Abstract
OBJECTIVE.
The purpose of our study was to determine the efficacy of using the Choyke questionnaire to stratify the potential risk for nephrogenic systemic fibrosis (NSF) before a gadolinium-enhanced MRI examination and to compare the economic impact of such an approach with universal blood sampling before gadolinium-enhanced MRI.
MATERIALS AND METHODS.
We retrospectively reviewed the records of 1,086 consecutive outpatients referred for gadolinium-enhanced MRI. For inclusion, patients were required to have an estimated glomerular filtration rate (eGFR) result within 30 days before gadolinium-enhanced MRI and a completed Choyke questionnaire, first when the order was placed and again at the point of service. Questionnaire results were dichotomized into “No” (all no responses in both questionnaires) or “Yes” (one or more yes responses in either questionnaire) response groups for comparison with the corresponding eGFR. An economic impact assessment for using the questionnaire to identify “at risk” patients was performed using a decision–analytic model.
RESULTS.
Entry criteria were met in 665 of 1,086 (61.2%) patients. Zero patients in the No (n = 287) and seven in the Yes (1.9%) group (n = 378) had an eGFR of less than 30 mL/ min/1.73 m2. Using the 100% sensitivity achieved in identifying higher risk patients (seven patients; 95% CI, 59–100%), the questionnaire could save $4.52 per patient. At the lower boundary of the 95% CI for sensitivity, the savings incurred would come at the expense of administering gadolinium to 0.4% of patients with an eGFR less than 30 mL/min/1.73 m2.
CONCLUSION.
The Choyke questionnaire effectively stratifies patients for NSF risk before gadolinium-enhanced MRI, offering potential cost savings and streamlined care.
Keywords: efficacy, gadolinium chelates, MRI, nephrogenic systemic fibrosis, renal insufficiency, screening
Nephrogenic systemic fibrosis (NSF) is a systemic fibrosing disorder strongly associated with the administration of gadolinium-based contrast agents in patients with substantial renal disease when the estimated glomerular filtration rate (eGFR) is less than 30 mL/min/ 1.73 m2, with a clear majority noted in dialysis patients [1–4].
In May 2007, the United States Food and Drug Administration (FDA) notified healthcare professionals of their request for the addition of a boxed warning to highlight that exposure to gadolinium-based contrast agents increases the risk for NSF in patients with acute or chronic severe renal insufficiency (GFR < 30 mL/min/1.73 m2) or acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period [5].
The FDA Web site recommends an evaluation of patients “….for renal dysfunction by assessing their renal function, either by obtaining a medical history or conducting laboratory tests that measure renal function” [6]. However, no specific recommendations regarding the approach to patients have been provided.
A variety of strategies, including universal blood sampling before MRI for determining renal function, a host of questionnaires (some validated, some empirical), and combined approaches have been used in clinical practice [7, 8]. Most questionnaires are derived from those used before the administration of iodinated contrast media [9–11]. However, to the best of our knowledge, no studies have been conducted assessing the efficacy of screening questionnaires to identify higher-risk patients before the administration of gadolinium-based contrast agents.
Choyke et al. [9] found that when all responses to a six-question survey inquiring about a history of renal disease, renal surgery, proteinuria, diabetes, hypertension, or gout, hereafter referred to as the Choyke questionnaire, were “No,” 94% of patients had a normal serum creatinine (< 114 µmol/L for women, 123 µmol/L for men), and 99% of patients had creatinine levels less than 150 µmol/L. From that original article, those were the six questions derived from 11 questions posed to 673 consecutive patients. Those six questions were shown to have the strongest predictive value for an elevated creatinine level and ultimately form the Choyke questionnaire on the basis of an improved ability to reduce the need for serum creatinine determinations compared with the full 11-question survey [9].
It has subsequently been reported that the GFR, either measured or estimated (eGFR), is a better determinant of kidney function than the serum creatinine level and is preferred as a means to identify individuals at risk for contrast-induced nephropathy [12–14].
