miRNAs, cel-miR-39. The data were shown as mean ± SE representative of three independent experiments. *P < 0.05, †P < 0.01 compared with the normoxia group. miR-19b: MicroRNA-19b; SE: Standard error; EMPs: Endothelial microparticles; RT-PCR: Real-time polymerase chain reaction.
The levels of overexpressed miR-19b in HUVECs and EMPs. (a) The expression levels of miR-19b in HUVECs transfected with miR-19b mimic or NC mimic were measured by RT-PCR and normalized to RNU6B. (b) The expression levels of miR-19b in EMPvehicle, EMPNC mimic, and EMPmiR-19b mimic were measured by RT-PCR and normalized to the spiked-in Caenorhabditis elegans miRNAs, cel-miR-39. The data were shown as mean ± SE representative of three independent experiments. *P < 0.01 compared with the NC mimic group. miR-19b: MicroRNA-19b; NC: Negative control; Vehicle: Lipofectamine 2000; SE: Standard error; HUVECs: Human umbilical vein endothelial cells; EMPs: Endothelial microparticles; RT-PCR: Real-time polymerase chain reaction.