Abstract
Background/Aims:
The mitochondrial permeability transition pore opening plays a critical role in the pathogenesis of myocardial infarction. Inhibition of cyclophilin-D (CyP-D), a key regulator of the mitochondrial permeability transition pore, has been shown to exert cardioprotective effects against ischemia-reperfusion injury on various animal models, mostly in males. However, failure of recent clinical trials requires a detailed elucidation of the cardioprotective efficacy of CyP-D inhibition. The aim of this study was to examine whether cardioprotective effects of sanglifehrin A, a potent inhibitor of CyP-D, on post-infarcted hearts depends on reperfusion.
Methods:
Acute or chronic myocardial infarction was induced by coronary artery ligation with/without subsequent reperfusion for 2 and 28 days in female Sprague-Dawley rats. Cardiac function was estimated by echocardiography. Oxygen consumption rates, ROS production, permeability transition pore opening, protein carbonylation and respiratory supercomplexes were analyzed in isolated cardiac mitochondria.
Results:
Sanglifehrin A significantly improved cardiac function of reperfused hearts at 2 days but failed to protect after 28 days. No protection was observed in non-reperfused post-infarcted hearts. The respiratory control index of mitochondria was significantly reduced in reperfused infarcted hearts at 2-days with no effect at 28-days post-infarction on reperfused and non-reperfused hearts. Likewise, only a minor increase in reactive oxygen species production was observed at 2-days in non-reperfused post-infarcted hearts.
Conclusion:
This study demonstrates that CyP-D inhibition exerts cardioprotective effects in reperfused but not in non-reperfused infarcted hearts of female rats, and the effects are observed only during acute post-infarction injury.
Keywords: Myocardial infarction, Reperfusion, Mitochondrial permeability transition, Cyclophilin D, Cardioprotection, Sanglifehrin A
Introduction
Cardiovascular disease is the leading cause of death in the United States [1] and worldwide, and myocardial ischemia (MI) is the most common [2]. Current therapy focuses on rapid restoration of blood flow to the ischemic area known as reperfusion (R). However, reperfusion exacerbates oxidative and calcium stress which induces additional damages to the myocardium and a ~ 50% increase in the infarct size [3]. Myocardial ischemia-reperfusion (IR) causes com pensatory myocardial remodeling which, over time, leads to heart failure (HF) [4].
The heart is the most energy consuming organ in the body. Mitochondria occupy up to 30% of cardiomyocyte volume; they are responsible for supplying ~ 90% of the ATP needed for its normal function [5]. Importantly, oxidative stress-induced mitochondrial dysfunction and ATP depletion are hallmarks of HF [6]. Mitochondria play an important role in post-infarction remodeling and its progression to HF. During ischemia, anaerobic metabolism is primarily favored over aerobic metabolism due to oxygen deficiency, resulting in an increase of intracellular Ca2+. At reperfusion, Ca2+ accumulates in the mitochondria. Additionally, dysfunctional electron transport chain (ETC) complexes increase reactive oxygen species (ROS) production. This results in an increase in mitochondrial membrane permeabilization known as the mitochondria permeability transition (mPT) accompanied by the opening of the mitochondrial permeability transition pore (mPTP) [7].
The mPTP is a non-selective pore with an unknown molecular identity that allows passage of molecules with molecular weight less than 1.5 kDa through the inner mitochondrial membrane. The pathological opening of the mPTP causes loss of mitochondrial membrane potential, m atrix swelling, and ROS generation leading to rupture of outer mitochondrial membrane and cell death. Inhibition of the mPTP at reperfusion could offer cardioprotective advantages delaying the development of post-MI HF. Nevertheless, due to the lack of knowledge of the molecular components of the mPTP, pharmacological inhibition without a specific target has proven to be impossible. Importantly, previous studies reported a major regulatory role of cyclophilin D (CyP-D), a matrix-located peptidyl-prolyl cis-trans isomerase (PPIase), in pore formation [8]. Genetic ablation or pharmacological inhibition of CyP-D increased the resistance of mitochondria to Ca2+-induced swelling as a m arker of mPTP opening. Although many studies have shown the beneficial effects of CyP-D inhibition in IR, recent failure of Cyclosporine Improve Clinical Outcome in ST Elevation Myocardial Infarction Patients (CIRCUS) clinical trials [9] using cyclosporine A (CsA), a CyP-D inhibitor, highlight the necessity to further study the role of CyP-D inhibition in reperfusion injury.
