Abstract
Background:
Type 2 diabetes (T2DM) patients are susceptible to Helicobacter pylori (HP), and it has been reported that the occurrence of proteinuria is associated with HP infection in T2DM patients; however, this view remains controversial. This meta-analysis aimed to explore the association between HP infection and the occurrence of proteinuria in T2DM patients. In addition, we hope to provide some recommendations to readers in clinical or related fields.
Methods:
Our meta-analysis was conducted with the methodology of the Cochrane Collaboration. Search strategies were formulated by relevant professionals. Case–control studies that compared the occurrence of proteinuria in T2DM patients with and without HP infection were involved in our meta-analysis. Relevant English or Chinese studies were searched on online databases before 2018, including PubMed, the Cochrane library, Medline, Google Scholar, the China National Infrastructure, and Wanfang database. The search strategies were “diabetic proteinuria, diabetic microalbuminuria, diabetic albuminuria, diabetic kidney disease, diabetic renal dysfunction, diabetic renal disease, diabetic nephropathy, diabetic complications, and diabetic mellitus, combined with HP.” The quality of these involved articles was separately assessed by two investigators using the Newcastle–Ottawa Scale (NOS). Odds ratios (ORs) and associated 95% confidence intervals (CIs) were extracted and pooled using fixed-effects models.
Results:
Seven studies involving 1029 participants were included. The quality of these seven articles was all above five stars as assessed by NOS, and there was no significant publication bias in our meta-analysis. We found that T2DM patients with HP infection had a 2.00 times higher risk of the occurrence of proteinuria than patients without HP infection (OR: 2.00, 95% CI: 1.48–2.69).
Conclusions:
Our analysis showed that HP infection was associated with the occurrence of proteinuria in T2DM patients. HP radical surgery might be a therapeutic option for protecting kidney function in patients with T2DM.
Keywords: Helicobacter pylori, Kidney, Meta-Analysis, Proteinuria, Type 2 Diabetes
摘要
背景:
2型糖尿病患者易出现幽门螺旋杆菌感染,并且有学者报道2型糖尿病患者出现蛋白尿和幽门螺旋杆菌感染有关,但此 观点仍然存在争议。
目的:
本荟萃分析的目的主要是探求2型糖尿病肾病患者出现蛋白尿和幽门螺旋杆菌感染的关系,并为临床工作者及相关学 者提供建议。
方法:
本荟萃分析纳入与2型糖尿病患者出现蛋白尿和HP感染有关的病例对照研究。在以下数据库搜索了2018年以前的英文 或中文研究:Pubmed,Cochrane图书馆,Medline,谷歌学术,中国知网(CNKI)和万方数据库,检索策略为:糖尿病蛋白 尿,糖尿病微量白蛋白尿,糖尿病白蛋白尿,糖尿病肾病,糖尿病肾功能不全,糖尿病并发症和糖尿病,分别结合幽门螺旋 杆菌。纳入文章的质量由2名实验人员分别使用NOS量表进行评估。 本研究采用固定效应模型分析整合相关数据。
结果:
本荟萃分析纳入了7个病例对照研究共1029名参与者。7个病例对照研究的NOS评分均超过5颗星,且没有显著的发表偏 倚。分析结果显示,2型糖尿病患者合并幽门螺旋杆菌感染出现蛋白尿的风险是无幽门螺旋杆菌感染者的2倍(OR=2.00,95%CI (1.48,2.69))。
结论:
本荟萃分析结果提示2型糖尿病患者出现蛋白尿和幽门螺旋杆菌的感染有关,幽门螺旋杆菌根治术可能是保护合并幽门 螺旋杆菌感染的糖尿病患者肾功能的有效方法。
INTRODUCTION
Helicobacter pylori (HP) is a gram-negative bacterium, and it mainly colonizes in the mucosa. With the expression of two genes named VacA and CagA, HP is divided into two main types: type I contains both genes, and type II only contains VacA.[1]
At present, the rate of HP infection exceeds 50% worldwide and 80% in Africa.[2] Innate immunity and adaptive immunity are activated after HP infection, and multiple organs are affected.[3] The research on HP infection has put most of the attention on gastrointestinal diseases, such as chronic gastritis and peptic ulcers, in the past years.[4,5] However, in recent years, the association between HP infection and extragastric diseases has garnered more attention in recent years, as shown in Figure 1.
Figure 1.
