Abstract
The microenvironment of follicular lymphoma (FL) is composed of tumor-infiltrating CD8+ T cells, follicular regulatory T cells, lymphoma-associated macrophages and mast cells, follicular helper T cells, follicular dendritic cells, and follicular reticular cells, all of which have been reported to have relevance in the prognosis of FL patients. In addition, some of these cells play a role in the histologic transformation of FL. Macrophages contribute to a poor prognosis in FL patients treated in the pre-rituximab era, but are associated with good prognosis in those treated in the rituximab era. T-cell immunoglobulin and mucin domain protein (TIM) 3 are markers of T-cell exhaustion, and T cells co-expressing programed death 1 (PD1) in peripheral blood and lymph nodes secrete interleukin (IL)-12 in the serum. Serum CXCL9, IL-2 receptor, and IL-1 receptor agonist are associated with shorter survival of FL patients. Agents for manipulation of the microenvironment surrounding FL cells include the immunomodulatory drug lenalidomide, immune check-point inhibitors, and cyclophosphamide prior to rituximab. To battle FL and to improve the outcomes of FL patients, understanding the relationship between neoplastic cells and the various microenvironmental cellular components is crucial for developing therapeutics against the microenvironment.
Keywords: lenalidomide, PD1, follicular helper T cell, regulatory T cell, tumor-associated macrophage
INTRODUCTION
Follicular lymphoma (FL) accounts for 7-15% of all lymphomas worldwide.1 FL arises from malignant transformation of normal germinal center (GC) B cells and, in approximately 85% of cases, harbors the t(14;18)(q32;q21) translocation, resulting in an inability to down-regulate expression of anti-apoptotic protein B-cell lymphoma 2 (BCL2), which is absent in normal GC B cells.2 Most tumors are characterized by recurrent secondary genetic alterations that may provide a growth advantage, including genomic gains, losses, and mutations, and have been reported in MLL2,3 EPHA7,4 TNFRSF14,5,6 and EZH2.7
FL is a low grade lymphoma with variable clinical course; in some patients, the disease is indolent and slowly progressive over a period of many years, with waxing and waning lymphadenopathy, whereas in others the disease progresses rapidly, often with transformation to aggressive lymphoma and early death.8 Despite advances in the treatment of FL, most FL patients remain incurable and, at 5 years approximately 11%9 to 13% of cases,10 and at 10 years 15%10 to 28%11 of cases will transform to an aggressive phenotype, typically diffuse large B-cell lymphoma (DLBCL). Management strategies include watch and wait, immunochemotherapy, and new targeted treatment options.12
The prognosis of FL remains heterogeneous, as does its treatment options. Thus, prognostic indices are necessary to help the physician’s decision making process and to design clinical trials. Thus far, several prognostic factors have been identified in patients with FL, including age, stage, tumor burden, bone marrow (BM) involvement, systemic symptoms, performance status, serum lactate dehydrogenase, hemoglobin, erythrocyte sedimentation rate, and β2-microglobulin.13-17 As a result of international cooperative study, the FL International Prognostic Index (FLIPI) was established in 2004.18 However, the FLIPI was created before the era of rituximab, and was based on retrospective analyses. For these reasons, the International FL Prognostic Factor Project launched the F2 study, which aimed to verify whether a prospective collection of data would enable the development of a more accurate prognostic index, leading to the establishment of FLIPI2 in 2009.19 However, both FLIPI and FLIPI2 have several limitations. These indices focus on clinical factors, and do not take into account biological factors, such as the tumor microenvironment and the host response.
The neoplastic follicles in FL contain not only neoplastic GC B cells, but also non-neoplastic T cells, macrophages, and dendritic cells that comprise the microenvironment (Table 1, Fig. 1). Recently, many studies have reported that the microenvironment plays a critical role in FL progression. FL histology is characterized by varying proportions of nonmalignant immune-related cells. The importance of the microenvironment in FL is further highlighted by the fact that investigators have been unable to propagate FL cell lines, and even short-term growth in vitro requires survival signals derived from either feeder cells or cytokines.
Table 1. The impact of cell components of the microenvironment of follicular lymphoma on the outcomes of follicular lymphoma patients.
| Cell | Phenotype | Secreted cytokine | Outcome* (Pre-R era) |
Outcomae* (R era) |
|---|---|---|---|---|
| TIL (CTL) | CD8+ GrzB+ (21) | F (32,33,72) | F (34) | |
| TFR | CD4+ CD25+ FOXP3+ CXCR5high ICOShigh BCL6low BLIMP1+ PD1high | F (35,39) | ||
| (M1) TAM (= LAM) | CD68+ | IL-12 | P (19,36,52,53,54) F (52) |
No (53) |
| (M2) TAM (= LAM) | CD163+ CD68- | IL-10 | NA | P (81)† F (81)‡ |
| TA-mast cell | naphthol-ASD-chroloacetate esterase+ | No (55) | P (55) | |
| TFH | CD4+ CD25- CD200+ CXCR5high ICOShigh BCL6+ PD1high CXCL13+ TIM3- | IL-4, IL-17, IL-21, IFNγ | P# | No (66,67,68) |
| FDC | CD21+ CD23+ CD35+ CD14+ | CXCL13 | P# | |
| FRC | CCL2, CXCL12 | P# |
Pre-R era, pre-rituximab era; R era, rituximab era; TIL, tumor-infiltrating lymphocyte; CTL, cytotoxic T cell; GrzB, granzyme B; F, favorable; TFR, follicular regulatory T cell; M1, M1 polarized; TAM, tumor-associated macrophage; LAM, lymphoma-associated macrophage; P, poor; No, no impact on outcome; M2, M2 polarized; NA, no available data; TA-mast cell; tumor-associated mast cell; TFH, follicular helper T cell; FDC, follicular dendritic cell; FRC, fibroblastic reticular cell.
*In case of increase number of the infiltrating cells.
† In case of the patients treated with R-CVP. ‡ In case of the patients treated with R-CHOP. #Theoretically. In case of the patients treated with chemotherapy alone. In case of the patients treated with R-CHOP.
Fig. 1.
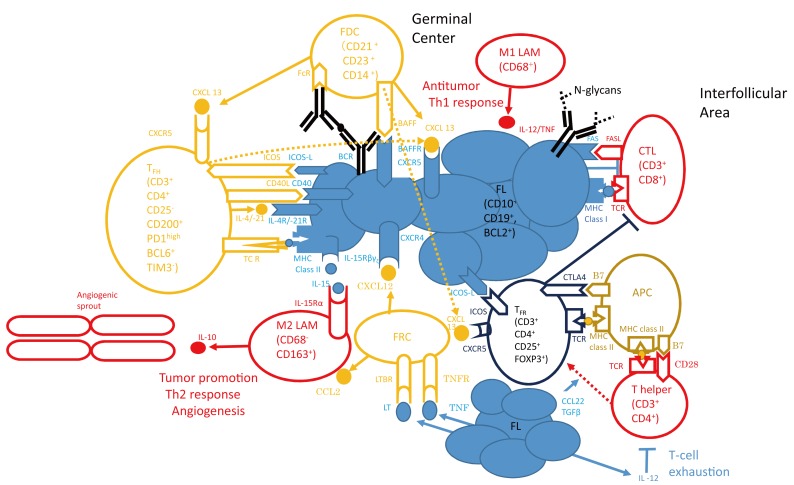
Tumor cells are nurtured by a variety of cells (orange open circles), such as follicular helper T (TFH) cells, follicular dendritic cells (FDCs), and follicular reticular cells (FRCs). Beneficial signals for growth and survival include cytokines, such as interleukin (IL)-4 and IL-21, which bind to interleukin receptors on lymphoma cells (IL-4 receptor/IL-21 receptor) (left middle side of the light blue filled circles) or CXCL12 and CXCL13 secreted by stromal cell subsets. B-cell receptor (BCR) signaling occurs through stimulation of the BCR by the innate immune system through N-glycans (right side of the light blue filled circles) or by specific antigen presentation through professional antigen presenting cells, such as FDCs (left upper side of the light blue filled circles). Tumor cells overcome the antitumor immune response from T helper cells, cytotoxic T cells (CTLs), and macrophages (red open circles). For example, follicular lymphoma (FL) cells prevent lysis via CTLs by inducing a T-cell immunologic synapse dysfunction (left upper corner of “non-Hodgkin lymphoma” in Fig. 2), or by secretion of IL-12, they induce T-cell exhaustion (right lower corner, below the red open circle of the “T helper” cell). Immune cell subsets that suppress an efficient immunological response against the tumor include regulatory T cells (Tregs) and M2 polarized macrophages (TAMs) (blue open circles). The former are enriched in the FL microenvironment and diminish the T-cell response. FL (light blue filled circles) contributes to the preferential conversion of T helper cells into Tregs by proteins such as transforming growth factor (TGF)-β or CCL22. Whereas classically activated TAM (M1 polarized) control malignant growth through induction of a Th1 response, M2-polarized TAM exert a tumor-promoting function through angiogenesis and induction of an immunosuppressive Th2 response. (A modification of Fig. 1 of Ref. 21).
APC, antigen-presenting cells; CTL, cytotoxic T cells; FDC, follicular dendritic cell; FL, follicular lymphoma; FRC, follicular reticular cell; TFH, follicular helper T cell; LAM, lymphoma-associated macrophage; TFR, follicular regulatory T cell; PD1, programed death 1; TIM3, T-cell immunoglobulin and mucin domain protein 3; ICOS, inducible costimulatory; TCR, T-cell receptor; TNF, tumor necrosis factor; TNFR, TNF receptor; LT, lymphotoxin; BAFF, B-cell activating factor belonging to the tumor necrosis factor family; BAFFR, BAFF receptor
In this review we focus on the perturbations of FL in the milieu of lymph nodes (LNs), that is, the so-called “microenvironment”, and its relevance to the prognosis of FL patients. The results from many studies on the impact of the microenvironment on FL reported thus far are variable and sometimes even contradictory (Table 1). This may be partly explained by poor reproducibility of immunohistochemical marker quantification,20 and treatment heterogeneity within small studies, and the correlation between biomarkers and outcome may result from statistical issues, such as failure to control for multiple hypothesis testing. The role of the microenvironment in FL can be said to act in two directions; one supporting tumor growth and survival and the other suppressing the antitumoral immune response (Fig. 1).21
However, understanding the relationship between the neoplastic cells and the various cellular components of the microenvironment will be crucial for developing therapeutics aimed at the microenvironment in the battle against FL. At present, the proposed classes of new agents for manipulation of the microenvironment surrounding FL cells include immunomodulatory drugs, that is, lenalidomide,22-28 as well as immune checkpoint blockers, such as those targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA4)29 or the programed death 1 (PD1)30 axes. Further investigations will be needed to improve the outcomes of FL patients who have been treated with the chimeric anti-CD20 monoclonal antibody during the rituximab era.
SURVIVAL PREDICTION OF FL PATIENTS BASED ON GENE EXPRESSION PROFILING (GEP)
Gene expression profiling (GEP) is the simultaneous measurement of the expression of thousands of genes to create a global picture of cellular function. With appropriate statistical and bioinformatic analyses, GEP provides valuable insights into lymphoma biology. Several gene expression profiling studies have highlighted that the gene expression signatures derived from non-neoplastic immune cells are associated with disease behavior.