In response to the concerns regarding NSF, we have used the evidenced-based, Choyke questionnaire in our clinical MRI practice to screen for renal function before the administration of gadolinium-based contrast agents implemented at two time points: at the time of ordering the examination and at the time of service.
In this study we seek to determine the efficacy of using the Choyke questionnaire to stratify patients for the potential risk of renal insufficiency, and hence NSF, before a gadolinium-enhanced MRI examination and to compare the economic impact of such an approach to one that uses universal blood sampling before gadolinium-enhanced MRI.
Materials and Methods
Patients and Data Collection
This retrospective study was approved by our institutional review board, was compliant with HIPAA, and the requirement for informed consent was waived. A query of the radiology information system database at our institution between May 6, 2008, and June 11, 2008, identified 1,086 outpatients for whom a gadolinium-enhanced MRI was re quested. The electronic medical records of these patients were reviewed to tabulate clinical history, sex, age, creatinine and eGFR results, prior exposure to gadolinium-based contrast agents, and responses to the Choyke questionnaire completed at the time of scheduling [9].
Reflecting our clinical practice, the questionnaire was answered at two different times during the screening process: first at the time of order of the MRI (by the referring physician or a designee) and then at the time of the scan (by the patient). Six questions, as described by Choyke et al. [9], were asked about the patient: Have you ever been told you have renal problems? Have you ever been told you have protein in your urine? Do you have high blood pressure? Do you have diabetes mellitus? Do you have gout? Have you ever had kidney surgery?
Using information obtained from our PACS (Centricity, GE Healthcare) we determined the type of examination completed, the results of outside blood work, and the patient’s answers to the questionnaire completed again at the point of service. Creatinine and eGFR data obtained outside our institution and the questionnaire results obtained on the day of the MRI were electronically scanned into the PACS as is routine clinical practice in our department.
Only those outpatients with creatinine levels determined from blood samples obtained ≤ 30 days before the MRI study were included. On the basis of the patient’s serum creatinine concentration, the eGFR was calculated using the Modification of Diet in Renal Disease equation [15].
Of the 1,086 individuals, 757 patients had a creatinine and eGFR result available within the designated time frame. Of these remaining 757 patients, 665 (87.8%) had completed questionnaires available at both time points, forming the cohort for this study.
These 665 individuals (369 women and 296 men) ranged from 19 to 94 years in age (mean, 54 years; median, 55 years). MRI referrals were for neurologic indications in 261 (39.3%), for gastrointestinal or genitourinary indications in 177 (26.6%), for oncologic indications in 126 (18.9%), for musculoskeletal indications in 64 (9.6%), and for vascular indications in 37 (5.6%).
Data Analysis
The questionnaire results were dichotomized, as either “No” to all or “Yes” to one or more questions. A No to all was considered the test result that would predict patients to have eGFR values ≥ 30 mL/ min/1.73 m2, whereas a Yes to one was considered the test result that could predict patients at risk for eGFR values < 30 mL/min/1.73 m2.
The primary outcome to be assessed was the prevalence of preexisting significant renal insufficiency (identified with a calculated eGFR of < 30 mL/min/1.73 m2 derived from serum creatinine assays), in the dichotomized groups according to Choyke questionnaire results. An analysis that includes sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) was conducted for the data obtained at each time point separately and then in combination along with 95% CI for sensitivity and specificity estimates.
Economic Impact
A preliminary economic analysis estimated the potential savings afforded by implementing a double-questionnaire for triaging patients into high-(eGFR< 30 mL/min/1.73 m2) and low-(eGFR ≥ 30 mL/min/1.73 m2) risk categories. The horizon of this analysis encompassed only the decision-making point (regarding contrast administration) before MRI
We considered two potential strategies in our analysis: all patients undergo serum creatinine assessment (for eGFR estimation) before gadolinium-enhanced MRI to designate patients as high- or low-risk or all patients undergo the doublequestionnaire approach detailed in this study. Patients who test positive—that is, have a Yes result in either questionnaire—are then further triaged to serum creatinine assessment. Patients with a No–No response are considered to test negative and receive no subsequent blood work.