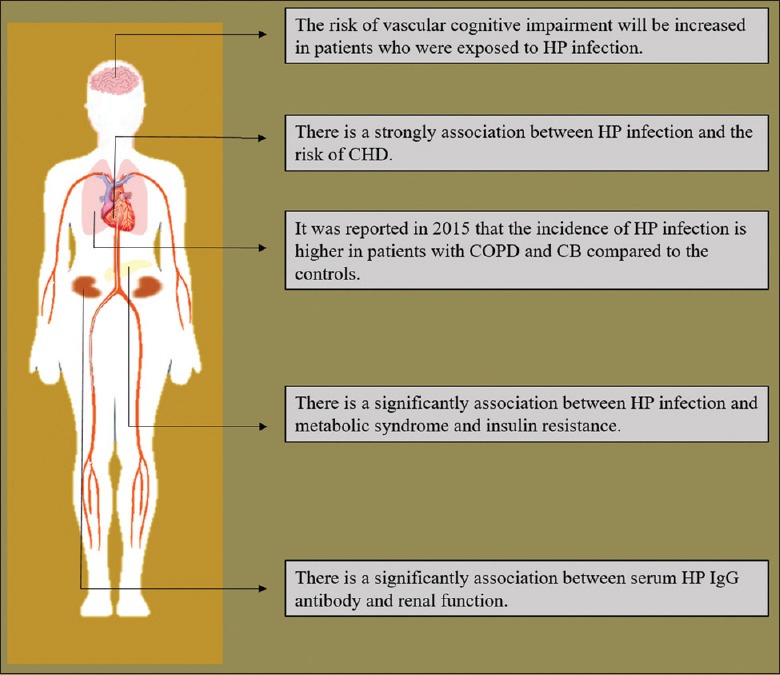
The association between HP infection and extragastric diseases. The association between HP infection and extragastric diseases has garnered more attention in recent years, as related to cognitive impairment, CHD, COPD, CB, metabolic syndrome and renal disease. HP: Helicobacter pylori; CHD: Coronary heart disease; COPD: Chronic obstructive pulmonary disease; CB: Chronic bronchitis.
For example, the association between neurological disease and HP infection has been of interest. In 2015, it was reported by Wright et al.[6] that the risk of vascular cognitive impairment increased in patients exposed to HP. In addition, Sun et al.[7] conducted a meta-analysis of the association between HP infection and the risk of coronary heart disease (CHD) in 2016, showing that HP infection increased the risk of CHD by 11% (risk ratio: 1.11; confidence interval [CI] 1.01–1.22), especially in the early life of patients.
The incidence of HP infection was higher in patients with chronic obstructive pulmonary disease and chronic bronchitis than that in controls (odds ratio [OR] 2.07, 95% CI 1.81–2.36, P for heterogeneity = 0.05; and OR 1.57, 95% CI: 1.33–1.86, P for heterogeneity = 0.08).[8] In 2016, it was reported that the prevalence of type 2 diabetes (T2DM) was significantly higher in patients with HP infection than in HP-negative patients (21.3% vs. 20.2%, P = 0.026).[9] A significant association between serum HP IgG antibody and renal function was also reported in 2013.[10] The association between HP infection and extragastric diseases was shown in Figure 1.
In 2013, there were 382 million people with diabetes in the world, and this number is expected to reach 592 million by 2035.[11] Diabetic nephropathy is a serious complication of diabetes, and it is the leading cause of end-stage renal disease in developed countries.[12] The mechanism of T2DM nephropathy is multifactorial.[13,14] Proteinuria is a significant marker in the early diagnosis of T2DM nephropathy and can be used as a sensitive indicator of renal function in patients with T2DM nephropathy,[15] having a certain reference value for the clinical treatment of T2DM patients.
In T2DM patients, proteinuria not only marks the glomerular filtration barrier injury but also implicates vascular endothelial dysfunction.[16] Various complications of diabetes are associated with the occurrence of proteinuria, such as hypertension, hypercholesterolemia, diabetic retinopathy, atherosclerosis, and cardiovascular complications.[17,18,19]
Within a large diabetic population, the prognosis of patients with proteinuria is serious, and there is a certain coping strategy for HP infection.[20,21] An increasing amount of research focuses on the interaction between HP infection and renal function. However, controversy still exists about the association between HP infection and the occurrence of proteinuria in T2DM patients. This controversy may be due to the small sample size, diversity of research methods, and differences in the epidemiological distribution of HP. To overcome these difficulties, we conducted a meta-analysis to further explore the association between HP infection and the occurrence of proteinuria in T2DM patients.
METHODS
Search strategies
This study was conducted with the Cochrane Collaboration methodology.[22] The reporting utilized the meta-analysis of observational studies in epidemiology group methodology.[23]
Search strategies were formulated by relevant professionals. The search strategies included “diabetic proteinuria, diabetic microalbuminuria, diabetic albuminuria, diabetic kidney disease, diabetic renal dysfunction, diabetic renal disease, diabetic nephropathy, diabetic complications, and diabetic mellitus, combined with HP.” Relevant English or Chinese studies were searched on online databases before 2018, including PubMed, the Cochrane library, Medline, Google Scholar, the China National Infrastructure, and Wanfang database.