In a landmark study, Dave et al.31 conducted GEP analysis using whole tumor biopsies from 191 untreated FL patients diagnosed between 1974 and 2001, who were divided into a training set (n = 95) and a test set (n = 96). The patients underwent a variety of standard treatments after biopsy, including various chemotherapy regimens, such as anthracycline- or purine analogue-containing chemotherapies, and autologous stem-cell transplantation, or were followed without any treatment by a watchful waiting policy. On the basis of the expression profile of the training set, individual genes that predicted the length of survival were grouped into two gene expression signatures that strongly correlated with patient prognosis. The two gene expression signatures were defined as the immune-response 1 signature, which was associated with a favorable prognosis, and the immune-response 2 signature, which was associated with a poor prognosis. The authors then generated a survival-predictor score (2.71 × immune-response 2 signature average) - (2.36 × immune-response 1 signature average), which was used to divide the patients in the test set into four quartiles; these groups showed widely disparate median lengths of survival (13.6, 11.1, 10.8, and 3.9 years), independent of clinical prognostic variables.
Interestingly, these gene signatures were based on the molecular features of nonmalignant cells; the immune-response 1 signature included several T-cell-restricted genes as well as genes more highly expressed in macrophages than in T cells; on the other hand, the immune-response 2 signature did not include T-cell-restricted genes, but genes that were preferentially expressed in macrophages, dendritic cells, or both. These results suggested an important interplay between the host immune system and tumor cells.31
IMPLICATIONS OF THE TUMOR MICROENVIRONMENT ON SURVIVAL AND DISEASE RESPONSE IN FL
CD8+ T cells
Using different methods, a greater number of infiltrating CD8+ T cells was shown to be significantly associated with favorable overall survival (OS).32,33 A Spanish group used an image analysis program, in which the number of positively stained cells was automatically scored, and then demonstrated that the median OS was 181 months in the patients with more than 108 cells/field, compared with 154 months in the patients with ≤ 108 cells /field (P = 0.009), using a cutoff value based on the mean value of the infiltrating cells.32 Furthermore, using flow cytometry, a Swedish group demonstrated that higher CD8+ T-cell levels correlated with longer OS as well as disease-specific survival, independent of FLIPI. Patients with > 8.6% CD8+ cells had a five-fold lower risk of death, and patients with 4.2% to 8.6% CD8+ cells had a two-fold lower risk, compared with patients with < 4.2% CD8+ cells. This biomarker had no impact on the patients at low risk according to FLIPI, but did in those of the intermediate- or high-risk FLIPI groups. Furthermore, patients who did not require treatment within 6 months from diagnosis were observed to have more CD8+ T cells (P = 0.011).33
A French group analyzed pretreatment lymph nodes from FL patients by immunohistochemistry (IHC) (n = 80) or by 3-color confocal microscopy (n = 10). IHC revealed a rich infiltration of CD8+ granzyme B (GrzB) cells in the FL interfollicular spaces. Accordingly, confocal microscopy showed an increased number of CD3+CD8+GrzB+ cytotoxic T cells (CTLs) and brighter GrzB staining in CTLs from FL samples compared with those from reactive lymph nodes. In 3-dimensional image reconstructions, CTLs were detected at the FL follicle border, where they formed lytic synapse-like structures with FL B cells and apoptotic cells, suggesting an in situ cytotoxic function. Finally, although GrzB expression in CTLs was not correlated with risk factors, high GrzB content was correlated with prolonged progression-free survival (PFS) after rituximab-combined chemotherapy.34
The immune microenvironment plays an important role in FL outcomes, and genes and proteins expressed by infiltrating T cells and macrophages are among the most important predictors of outcome.31,32,35,36 CD8+ and CD4+ tumor-infiltrating lymphocytes (TILs) in FL were shown to impair the function and suppress the recruitment of critical signaling proteins to the immunologic synapse.27 However, these studies shed little light on how FL cells alter the immune environment heterogeneity. To examine the mechanisms through which FL TILs affect outcome, a group from the United Kingdom (UK) analyzed the global gene expression profiles of highly purified CD8+ and CD4+ TILs from FL and compared these with profiles from reactive tonsil tissue. Both CD8+ and CD4+ TILs from FL patients showed significantly impaired motility compared with that of healthy TILs from reactive tonsils, and FL cells could induce this impairment in healthy R cells.37 The authors demonstrated altered protein expression levels of pro-melanin-concentrating hormone (PMCH), PMCH variant 1 (ETV1), and nicotinamide phosphoribosyltransferase (NAMPT) by dual-staining IHC, using tissue microarrays from a well-characterized independent cohort of 172 treatment-naïve FL patients. Furthermore, they examined the clinical significance of the altered expression levels of PMCH, ETV1, and NAMPT in FL CD4+ and CD8+ TILs. A high number of TILs expressing PMCH in the intrafollicular (P = 0.03) or interfollicular (P = 0.0003) areas was associated with improved OS; this difference was maintained independent of previous rituximab treatment. A high number of NAPMT-expressing TILs was associated with improved OS in both the intrafollicular (P = 0.02) and interfollicular (P = 0.045) areas. Patients with a high number of intrafollicular ETV1-expressing TILs had poor OS (P = 0.045), whereas patients who had a high number of interfollicular ETV1-expressing TILs had improved OS (P = 0.03). In multivariate analysis, none of the proteins examined alone retained independent significance for OS. However, the authors were able to build a model based on a combination of these biomarkers using the number of PMCH- and NAPMT-expressing cells in the interfollicular/intrafollicular area; a high combined score identified patients with improved OS (hazard ratio [HR), 0.32, 95% confidence interval [CI] = 0.1 to 0.61; P = 0.007].37
Regulatory T (Treg) cells
In an in vitro study using non-Hodgkin lymphoma (NHL) B-cells, a Mayo Clinic group showed that intratumoral Treg cells inhibit the proliferation and granule production of activated autologous infiltrating CD8+ T cells. Their results also showed that the degranulation and subsequent cytotoxic activity of infiltrating CD8+ T cells exposed to lymphoma B cells were completely attenuated by the presence of intratumoral Treg cells. Furthermore, the authors showed increased numbers of intratumoral Treg cells in biopsy specimens from patients with B-cell NHL.38 However, previously published data on the prognostic significance of the number and localization of tumor-infiltrating cells are highly conflicting. Although the findings were contradictory with non-hematopoietic cancer, several retrospective studies have reported that interfollicular infiltration of forkhead/winged helix transcription factor 3-positive (FOXP3+) T cells is a good prognostic sign in FL,35,39-42 germinal center type DLBCL, and Hodgkin lymphoma,40 irrespective of treatment,35,39-42 but is a poor prognostic sign in non-GC type DLBCL.40 Furthermore, contradictory results regarding treatment heterogeneity correlation with clinical impact have been occasionally reported.27 One study reported a trend of dense infiltration of FOXP3+ T cells, dense and interfollicular infiltration of CD68+ macrophages, and complete follicular dendritic meshwork being associated with a favorable time to progression in patients treated with cyclophosphamide, vincristine, and prednisone (CVP), whereas these were poor prognostic signs in patients treated with fludarabine.42 The same may be said for the results obtained from tissue microarrays (TMAs) constructed from archived tissues from the FL patients enrolled in three sequential Southwest Oncology Group (SWOG) trials (S8809, S9800, and S9911), in which rituximab was included in the treatment in the latter two, showing that the number or pattern of infiltrating FOXP3+ cells was not correlated with OS.43 The British Colombia Cancer Agency (BCCA) Center for Lymphoid Cancer also reported that FL patients with a diffuse pattern of infiltrating FOXP3+ T cells showed more significantly favorable overall survival (P < 0.0001) than those with a follicular pattern of FOXP3+ T-cell infiltration (present either mostly within the follicle [true follicular] or around the follicle and in the mantle zone [perifollicular]); the former also showed significantly less risk of histologic transformation than the latter (P = 0.002).44
Interaction between intratumoral CD4+CD25- T cells and CD70+ non-Hodgkin lymphoma B cells
FOXP3 expression can also be induced in CD4+CD25- T cells by corticosteroids, estrogen, and transforming growth factor-β.45-47 CD4+CD25- T cells expressing FOXP3 was reported in aging mice.48 The Mayo Clinic group described in the previous section found that in B-cell NHL, a subset of intratumoral, but not peripheral blood, CD4+CD25- T cells comprising approximately 15% of the intratumoral CD4+ T cells expressed FOXP3, and were capable of suppressing the proliferation of autologous infiltrating CD8+ T cells.49 Additionally, they also found that the presence of lymphoma B cells during activation augmented activation-induced FOXP3 expression in CD4+CD25- T cells. Furthermore, CD70+ lymphoma B cells significantly contributed to the activation-induced FOXP3 expression in intratumoral CD4+CD25- T cells. The authors further demonstrated that the inhibition of CD27-CD70 interaction by anti-CD70 antibody abrogated lymphoma B-cell-mediated induction of FOXP3 expression in intratumoral CD4+CD25- T cells.49
Follicular regulatory T (TFR) cells
Because Treg cells are specifically recruited to follicles in FL, a French group evaluated the expression of CD25 and FOXP3 in CD4+ cells within the secondary lymphoid organs. They reported a higher frequency of FOXP3+CD25+ Treg cells among the CD4+ T cells in FL samples compared with tonsil, reactive LN, and DLBCL samples.65 Interestingly, they noticed that the LNs of FL patients were particularly enriched for CD4+ T cells harboring both CXCR5highICOShigh and FOXP3+CD25+ phenotypes, which were called thereafter follicular regulatory T (TFR) cells.65 In order to evaluate whether this phenotype was really associated with a follicular localization, double-immunostaining was performed on FL biopsy samples, confirming the presence of numerous FOXP3+ cells co-expressing ICOS within FL neoplastic follicles. On the contrary, these cells were rare in the GCs of follicular hyperplasia, and were localized in the interfollicular areas or at the periphery of the GCs, in accordance with their homogeneous low expression of CXCR5 in the tonsils.65
Of note, TFR co-expressed Prdm1, the gene encoding BLIMP1, and its expression in TFR was higher than in any other CD4+ T-cell subset. Expression of BCL6 and BLIMP1 proteins in FOXP3+ cells within the GC was also confirmed by immunofluorescence staining of spleen sections from sheep red blood cell-immunized mice. All FOXP3+ TFR within the GC expressed BCL6, albeit at low levels, and 75% stained positive for BLIMP1 after 7 days immunization. Loss of BLIMP1 resulted in the doubling of TFR number, suggesting that BLIMP1 limits the size of the TFR population. TFR cells share phenotypic characteristics with follicular helper T (TFH) and conventional FOXP3+ Treg cells. Similar to TFH cells, TFR development depends on BCL6, signaling lymphocyte activation molecule-associated protein, CD28, and B cells.50 However, it was demonstrated that TFR originated from thymic-derived FOXP3+ precursors, not naïve or TFH cells. TFR were suppressive in vitro and limited TFH and GC B-cell numbers in vivo.50
In addition, the investigation performed by MD Anderson Cancer Center identified a subset of Treg cells expressing CXCR5 and BCL6 localized in the GCs in mice, as well as in humans. The expression of CXCR5 in Treg cells depends on BCL6. These CXCR5+BCL6+ Treg cells are absent in the thymus, but can be generated de novo from CXCR5-FOXP3+ natural Treg precursors. Lack of CXCR5+ Treg cells leads to greater GC reactions. Thus, these results unveiled a BCL6-CXCR5 axis in Treg cells that undermines the development of TFR cells, functioning to inhibit GC reactions.51
Tumor (Lymphoma)-associated macrophage (TAM or LAM)
The BC group also demonstrated in a multivariate analysis that a higher content of CD68+ macrophages was an independent predictor of worse OS in patients treated homogeneously with a multiagent chemotherapy comprised of bleomycin, cisplatin, etoposide, doxorubicin, cyclophosphamide, vincristine, and prednisone, followed by involved-field radiotherapy.36 However, contradictory results regarding the correlation between treatment heterogeneity and clinical impact have been reported by a Finnish group52 and the d’Etude des Lymphomes de I’Adulte (GELA).53
The Finnish group showed that addition of rituximab to chemotherapy reversed the negative prognostic impact of high macrophage content to favorable.52 Consistent with previous studies, a high proportion of tumor-associated macrophages (TAM) (> 67%) (n = 14) detected by anti-CD68 (clone KP1) antibody was associated with adverse outcome in chemotherapy-treated patients (compared with low TAM ≤ 67%; n = 31) (P = 0.026). In contrast, after treatment with a combination of rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy (R-CHOP), high TAM content (> 67%; n = 32) was correlated with longer survival rates (compared with low TAM ≤ 67%; n = 64). According to Kaplan-Meier estimates, the median PFS was not reached in patients with high TAM content, compared with 45 months for patients with low TAM content (≤ 67%) (P = 0.006). A trend toward a better OS at 5 years was also observed in patients with higher TAM content (OS, 97% vs. 90%, P = 0.116). The positive prognostic value of TAM was observed at both diagnosis and at relapse. In multivariate analyses, TAM content remained an independent prognostic factor for OS and PFS.48 However, the relative number of lymphoma-associated macrophages (LAMs) in their cohort48 was higher than that reported previously.36 Considering that the younger patients tended to have somewhat lower LAM levels in the Finnish group’s cohort,36 and the fact that the median age of the patients was only 44 years in the previous study,36 this difference may be partially age-related.36 However, different patient cohorts have many intrinsic properties, which may affect outcomes. Thus, extreme caution should be exercised in making comparisons of data between series of different reports.52 The mechanism by which the addition of rituximab to chemotherapy improves the outcome of patients with high LAM content is unknown; however, it is likely to be associated with antibody-dependent cell-mediated cytotoxicity (ADCC).52 The antitumoral activity of rituximab is dependent on interactions with the effector cells that have Fc receptors; namely, neutrophils, natural killer cells, and macrophages. Because tissue macrophages are critical for B-cell depletion after anti-CD20 antibody therapy, it is plausible that there is a relationship between LAM content and the efficacy of rituximab.