A decision–analytic model was constructed to test these strategies in a hypothetical population of patients for whom gadolinium-enhanced MRI was planned (TreeAge Pro 2009, TreeAge Software). We used the following input data from our study: double-questionnaire sensitivity and specificity and the underlying prevalence of patients with eGFR < 30 mL/min/1.73 m2. The estimated cost of serum eGFR assessment included the costs of serum creatinine assessment and venipuncture ($10.48), which were estimated using associated Current Procedural Terminology (CPT) codes (CPT codes 82565 and 36415, 2009) [16].
We calculated the estimated per-patient cost savings that would occur if the double-questionnaire approach were used in lieu of empirical serum creatinine assessment. We also calculated the projected yearly institutional savings expected, on the basis of information obtained from a computerized database identifying all outpatients who underwent MRI at our institution for a 12-month period between June 2008 and May 2009.
Importantly, this preliminary economic analysis did not incorporate downstream sequelae of missed cases of patients with eGFR < 30 mL/min/1.73 m2 (i.e., false-negative results). Thus, using the lower boundary calculated for the 95% CI of the doublequestionnaire’s sensitivity and the underlying prevalence of patients with eGFR < 30 mL/min/1.73 m2 in our study population, we also estimated the negative consequences of a reduced sensitivity value—specifically, the percentage of patients with an eGFR < 30 mL/min/1.73 m2 who would receive a gadolinium-based contrast agent. This enabled us to assess the potential scope of negative consequences inherent to false-negative results alongside our analysis of potential cost savings.
Results
Figure 1 is a flow chart depicting the results from the questionnaire answers and the subsequently determined eGFR levels. Of the 665 patients, the initial Choyke questionnaire responses indicated a No to all questions in 490 (74%) cases and a Yes to at least one question in 175 (26%) cases. Of those with an initial Yes answer to at least one question obtained at the time of order, 153 (87%) individuals responded Yes to at least one question at the point of service, whereas 22 (13%) answered No to all questions at the point of service.
Fig. 1.
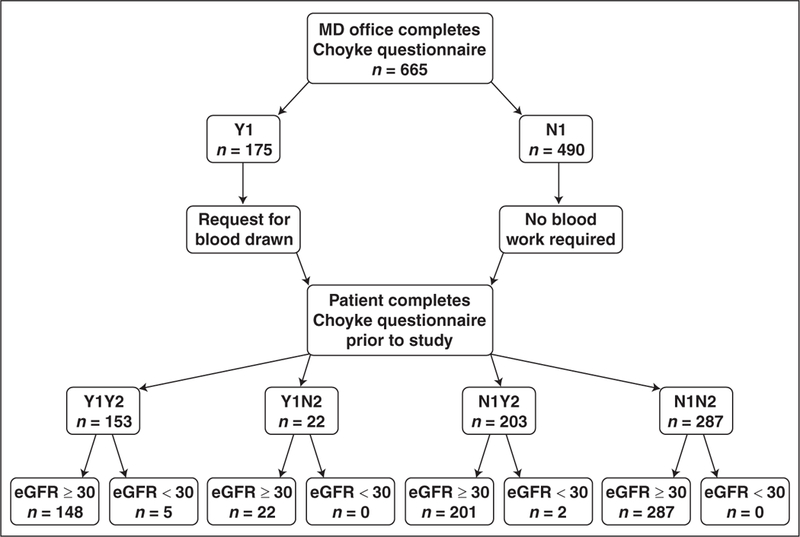
Flowchart of response results and corresponding estimated glomerular filtration rate (eGFR) values shows Yes (Y) and No (N) response results obtained at initial time point (Y1 or N1) and at second time point (Y2 or N2). Each box with response details number of patients in group and lowest level data correlates combined responses at both time points with eGFR results. All eGFR values were derived from serum creatinine measurements obtained within 30 days before MRI and are in units of mL/min/1.73 m2.