Articles selection
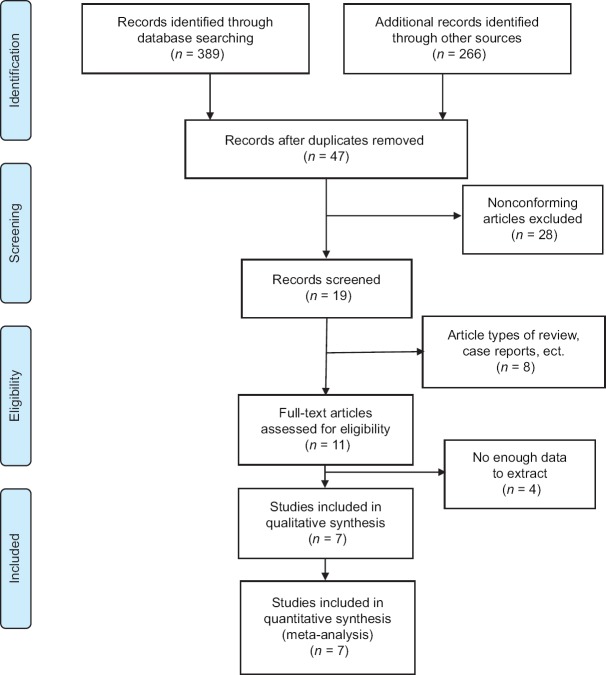
We screened a total of 655 related articles. After removing articles after identifying duplicate and apparently nonconforming articles, eleven articles involved in this meta-analysis met the inclusion and exclusion criteria; however, after reading the abstract and full text, four of these articles did not provide the relevant data, and we were unable to obtain the data after contacting the corresponding authors. Thus, seven articles were ultimately included.[24,25,26,27,28,29,30] The process of articles selection is shown in Figure 2.
Figure 2.
The process of article selection. We screened a total of 655 related articles, with 47 articles excluded because of repetition. After utilizing the inclusion and exclusion criteria for the abstract and full text of the remaining articles, seven were finally included in our meta-analysis.
Inclusion criteria
Inclusion criteria included: (1) the study type was case-control, (2) Chinese or English articles, (3) participants with T2DM, (4) participants with HP infection, and (5) participants with the diagnostic criteria for proteinuria.
Exclusion criteria
Exclusion criteria included: (1) articles’ full texts were unobtainable, (2) proteinuria diagnosis was not clear, (3) numbers of T2DM patients with and without HP infection were not provided, and (4) animal studies.
Definitions of proteinuria and Helicobacter pylori infection
The urinary albumin excretion of more than 30 mg/24 h or a urinary albumin to creatinine ratio of >30 mg/g was considered proteinuria. HP infection was diagnosed by serum HP IgG antibodies, a 14C-urea breath test, a rapid urease test and gastroduodenoscopy mucosal biopsies.
Data extraction
The quality of the seven included articles was separately assessed by two investigators using the Newcastle–Ottawa Scale (NOS).[31] A third person was involved in the discussions and decisions regarding the articles that had different scores. The qualities of these seven articles were all above five stars as assessed by NOS. The four-fold table was then drafted, and the data from each article were extracted by two investigators. The basic information of each article, such as the first author's name, country of origin, sample size, and published time of each article, was also extracted by two investigators, as shown in Table 1.
Table 1.
Basic characteristics of the included seven articles
| Studies | Year | Country | Study design | Case/control (n) | Ethnicity (mean age of case group and control group, years) | NOS quality assessment |
|---|---|---|---|---|---|---|
| Gulcelik et al.[24] | 2005 | Turkey | Case-control study | 31/71 | The population of Ankara Training Hospital (51.9 ± 10.6, 48.2 ± 8.3) | ★★★★★ |
| Kayar et al.[25] | 2014 | Turkey | Case-control study | 23/39 | The population of internal medicine outpatient clinic. (48.6 ± 12.1, 47.3 ± 12.6) | ★★★★★★ |
| Ohnishi et al.[26] | 2008 | Japan | Case-control study | 61/69 | The population of Matsushita Memoril Hospital (64.2 ± 11.6, 71.3 ± 14.4) | ★★★★★ |
| Zhou et al.[27] | 2015 | China | Case-control study | 125/65 | The population of the hospital in Qingdao. (50.2 ± 12.6, 45.6 ± 12.1) | ★★★★★★ |
| Demir et al.[28] | 2008 | Turkey | Case-control study | 21/120 | The population of Baskent University Konya Hospital. (52.8 ± 10.1, 52.6 ± 10.2) | ★★★★★ |
| Chung et al.[29] | 2013 | South Korea | Case-control study | 68/256 | The population of Seoul National University Healthcare System Gangnam Center. (57.7 ± 10.5, 54.6 ± 9.1) | ★★★★★ |
| Hamed et al.[30] | 2007 | Egypt | Case-control study | 37/43 | The population of Assiut University Hospital. (47.7 ± 9.2, 47.7 ± 9.1) | ★★★★★ |
Data analysis
Data analysis used RevMan 5.3 software (The Cochrane Collaboration, Copenhagen, Denmark) as recommended by the Cochrane Library. I-square (I2) test was used to assess the heterogeneity of the studies involved in our meta-analysis.[32] When severe heterogeneity, which was defined as I2 ≥50%, was found, a random-effects model was used; otherwise, a fixed-effects model was used according to the Cochrane review guidelines. I2 = 46% in this study; thus, a fixed-effects model was adopted.
