Abstract
Background
MMR-D pancreatic cancer have been reported to respond to checkpoint inhibitor therapy. Here, we report the first case of acquired resistance to immunotherapy in MMR-D pancreatic cancer.
Case presentation
A 45-year-old woman with unresectable MMR-D pancreatic cancer was initially treated with FOLFIRINOX, FOLFIRI, and stereotactic body radiation with stable disease burden. After 3 months, imaging showed progression of disease with an increase in CA19-9. She was subsequently enrolled in a clinical trial of an anti-PD-L1 antibody in combination with an IDO1 inhibitor. She demonstrated a partial response to therapy by RECIST 1.1 criteria with declining tumor markers. Twenty-two months after beginning immunotherapy, imaging revealed an increasing left ovarian cystic mass. There were no other sites of progressive disease. The patient underwent a total hysterectomy and bilateral salpingo-oophorectomy, appendectomy, omentectomy and pelvic lymphadenopathy. Pathology was consistent with a metastasis from the pancreas involving the endometrium and left ovary. Thereafter, the patient continued with PD-1 blockade therapy off protocol with no further progressive disease. Immune profiling showed high levels of CD8+ T cells and PD-1 positive immune cells infiltrating the tumor, with a moderate level of PD-L1 expression in both the immune cells and the tumor cells. Next generation sequencing found only the KRAS G12D and RNF43 G659Vfs*41 mutations were retained from the pre-treatment tumor in the treatment-resistant tumor.
Conclusions
This is the first report describing acquired resistance to immunotherapy in MMR-D pancreatic cancer with accompanying genomic and immune profiling. This case of oligoprogression in the setting of immunotherapy demonstrates the feasibility of localized treatment followed by continuation of immunotherapy to sustain ongoing response.
Keywords: Pancreatic cancer, Acquired resistance, Immunotherapy, Mismatch repair deficiency
Background
As checkpoint inhibitors have now entered broad use for the treatment of solid tumors, an increasing number of patients who initially respond to immunotherapy have been identified to develop acquired resistance. Such reports have been described in individuals with melanoma, non-small cell lung cancer (NSCLC), uterine leiomyosarcoma, and mismatch repair deficient (MMR-D) colorectal cancer (CRC) patients [1–7].
Pancreatic ductal adenocarcinoma (PDAC) has been largely refractory to single and combination checkpoint inhibitor therapy [8–10]. The tumor microenvironment of PDAC have been described to be largely immunosuppressive, with involvement of regulatory T cells, tumor-associated macrophages (TAMs), and myeloid-derived suppressive cells (MDSCs) [11–13]. Another contributing factor to PDAC’s immunotherapy resistance may be PDAC’s relatively low tumor mutation burden (TMB) and poor antigenicity, leading to impaired endogenous T cell response to the tumor [14]. TMB, in general, has been reported to have a significant correlation with objective response rate to PD-1 inhibition [15]. However, a rare subset of PDAC patients with MMR-D has been reported to have partial and complete responses to immunotherapy [1, 14]. MMR-D occurs at a frequency of < 1% of all PDAC patients and is typically associated with germline mutations in MMR genes, IHC loss of MMR expression, an elevated MSIsensor score, significantly prolonged survival times, and high TMB.
Here, we describe a patient with locally advanced MMR-D PDAC who had a partial response to checkpoint inhibitor therapy, but subsequently acquired resistance to therapy and developed a metastasis to the ovary. We evaluated tumor cell-intrinsic and extrinsic causes of acquired resistance in the metastatic tumor. We determined the tumor mutational profile before and after acquired resistance using next generation sequencing (NGS) and assessed PD-1, PD-L1, and CD8+ T cell levels in the immunotherapy-resistant tumor specimen.