Of the 490 individuals with an initial No to all questions, 203 (41%) replied Yes to at least one question at the point of service, whereas 287 (59%) answered No to all questions during subsequent questionnaire screening. For those individuals with a Yes result to both questionnaires (Yes–Yes), 148 exhibited eGFR ≥ 30 mL/min/1.73 m2 and five exhibited eGFR < 30 mL/min/1.73 m2. All of the 22 individuals with a Yes–No response had eGFR ≥ 30 mL/min/1.73 m2. Of those who had No–Yes responses, two of 203 had an eGFR < 30 mL/min/1.73 m2 and 201 had eGFR ≥ 30 mL/min/1.73 m2. Finally, all individuals with a No to all responses to the questionnaire at both time points had an eGFR ≥ 30 mL/min/1.73 m2.
The dichotomized results showed that none of the 287 patients with all No responses at both time points had an eGFR < 30 mL/min/1.73 m2. Furthermore, any Yes answer provided by a patient yielded a seven of 378 (1.9%) prevalence of eGFR < 30 mL/ min/1.73 m2. Discrepant results between the questionnaires offered at the two time points occurred in 225 of 665 (34%) of cases.
Descriptive statistics summating the results at both time points are shown in Table 1. Seven of 665 (1%) were designated as atrisk patients on the basis of an eGFR < 30 mL/min/1.73 m2 and all seven were captured in the “Any Yes” subgroup; no at-risk individuals were found among the No to all subgroup. Consequently, 658 of the total 665 (98.9%) might be considered as not-at-risk (eGFR ≥ 30 mL/min/1.73 m2).
TABLE 1:
Descriptive Statistics
| Questionnaire Result | eGFR < 30 mL/min/1.73 m2 | eGFR ≥ 30 mL/min/1.73 m2 | Total |
|---|---|---|---|
| Yes (any yes) | 7 | 371 | 378 |
| No (no–no) | 0 | 287 | 287 |
| Total | 7 | 658 | 665 |
Note—Responses are all No versus any Yes at both time points. eGFR = estimated glomerular filtration rate.
Sensitivity, specificity, PPV, and NPV values are compared in Tables 2–4. When gadolinium administration is based solely on questionnaire responses during the time of order for the examination (first time point), the questionnaire has sensitivity of detecting patients with an eGFR < 30 mL/min/1.73 m2 of 71.4% (5/7; 95% CI, 29.0–96.3%), specificity of 74.2% (488/658; 95% CI, 70.6–77.5%), PPV of 2.86% (5/175), and NPV of 99.6% (488/490), as shown in Table 2. This method by itself failed to identify two patients at risk for NSF.
TABLE 2:
Questionnaire at Time of Order (First Time Point): Ability to Detect Patients With an Estimated Glomerular Filtration Rate (eGFR) < 30 mL/min/1.73 m2
| Parameter | No./Total | Value | |
|---|---|---|---|
| Percentage | 95% CI (%) | ||
| Sensitivity | 5/7 | 71.4 | 29.0–96.3 |
| Specificity | 488/658 | 74.2 | 70.6–77.5 |
| PPV | 5/175 | 2.9 | |
| NPV | 488/490 | 99.6 | |
Note—All “No” predicts eGFR ≥ 30 mL/min/1.73 m2, and any “Yes” predicts eGFR < 30 mL/min/1.73 m2. PPV = positive predictive value, NPV = negative predictive value.
TABLE 4:
Double-Questionnaire Approach: Ability to Detect Patients With an Estimated Glomerular Filtration Rate (eGFR) < 30 mL/min/1.73 m2
| Parameter | No./Total | Value | |
|---|---|---|---|
| Percentage | 95% CI (%) | ||
| Sensitivity | 7/7 | 100 | 59.0–100 |
| Specificity | 287/658 | 43.6 | 39.8–47.5 |
| PPV | 7/378 | 1.9 | |
| NPV | 287/287 | 100 | |
Note—All “No” predicts eGFR ≥ 30 mL/min/1.73 m2, and any “Yes” predicts eGFR < 30 mL/min/1.73 m2. PPV = positive predictive value, NPV = negative predictive value.