The OR with 95% CI was used to evaluate the association between HP infection and the occurrence of proteinuria in T2DM patients. A statistically significant association was defined as P < 0.05. Publication bias was evaluated by funnel plots. Omitting one study in each turn was used to estimate the sensitivity of our meta-analysis.
RESULTS
Basic characteristics of included articles
There were seven case–control studies involved in our study, those were in accordance with the inclusion and exclusion criteria. A total of 1029 participants were involved in our meta-analysis, including 366 patients in the case group and 663 in the control group. The basic characteristics and the detailed NOS score of these included seven articles are shown in Table 1 and Supplementary Table 1, respectively.
Supplementary Table 1.
NOS score of the included articles
| Author | Is the case definition adequate | Representativeness of the cases | Selection of controls | Definition of controls | |
|---|---|---|---|---|---|
| N.E,.Gulcelik | Yes, with independent validation★ | Consecutive or obviously representative series of cases★ | No description | No history of disease (endpoint)★ | |
| Goh Eun Chung | Yes, with independent validation★ | Potential for selection biases or not stated | Hospital controls | No history of disease (endpoint)★ | |
| Masayoshi Ohnishi | Yes, with independent validation★ | Potential for selection biases or not stated | Hospital controls | No history of disease (endpoint)★ | |
| Fei Zhou | Yes, with independent validation★ | Consecutive or obviously representative series of cases★ | No description | No history of disease (endpoint)★ | |
| Mehmet Demir | Yes, with independent validation★ | Potential for selection biases or not stated | Hospital controls | No history of disease (endpoint)★ | |
| Yusuf Kayar | Yes, with independent validation★ | Consecutive or obviously representative series of cases★ | No description | No history of disease (endpoint)★ | |
| Sherifa Ahmed Hamed | Yes, with independent validation★ | Potential for selection biases or not stated | No description | No history of disease (endpoint)★ | |
| Author | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Nonresponse rate | NOS quality assessment |
| N.E,.Gulcelik | Select the most important factor★ | Secure record (e.g., surgical records)★ | Yes★ | Nonrespondents described | ★★★★★★ |
| Goh Eun Chung | Select the most important factor★ | Secure record (e.g., surgical records)★ | Yes★ | Nonrespondents described | ★★★★★ |
| Masayoshi Ohnishi | Select the most important factor★ | Secure record (e.g., surgical records)★ | Yes★ | Nonrespondents described | ★★★★★ |
| Fei Zhou | Select the most important factor★ | Secure record (e.g., surgical records)★ | Yes★ | Nonrespondents described | ★★★★★★ |
| Mehmet Demir | Select the most important factor★ | Secure record (e.g., surgical records)★ | Yes★ | Nonrespondents described | ★★★★★ |
| Yusuf Kayar | Select the most important factor★ | Secure record (e.g., surgical records)★ | Yes★ | Nonrespondents described | ★★★★★★ |
| Sherifa Ahmed Hamed | Select the most important factor★ | Secure record (e.g., surgical records)★ | Yes★ | Nonrespondents described | ★★★★★ |
NOS: Newcastle–Ottawa Scale.
Results of meta-analysis
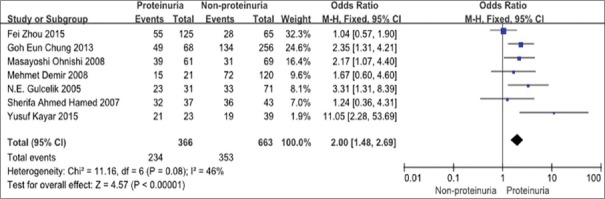
There was a significant correlation between HP infection and the occurrence of proteinuria in T2DM patients. The rate of HP infection in T2DM patients with proteinuria was significantly higher than the rate without HP infection (OR = 2.00, 95% CI: 1.48–2.69), as shown in Figure 3.
Figure 3.
Forest plot. The rate of HP infection in T2DM patients with proteinuria was significantly higher than that in patients without HP infection (OR = 2.00, 95% CI [1.48, 2.69]). HP: Helicobacter pylori; T2DM: Type 2 diabetes; OR: Odds ratio; CI: Confidence interval.
Assessment of publication bias
The publication bias of the involved studies was assessed by a funnel plot conducted by the use of RevMan. Each point in the funnel plot represents an article. The distribution of these points was roughly symmetrical on the funnel plot, as shown in Figure 4, meaning that there was no significant publication bias in our meta-analysis.
Figure 4.