Case presentation
Clinical course
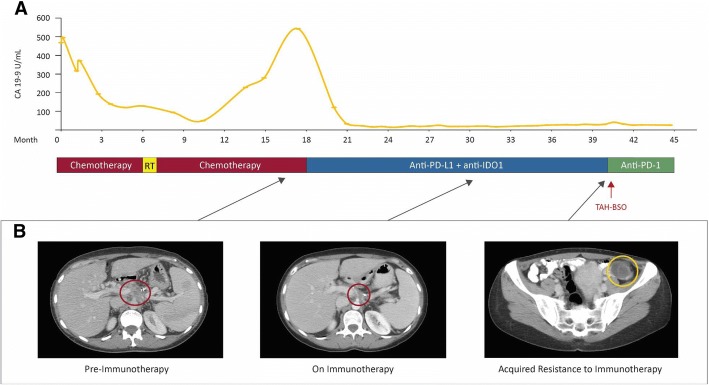
An otherwise healthy 45-year-old woman with known Lynch syndrome (germline mutation in MLH1) presented in 2014 with abdominal bloating. Computed tomography (CT) showed a 4 cm pancreatic body mass encasing the portal vein, splenomesenteric confluence, and common hepatic artery with enlarged periportal lymph nodes present. Biopsy revealed pancreatic adenocarcinoma. The patient was deemed to have unresectable disease and treated with FOLFIRINOX (5-fluorouracil, folinic acid, irinotecan, oxaliplatin) and FOLFIRI with stable disease burden and declining tumor markers (Fig. 1). She also received stereotactic body radiation therapy (SBRT) 3300 cGy in five fractions.
Fig. 1.
Clinical Pattern of Acquired Resistance. Panel a shows CA 19–9 levels corresponding to the timeline showing therapy. Panel b shows axial CT images corresponding to the primary pancreatic mass before treatment with immunotherapy and during immunotherapy, and the ovarian mass that developed after 22 months of immunotherapy. Red circles indicate the pancreatic mass and the yellow circle indicates the ovarian mass. Panel b shows the pancreatic mass after chemotherapy and RT
In 2015, CT scan revealed progression of disease, along with a rise in CA19-9 and clinical symptoms. The patient was enrolled in a clinical trial (NCT 02471846) of an anti-PD-L1 antibody in combination with an IDO1 inhibitor (navoximod). She demonstrated a partial response as defined by RECIST 1.1 criteria with declining tumor markers and prompt resolution of symptoms. In 2017, 22 months after beginning therapy, CT scan revealed an increasing left ovarian cystic mass. There were no other sites of progressive disease. The patient underwent a total hysterectomy and bilateral salpingo-oophorectomy, appendectomy, omentectomy and pelvic lymphadenopathy. Pathology was consistent with a metastasis from the pancreas involving the endometrium and left ovary. Thereafter, the patient continued with PD-1 blockade therapy off protocol with no further progressive disease.
Genomic features of pre-treatment and treatment-resistant tumors
Tumor mutation profile and burden were determined through MSK-IMPACT, a next generation sequencing assay of somatic mutations in key cancer genes [16]. TMB was 50.2 mutations per megabase (mt/Mb) in the pretreatment sample and 21.1 mt/Mb in the acquired resistance sample (Table 1); both tumors were computationally consistent with microsatellite-instability high. Only the KRAS G12D and RNF43 G659Vfs*41 mutations were retained from the pre-treatment tumor in the treatment-resistant tumor. No copy number alterations were detected in either the pre-treatment or the acquired resistance tumor sample. There was no loss-of-function mutations or loss of heterozygosity (LOH) in the HLA genes, B2M, PTEN, JAK1, JAK2, or TAP1.
Table 1.