So far, the results from studies of the prognostic effect of CD68+ macrophages in diagnostic FL tissues have been inconsistent. The discrepancies could be due to different patient populations and technical factors, such as quantification methods and different cutoff values used to distinguish the high and low subgroups; the cutoff values chosen to best discriminate between the low and high subgroups differed even between the patients treated with chemotherapy and those treated with IHC.
In a larger scale GELA study, immunohistochemical CD68 expression was analyzed in 194 FL patients enrolled in the FL-2000 trial, in which they were randomly assigned to receive cyclophosphamide, doxorubicin, etoposide, prednisolone and interferon (CHVP-I) or rituximab plus CHVP-I.53 IHC was performed on paraffin sections using the same method as that in the previous study, and stained macrophages in high-power fields (hpf) were scored in the intrafollicular or extrafollicular areas.53 For intrafollicular macrophage count, the best cutoff value was estimated to be 10 macrophages/hpf. Low intrafollicular macrophage count was significantly associated with a better event-free survival (EFS) (P = 0.011). However, this effect was observed only in the CHVP-I arm (relative risk [RR] = 1.7; P = 0.012) and not in the rituximab plus CHVP-I arm (RR = 1.4; P = 0.156). Instead, when a cutoff value of 15 macrophages/hpf was used, they found no significant association with EFS. For extrafollicular macrophage count, fewer than 22 macrophages/hpf was associated with better EFS in the CHVP-I arm (RR = 1.6; P = 0.023), but not in the rituximab plus CHVP-I arm (RR = 1.1; P = 0.315).53 The same considerations may apply to the results of the aforementioned SWOG study concerning LAM, as well as regulatory T cells.43
The anti-CD68 antibody (clone KPI) is an ideal candidate for routine immunohistochemical prognostic use because the corresponding epitope is highly resistant to usual fixatives. Canioni et al.53 counted TAM under x400 magnification with a dry lens, which is recommended for the histologic grading of FL according to the WHO classification.
Signal transducers and activators of transcription (STAT) 1-positive TAM
A Spanish group showed that positive Signal Transducers and Activators of Transcription (STAT) 1 protein expression, defined by CD68+ cell count (dichotomized into ≤ 58 or > 58 positive cells/field), as well as the FLIPI, was a strong predictor of worse OS (RR = 3.47, 95% CI = 1.43-8.39; P = 0.006) in a multivariate Cox model.52 Double-fluorescence staining confirmed that STAT1 protein co-localized exclusively with CD68, indicating the presence of a subset of STAT1- expressing TAM localized principally in the vicinity of tumor cells. CD68+ macrophages were mainly localized outside the neoplastic follicles, whereas fewer cells were detected within the follicles. However, the cells expressing STAT1 protein, principally in the cytoplasm but also with nuclear staining, had a different distribution, with more positive cells inside the neoplastic follicles, particularly at the GC edge of the tumoral follicles. The majority of the STAT1+ cells outside the follicles were smaller and more regularly shaped than those present inside the tumoral follicles, where the cells had a more voluminous nucleus and cytoplasm and longer cytoplasmic extensions. STAT1 was also observed to be expressed in CD68+ macrophages, but not in CD21+ dendritic cells. The presence of STAT1+ macrophages was not restricted to neoplastic follicles.54 Immunohistochemical staining of tonsils or lymph nodes with reactive follicular hyperplasia revealed the occasional presence of STAT1+ macrophages.54
Tumor-associated mast cells
The Finnish group mentioned in the “TAM or LAM” section that demonstrated that the addition of rituximab to chemotherapy reversed the negative prognostic impact of high macrophage content to favorable55 in turn analyzed 98 patients treated with R-CHOP, 70 at diagnosis and 28 at relapse. Mast cells were detected by a histochemical Leder stain for naphthol-ASD-chloroacetate esterase. The patients with high mast cell content (> 1.5, cutoff value set to observed median value; n = 47) had a worse 4-year PFS than the patients with low mast cell content (≤ 1.5; n = 51) after R-CHOP therapy (34% vs. 74%, P = 0.002). The adverse prognostic value of mast cells was demonstrated both in the treated patients and at diagnosis (median PFS: high, 46 months [n = 34] vs. low, not reached [n = 36], P = 0.009) and at relapse (median PFS: high, 20 months [n = 13] vs. low, 54 months [n = 15], P = 0.004). On the contrary, no such impact on PFS was observed in the control patients treated with chemotherapy alone (P = 0.4). Furthermore, they investigated whether the negative impact of high monocyte content on survival was confounded with the previously identified predictive value of TAM, which had been defined by CD68 positivity.52 Of note, when the TAM-related PFS was analyzed separately in patients with high and low mast cell contents, the positive prognostic effect of TAM on the outcome of the FL patients treated with R-CHOP was seen only in patients with few mast cells. Among the patients with low mast cell contents, PFS was found to be worse in the group with low TAM scores (≤ upper tertile, n = 34) than in the group with high TAM scores (> upper tertile, n = 17) (4-year PFS 60% vs. 100%, median PFS 64 months vs. not reached, P = 0.006).55
Follicular helper T (TFH) cells, follicular dendritic cells (FDCs), and programed death 1 (PD1)-positive cells
TFH cell are defined by their capacity to support antigen-specific B-cell response by providing survival, activation, differentiation, and class switch recombination signals to normal B cells.56 FL cells express high levels of CXCR4 (bottom of the light blue filled circles, “FL” cells, Fig. 1) and CXCR5 (top of the light blue filled circles, “FL” cells, Fig. 1) and are attracted into follicles by cytokines, such as CXCL13, which are released by TFH cells or follicular dendritic cells (FDCs) (orange open circle, “FDC” cell, left upper corner in Fig. 1). As a result of their localization and the expression of Fc and complement receptors, FDCs are ideal antigen presenting cells, thus contributing to B-cell receptor (BCR) signaling (left upper corner, between “FDC” and “FL” cells in Fig. 1). TFH cells engage in cellular interaction with FL through their T-cell receptors via MHC class II, as well as CD40L/CD40 (left side of light blue filled circles of “FL” cells in Fig. 1). TFH cells are enriched in FL biopsies and secrete cytokines such as IL-4, which binds to the IL-4 receptor on lymphoma cells (left side of light blue filled circles of “FL” cells in Fig. 1) and triggers signaling by phosphorylation of extracellular signal-regulated kinase and STAT6.57 The role of stromal cells in FL pathogenesis has begun to emerge; in vitro studies have shown that stromal cells increase neoplastic B-cell survival, contribute to monocyte recruitment via the secretion of CCL258,59 (left lower side of the orange open circle of “follicular reticular cell FRC” cell in Fig.1), and contribute to macrophage polarization (the blue open circle of “M2 LAM” on the left lower corner in Fig.1). Data from co-culture experiments with bone marrow-derived mesenchymal stromal cells suggest that the malignant cells induce changes in stromal cells toward lymphoid differentiation and overexpression of CCL2, which in turn leads to the recruitment of monocytes that are differentiated into a TAM phenotype.59
The localization of CD4+ T cells within neoplastic follicles, unlike their absolute number, was associated with poor survival and rapid transformation. TFH provide survival signals to antigen-selected GC B cells, and help them achieve class-switch recombination and differentiation into antibody-secreting plasma cells. Highly controversial findings have been reported concerning the prognostic value of the number and localization of PD1+ and CD57+ T cells.32,41,60,61 TFH are characterized by strong expression of CXCR5 (top of “TFH” cell on the left side of Fig. 1) associated with a lack of CCR7, allowing their migration and retention into the CXCL13-rich light zone of the GC (upper left corner of Fig. 1). In addition, they express high levels of inducible costimulatory (ICOS) molecule (right side of the orange open circle of the “TFH” cell on the left side of Fig. 1), CD200, PD1, and produced IL-21 (in the interspace between a “TFH” cell and “FL” cells on the left side of Fig. 1) and CXCL1356 (on the top of a “TFH” cell on the left side of Fig. 1). These features are associated with the expression of the transcription factor BCL6, the master regulator of TFH differentiation.62 Furthermore, TFH secrete interferon (IFN)γ, IL-4, and IL-17, the prototypic Th1, Th2, and Th17 cytokines.57,63,64
The French group mentioned above demonstrated that CXCR5highICOShigh CD4+ T cells sorted from FL biopsies comprised at least two separate cell populations with distinct genetic and functional features: (i) CD25+ TFR and (ii) CD25- TFH, displaying a FL B-cell supportive activity without regulatory functions. They demonstrated that FL B cells upregulated the expression of the CD86 activation antigen when co-cultured with autologous TFH cells, and not with CXCR5-ICOS-CD4+ non-TFH cells.65 In turn, TFH were able to rescue autologous malignant B cells from spontaneous apoptosis in vitro. In addition, functional studies revealed that TFR did not display a malignant B-cell supportive effect, and exerted strong regulatory potential, as demonstrated by their capacity to inhibit CD4+CD25- effector T-cell proliferation as efficiently as the control tonsil Treg cells. On the contrary, paired FL TFH cells displayed no regulatory properties in similar experiments. These results convincingly demonstrated that TFR could be considered as bona fide Treg expressing the GC-specific receptor CXCR5 (blue open circle of the “TFR” in the right lower side of the “FL” cells in Fig. 1).65 Moreover, despite their strong similarities to tonsil-derived TFH, purified FL-derived TFH had a unique gene expression profile, including overexpression of several genes potentially involved directly or indirectly in lymphomagenesis, in particular TNF, LTA, IL-4, and CD40LG. Interestingly, the authors further demonstrated that IL-4, and CD40LG efficiently rescued malignant B cells from spontaneous and rituximab-induced apoptosis.65
Recently, the same Mayo Clinic group described in the “Treg cells” section reported that PD1 was expressed in intratumoral CD4+ T cells with both bright and dim intensity, representing two different sub-populations of cells.66 By IHC, CD4+PD1high T cells were found predominantly localized to the LN follicles, whereas PD1low T-cells were mainly in interfollicular areas. Intratumoral CD4+PD1high T cells showed a TFH-cell phenotype: CXCR5 expression and secretion of IL-21 (left upper corner of Fig. 1). Moreover, these cells were BCL6-positive, but T-cell immunoglobulin and mucin domain protein (TIM) 3-negative (left upper corner of Fig. 1). On the other hand, CD4+PD1low T cells had an exhausted phenotype, showing TIM3 expression. These cells did not express either BCL6 or CXCR5. CD4+PD1high T cells, otherwise TFH cells, actively supported B-cell growth, whereas CD4+PD1low T cells displayed reduced cytokine production as well as cell-signal transduction. The proportion of CD4+PD1low T cells (cutoff value set to mean, 26%) or CD8+PD1low T cells (cutoff value, 45%) was observed to be significantly correlated with reduced OS in FL patients (P = 0.007 and 0.026, respectively; n = 32). On the contrary, the proportion of CD4+PD1high T cells (cutoff value, 25%) was not associated with patient outcome.66 Furthermore, in the 14th International Conference on Malignant Lymphoma, the French group reported that PD1+ TFH cells did not have any prognostic significance in FL patients enrolled in the PRIMA trial.67