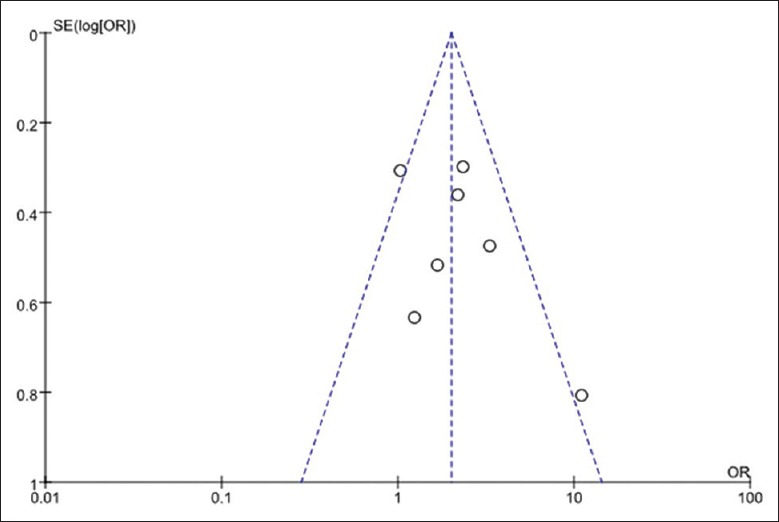
Funnel plot. The distribution of these articles is roughly symmetrical on the funnel plot, meaning that there was no significant publication bias in our meta-analysis.
DISCUSSION
After our meta-analysis, we found that HP infection was significantly associated with the occurrence of proteinuria in T2DM patients. This result suggested that HP infection played an important role in the process of renal dysfunction in T2DM patients. However, to the best of our knowledge, studies on the mechanism of this role currently remain scarce. The main focus of current articles is as follows.
First, people with diabetes are susceptible to HP. It has been reported that the rate of HP infection is as high as 75.6% in diabetes patients.[24] Moreover, the longer the diabetes duration is, the higher the rate of HP infection.[33] The mechanism of the high incidence of HP in T2DM patients is very complex; there are many factors involved in this process, and it is believed that cellular and humoral disorders are the main causes. The gastric mucosal surface is altered in T2DM patients.[31] The HP infection will be initiated by the overgrowth of upper gastrointestinal bacteria that is caused by delayed gastric emptying and dysfunction of glucose regulation in the T2DM patient.[34] It is speculated that the mechanism may be related to insulin resistance caused by HP infection in T2DM patients.[35,36]
HP infection often occurs in early childhood and will last a patient's whole life, whereas only a small percentage of HP-infected patients have obvious symptoms.[37] A complex balance between the human immune system and the HP after HP infection develops, resulting in a long state of chronic infection.[3,38]
The innate and adaptive immune system can be activated after HP infection, and there is a significant increase in interleukin-6 (IL-6), IL-8, IL-10, and tumor necrosis factor α (TNF-α).[3] A large number of immune complexes will subsequently be formed in a long-term inflammation.[39] Many organs and tissues can be affected, especially the kidney, which can be damaged by inflammatory cytokines, inflammatory cells, and immune complexes in T2DM patients.[40]
TNF-α is considered the main factor that is responsible for the increase in urinary albumin in T2DM patients.[41] The glomerular epithelial cells are directly damaged by the elevation of TNF-α. TNF-α can also promote the damage of the renal interstitium through the formation of peroxide.[42] After HP infection, IL-6 can promote glomerular sclerosis by increased production of the extracellular matrix and mesangial proliferation.[43]
In addition, it has been reported that, after HP infection, cross-reactivity appears between the host antigens and the inflammatory factors that are generated.[44] The permeability of glomerular basement membrane can also be changed by an increase in TNF-α and IL-1 after HP infection, leading to proteinuria.[45] In addition, granular deposits have been found in glomerular capillaries in patients with membranous nephropathy (MN), and these granular deposits were positive for HP-specific antibodies.[46] Although there are few relevant reports on T2DM patients, we speculated that immunoprecipitation can be caused by a reaction between HP antibodies and podocyte antigens in the glomerulus.
The mechanism of the increased urinary protein excretion in T2DM patients is complex, but endothelial cell dysfunction is one of the major reasons.[47] Some studies have shown that HP infection is associated with endothelial dysfunction and injury of the vascular system.[48,49] Cellular and humoral immunity is impaired in patients with T2DM; thus, bacteria invade the vascular wall more often in T2DM patients than that in patients without T2DM. If the level of B12 and folic acid is decreased after HP infection, the T2DM patients are then susceptible to atrophic gastritis; furthermore, the level of homocysteine will then be increased after HP infection in T2DM patients, risking injury of the vascular system.[45]
As far as we know, there is a close correlation between proteinuria and markers of endothelial cell dysfunction, such as von Willebrand factor, endothelin, tissue plasminogen activator, and fibrinogen, in T2DM patients.[50] Considering that the increase in inflammatory factors after HP infection in T2DM patients may be closely related to the dysfunction of endothelial and vascular injury, the promotion of the occurrence of proteinuria in T2DM patients with HP infection may occur.