Mutations in primary and metastatic lesions
| Gene | Protein change | Mutation type |
|---|---|---|
| Primary Pancreas Lesion | ||
| KRAS | G12D | Missense_Mutation |
| RNF43 | G659Vfs*41 | Frame_Shift_Del |
| ARID1A | P1326Rfs*155 | Frame_Shift_Del |
| STK11 | P281Rfs*6 | Frame_Shift_Del |
| B2M | S14Ffs*29 | Frame_Shift_Del |
| MAP3K1 | N1079Ifs*3 | Frame_Shift_Del |
| ARID1A | A339Lfs*24 | Frame_Shift_Del |
| CIC | P1529Lfs*91 | Frame_Shift_Del |
| ERCC4 | R689C | Missense_Mutation |
| PIK3R1 | M1? | Translation_Start_Site |
| KMT2A | S2872* | Nonsense_Mutation |
| BCL2L11 | S118Kfs*21 | Frame_Shift_Ins |
| MLH1 | X556_splice | Splice_Site |
| FANCA | P1444Rfs*3 | Frame_Shift_Del |
| BRCA2 | R2502C | Missense_Mutation |
| BCOR | S526 L | Missense_Mutation |
| BRCA2 | K1191 M | Missense_Mutation |
| ALK | G1202Efs*56 | Frame_Shift_Del |
| ZFHX3 | A3407Lfs*78 | Frame_Shift_Del |
| PTPRS | R1102H | Missense_Mutation |
| FLT1 | R1305H | Missense_Mutation |
| AR | R280C | Missense_Mutation |
| ROS1 | R1592C | Missense_Mutation |
| JAK3 | P84Rfs*63 | Frame_Shift_Del |
| PTPRT | I502T | Missense_Mutation |
| RECQL4 | V155Sfs*25 | Frame_Shift_Del |
| VTCN1 | Y145H | Missense_Mutation |
| RAF1 | Y458F | Missense_Mutation |
| SETD2 | D1057N | Missense_Mutation |
| GATA2 | P385S | Missense_Mutation |
| APC | R259Q | Missense_Mutation |
| APC | T1932A | Missense_Mutation |
| ARID1B | G314R | Missense_Mutation |
| ARID1B | A1002V | Missense_Mutation |
| RECQL4 | G1166S | Missense_Mutation |
| RECQL4 | A33V | Missense_Mutation |
| PAX5 | R38C | Missense_Mutation |
| TGFBR1 | V229D | Missense_Mutation |
| H3F3C | M120 V | Missense_Mutation |
| KMT2D | S1555F | Missense_Mutation |
| CREBBP | P2311L | Missense_Mutation |
| ZFHX3 | T2667A | Missense_Mutation |
| PLCG2 | L631R | Missense_Mutation |
| SPOP | N196K | Missense_Mutation |
| STK11 | L285R | Missense_Mutation |
| INSR | R279H | Missense_Mutation |
| MEF2B | P106H | Missense_Mutation |
| TOP1 | I457T | Missense_Mutation |
| NF2 | K469R | Missense_Mutation |
| AR | V139M | Missense_Mutation |
| TRAF7 | N174del | In_Frame_Del |
| Metastatic Site: Ovary/Endometrium | ||
| KRAS | G12D | Missense_Mutation |
| RNF43 | G659Vfs*41 | Frame_Shift_Del |
| TSC2 | Q35* | Nonsense_Mutation |
| TP53 | R273C | Missense_Mutation |
| STK11 | X245_splice | Splice_Site |
| PBRM1 | P1411Lfs*21 | Frame_Shift_Del |
| BARD1 | K208Rfs*4 | Frame_Shift_Del |
| ATRX | D1940Ifs*15 | Frame_Shift_Del |
| KMT2D | K4318Efs*15 | Frame_Shift_Del |
| KMT2B | G1879Vfs*16 | Frame_Shift_Del |
| NRAS | A66V | Missense_Mutation |
| CIC | R440H | Missense_Mutation |
| INSR | R1331C | Missense_Mutation |
| MYCN | R285Q | Missense_Mutation |
| BCL6 | S434 N | Missense_Mutation |
| TNFAIP3 | K759Qfs*10 | Frame_Shift_Ins |
| RPS6KA4 | RPS6KA4-BAD fusion | Fusion |
| FLT3 | S188R | Missense_Mutation |
| ERBB2 | H193N | Missense_Mutation |
| DOT1L | V170 L | Missense_Mutation |
| PTPRT | E917V | Missense_Mutation |
| HIST1H3F | T119A | Missense_Mutation |
| INHBA | A41T | Missense_Mutation |
| RXRA | G73C | Missense_Mutation |
| ARAF | E556G | Missense_Mutation |
| BAD | RPS6KA4-BAD fusion | Fusion |
Pathological features of the treatment-resistant tumor
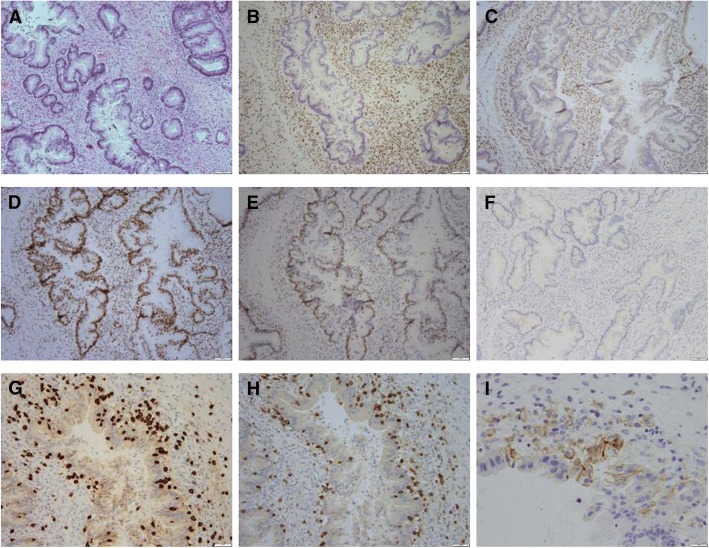
Immunohistochemistry (IHC) of the metastatic sample confirmed that the tumor was MMR-D, with loss of MLH1 and PMS2 expression (Fig. 2a-e). Histologically and immunophenotypically, the tumor exhibited features consistent with a metastasis of pancreatic origin including negative IHC staining for PAX8 (Fig. 2f), a marker typically associated with a Mullerian primary.