Another Mayo Clinic group analyzed serial biopsy specimens of 58 FL patients by localization pattern and cell content.68 No associations with time to transformation (TTT) or OS were found between the number or distribution of cells that expressed FOXP3, CXCL3, CD21, CD68, or CD11c. Although CD14+ cell number was also not associated with TTT or OS, patients with follicular (n = 13) localization of CD14+ cells had a median TTT of 3.8 years compared with 5.9 years in those with CD14+ cells localized to non-follicular areas (n = 41) (P = 0.027). However, no association between localization of CD14+ cells and OS was observed (P = 0.66).68 A substantial portion of CD14+ cells co-expressed CD21 and CD23, which are markers typically associated with FDC. Multicolor IHC demonstrated that CD21 and CD23 were co-expressed in nearly all CD14+ cells, and were absent in CD68+ and CD163+ cells. These results were confirmed by flow cytometry findings suggesting that the CD14+ cells were FDCs rather than monocytes or macrophages.68 Their results suggested the association between inferior outcome and increase in CD14+ cells number localizing to the follicle and whose immunophenotype was consistent with FDC.68
Lymphoma-associated monocytes have been associated with poor survival in FL,36 and it was initially assumed the CD14+ cells represented intratumoral monocytes. However, further analysis with flow cytometry and multicolor IHC confirmed that the CD14+ intrafollicular cells were distinct from monocytes and macrophages, and represented FDCs. Although FDCs are typically identified by CD21, CD23, and CD35 surface markers, they have also been reported to be associated with CD14 as well.69 CD14+ monocytes were thought to be immunosuppressive, and their presence was associated with more aggressive tumors.70
In addition to TFH, PD1 expression has also been described in exhausted T cells.71 Because these exhausted cells characteristically express TIM3, this can act as a marker for the presence of exhausted T cells.71 However, multicolor IHC with CD3, PD1, and TIM3 confirmed that PD1+ cells in the follicle were positive for CD3, but completely negative for TIM3, whereas PD1+ cells in the interfollicular space showed co-expression of TIM3 (Table 1).68 Cells in the follicle stained positive for both CXCR5 and PD1.68 These results confirmed that the PD1+ cells in the follicle were TIM3 negative, and did not represent exhausted T cells. Thus, the improved clinical outcomes associated with the follicular pattern of PD1+ cells, such as delay in TTT and improved survival, could be attributed to the maintained presence of TFH cells.68 PD1+ cells outside the follicle are a distinct cell type compared with follicular PD1+ cells; these PD1+TIM3+ cells appear to represent exhausted T cells, and were associated with inferior clinical outcomes. The role of PD1 in solid tumors has been well studied, with some solid tumors expressing PD-L1, a ligand of PD1, associated with a poor prognosis, possibly due to a decrease in tumor immune surveillance. Previous analyses of PD1+ cells in FL have yielded conflicting results. Two studies supported that increased levels of PD1+ cells were associated with superior outcomes, including a decreased risk of transformation.41,72 However, additional studies have not confirmed this correlation,73 and instead reported that increased PD1+ cells were associated with inferior survival in FL.61 In one of these studies, > 90% of PD1+ cells were localized to the follicle, and in the other, limited to the PD1+ cells localized in follicle.72
M2 polarization of macrophages
In most studies using specimens from those treated in the pre-rituximab era, elevated numbers of CD68+ TAM were shown to be associated with poor prognosis.19,36,53,54 These findings are usually interpreted in the light of alternative or M2 polarization of macrophages, a phenotype that is associated with tumor dissemination, immunosuppression, and angiogenesis.74,75 Two studies showed that increased microvessel density and angiogenic sprouting was correlated with increased numbers of macrophages and poor outcome in FL.76,77 FL B cells divert the classical activation of the innate immune system and subvert the function of the adaptive immune response. For example, immune synapses between malignant B cells and T cells are defective, although CD8+ CTLs have been shown to localize at the follicle border and enter into contact with tumor cells.27,34 It was also shown that IL-12 secretion by malignant B cells induces T-cell exhaustion (right lower corner of Fig. 1) via expression of TIM3.71 Moreover, FL cells induce the conversion of effector T cells into FOXP3-expressing Tregs, which suppresses the proliferation and activity of both CD4+CD25- and CD8+ T cells.38,78,79 In conclusion, these observation illustrate how malignant B cells disable the function of various T-cell subsets in the FL microenvironment to escape immune surveillance.
CD163, a member of the scavenger receptor cysteine-rich superfamily restricted to the monocyte/macrophage lineage, is a useful marker for anti-inflammatory or alternatively activated macrophages (M2 macrophages).80 Clear et al.76 observed a correlation between the number of CD163+ TAM and angiogenic sprouts (left lower corner in Fig. 1) in the poor prognostic group, assessed by both histopathologists (Spearman r = 0.4263, 95% CI, 0.1880-0.6171; P < 0.01) and an automated system (Spearman r = 0.3145, 95% CI, 0.1349-0.4742; P < 0.01). Analysis of samples restricted to 200-micrometer areas immediately surrounding these vascular sprouts revealed increased numbers of CD163+ cells compared with reactive control samples and those from the good prognostic group (P = 0.05). This analysis demonstrated an association between elevated numbers of infiltrating CD163+ macrophages within the immediate microenvironment surrounding the neovascular sprout and increased angiogenic sprouting in the interfollicular area in FL.76
Recently, the BCCA and Lymphoma Study Association assessed CD68 and CD163 by IHC in two large TMA studies. The first TMA cohort included samples from 186 patients obtained from the BCCA who had been treated with first-line systemic treatment, including rituximab and CVP. The second TMA cohort contained 395 samples from Primary RItuximab and MAintenance (PRIMA) trial patients treated with R-CHOP, who were then randomized to either rituximab maintenance or observation group. Macrophage infiltration was assessed using Aperion image analysis. Both of the cohorts were randomly split into training/validation sets. As a result, an increased CD163+ pixel count was shown to be predictive of adverse outcome in the BCCA dataset (5-year PFS 36% vs. 72%, respectively, P = 0.004 in the training cohort and 5-year PFS 29% vs. 61%, respectively, P = 0.004 in the validation cohort). In the PRIMA trial, an increased CD163+ pixel count was associated with favorable outcome (5-year PFS 60% vs. 44%, respectively, P = 0.001 in the training cohort and 5-year PFS 55% vs. 37%, respectively, P = 0.030 in the validation cohort). Consequently, CD163+ macrophages predicted outcome in FL, but their prognostic impact was highly dependent on the treatment received.81 It is noteworthy that one of the main differences in terms of treatment resided in the administration of doxorubicin as part of the R-CHOP regimen, which was administered to some of the PRIMA patients evaluated in their study, whereas the BCCA patients were invariably managed without an anthracycline as part of their first-line therapy. Depletion of macrophages was shown to reduce the efficacy of doxorubicin in an allograft mouse model, and conversely, prior macrophage activation enhanced the efficacy of doxorubicin.82,83 A study also indicated that anthracyclines were able to modulate the differentiation and function of cells involved in innate immunity toward a tumoricidal phenotype.84 Macrophages were shown to positively modulate rituximab efficacy, demonstrated by the significant association of elevated CD163 staining with favorable PFS only in the rituximab maintenance arm.81 These findings mirror the findings of the FL-2000 trial in which CD68+ macrophages predicted poor EFS in the CHVP-I arm but not in the rituximab plus CHVP-I arm.53 Moreover, the study by Taskinen and colleagues52 showed that elevated numbers of macrophages correlated with favorable PFS in patients treated with R-CHOP but not with CHOP alone. Furthermore, M2-skewed macrophages showed increased in vitro phagocytic capacity of rituximab-opsonized chronic lymphocytic leukemia cells in contrast with M1 macrophages, suggesting that CD163 is a rational marker to identify TAM that participate in rituximab-mediated antitumor responses.85 However, it is notable that the recent study by Pallasch et al.86 reported that antitumor responses to an anti-CD20 antibody were achieved in the macrophage-rich environment in the spleen, but not in the macrophage-poor bone marrow.
Role of stromal cells in FL pathogenesis: Fibroblastic reticular cells (FRCs) and human mesenchymal stem cells (MSCs)
Fibroblastic reticular cells (FRCs) (lower center part of Fig. 1, open circle “FRC” below the filled light blue circles of “FL” in Fig. 1) mediate immune cell migration, adhesion, and reciprocal interactions. The French group mentioned above in the “TFH and FDCs, and PD1-positive cells” section investigated the relationship between FRCs and their postulated progenitors; in other words, BM mesenchymal stem cells (MSCs) and their capacity to sustain malignant B-cell growth. In vitro, BM-derived MSCs (BM-MSCs) acquired a complete FRC phenotype in response to a combination of tumor necrosis factor (TNF)-α and lymphotoxin (LT)-α1β2 (lower part of Fig. 1). Moreover, MSCs recruited primary FL cells that, in turn, triggered their differentiation into FRCs, supporting malignant B-cell survival.58 They described a bidirectional interaction between BM-MSCs and FL B cells. BM-MSCs mediated primary FL cell migration and adhesion. CXCL12 (the orange closed small circle below the light blue filled circles of the “FL” in Fig. 1) was a pivotal factor in the recruitment of malignant GC-derived B cells, because CXCR4 (bottom of the light blue filled circles of the “FL” in Fig. 1) inhibition completely abrogated their migration toward BM-MSCs. However, lymphoma cell migration in vivo is a complex process resulting from the integration of multiple signals, and other chemokines present in stromal cell supernatant may modulate CXCL12-induced chemotaxis.58 For example, CCL2 (lower left side of the orange open circle of the “FRC” in Fig. 1) has been described as a potent FL cell chemoattractant only in combination with CXCL12 (lower center of Fig. 1, below the light blue circles of the “FL”, the small orange closed circle from the orange open circle of the “FRC”)87 and the authors were able to detect high levels of CCL2 mRNA in LN- and BM-derived stromal cells. Similarly, within LNs, production of CXCL13 by FDCs (upper center of Fig. 1, above the light blue circles of “FL”, the small orange closed circle from the orange open circle of the “FDC”) and CXCL12 by FRCs (lower center of Fig. 1, below the light blue circles of “FL”, the small orange closed circle from the orange open circle of the “FRC”) synergistically directs the accumulation of CXCR4+ (bottom of the light blue filled circles of “FL” in Fig. 1) CXCR5+ (top of the light blue filled circles of “FL” in Fig. 1) FL cells.88 Tumor B cells, unlike normal B cells, were able to induce an FRC phenotype in BM-MSCs. Using quantitative reverse transcription-polymerase chain reaction, purified FL B cells, unlike stromal cells, expressed high levels of transcripts for TNF, LTα, and LTβ (bottom of Fig. 1, in the space between the orange open circle of “FRC” and the light blue circles of “FL”). Therefore, these cytokines cooperated in vivo for the induction of FRC differentiation.58 Collectively, these new insights into the cross talk between lymphoma cells and their microenvironment could offer original therapeutic strategies.