In 2016, it was reported that early HP eradication is beneficial to patients with HP infection to avoid the occurrence of chronic kidney diseases.[51] Since proteinuria in patients with MN is decreased after HP eradication,[52] we speculated that HP eradication may be useful for T2DM patients in an effort to reduce the risk of proteinuria.
In summary, T2DM patients are susceptible to HP infection. The dysfunction of vascular endothelial cells and chronic inflammation after HP infection may be the main reasons for the occurrence of proteinuria in T2DM patients. Thus, there may be a breakthrough of the protection of renal function in T2DM patients if we place more focus on the research of inflammation markers and vascular injury after HP infection.
HP eradication therapies could be recommended to reduce the risk of proteinuria in diabetes patients with HP infection, especially for those patients with a higher risk of diabetic complications.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by grants from National Key R&D Program of China (No. 2016YFC1305500), National Natural Science Foundation of China (Nos. 61471399, 61671479, U1604284, and 81670663), the National Key Research and Development Program (No. 2016YFC1305404), and Special Research Project on Health Care of the Chinese People's Liberation Army (No. 15BJZ35).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Xiang Z, Censini S, Bayeli PF, Telford JL, Figura N, Rappuoli R, et al. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–8. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(Suppl 1):1–5. doi: 10.1111/hel.12165. doi: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 3.D’Elios MM, Czinn SJ. Immunity, inflammation, and vaccines for Helicobacter pylori. Helicobacter. 2014;19(Suppl 1):19–26. doi: 10.1111/hel.12156. doi: 10.1111/hel.12156. [DOI] [PubMed] [Google Scholar]
- 4.Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1457–64. doi: 10.1016/S0140-6736(15)60310-5. doi: 10.1016/S0140-6736(15)60310-5. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168–74. doi: 10.1038/nrgastro.2013.9. doi: 10.1038/nrgastro.2013.9. [DOI] [PubMed] [Google Scholar]
- 6.Wright CB, Gardener H, Dong C, Yoshita M, DeCarli C, Sacco RL, et al. Infectious burden and cognitive decline in the Northern Manhattan study. J Am Geriatr Soc. 2015;63:1540–5. doi: 10.1111/jgs.13557. doi: 10.1111/jgs.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Rangan P, Bhat SS, Liu L. A meta-analysis of the association between Helicobacter pylori infection and risk of coronary heart disease from published prospective studies. Helicobacter. 2016;21:11–23. doi: 10.1111/hel.12234. doi: 10.1111/hel.12234. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Guan Y, Li Y, Liu X, Zhang Y, Wang F, et al. Association between chronic respiratory diseases and Helicobacter pylori: A meta-analysis. Arch Bronconeumol. 2015;51:273–8. doi: 10.1016/j.arbres.2014.03.019. doi: 10.1016/j.arbres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Han X, Li Y, Wang J, Liu B, Hu H, Li X, et al. Helicobacter pylori infection is associated with type 2 diabetes among a middle- and old-age chinese population. Diabetes Metab Res Rev. 2016;32:95–101. doi: 10.1002/dmrr.2677. doi: 10.1002/dmrr.2677. [DOI] [PubMed] [Google Scholar]
- 10.Nasri H, Rafieian-Kopaei M. Significant association of serum H. pylori IgG antibody titer with kidney function in renal transplanted patients. J Renal Inj Prev. 2013;2:23–5. doi: 10.12861/jrip.2013.08. doi: 10.12861/jrip.2013.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375:905–6. doi: 10.1056/NEJMc1602469. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 12.Kato M, Natarajan R. Diabetic nephropathy – Emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10:517–30. doi: 10.1038/nrneph.2014.116. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Q, Zhu H, Chen X, Liu Z. Non-genetic mechanisms of diabetic nephropathy. Front Med. 2017;11:319–32. doi: 10.1007/s11684-017-0569-9. doi: 10.1007/s11684-017-0569-9. [DOI] [PubMed] [Google Scholar]