Fig. 2.
Immune Profiling of Metastatic Lesion. Metastatic pancreatic adenocarcinoma showing loss of MLH1 and PMS2 and increased immune cell infiltration. H&E section demonstrates a gland forming adenocarcinoma, morphologically compatible with pancreatic origin (a). By immunohistochemistry, the tumor cells show loss of staining for MLH1 (b) and PMS2 (c) and retained staining for MSH2 (d) and MSH6 (e). The tumor cells are also negative for PAX8 (f), in keeping with its non-Mullerian origin. Assessment of immune cell infiltration demonstrates florid CD8 positive T cells infiltrating the tumor epithelium and in the stroma surrounding the tumor epithelium (g). There is also prominent PD-1 positive immune cells (h) distributed similarly as the CD8 positive cells. PD-L1 expression is focally present in immune cells and in some tumor cells (i)
We were unable to assess immune cell infiltration with IHC in the pre-treatment tumor due to insufficient tissue. However, for the resected treatment-resistant metastasis, we found high levels of CD8+ T cells and PD-1 positive immune cells, with a moderate level of PD-L1 expression in both the immune cells and the tumor cells (Fig. 2g-i).
Discussion
Patients treated with immunotherapy may respond durably, fail to respond, or initially respond but subsequently develop acquired resistance. Acquired resistance to immunotherapy is a consequence of a number of tumor-extrinsic and tumor cell-intrinsic factors [17]. Tumor-extrinsic acquired resistance can be due to insufficient CD8+ T cell infiltration at the tumor microenvironment (TME) and immunosuppression in the TME by regulatory T cells, MDSCs, and TAMs [18]. Mechanisms of tumor-intrinsic acquired resistance include decreases in and loss of neoantigens [2, 4, 19], disruption of neoantigen presentation [3, 5, 20, 21], and resistance to interferon gamma [5].
The ovaries have been previously reported as a potential sanctuary site for malignant gastrointestinal metastases given their resistance to chemotherapy [22]. In this case, however, we did not see a deficit in immune cell infiltration at the ovarian site. Given the abundant CD8+ T cell infiltration, PD-1, and PD-L1 expression in the ovarian site, we speculate that the resistance mechanism is driven less by tumor-extrinsic factors and more by tumor-intrinsic factors.
In this case of acquired resistance to PDAC, the decrease in tumor mutation burden after treatment is likely reflective of immunoediting [23–25]. However, the robust T cell infiltration within the resistant tumor microenvironment suggests a potential alternate mechanism restraining productive anti-tumor immunity. Through genomic profiling, we found no changes in loss of function or loss of heterozygosity in previously reported mechanisms of intrinsic resistance, including the HLA genes, B2M, PTEN, JAK1, JAK2, or TAP1. Similar cases in which the driver of resistance is unknown have been reported, and highlight the complexity of resistance in the context of immunotherapy and the need for larger, cooperative efforts to integrate analyses of these uncommon cases in order to reveal mechanistic insight [26].