IL-12 AND TIM3 MEDIATED EXHAUSTION OF T CELLS IN FL
IL-12 induces IFNγ production by T and NK cells, and contributes to antitumor activity.89 However, a clinical trial of IL-12 in combination with rituximab in FL showed a lower response rate in patients treated with the combination than in patients treated with rituximab alone,90 suggesting that IL-12 actually plays a detrimental role in FL patients. Therefore, a group from the Mayo Clinic investigated the mechanism mediating this observed inferior effect of IL-12 administration in FL patients.71
First, they performed a multiplex enzyme-linked immunosorbent assay (ELISA) to determine which cells were producing IL-12. T cells, B cells, or monocytes were freshly isolated from the peripheral blood of healthy individuals or biopsy specimens of FL patients, and were cultured in the absence or presence of IFNγ followed by treatment with lipopolysaccharide (LPS). The culture supernatants were collected, and IL-12 levels were measured by ELISA. B cells from FL stimulated with IFNγ plus LPS produced higher levels of IL-12 than normal B cells obtained from healthy individuals. In addition, using flow cytometry and intracellular cytokine staining, they found that the cells able to produce IL-12 after stimulation with IFNγ plus LPS were monocytes obtained from both healthy individuals and FL patients, and B cells obtained only from FL patients. These results indicated that, in FL patients, both B cells and monocytes were able to produce IL-12, whereas in healthy individuals, monocytes were the primary source of IL-12. The results of this study implied that lymphoma B cells played an important role in contributing to elevated IL-12 levels.71
It is well known that IL-12 induces IFNγ expression in T cells. Short-term incubation with IL-12 increased IFNγ production in CD4+ T cells compared with IL-12-untreated cells. However, they observed that long-term culture with IL-12 inhibited T-cell secretion of IL-2 and impaired T-cell function, in other words, inducing T-cell exhaustion.71 T-cell exhaustion, a condition in which T cells exhibit reduced differentiation, proliferation, and effector function, has been characterized and validated in chronic viral infections and tumors.91-95
Next, the authors determined the expression and function of TIM3/PD1, expression of which has been shown to be associated with impaired T cell function and T-cell exhaustion.96-98 They showed that TIM3 was more highly expressed in a subset of T cells from peripheral blood mononuclear cells or LNs from FL patients compared with healthy individuals. Additionally, they observed that TIM3+ T cells coexpressed PD1. The authors were able to confirm that TIM3 signaling played a crucial role in T-cell dysfunction mediated by long-term exposure to IL-12, by inhibiting TIM3 with an anti-TIM3 antibody, which then demonstrated recovery of IL-2 or IFNγ production in TIM3+CD4+ T cells.71
Furthermore, the authors demonstrated that elevated numbers of TIM3+ T cells were associated with a higher histological grade, higher serum lactate dehydrogenase levels, and poorer survival in FL patients.71 The IL-12/TIM3 pathway may have therapeutic potential for FL patients in which IL-12-mediated T cell-exhaustion could be targeted.
THE PROGNOSTIC IMPORTANCE OF SERUM CYTOKINES
Serum cytokines play a vital role in physiological and pathological immune pathways, and have been studied as markers of biological activity in FL. Several groups have studied cytokines, such as IFNα,99 IL-2,100 IL-12,90 and IL-21101 as potential interventional targets in FL, with mixed results. However, these studies have typically been reported in a variety of lymphoma subtypes, and have often evaluated small numbers of patients and samples. Therefore, Mir et al.102 conducted a study using 30 cytokines and chemokines in a large cohort of patients to assess which serum cytokines and chemokines had predictive value independent of FLIPI, and thus, highlighted their potential for incorporation into a risk-stratified approach.102
The study populations consisted of the University of Iowa and Mayo Clinic Specialized Programs of Research Excellence Molecular Epidemiology Resource (MER) cohort, the SWOG cohort, and clinic-based controls from an ongoing case-control study of lymphoma patients who visited the Mayo Clinic Department of Medicine for a prescheduled general medical examination.102 The MER cohort included newly diagnosed, prospectively enrolled FL patients. The SWOG cohort was assembled from patients with available pretreatment serum from 3 SWOG clinical trials for newly diagnosed FL: S9800 (n = 27, a phase 2 trial of CHOP followed by rituximab),103 S9911 (n = 29, CHOP followed by 131I tositumomab),104 and S0016 (n = 127, CHOP + rituximab vs. CHOP + 131I tositumomab).105 This chemotherapy-treated SWOG cohort was then combined with the chemotherapy-treated MER cohort in a meta-analysis.
Because serum cytokines may differ in patients with lower or higher disease burdens, and in asymptomatic vs. symptomatic patients, the authors subsequently classified patients in the MER cohort into two groups: 1) patients initially followed up by watchful waiting policies and those initially treated with rituximab and 2) chemotherapy-treated patients. In total, 6 cytokines were elevated in 10% or more of the FL patients: hepatocyte growth factor, IL-8, IL-1 receptor antagonist (IL-1RA), CXCL9, IL-12, and IL-2 receptor (R). Furthermore, elevated serum levels of IL-1RA, CXCL9, and IL-2R were all associated with an inferior EFS (P = 0.042, 0.0012, and 0.013, respectively).102
Moreover, the authors examined these 6 cytokines in the subset of 103 MER patients with less aggressive disease, and therefore, who were initially followed up with watchful waiting policies or treated with rituximab alone. They found that increased IL-12 (HR = 1.96, 95% CI, 1.23-3.15; P = 0.005) and IL-1RA (HR = 1.97, 95% CI, 1.11-3.48; P = 0.021) were significantly associated with a shorter EFS.102 They further examined these 6 cytokines in the subset of 81 MER patients with aggressive disease, and who therefore were initially treated with alkylator- or anthracycline-containing chemotherapy, such as R-CVP or R-CHOP. After adjusting for FLIPI and treatment (anthracycline, yes vs. no), CXCL9 (HR = 3.96, 95% CI, 1.82-8.59; P = 0.0005), IL-1RA (HR = 2.96, 95% CI, 1.40-6.26; P = 0.0045), hepatocyte growth factor (HR = 3.03, 95% CI, 1.24-7.41; P = 0.015), and IL-8 (HR = 3.19, 95% CI, 1.01-10.05; P = 0.048) were significantly associated with shorter survival. Finally, in the meta-analysis combining the MER chemotherapy cohort (n = 81) and the SWOG cohort (n = 183), elevated CXCL9 (HR = 1.96, 95% CI, 1.30-2.95; P = 0.0012), IL-2R (HR = 2.05, 95% CI, 1.16-3.61; P = 0.013), and IL-1RA (HR = 1.57, 95% CI, 1.02-2.42; P = 0.042) were significantly associated with shorter EFS.102 Thus, the authors suggested that incorporation of these cytokines into future prognostic models should be considered.
Histologic transformation and the tumor microenvironment
Transformation has also been attributed to changes in the tumor microenvironment, which include the disruption of the FDC meshwork,60 increased intrafollicular CD4+ T cells,60 a follicular, not diffuse, localization of FOXP3+ Tregs,44 and increased microvessel density.77
A Mayo Clinic group68 recently identified the presence of PD1+ T cells and CD14+ FDC as independent predictors of transformation in FL. In their study, serial biopsy specimens of 58 FL patients were analyzed by pattern of localization and cell content. No associations between TTT or OS were found between the number or distribution of cells expressing FOXP3, CXCL3, CD21, CD68, or CD11c. The quantity of CD14+ cells was also not associated with TTT or OS; however, the localization of CD14+ cells was predictive of TTT. CD14+ cells were categorized based on pattern of localization, either follicular (n = 13) or non-follicular (n = 41). Patients with follicular localization of CD14+ cells had a median TTT of 3.8 years, compared with 5.9 years in those with a non-follicular staining pattern (P = 0.027). The localization of CD14+ cells was not associated with a significant difference in OS (P = 0.66).
Forty-five patients, with median TTT of 4.7 years, were analyzed and demonstrated that the pattern of PD1+ and CD14+ cells, rather than the quantity of cells, was predictive of clinical outcomes. On multivariate analysis including the FLIPI score, CD14+ cells localized in the follicle were associated with a shorter TTT (HR = 1.9, P = 0.045) and inferior OS (HR = 2.5, P = 0.012). Multicolor IHC and flow cytometry identified the CD14+ cells as FDCs, whereas the PD1+ cells represented two separate populations, TFH and exhausted T-cells.68 Similar to CD14+ cells, the relative quantity of PD1+ cells was not associated with either OS or TTT; however, the localization of the PD1+ cells was predictive of clinical outcomes. Thirty-eight patients had a follicular pattern of PD1+ cells, and 19 had a diffuse pattern. The patients with PD1+ cells localized to the follicle had a median TTT of 6.1 years, compared with 3.6 years in those with a diffuse pattern (P = 0.033). The median OS for the patients with PD1+ cells localized to the follicle was 9.7 years vs. 4.6 years in those with a diffuse distribution of PD1+ cells (P = 0.009). Categorizing the samples by early (< 1 year) vs. late (> 5 years) transformation confirmed that early transformation exclusively involved the patients with a diffuse pattern of PD1+ cell expression, whereas late transformation predominantly involved the patients with a follicular pattern. Notably, the pattern of PD1+ and CD14+ cells showed no correlation with each other (P = 0.36). Instead, the localization of PD1+ and CD14+ cells appeared to have an inverse relationship, because the patients with PD1+ cells localized to the follicle had superior outcomes, whereas those with CD14+ cells localized to the follicle had inferior outcomes. The patients with both follicular PD1+ and diffuse CD14+ cells had a delay in TTT (6.4 vs. 3.2 years) compared with the patients with follicular CD14+ cells and diffuse PD1+ cells (P = 0.01). The FLIPI score was included in their multivariate analysis, and the pattern of PD1+ (HR = 1.9, 95% CI 1.0-3.5; P = 0.045) and CD14+ (HR = 3.0, 95% CI 1.5-6.1; P = 0.004) cells remained significantly associated with inferior TTT. After taking the FLIPI score into consideration, the diffuse pattern of PD1+ cells remained significantly associated with an inferior OS (HR = 2.5, 95% CI 1.2-4.8; P = 0.012), whereas the pattern of CD14+ cells was not (HR = 1.4, 95% CI 0.6-2.9; P = 0.37).68
On the other hand, the same UK group mentioned in the “CD8+ T cells” section examined the relationship between TTT and altered expression levels of PMCH, ETV1, and NAMPT in FL CD4 and CD8 TILs.37 A high number of PMCH-expressing TILs in the intrafollicular area (P = 0.029) and a low number in the interfollicular area (P = 0.033) was associated with shorter TTT. High mean intensity (MI) level of NAMPT expression in the intrafollicular area was associated with longer TTT (P = 0.0034). A higher number of ETV1-expressing cells in the intrafollicular area (P = 0.02) and higher MI level in the interfollicular area (P = 0.0005) were associated with shorter TTT. In multivariate analysis, the number of PMCH-expressing cells in the interfollicular area (95% CI = 0.1-0.71; P = 0.008) and MI of NAMPT in the intrafollicular area (95% CI = 0.15-0.86; P = 0.021) were independently associated with the predicted prognosis of TTT. A model combining the ratio of PMCH-expressing cells in the interfollicular/intrafollicular area plus a high level of expression of NAMPT and a low level of ETV1 expression in the intrafollicular area best identified patients with longer TTT (HR, 0.19, 95% CI = 0.09-0.47; P = 0.003).37
THERAPEUTIC AVENUES THROUGH WHICH THE INTERACTION BETWEEN LYMPHOMA CELLS AND THE MICROENVIRONMENT MAY BE TARGETED
Knowledge of the supportive interactions between lymphoma cells and various cell components of the microenvironment has revealed multiple avenues through which these interactions may be targeted to shift the balance towards lymphoma eradication. Considerable successes have been observed using passive and active immunotherapies to produce antitumor immune responses. Other approaches that aim to reduce the microenvironmental support of tumor cells include inhibition of cell surface molecules and their downstream intracellular pathways, improvement of the function of immune effectors, and disruption of angiogenesis.