Using solely questionnaire results during the time of the MRI (second time point), the questionnaire has sensitivity of 100% (7/7; 95% CI, 59.0–100%), specificity of 47.0% (309/658; 95% CI, 43.1–50.9%), PPV of 1.97% (7/356), and NPV of 100% (309/309), as shown in Table 3.
TABLE 3:
Questionnaire at Time of Scanning (Second Time Point): Ability to Detect Patients With an Estimated Glomerular Filtration Rate (eGFR) < 30 mL/min/1.73 m2
| Parameter | No./Total | Value | |
|---|---|---|---|
| Percentage | 95% CI (%) | ||
| Sensitivity | 7/7 | 100 | 59.0–100 |
| Specificity | 309/658 | 47.0 | 43.1–50.9 |
| PPV | 7/356 | 2.0 | |
| NPV | 309/309 | 100 | |
Note—All “No” predicts eGFR ≥ 30 mL/min/1.73 m2, and any “Yes” predicts eGFR < 30 mL/min/1.73 m2. PPV = positive predictive value, NPV = negative predictive value.
Using the double-questionnaire approach, sensitivity of 100% (7/7; 95% CI, 59.0– 100%), specificity of 43.6% (287/658; 95% CI, 39.5–47.5%), PPV of 1.85% (7/378), and NPV of 100% (287/287) were found, as shown in Table 4.
Assuming test sensitivity of 100%, specificity of 43.6%, and an underlying prevalence of patients with eGFR < 30 mL/min/1.73 m2 of 1.0% (7/665 patients), we found that the double-questionnaire approach was $4.52 less expensive per patient than a strategy that required empirical serum creatinine assessment in all patients. A search in our computerized database identified a total of 11,401 outpatients who underwent gadolinium-enhanced MRI at our institution in the 12-month period ending June 30, 2009. Therefore, at the institutional level, a yearly savings of $51,533 would be expected.
Given that the 95% CI for sensitivity ranged from 59% to 100%, we considered the trade-off that might result with imperfect sensitivity. In this scenario, any expenses saved would come at the cost of administering gadolinium to at-risk patients with an eGFR < 30 mL/min/1.73 m2. Using the lower limit of sensitivity of 59%, any cost savings incurred would come at an expense of administering gadolinium-based contrast to four at-risk patients per 1,000 patients for whom gadolinium-enhanced MRI had been requested (rate, 0.43%).
Discussion
The discovery of NSF has prompted new concerns for identifying patients at risk for developing this disorder. The strongest risk factor is substantial underlying renal insufficiency [1–4]. Although a variety of screening procedures exist at institutions, to the best of our knowledge, no firm recommendations for a particular procedure have been provided by the FDA, the American College of Radiology, or other organizations, and there are no prior reports addressing the efficacy and cost implications of these strategies.
At some hospitals, in our locale and elsewhere, questionnaires and universal blood sampling have been used [7, 8]. Because most outpatients do not have severe renal insufficiency [15], the time, resources, and costs associated with obtaining a blood sample for each patient before performing a contrastenhanced MRI might be excessive. Such a concern has been previously addressed in patients before the administration of iodinated contrast media for whom questionnaires intended to stratify at-risk patients, emphasizing the capture of patients with underlying renal insufficiency, have served as functional screening tools [9–11].
For our study, the Choyke questionnaire was selected on the basis of its simplicity and its documented ability to stratify patients according to renal function [9]. Of 11 questions posed to patients, the six with the strongest odds ratios for being associated with abnormal serum creatinine levels were selected for use in the final questionnaire. Interestingly Choyke et al. [9] found that some commonly assumed risk factors for renal insufficiency (e.g., age > 60 years) did not achieve sufficient strength to be included in the final questionnaire.
In the original work, the Choyke questionnaire reduced the number of patients in need of a blood sample by 67% before administration of iodinated contrast agents, whereas our use of the questionnaire could reduce the need by 44% before administering gadolinium-based contrast agents. The difference might be at least partially explained by the use of serum creatinine levels in the original study, whereas eGFR levels were used in ours, the latter a more reliable way to assess renal function [12–14].