In this PDAC patient, disease progression only occurred in the ovary, an uncommon site of metastases in PDAC [27]. The phenomenon and management of oligoprogression in the setting of acquired resistance to targeted therapy have been previously described in NSCLC [28]. But oligoprogression in the setting of acquired resistance to immunotherapy is less well described. A case series of acquired resistance to PD-1 axis inhibitors in 26 NSCLC patients found that a majority (89%) of these patients had recurrence limited to one or two sites of disease [7]. Isolated progression was also reported in the majority (78%) of 36 melanoma patients with acquired resistance to PD-1 blockade [29]. MMR-D patients under PD-1 blockade have been reported to develop acquired resistance, with tumors developing from occult sites such as the brain and the bone [1].
The present report has notable limitations. No clear mechanism of resistance was determined, although we speculate that immunoediting is a primary driving mechanism. Immunoediting is a dynamic dialogue between the immune system and the invading system that consists of elimination, equilibrium, and escape phases [30]. In the elimination phase, tumor cells are identified and eliminated by the immune system. In the equilibrium phase, the immune system is unable to eliminate all cancer cells but is able to contain further growth. In the escape phase, tumor cells variants are selected to proliferate in an immunologically intact environment. Genetic and epigenetic changes within these tumor cells grant additional resistance to immune elimination, allowing the tumor cells to grow. Further in vitro studies are needed to determine the specific acquired changes within the tumor and the selection pressure exerted by PD-L1 therapy. We also had insufficient pre-treatment tissue for immunopathologic testing to directly compare the phenotypic changes.
This is the first reported case, to our knowledge, of acquired immunotherapy resistance in PDAC with accompanying genomic and immune profiling of the metastasis. This case of oligoprogression in the setting of immunotherapy also highlights the feasibility of localized treatment followed by continuation of immunotherapy to sustain ongoing response elsewhere. A number of factors, including tumor heterogeneity, the specific resistance mechanism, and tissue-specific immunoregulation, likely influence the sites, extent, and rate of disease progression in acquired resistance to immunotherapy, and remain to be fully characterized [31].
Acknowledgments
The authors wish to gratefully acknowledge the patient and her family for allowing us to publish her case report.
Funding
This work was supported in part by the National Cancer Institute Cancer Center Core Grant No. P30-17 CA008748.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRC
Colorectal cancer
- CT
Computed tomography
- IHC
Immunohistochemistry
- LOH
Loss of heterozygosity
- MDSC
Myeloid-derived suppressive cell
- MMR-D
Mismatch repair deficient
- NGS
Next generation sequencing
- NSCLC
Non-small cell lung cancer
- PDAC
Pancreatic ductal adenocarcinoma
- SBRT
Stereotactic body radiation
- TAM
Tumor-associated macrophages
- TMB
Tumor mutation burden
- TME
Tumor microenvironment
- T-regs
T-regulatory cells
Authors’ contributions
All authors were involved in the generation of figures and writing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The patient described in this case signed a case report informed consent form which is available for review.
Consent for publication
The patient consented to publication of the case report.
Informed written consent was obtained from patient for publication of this case. Consent is available upon request.
Competing interests
Eileen M. O'Reilly (EOR): Research Funding Celgene, Sanofi, ActaBiologica, AstraZenica, Silenseed, Genentech-Roche. Consulting: Targovax, Celgene, Bayer. Matthew D. Hellman (MDH) receives research funding from Bristol-Myers Squibb; is paid consultant to Merck, Bristol-Myers Squibb, AztraZeneca, Genentech/Roche, Janssen, Nektar, Syndax, Mirati, and Shattuck Labs; receives travel support/honoraria from AztraZeneca and BMS; and a patent has been filed by MSK related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx.Luis A. Diaz (LAD) is a member of the board of directors of Personal Genome Diagnostics (PGDx) and Jounce Therapeutics. LAD holds equity in PapGene, Personal Genome Diagnostics (PGDx) and Phoremost. He is a paid consultant for Merck, PGDx and Phoremost. LAD is an inventor of licensed intellectual property related to technology for circulating tumor DNA analyses and mismatch repair deficiency for diagnosis and therapy (WO2016077553A1) from Johns Hopkins University. These licenses and relationships