A major focus on approaches that target the microenvironment is to improve the function of immune effector cells, thereby increasing the efficacy of immunotherapies and/or unleashing existing endogenous antitumor immunity. Approaches that aim to improve T-cell function include immune checkpoint inhibition with antibodies against CTLA4 and PD1 or CD274, and amelioration of T-cell synapse defect (left upper surface of the light blue filled circle of “NHL” in the left upper field of Fig. 2) using the immune modulatory drug lenalidomide.
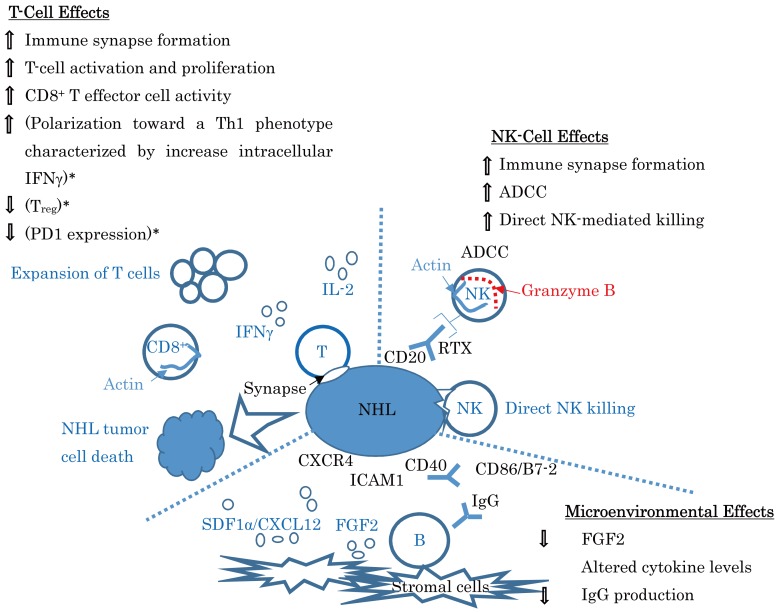
Fig. 2.
Lenalidomide: mechanism of action in lymphoma. (A modification of a figure from ASH 2012 Annual Meeting Abstract #901, Fowler N, et al.106) * are derived from the results of the study of multiple myeloma patients (Ref. 107).
RTX, rituximab; ADCC, antibody-dependent cellular cytotoxicity; B, B cells; FGF2, fibroblast growth factor 2; ICAM1, intracellular adhesion molecule 1; IFN, interferon; IL, interleukin; NHL, non-Hodgkin lymphoma; NK, natural killer cells; RTX, rituximab; SDF1, stromal cell-derived factor 1; T, T cells; Treg, Regulatory T cells; PD1, programed death 1
Immunomodulatory drug: lenalidomide
In a phase II multicenter trial of patients with relapsed or refractory indolent NHL, lenalidomide monotherapy produced a modest overall response rate (ORR) of 27% in FL patients.22 Although the median PFS for the entire cohort was 4.4 months, the median duration of response was longer than 16.5 months, with 7 of the 10 responses maintained, at 15 to 28 months, at the time of analysis. These patients received a median of three prior systemic therapies, 91% of the patients had received prior rituximab, and 67% were rituximab refractory. In addition, 59% of the patients were refractory to their last chemotherapy. Of note, none of these factors appeared to affect the ability of patients to respond to lenalidomide. In contrast, age ≤ 65 years, low/intermediate FLIPI score, and an Eastern Cooperative Oncology Group performance status score of 0 were significantly associated with an improved likelihood of response.22 Lenalidomide was shown to have the potential to enhance the rituximab-induced killing of NHL cell lines via a natural killer cell-mediated and monocyte-mediated ADCC mechanism in vitro (right upper field of Fig. 2).23 Using human B-lymphoblastic lymphoma-bearing SCID mice, it was demonstrated that the natural killer cell expansion by lenalidomide was mediated by stimulation of dendritic cells and alteration of the cytokine microenvironment, associated with an increase in monocyte chemotactic protein-1, TNFα, and IFNγ (left upper field of Fig. 2), contributing to the augmentation of rituximab-associated ADCC. In addition, lenalidomide had significant anti-angiogenic effects.24 These results provided a strong rationale for the combination of lenalidomide with IgG1 antibodies to target tumor-specific antigens in FL patients. In a phase II MD Anderson Cancer Center study of lenalidomide plus rituximab (R2),25 in previously untreated, advanced-stage indolent NHL, the ORR in FL patients was 98% (45/46 evaluable patients), with a complete (CR)/CR unconfirmed (CRu) rate of 87% (40/46 patients). Responses were independent of FLIPI score, tumor bulk, and GELF (Groupe d’Etude des Lymphomes Folliculaires) criteria in the FL subset. Estimated 3-year PFS was 79% in the FL patients. In a single-center phase II study of relapsed/refractory indolent NHL,26 27 evaluable patients (22 patients with FL) with a median of three prior therapies responded to R2. Twenty patients with FL showed a 77% ORR and a 41% CR/CRu. At a median follow-up of 43 months, median duration of response and PFS were 15.4 months and 12.4 months, respectively. To understand the mechanisms of FL-induced T-cell dysfunction, a UK group27 investigated immunologic synapse function in TILs (left upper field of Fig. 2) and identified a major tumor-induced defect, reversible with the immunomodulatory drug lenalidomide. They found a significant reduction in the formation of the F-actin (inside the light blue open circle of “CD8+” in the left upper field of Fig. 2) immune synapse in TILs (P < 0.01) from lymphoma patients compared with age-matched healthy donor cells. After 24-hour co-culture with FL cells, previously healthy T cells showed suppressed recruitment of critical signaling proteins to the synapse. They further demonstrated repair of this defect after treatment of both FL cells and T cells with lenalidomide. TMA analysis identified reduced expression of T-cell synapse signature proteins (left upper surface of the light blue filled circle of “NHL” in the left upper field of Fig. 2), including the cytolytic effector molecule Rab27A, which were associated with poor prognosis, reduced T-cell numbers and activity, and disease transformation.
Immune checkpoint blockade
Eighteen patients with relapsed or refractory B-cell NHL were enrolled in a phase 1 study of ipilimumab, an antibody against CTLA4 that is a negative regulator of T-cell activation, and serves to diminish antitumor immune responses (upper right side of the dark blue open circle “TFR”, between the light blue filled circles of “FL” in Fig. 1). Of the 14 patients with FL, one had a partial response lasting 19 months. In 5 of the 16 patients tested (31%), T-cell proliferation to recall antigens was significantly increased (> 2-fold) after ipilimumab therapy.29
A phase 2 trial at the MD Anderson Cancer Center was performed to investigate the activity of pidilizumab, a humanized anti-PD1 monoclonal antibody, with rituximab in patients with relapsed FL. Thirty-two patients were enrolled, and the combination was well tolerated. Of the 29 patients evaluable for efficacy, 19 patients (66%) achieved an objective response with CRs in 15 patients (52%).30
Cyclophosphamide sensitizing protective tumor microenvironments (macrophages) to anti-CD20 antibody-mediated therapy
Improving macrophage function is also an attractive approach, with these cells having a central role in the action of antibody therapies via ADCC. Using a humanized mouse model of B-cell lymphoma/leukemia, it has recently been shown that treatment with cyclophosphamide induces the release of stress-related cytokines from the malignant cells, thereby attracting macrophages and potentiating antibody-specific killing.