The discrepancies found between the Choyke questionnaire results obtained at the time of scheduling and at the point of care might be due to an emphasis on expediency by the office site or the patients’ unawareness of their own medical histories. Either could contribute to inaccuracies. Using the questionnaire at two different entry points would be expected to offer perspectives from each and thus serve a complementary role to the other. However, from a sensitivity perspective, the questionnaire posed to the patient only at the time of care was sufficient, whereas two atrisk patients would have been missed by relying exclusively on the initial questionnaire.
Despite those differences, there still may be a role in clinical practice for a questionnaire obtained at the time of ordering. Patients identified as having an initial Yes answer could undergo a serum blood test in advance of the MR examination and thus offer a throughput advantage. In those facilities in which a point-of-care device capable of ascertaining an eGFR rapidly is available, a single questionnaire obtained from the patient might be preferable.
It is recognized that other questions geared toward specific conditions associated with NSF seem appealing and could have been added to a questionnaire strategy. However, without a priori testing of new questions, their potential impact is merely speculative. We elected to use the Choyke questionnaire in its original format on the basis of its previously proven success in a relevant patient population.
The resulting cost savings we have shown go hand-in-hand with the risks of false-negative results. The conservative estimate that, in a referral base such as ours, four of 1,000 patients with an eGFR < 30 mL/min/1.73 m2could potentially receive a gadoliniumbased contrast agent is based on the lower limits of a 95% CI for sensitivity and, importantly, does not predict risk for NSF per se. It is notable that most NSF cases (approximately 90%) have occurred in dialysis patients; that it is difficult to induce NSF, even when high doses are administered to at-risk patients; and that some types of gadolinium-based contrast agents may be safe even in patients with eGFR < 30 mL/min/1.73 m2 [4, 17–21].
Therefore, the actual chance of NSF occurring in an outpatient “erroneously” assigned to a lower-risk group on the basis of Choyke questionnaire results is likely to be far less than the conservative four in 1,000 figure.
Although in our study all cases with an eGFR < 30 mL/min/1.73 m2 were previously known to have renal insufficiency, the incidental discovery of patients with an eGFR< 60 mL/min/1.73 m2 represents an opportunity to impact clinical care. In this regard, a mechanism by which the referring physician is specifically notified of such a finding should be a part of the care provided by radiologists. We have instituted a policy that requires direct communication, separate from the radiologic report. Such rigor may not be essential in all practices.
Our study has some limitations. First, the retrospective nature of the study could have resulted in a selection bias. A substantial number of patients were excluded from our study because creatinine levels or completed questionnaires were not available. The use of strict inclusion criteria, however, provides a framework for future studies and potential evaluation of reproducibility in other institutions. Second, inpatients were excluded from our analysis. The common availability of creatinine and eGFR levels in hospitalized patients mitigates the need for implementation of additional screening methods in this setting.
Third, we simplified our economic analysis by incorporating costs of eGFR assessment on the basis of related CPT codes and by assuming that these costs would be the same for all patients. In reality, the costs of obtaining and analyzing blood samples are likely less uniform across different health care settings. Our analysis nonetheless provides an initial framework for estimating cost savings that could be incurred using a questionnaire approach and provides groundwork for more detailed evaluations of costs relevant to patient triage before gadolinium-enhanced MRI.
Fourth, although our study shows the successful stratification of patients at greater and lesser risk for renal insufficiency by using the Choyke questionnaire, the small number of cases with eGFR < 30 mL/min/1.73 m2 results in a wide CI around the questionnaire’s calculated sensitivity. Further, larger-scale investigations would offer an opportunity to more precisely estimate the trade-offs associated with false-negative results. Finally, further studies that quantify the downstream economic and health consequences of falsenegative results also are needed to improve assessment of such trade-offs.
By using the Choyke questionnaire to identify patients at a low risk for NSF, the need for blood sampling can be reduced, offering a potential savings in health care costs as well as streamlined patient care.