- 14.Yang XL, Yu HJ, Zhu HY, Zheng Y, Han QX, Cai GY, et al. Potential value of Datura stramonium agglutinin-recognized glycopatterns in urinary protein on differential diagnosis of diabetic nephropathy and nondiabetic renal disease. Chin Med J. 2018;131:180–7. doi: 10.4103/0366-6999.222328. doi: 10.4103/0366-6999.222328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parving HH, Persson F, Rossing P. Microalbuminuria: A parameter that has changed diabetes care. Diabetes Res Clin Pract. 2015;107:1–8. doi: 10.1016/j.diabres.2014.10.014. doi: 10.1016/j.diabres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: Is albumin excretion rate sufficient? Diabetes. 2000;49:1399–408. doi: 10.2337/diabetes.49.9.1399. doi: 10.2337/diabetes.49.9.1399. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad T, Ulhaq I, Mawani M, Islam N. Microalbuminuria in type-2 diabetes mellitus; the tip of iceberg of diabetic complications. Pak J Med Sci. 2017;33:519–23. doi: 10.12669/pjms.333.12537. doi: 10.12669/pjms.333.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Sharma R, Paul J, Tyagi A, Khari S, Prasad K. A study of microalbuminuria levels among patients with type 2 diabetic complications in Western UP. Int J Contemp Med. 2017;5:84–9. doi: 10.5958/2321-1032.2017.00017.1. [Google Scholar]
- 19.Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in thai patients with type 2 diabetes. J Diabetes Complications. 2014;28:124–9. doi: 10.1016/j.jdiacomp.2013.12.002. doi: 10.1016/j.jdiacomp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Huang J. Analysis of the relationship between Helicobacter pylori infection and diabetic gastroparesis. Chin Med J. 2017;130:2680–5. doi: 10.4103/0366-6999.218012. doi: 10.4103/0366-6999.218012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song ZQ, Liu J, Zhou LY. Hybrid therapy regimen for Helicobacter pylori eradication. Chin Med J. 2016;129:992–9. doi: 10.4103/0366-6999.179803. doi: 10.4103/0366-6999.179803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 4.2.5. The Cochrane library. 2005;3 [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting.Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Gulcelik NE, Kaya E, Demirbas B, Culha C, Koc G, Ozkaya M, et al. Helicobacter pylori prevalence in diabetic patients and its relationship with dyspepsia and autonomic neuropathy. J Endocrinol Invest. 2005;28:214–7. doi: 10.1007/BF03345375. doi: 10.1007/BF03345375. [DOI] [PubMed] [Google Scholar]
- 25.Kayar Y, Pamukçu Ö, Eroğlu H, Kalkan Erol K, Ilhan A, Kocaman O. Relationship between Helicobacter pylori infections in diabetic patients and inflammations, metabolic syndrome, and complications. Int J Chronic Dis 2015. 2015:290128. doi: 10.1155/2015/290128. doi: 10.1155/2015/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohnishi M, Fukui M, Ishikawa T, Ohnishi N, Ishigami N, Yoshioka K, et al. Helicobacter pylori infection and arterial stiffness in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1760–4. doi: 10.1016/j.metabol.2008.08.001. doi: 10.1016/j.metabol.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Zhong X, Chen J, Li C, Shang M, Jiang C, et al. Helicobacter pylori infection associated with type 2 diabetic nephropathy in patients with dyspeptic symptoms. Diabetes Res Clin Pract. 2015;110:328–34. doi: 10.1016/j.diabres.2015.09.008. doi: 10.1016/j.diabres.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Demir M, Gokturk HS, Ozturk NA, Kulaksizoglu M, Serin E, Yilmaz U. Helicobacter pylori prevalence in diabetes mellitus patients with dyspeptic symptoms and its relationship to glycemic control and late complications. Dig Dis Sci. 2008;53:2646–9. doi: 10.1007/s10620-007-0185-7. doi: 10.1007/s10620-007-0185-7. [DOI] [PubMed] [Google Scholar]
- 29.Chung GE, Heo NJ, Park MJ, Chung SJ, Kang HY, Kang SJ, et al. Helicobacter pylori seropositivity in diabetic patients is associated with microalbuminuria. World J Gastroenterol. 2013;19:97–102. doi: 10.3748/wjg.v19.i1.97. doi: 10.3748/wjg.v19.i1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamed SA, Amine NF, Galal GM, Helal SR, Tag El-Din LM, Shawky OA, et al. Vascular risks and complications in diabetes mellitus: The role of Helicobacter pylori infection. J Stroke Cerebrovasc Dis. 2008;17:86–94. doi: 10.1016/j.jstrokecerebrovasdis.2007.10.006. doi: 10.1016/j.jstrokecerebrovasdis.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520–5. doi: 10.2337/dc11-1043. doi: 10.2337/dc11-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He C, Yang Z, Lu NH. Helicobacter pylori infection and diabetes: Is it a myth or fact? World J Gastroenterol. 2014;20:4607–17. doi: 10.3748/wjg.v20.i16.4607. doi: 10.3748/wjg.v20.i16.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vafaeimanesh J, Parham M, Seyyedmajidi M, Bagherzadeh M. Helicobacter pylori infection and insulin resistance in diabetic and nondiabetic population. ScientificWorldJournal 2014. 