Infiltration of leukemia cells into the BM rewires the tumor microenvironment to inhibit engulfment of antibody-targeted tumor cells. Resistance to macrophage-mediated killing can be overcome by combination regimens involving therapeutic antibodies and chemotherapy. Specifically, the nitrogen mustard cyclophosphamide was shown to induce an acute secretory activating phenotype (ASAP) in treated tumor cells; that is, they released CCL4, IL-8, vascular endothelial growth factor, and TNFα, which induced macrophage infiltration and phagocytic activity in the BM.86 A German group86 demonstrated that the combination of alemtuzumab and cyclophosphamide (300 mg/kg) yielded a strikingly synergistic therapeutic effect, which led to near-complete elimination of disease in the BM, using human disseminated and aggressive lymphoma/leukemia cells caused by transduction with the lentiviral CD19-promoter/Eμ-enhancer GFP-MYC-BCL2 in sublethally irradiated NOD-SCID Il2rg-/- mice with reconstitution of human hematopoietic stem cells. Furthermore, they were able to reduce the cyclophosphamide dose to a minimal dose of 100 mg/kg and still maintain comparable drug synergism, which suggested that the levels of DNA damage may not account for the antitumor activity. Of note, most mice treated with a combination of cyclophosphamide and alemtuzumab showed CR and remained alive > 6 months, whereas lymphoma/leukemia-bearing mice treated with either alemtuzumab or cyclophosphamide alone survived on average 10 days longer than untreated mice. The synergism between alemtuzumab and cyclophosphamide was very specific to this drug combination. On the contrary, coadministration of alemtuzumab with Ara-C, chlorambucil, or bendamustine failed to produce a synergistic effect. Moreover, they showed that cyclophosphamide promoted a progressive increase in the concentration of CD11b+/Gr-1low/CD11c-/F4/80+ BM macrophages as early as 24 hours after treatment. Examination of the BM and spleen by multiphoton confocal microscopy for Texas-Red-positive macrophages (indicating particle phagocytosis) showed dramatically increased numbers of Texas-Red-positive cells at day 5 after cyclophosphamide treatment, although numerous blastoid cells were still present due to partial response to cyclophosphamide monotherapy. Leukemic infiltration significantly induced the expression of CD86, CXCL9, CXCR4, Dectin-1, and IL-12, whereas it decreased levels of TNFα, CD68, and IL-4. These changes reflected a loss of macrophage effector function during leukemia progression, because they revealed a differentiation state distinct from both the naïve macrophage and the classic M1-M2 polarized states. Conversely, cyclophosphamide treatment of leukemic mice significantly induced expression of toll-like receptor 2, arginase-1, and FCGR1α, whereas it decreased IL-10 levels; in other words, these changes were consistent with macrophage activation. Notably, the cyclophosphamide-induced secretory crosstalk between lymphoma/leukemia cells and their microenvironment is the major contributor to antibody efficacy. The ASAP response showed a maximum peak of cytokine release within 24 hours of treatment. This short window of cytokine release was consistent with the transient susceptibility of tumor cells to antibody-dependent killing. These data provided important practical implications for current chemoimmunotherapy regimens, such as R-CHOP, although antibody administration prior to the application of cyclophosphamide along with additional genotoxic drugs has been adopted in most chemoimmunotherapy regimens. This is also true for the reverse order, because their data suggested that pretreatment of lymphoma/leukemic cells with cyclophosphamide shortly before antibody administration was necessary to yield optimal effects. Therefore, they proposed that the DNA-damage induced ASAP of effector cells was the major synergistic mechanism of chemoimmunotherapy, and new optimized clinical protocols based on the temporal kinetics of this secretory response are warranted.86
CONCLUSION
The role of the microenvironment in FL can be said to act in two directions; one supporting tumor growth and survival and the other suppressing the antitumoral immune response. Understanding the relationship between the neoplastic cells and the various cellular components of the microenvironment will be crucial for developing therapies aimed at the microenvironment to battle against FL.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Izumo T, Maseki N, Mori S, Tsuchiya E: Practical utility of the revised European-American classification of lymphoid neoplasms for Japanese non-Hodgkin’s lymphomas. Jpn J Cancer Res 91: 351-360, 2000. 10.1111/j.1349-7006.2000.tb00952.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roulland S, Faroudi M, Mamessier E, Sungalee S, Salles G, et al. : Early steps of follicular lymphoma pathogenesis. Adv Immunol 111: 1-46, 2011. 10.1016/B978-0-12-385991-4.00001-5 [DOI] [PubMed] [Google Scholar]
- 3.Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, et al. : Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476: 298-303, 2011. 10.1038/nature10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oricchio E, Nanjangud G, Wolfe AL, Schatz JH, Mavrakis KJ, et al. : The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell 147: 554-564, 2011. 10.1016/j.cell.2011.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung KJ, Johnson NA, Affleck JG, Severson T, Steidl C, et al. : Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res 70: 9166-9174, 2010. 10.1158/0008-5472.CAN-10-2460 [DOI] [PubMed] [Google Scholar]
- 6.Launay E, Pangault C, Bertrand P, Jardin F, Lamy T, et al. : High rate of TNFRSF14 gene alterations related to 1p36 region in de novo follicular lymphoma and impact on prognosis. Leukemia 26: 559-562, 2012. 10.1038/leu.2011.266 [DOI] [PubMed] [Google Scholar]
- 7.Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, et al. : Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42: 181-185, 2010. 10.1038/ng.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson PW, Rohatiner AZ, Whelan JS, Price CG, Love S, et al. : Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol 13: 140-147, 1995. 10.1200/JCO.1995.13.1.140 [DOI] [PubMed] [Google Scholar]
- 9.Link BK, Maurer MJ, Nowakowski GS, Ansell SM, Macon WR, et al. : Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/Mayo Clinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol 31: 3272-3278, 2013. 10.1200/JCO.2012.48.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conconi A, Ponzio C, Lobetti-Bodoni C, Motta M, Rancoita PM, et al. : Incidence, risk factors and outcome of histologic transformation in follicular lymphoma. Br J Haematol 157: 188-196, 2012. 10.1111/j.1365-2141.2012.09054.x [DOI] [PubMed] [Google Scholar]
- 11.Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, et al. : Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol 17: 2426-2433, 2007. 10.1200/JCO.2006.09.3260 [DOI] [PubMed] [Google Scholar]
- 12.Abramson JS, Zelenetz AD: Recent advances in the treatment of non-Hodgkin’s lymphomas. J Natl Compr Canc Netw 11(suppl): 671-675, 2013. 10.6004/jnccn.2013.0198 [DOI] [PubMed] [Google Scholar]
- 13.Bastion Y, Berger F, Bryon PA, Felman P, Ffrench M, et al. : Follicular lymphomas: assessment of prognostic factors in 127 patients followed for 10 years. Ann Oncol 2(suppl 2): 123-129, 1991. 10.1093/annonc/2.suppl_2.123 [DOI] [PubMed] [Google Scholar]
- 14.Leonard RC, Hayward RL, Prescott RJ, Wang JX: The identification of discrete prognostic groups in low grade non-Hodgkin’s lymphoma: The Scotland and Newcastle Lymphoma Group Therapy Working Party. Ann Oncol 2: 655-662, 1991. 10.1093/oxfordjournals.annonc.a058044 [DOI] [PubMed] [Google Scholar]
- 15.Decaudin D, Lepage E, Brousse N, Brice P, Harousseau JL, et al. : Low-grade stage III-IV follicular lymphoma: multivariate analysis of prognostic factors in 484 patients – a study of the groupe d’Etude des lymphomes de l’Adulte. J Clin Oncol 17: 2499-2505, 1999. 10.1200/JCO.1999.17.8.2499 [DOI] [PubMed] [Google Scholar]
- 16.Federico M, Vitolo U, Zinzani PL, Chisesi T, Clò V, et al. : Prognosis of follicular lymphoma: A predictive model based on a retrospective analysis of 987 cases. Intergruppo Italiano Linfomi. Blood 95: 783-789, 2000 [PubMed] [Google Scholar]
- 17.Federico M, Guglielmi C, Luminari S, Mammi C, Marcheselli L, et al. : Prognostic relevance of serum β2 microglobulin in patients with follicular lymphoma treated with anthracycline-containing regimens: A GISL study. Haematologica 92: 1482-1488, 2007. 10.3324/haematol.11502 [DOI] [PubMed] [Google Scholar]
- 18.Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, et al. : Follicular lymphoma international prognostic index. Blood 104: 1258-1265, 2004. 10.1182/blood-2003-12-4434 [DOI] [PubMed] [Google Scholar]
- 19.Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, et al. : Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 27: 4555-4562, 2009. 10.1200/JCO.2008.21.3991 [DOI] [PubMed] [Google Scholar]
- 20.Sander B, de Jong D, Rosenwald A, Xie W, Balagué O, et al. : The reliability of immunohistochemical analysis of the tumor microenvironment in follicular lymphoma: a validation study from the Lunenburg Lymphoma Biomarker Consortium. Haematologica 99: 715-725, 2014. 10.3324/haematol.2013.095257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kridel R, Sehn LH, Gascoyne RD: Pathogenesis of follicular lymphoma. J Clin Invest 122: 3424-3431, 2012. 10.1172/JCI63186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, et al. : Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s Lymphoma. J Clin Oncol 27: 5404-5409, 2009. 10.1200/JCO.2008.21.1169 [DOI] [PubMed] [Google Scholar]
- 23.Wu L, Adams M, Carter T, Chen R, Muller G, et al. : Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 14: 4650-4657, 2008. 10.1158/1078-0432.CCR-07-4405 [DOI] [PubMed] [Google Scholar]
- 24.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, et al. : Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol 140: 36-45, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Fowler NH, Davis RE, Rawal S, Nastoupil L, Hagemeister FB, et al. : Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol 15: 1311-1318, 2014. 10.1016/S1470-2045(14)70455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuscano JM, Dutia M, Chee K, Brunson A, Reed-Pease C, et al. : Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol 165: 375-381, 2014. 10.1111/bjh.12755 [DOI] [PubMed] [Google Scholar]
- 27.Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, et al. : Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood 114: 4713-4720, 2009. 10.1182/blood-2009-04-217687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilly H, Morschhauser F, Salles G, Casasnovas RO, Feugier P, et al. : Phase 1b study of lenalidomide in combination with rituximab-CHOP (R2-CHOP) in patients with B-cell lymphoma. Leukemia 27: 252-255, 2013. 10.1038/leu.2012.172 [DOI] [PubMed] [Google Scholar]
- 29.Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, et al. : Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 15: 6446-6453, 2009. 10.1158/1078-0432.CCR-09-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, et al. : Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 15: 69-77, 2014. 10.1016/S1470-2045(13)70551-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, et al. : Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 351: 2159-2169, 2004. 10.1056/NEJMoa041869 [DOI] [PubMed] [Google Scholar]
- 32.Álvaro T, Lejeune M, Salvadó MT, Lopez C, Jaén J, et al. : Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol 24: 5350-5357, 2006. 10.1200/JCO.2006.06.4766 [DOI] [PubMed] [Google Scholar]
- 33.Wahlin BE, Sander B, Christensson B, Kimby E: CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res 13: 388-397, 2007. 10.1158/1078-0432.CCR-06-1734 [DOI] [PubMed] [Google Scholar]
- 34.Laurent C, Müller S, Do C, Al-Saati T, Allart S, et al. : Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood 118: 5371-5379, 2011. 10.1182/blood-2011-04-345777 [DOI] [PubMed] [Google Scholar]
- 35.Lee AM, Clear AJ, Calaminici M, Davies AJ, Jordan S, et al. : Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol 24: 5052-5059, 2006. 10.1200/JCO.2006.06.4642 [DOI] [PubMed] [Google Scholar]
- 36.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, et al. : Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 106: 2169-2174, 2005. 10.1182/blood-2005-04-1565 [DOI] [PubMed] [Google Scholar]
- 37.Kiaii S, Clear AJ, Ramsay AG, Davies D, Sangaralingam A, et al. : Follicular lymphoma cells induce changes in T-cell gene expression and function: potential impact on survival and risk of transformation. J Clin Oncol 31: 2654-2661, 2013. 10.1200/JCO.2012.44.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z-Z, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM: Attenuation of CD8+ T-cell function by CD4+CD25+ regulatory T cells in B-cell non-Hodgkin’s lymphoma. Cancer Res 66: 10145-10152, 2006. 10.1158/0008-5472.CAN-06-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, et al. : High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108: 2957-2964, 2006. 10.1182/blood-2006-04-018218 [DOI] [PubMed] [Google Scholar]
- 40.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, et al. : Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 93: 193-200, 2008. 10.3324/haematol.11702 [DOI] [PubMed] [Google Scholar]
- 41.Carreras J, Lopez-Guillermo A, Roncador G, Villamor N, Colomo L, et al. : High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 27: 1470-1476, 2009. 10.1200/JCO.2008.18.0513 [DOI] [PubMed] [Google Scholar]
- 42.de Jong D, Koster A, Hagenbeek A, Raemaekers D, Veldhuizen D, et al. : Impact of the tumor microenvironment of prognosis in follicular lymphoma is dependent on specific treatment protocols. Haematologica 94: 70-77, 2009. 10.3324/haematol.13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweetenham JW, Goldman B, LeBlanc ML, Cook JR, Tubbs RR, et al. : Prognostic value of regulatory T cells, lymphoma-associated macrophages, and MUM-1 expression in follicular lymphoma treated before and after the induction of monoclonal antibody therapy: a Southwest Oncology Group Study. Ann Oncol 21: 1196-1202, 2010. 10.1093/annonc/mdp460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, et al. : The architectural pattern of FOXP3-possitive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood 115: 289-295, 2010. 10.1182/blood-2009-07-235598 [DOI] [PubMed] [Google Scholar]