are associated with equity or royalty payments to LAD. The terms of all these arrangements are being managed by Johns Hopkins and Memorial Sloan Kettering in accordance with their conflict of interest policies. In addition, in the past 5 years, LAD has participated as a paid consultant for one-time engagements with Caris, Lyndra, Genocea Biosciences, Illumina and Cell Design Labs. Jedd Wochok (JD) Consultant for: Adaptive Biotech; Advaxis; Amgen; Apricity; Array BioPharma; Ascentage Pharma;Astellas; Beigene; Bristol Myers Squibb; Celgene; Chugai; Elucida; Eli Lilly; F Star; Genentech; Imvaq; Kleo Pharma; MedImmune; Merck; Neon Therapuetics; Ono; Polaris Pharma; Polynoma; Psioxus; Puretech; Recepta; Trieza; Sellas Life Sciences; Serametrix; Surface Oncology; Syndax. Research support: Bristol Myers Squibb; Medimmune; Merck Pharmaceuticals; Genentech. Equity in: Potenza Therapeutics; Tizona Pharmaceuticals; Adaptive Biotechnologies; Elucida; Imvaq; Beigene; Trieza.Zsofia Stadler (ZK): Immediate Family Member, Ophthalmology Consulting/Advisory Role: Allergan, Adverum Biotechnologies, Alimera Sciences, Biomarin, Fortress Biotech, Genentech, Novartis, Optos, Regeneron, Regenxbio, Spark Therapeutics. The other authors (ZIH, MV, JS) declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7(3):264–276. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7(12):1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity. 2017;46(2):197–204. doi: 10.1016/j.immuni.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005;174(3):1462–1471. doi: 10.4049/jimmunol.174.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gettinger Scott N., Wurtz Anna, Goldberg Sarah B., Rimm David, Schalper Kurt, Kaech Susan, Kavathas Paula, Chiang Anne, Lilenbaum Rogerio, Zelterman Daniel, Politi Katerina, Herbst Roy S. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non–Small Cell Lung Cancer. Journal of Thoracic Oncology. 2018;13(6):831–839. doi: 10.1016/j.jtho.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Reilly EM, Oh D-Y, Dhani N, Renouf DJ, Lee MA, Sun W, et al. A randomized phase 2 study of durvalumab monotherapy and in combination with tremelimumab in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC): ALPS study. J Clin Oncol. 2018;36(4_suppl):217. [Google Scholar]
- 11.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 12.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61(9):1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24(6):1326–1336. doi: 10.1158/1078-0432.CCR-17-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachsmann MB, Pop LM, Vitetta ES. Pancreatic ductal adenocarcinoma: a review of immunologic aspects. J Investig Med. 2012;60(4):643–663. doi: 10.2310/JIM.0b013e31824a4d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375(23):2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88(2):100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donia M, Harbst K, van Buuren M, Kvistborg P, Lindberg MF, Andersen R, et al. Acquired immune resistance follows complete tumor regression without loss of target antigens or IFNgamma signaling. Cancer Res. 2017;77(17):4562–4566. doi: 10.1158/0008-5472.CAN-16-3172. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AE, Nicolson VM, Cunningham D. Ovarian metastases from primary gastrointestinal malignancies: the Royal Marsden Hospital experience and implications for adjuvant treatment. Br J Cancer. 1995;71(1):92–96. doi: 10.1038/bjc.1995.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171(4):934–949. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 25.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 26.Ascierto ML, Makohon-Moore A, Lipson EJ, Taube JM, McMiller TL, Berger AE, et al. Transcriptional mechanisms of resistance to anti-PD-1 therapy. Clin Cancer Res. 2017;23(12):3168–3180. doi: 10.1158/1078-0432.CCR-17-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133(3):413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 28.Gandara DR, Li T, Lara PN, Kelly K, Riess JW, Redman MW, et al. Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clin Lung Cancer. 2014;15(1):1–6. doi: 10.1016/j.cllc.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang DY, Eroglu Z, Ozgun A, Leger PD, Zhao S, Ye F, et al. Clinical features of acquired resistance to anti-PD-1 therapy in advanced melanoma. Cancer Immunol Res. 2017;5(5):357–362. doi: 10.1158/2326-6066.CIR-16-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 31.Pao W, Ooi CH, Birzele F, Ruefli-Brasse A, Cannarile MA, Reis B, et al. Tissue-specific immunoregulation: a call for better understanding of the “immunostat” in the context of cancer. Cancer Discov. 2018;8(4):395–402. doi: 10.1158/2159-8290.CD-17-1320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.