2014:391250. doi: 10.1155/2014/391250. doi: 10.1155/2014/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen LW, Chien CY, Yang KJ, Kuo SF, Chen CH, Chien RN. Helicobacter pylori infection increases insulin resistance and metabolic syndrome in residents younger than 50 years old: A community-based study. PLoS One. 2015;10:e0128671. doi: 10.1371/journal.pone.0128671. doi: 10.1371/journal.pone.0128671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson K, Kaneko K, Andersen L P. Helicobacter: Inflammation, immunology and vaccines. Helicobacter. 2017;22(Suppl 1) doi: 10.1111/hel.12406. doi: 10.1111/hel.12406. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Cui J. Evaluation of Helicobacter pylori infection in patients with chronic hepatic disease. Chin Med J. 2017;130:149–54. doi: 10.4103/0366-6999.197980. doi: 10.4103/0366-6999.197980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanriverdı O. Association of Helicobacter pylori infection with microalbuminuria in type 2 diabetic patients. Turk J Gastroenterol. 2011;22:569–74. doi: 10.4318/tjg.2011.0252. doi: 10.4318/tjg.2011.0252. [DOI] [PubMed] [Google Scholar]
- 40.Huang G, Lv J, Li T, Huai G, Li X, Xiang S, et al. Notoginsenoside R1 ameliorates podocyte injury in rats with diabetic nephropathy by activating the PI3K/Akt signaling pathway. Int J Mol Med. 2016;38:1179–89. doi: 10.3892/ijmm.2016.2713. doi: 10.3892/ijmm.2016.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanbay M, Kasapoglu B, Akcay A. An occult risk factor for proteinuria: Helicobacter pylori infection. Med Hypotheses. 2007;69:709–10. doi: 10.1016/j.mehy.2007.01.010. doi: 10.1016/j.mehy.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Sun L, Kanwar YS. Relevance of TNF-α in the context of other inflammatory cytokines in the progression of diabetic nephropathy. Kidney Int. 2015;88:662–5. doi: 10.1038/ki.2015.250. doi: 10.1038/ki.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Xiao C, Wang P, Xu W, Zhang A, Li Q, et al. The alteration of th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Hum Immunol. 2014;75:289–96. doi: 10.1016/j.humimm.2014.02.007. doi: 10.1016/j.humimm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Pietroiusti A, Giuliano M, Magrini A, Bergamaschi A, Galante A. Cytotoxin-associated gene A strains of Helicobacter pylori represent a risk factor for the development of microalbuminuria in type 2 diabetes. Diabetes Care. 2006;29:1399–401. doi: 10.2337/dc06-0404. doi: 10.2337/dc06-0404. [DOI] [PubMed] [Google Scholar]
- 45.Ozer B, Serin E, Gumurdulu Y, Kayaselcuk F, Anarat R, Gur G, et al. Helicobacter pylori eradication lowers serum homocysteine level in patients without gastric atrophy. World J Gastroenterol. 2005;11:2764–7. doi: 10.3748/wjg.v11.i18.2764. doi: 10.3748/wjg.v11.i18.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagashima R, Maeda K, Yuda F, Kudo K, Saitoh M, Takahashi T, et al. Helicobacter pylori antigen in the glomeruli of patients with membranous nephropathy. Virchows Arch. 1997;431:235–9. doi: 10.1007/s004280050094. doi: 10.1007/s004280050094. [DOI] [PubMed] [Google Scholar]
- 47.Brouwers O, Niessen PM, Miyata T, Østergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia. 2014;57:224–35. doi: 10.1007/s00125-013-3088-5. doi: 10.1007/s00125-013-3088-5. [DOI] [PubMed] [Google Scholar]
- 48.Kang MJ, Song EJ, Kim BY, Kim DJ, Park JH. Helicobacter pylori induces vascular endothelial growth factor production in gastric epithelial cells through hypoxia-inducible factor-1α-dependent pathway. Helicobacter. 2014;19:476–83. doi: 10.1111/hel.12169. doi: 10.1111/hel.12169. [DOI] [PubMed] [Google Scholar]
- 49.Liu N, Zhou N, Chai N, Liu X, Jiang H, Wu Q, et al. Helicobacter pylori promotes angiogenesis depending on wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer. 2016;16:321. doi: 10.1186/s12885-016-2351-9. doi: 10.1186/s12885-016-2351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanbay M, Kasapoglu B, Turgut F, Uz E, Bavbek N, Akcay A. Helicobacter pylori: A major risk factor for endothelial dysfunction? Med Hypotheses. 2007;69:227–8. doi: 10.1016/j.mehy.2006.12.001. doi: 10.1016/j.mehy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Wang JW, Hsu CN, Tai WC, Ku MK, Hung TH, Tseng KL, et al. The association of Helicobacter pylori eradication with the occurrences of chronic kidney diseases in patients with peptic ulcer diseases. PLoS One. 2016;11:e0164824. doi: 10.1371/journal.pone.0164824. doi: 10.1371/journal.pone.0164824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caliskan B, Yazici H, Caliskan Y, Ozluk Y, Gulluoglu M, Kilicaslan I, et al. The effects of Helicobacter pylori eradication on proteinuria in patients with primary glomerulonephritis. Int J Nephrol 2014. 2014:180690. doi: 10.1155/2014/180690. doi: 10.1155/2014/180690. [DOI] [PMC free article] [PubMed] [Google Scholar]