- 45.Chen W, Jin W, Hardegen N, Lei KJ, Li L, et al. : Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198: 1875-1886, 2003. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA: Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J Immunol 172: 5213-5221, 2004. 10.4049/jimmunol.172.9.5213 [DOI] [PubMed] [Google Scholar]
- 47.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, et al. : Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest 112: 1437-1443, 2003. 10.1172/JCI19441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S: CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol 176: 6586-6593, 2006. 10.4049/jimmunol.176.11.6586 [DOI] [PubMed] [Google Scholar]
- 49.Yang Z-Z, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM: CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25- T cells. Blood 110: 2537-2544, 2007. 10.1182/blood-2007-03-082578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, et al. : Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 17: 975-982, 2011. 10.1038/nm.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, et al. : Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 17: 983-988, 2011. 10.1038/nm.2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taskinen M, Karjalainen-Lindsberg M-L, Nyman H, Eerola L-M, Leppä S: A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res 13: 5784-5789, 2007. 10.1158/1078-0432.CCR-07-0778 [DOI] [PubMed] [Google Scholar]
- 53.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, et al. : High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol 26: 440-446, 2008. 10.1200/JCO.2007.12.8298 [DOI] [PubMed] [Google Scholar]
- 54.Álvaro T, Lejeune M, Camacho FI, Salvadó MT, Sánchez L, et al. : The presence of STAT1-positive tumor-associated macrophages and their relation to outcome in patients with follicular lymphoma. Haematologica 91: 1605-1612, 2006 [PubMed] [Google Scholar]
- 55.Taskinen M, Karjalaninen-Lindsberg M-L, Leppä S: Prognostic influence of tumor-infiltrating mast cells in patients with follicular lymphoma treated with rituximab and CHOP. Blood 111: 4664-4667, 2008. 10.1182/blood-2007-11-125823 [DOI] [PubMed] [Google Scholar]
- 56.Crotty S: Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29: 621-663, 2011. 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 57.Pangault C, Amé-Thomas P, Ruminy P, Rossille D, Caron G, et al. : Follicular lymphoma cell niche: identification of a preeminent IL-4-dependent TFH-B cell axis. Leukemia 24: 2080-2089, 2010. 10.1038/leu.2010.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amé-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, et al. : Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells on follicular lymphoma pathogenesis. Blood 109: 693-702, 2007. 10.1182/blood-2006-05-020800 [DOI] [PubMed] [Google Scholar]
- 59.Guilloton F1 , Caron G, Ménard C, Pangault C, Amé-Thomas P, et al.: Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood 119: 2556-2567, 2012. 10.1182/blood-2011-08-370908 [DOI] [PubMed] [Google Scholar]
- 60.Glas AM, Knoops L, Delahaye L, Kersten MJ, Kibbelaar RE, et al. : Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol 25: 390-398, 2007. 10.1200/JCO.2006.06.1648 [DOI] [PubMed] [Google Scholar]
- 61.Richendollar BG, Pohlman B, Elson P, Hsi ED: Follicular programmed death 1-positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Hum Pathol 42: 552-557, 2011. 10.1016/j.humpath.2010.08.015 [DOI] [PubMed] [Google Scholar]
- 62.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, et al. : Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325: 1006-1010, 2009. 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, et al. : Early commitment of naïve human CD4+ T cells to the T follicular helper (TFH) cell lineage is induced by IL-12. Immunol Cell Biol 87: 590-600, 2009. 10.1038/icb.2009.64 [DOI] [PubMed] [Google Scholar]
- 64.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, et al. : The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol 10: 167-175, 2009. 10.1038/ni.1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amé-Thomas P, Le Priol J, Yssel H, Caron G, Pangault C, et al. : Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia 26: 1053-1063, 2012. 10.1038/leu.2011.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Z-Z, Grote DM, Ziesmer SC, Xiu B, Novak AJ, et al. : PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J 5: e281, 2015. 10.1038/bcj.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xerri L, Bachy E, Fabiani B, Canioni D, Chassagne-Clément C, et al. : Rituximab treatment in follicular lymphoma circumvents the prognostic value of intra-tumoural T-cells: automated image analysis of the LYSA PRIMA trial. Hematol Oncol 33(suppl S1): 112, 2015. [Abstract 024] [Google Scholar]
- 68.Smeltzer JP, Jones JM, Ziesmer SC, Grote DM, Xiu B, et al. : Pattern of CD14+ follicular dendritic cells and PD1+ T cells independently predicts time to transformation in follicular lymphoma. Clin Cancer Res 20: 2862-2872, 2014. 10.1158/1078-0432.CCR-13-2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrasch S, Perez-Alvarez C, Schmitz J, Kosco M, Brittinger G: Antigenic phenotyping of human follicular dendritic cells isolated from nonmalignant and malignant lymphatic tissue. Eur J Immunol 20: 1013-1018, 1990. 10.1002/eji.1830200510 [DOI] [PubMed] [Google Scholar]
- 70.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, et al. : Immunosuppressive CD14+HLA-DRlow/- monocytes in B-cell non-Hodgkin lymphoma. Blood 117: 872-881, 2011. 10.1182/blood-2010-05-283820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Z-Z, Grote DM, Ziesmer SC, Niki T, Hirashima M, et al. : IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest 122: 1271-1282, 2012. 10.1172/JCI59806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wahlin BE, Aggarwal M, Montes-Moreno S, Gonzalez LF, Roncador G, et al. : A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1-positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin Cancer Res 16: 637-650, 2010. 10.1158/1078-0432.CCR-09-2487 [DOI] [PubMed] [Google Scholar]
- 73.Farinha P, Roncador G, Al-Tourah A, Connors JM, Gascoyne RD: Combined FOXP3+ and PD1+ T cell density and architectural patterns predict overall survival and risk of transformation in uniformly treated patients with follicular lymphoma. Blood 112: 2815, 2008. [ASH Annual Meeting Abstracts] [Google Scholar]
- 74.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A: Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549-555, 2002. 10.1016/S1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 75.Sica A, Mantovani A: Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787-795, 2012. 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clear AJ, Lee AM, Calaminici M, Ramsay AG, Morris KJ, et al. : Increased angiogenic sprouting in poor prognosis FL is associated with elevated numbers of CD163+ macrophages within the immediate sprouting microenvironment. Blood 115: 5053-5056, 2010. 10.1182/blood-2009-11-253260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farinha P, Kyle AH, Minchinton AI, Connors JM, Karsan A, et al. : Vascularization predicts overall survival and risk of transformation in follicular lymphoma. Haematologica 95: 2157-2160, 2010. 10.3324/haematol.2009.021766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Z-Z, Novak AJ, Stenson MJ, Witzig TE, Ansell SM: Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood 107: 3639-3646, 2006. 10.1182/blood-2005-08-3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ai WZ, Hou J-Z, Zeiser R, Czerwinski D, Negrin RS, et al. : Follicular lymphoma B cells induce the conversion of conventional CD4+ T cells to T-regulatory cells. Int J Cancer 124: 239-244, 2009. 10.1002/ijc.23881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, et al. : Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol 67: 97-103, 2000 [PubMed] [Google Scholar]
- 81.Kridel R, Xerri L, Gelas-Dore B, Tan K, Feugier P, et al. : The prognostic impact of CD163-positive macrophages in follicular lymphoma: A study from the BC Cancer Agency and the Lymphoma Association. Clin Cancer Res 21: 3428-3435, 2015. 10.1158/1078-0432.CCR-14-3253 [DOI] [PubMed] [Google Scholar]
- 82.De Palma M, Lewis CE: Macrophage regulation of tumor response to anticancer therapies. Cancer Cell 23: 277-286, 2013. 10.1016/j.ccr.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 83.Mantovani A, Polentarutti N, Luini W, Peri G, Spreafico F: Role of host defense mechanisms in the antitumor activity of adriamycin and daunomycin in mice. J Natl Cancer Inst 63: 61-66, 1979 [PubMed] [Google Scholar]
- 84.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, et al. : Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38: 729-741, 2013. 10.1016/j.immuni.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 85.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, et al. : M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M1 cells in vitro. J Immunol 182: 4415-4422, 2009. 10.4049/jimmunol.0713732 [DOI] [PubMed] [Google Scholar]
- 86.Pallasch CP, Leskov I, Braun CJ, Vorholt D, Drake A, et al. : Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 156: 590-602, 2014. 10.1016/j.cell.2013.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Husson H, Carideo EG, Cardoso AA, Lugli SM, Neuberg D, et al. : MCP-1 modulates chemotaxis by follicular lymphoma cells. Br J Haematol 115: 554-562, 2001. 10.1046/j.1365-2141.2001.03145.x [DOI] [PubMed] [Google Scholar]
- 88.Husson H, Freedman AS, Cardoso AA, Schultze L, Munoz O, et al. : CXCL13 (BCA-1) is produced by follicular lymphoma cells: role on the accumulation of malignant B cells. Br J Haematol 119: 492-495, 2002. 10.1046/j.1365-2141.2002.03832.x [DOI] [PubMed] [Google Scholar]
- 89.Colombo MP, Trinchieri G: Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 13: 155-168, 2002. 10.1016/S1359-6101(01)00032-6 [DOI] [PubMed] [Google Scholar]
- 90.Ansell SM, Geyer SM, Maurer MJ, Kurtin PJ, Micallef IN, et al. : Randomized phase II study of interleukin-12 in combination with rituximab in previously treated non-Hodgkin’s lymphoma patients. Clin Cancer Res 12: 6056-6063, 2006. 10.1158/1078-0432.CCR-06-1245 [DOI] [PubMed] [Google Scholar]
- 91.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, et al. : PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350-354, 2006. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 92.D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, et al. : Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol 179: 1979-1987, 2007. 10.4049/jimmunol.179.3.1979 [DOI] [PubMed] [Google Scholar]
- 93.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, et al. : PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol 80: 11398-11403, 2006. 10.1128/JVI.01177-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mumprecht S1 : Schürch C, Schwaller J, Solenthaler M, Ochsenbein AF: programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 114: 1528-1536, 2009. 10.1182/blood-2008-09-179697 [DOI] [PubMed] [Google Scholar]
- 95.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, et al. : Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114: 1537-1544, 2009. 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, et al. : Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA 107: 14733-14738, 2010. 10.1073/pnas.1009731107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, et al. : Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207: 2175-2186, 2010. 10.1084/jem.20100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, et al. : Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207: 2187-2194, 2010. 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rohatiner AZ, Gregory WM, Peterson B, Borden E, Solal-Celigny P, et al. : Meta-analysis to evaluate the role of interferon in follicular lymphoma. J Clin Oncol 23: 2215-2223, 2005. 10.1200/JCO.2005.06.146 [DOI] [PubMed] [Google Scholar]
- 100.Khan KD, Emmanouilides C, Benson DM, Jr, Hurst D, Garcia P, et al. : A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin’s lymphoma. Clin Cancer Res 12: 7046-7053, 2006. 10.1158/1078-0432.CCR-06-1571 [DOI] [PubMed] [Google Scholar]
- 101.Timmerman JM, Byrd JC, Andorsky DJ, Yamada RE, Kramer J, et al. : A phase I dose-finding trial of recombinant interleukin-21 and rituximab in relapsed and refractory low grade B-cell lymphoproliferative disorders. Clin Cancer Res 18: 5752-5760, 2012. 10.1158/1078-0432.CCR-12-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mir MA, Maurer MJ, Ziesmer SC, Slager SL, Habermann T, et al. : Elevated serum levels of IL-2R, IL-1RA, and CXCL9 are associated with a poor prognosis in follicular lymphoma. Blood 125: 992-998, 2015. 10.1182/blood-2014-06-583369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maloney DG, Press OW, Braziel RM, Rimsza LM, Friedberg JW, et al. : A phase II trial of CHOP followed by rituximab chimeric monoclonal anti-CD20 antibody for treatment of newly diagnosed follicular non-Hodgkin’s lymphoma: SWOG 9800. Blood 98: 843a, 2001. [Abstract 3502] [Google Scholar]
- 104.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, et al. : Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol 24: 4143-4149, 2006. 10.1200/JCO.2006.05.8198 [DOI] [PubMed] [Google Scholar]
- 105.Press OW, Unger JM, Rimsza LM, Friedberg JW, LeBlanc M, et al. : Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus 131iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol 31: 314-320, 2013. 10.1200/JCO.2012.42.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, et al. : Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother 62: 39-49, 2013. 10.1007/s00262-012-1308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fowler NH, Neelapu SS, Hagemeister FB, McLaughlin P, Kwak LW, et al.: Lenalidomide and rituximab for untreated indolent lymphoma: final results of a phase II study. Blood (ASH Annual Meeting Abstracts) 120:901, 2012 [Abstract] [Google Scholar]