References
- 1.Grobner T Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant, 2006; 21:1104–1108 [DOI] [PubMed] [Google Scholar]
- 2.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006; 17:2359–2362 [DOI] [PubMed] [Google Scholar]
- 3.Shellock FG, Spinazzi A. MRI safety update 2008. Part I. MRI contrast agents and nephrogenic systemic fibrosis. AJR 2008; 191:1129–1139 [DOI] [PubMed] [Google Scholar]
- 4.Perazella MA, Rodby RA. Gadolinium use in patients with kidney disease: a cause for concern. Semin Dial 2007; 20:179–185 [DOI] [PubMed] [Google Scholar]
- 5.United States Food and Drug Administration. FDA requests boxed warning for contrast agents used to improve MRI images www.fda.gov./NewsEvents/Newsroom/PressAnnouncements/2007/ucm108919.htm. Accessed December 15, 2009
- 6.U.S. Food and Drug Administration public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI)—Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance; www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm053112.htm. Published June 8, 2006. Accessed March 24, 2010 [Google Scholar]
- 7.Weinreb JC. Impact on hospital policy: Yale experience. J Am Coll Radiol 2008; 5:53–56 [DOI] [PubMed] [Google Scholar]
- 8.Juluru K, Vogel-Claussen J, Macura KJ, Kamel IR, Steever A, Bluemke DA. MR imaging in patients at risk for developing nephrogenic systemic fibrosis: protocols, practices, and imaging techniques to maximize patient safety. RadioGraphics 2009; 29:9–22 [DOI] [PubMed] [Google Scholar]
- 9.Choyke PL, Cady J, DePollar SL, Austin H. Determination of serum creatinine prior to iodinated contrast media: is it necessary in all patients? Tech Urol 1998; 4:65–69 [PubMed] [Google Scholar]
- 10.Tippins RB, Torres WE, Baumgartner BR, Baumgarten DA. Are screening serum creatinine levels necessary prior to outpatient CT examinations? Radiology 2000; 216:481–484 [DOI] [PubMed] [Google Scholar]
- 11.Olsen JC, Salomon B. Utility of the creatinine prior to intravenous contrast studies in the emergency department. J Emerg Med 1996; 14:543–546 [DOI] [PubMed] [Google Scholar]
- 12.Lameire N, Adam A, Becker CR, et al. Baseline renal function screening. Am J Cardiol 2006; 98:21K–26K [DOI] [PubMed] [Google Scholar]
- 13.Herts BR, Schneider E, Poggio ED, Obuchowski NA, Baker ME. Identifying outpatients with renal insufficiency before contrast-enhanced CT by using estimated glomerular filtration rates versus serum creatinine levels. Radiology 2008; 248:106–113 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation—Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139:137–147 [DOI] [PubMed] [Google Scholar]
- 16.Center for Medicare and Medicaid Services Website. Clinical laboratory fee schedule and physician fee schedule www.cms.hhs.gov/home/medicare.asp. Accessed March 24, 2010
- 17.Bridges MD St. Amant BS, McNeil RB, Cernigliaro JG, Dwyer JP, Fitzpatrick PM. High-dose gadodiamide for catheter angiography and CT in patients with varying degrees of renal insufficiency: prevalence of subsequent nephrogenic systemic fibrosis and decline in renal function. AJR 2009; 192:1538–1543 [DOI] [PubMed] [Google Scholar]
- 18.Weinreb JC. Which study when? Is gadoliniumenhanced MR imaging safer than iodine-enhanced CT? Radiology 2008; 249:3–8 [DOI] [PubMed] [Google Scholar]
- 19.Reilly RF. Risk for nephrogenic systemic fibrosis with gadoteridol (ProHance) in patients who are on long-term hemodialysis. Clin J Am Soc Nephrol 2008; 3:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rofsky NM, Sherry AD, Lenkinski RE. Nephrogenic systemic fibrosis: a chemical perspective. Radiology 2008; 247:608–612 [DOI] [PubMed] [Google Scholar]
- 21.Kanal E, Broome DR, Martin DR, Thomsen HS. Response to the FDA’s May 23, 2007, nephrogenic systemic fibrosis update. Radiology 2008; 246:11–14 [DOI] [PubMed] [Google Scholar]