In this study, we elucidate the divergent effects of CyP-D inhibition in reperfused and non-reperfused MI. Female rats were subjected to non-reperfused or reperfused MI in the presence or absence of a single intravenous bolus of sanglifehrin A (SfA), a potent CyP-D inhibitor similar to CsA [10], 25 min after MI. The rationale for utilizing SfA rather than CsA is that the later also inhibits calcineurin activity therefore making SfA a more specific CyP-D inhibitor [10]. Additionally, to understand if the beneficial effects of SfA are long-lasting, we examined post-MI cardiac function in an acute (2-days) and chronic (28-days) phase of post-infarction remodeling. Results demonstrated that the beneficial effects of SfA were only present in reperfused hearts, and this effect was no longer observed at 28-days post-MI. However, in contrast to our previous studies in male rats [11, 12], we demonstrate that cardiac dysfunction is not linked to mitochondrial dysfunction in female rats after 28-days post-MI perhaps due to a protective role of estrogen. Most importantly, the use of female rats in these experiments is concordant to NIH guidelines for the inclusion of females in biomedical research, especially in physiological studies where females represent only 21% of the data published [13]. The lack of studies in females may potentially limit the translational power of findings obtained in males to clinical studies.
Materials and Methods
Animals
Female Sprague-Dawley rats (200–250g) were purchased from Taconic (Rensselaer, NY). All experiments were performed according to protocols approved by the UPR Medical Sciences Campus Animal Care and Use Committee and conformed to the National Research Council Guide for the Care and Use of Laboratory Animals published by the NIH (2011, eighth edition).
In-vivo model of MI
Animals were randomly assigned to one of the following six treatment groups and followed for 2- or 28-days: 1) S, sham (2 days: n=8; 28 days: n=7); 2) SS, sham+SfA (2 days: n=9; 28 days: n=10); 3) I, MI by coronary artery ligation (CAL, 2 days: n=8; 28 days: n=9); 4) IS, MI+SfA (2 days: n=9; 28 days: n=10); 5) IR, MI+reperfusion (2 days: n=10; 28 days: n=8); 6) IRS, IR+SfA (2 days: n=11; 28 days: n=11). The design of experiments is shown in Fig. 1. The surgical procedure was performed as previously described [11, 12]. Briefly, rats were anesthetized with an anesthetic cocktail (179.2 mg/kg body weight, IP) containing 4.2 mg/kg xylazine, 87.5 mg/kg ketamine, and 0.88 mg/kg acepromazine. Then, they were intubated, and artificially ventilated with room air. The animal’s body temperature was maintained at 37°C and subcutaneous leads were position on the extremities to monitor cardiac electrical activity through ECG using the Rodent Surgical Monitor (Indus Instruments). After lateral thoracotomy the heart was gently exposed, and MI was induced by ligation of the left main coronary artery ~3 mm from its origin using a firmly tied silk suture (7–0). In the sham group, the ligature was placed in an identical fashion but not tied. To induce reperfusion, the ligature was released immediately after 30 min of CAL (MI). The animals were followed for 2 or 28 days. A single bolus of SfA (5 mg/kg, IV) was administered 25 min after the start of sham or CAL in both, reperfused and non-reperfused hearts. SfA was generously provided by Novartis Pharma AG (Basel, Switzerland). On the day of the experiment, rats were anesthetized, cardiac function was analyzed by echocardiography, then the rats were sacrificed, and their hearts were quickly removed for mitochondria isolation or infarction size assay (n=4 per each group).
Fig. 1.
The design of experiments. Animals were randomly assigned to one of the following six treatment groups and followed for 2 or 28 days: 1) S, sham; 2) SS, sham+SfA; 3) I, MI; 4) IS, MI+SfA; 5) IR, MI+reperfusion; 6) IRS, IR+SfA. See Materials and Methods for details.
Echocardiography and infarction size
Echocardiography measurements were performed by a technician blinded to the groups [11]. Infarction size was determined using tetrazolium chloride (TTC) staining [12].
Mitochondria isolation
To isolate mitochondria, the left ventricles were homogenized using a Polytron homogenizer in ice-cold sucrose buffer containing (in mM): 300 sucrose, 10 Tris-HCl, and 2 EGTA; pH 7.4. Mitochondria were isolated by centrifugation [14], and then used for subsequent analysis.
Enzymatic activity of citrate synthase (CS) was determined spectrophotometrically by measuring coenzyme A formation at 412 nm [11].
Mitochondrial respiratory function
Mitochondrial respiration rates were measured using a YSI Oxygraph (Yellow Springs, OH) equipped with a Clark-type oxygen electrode [11]. Mitochondria were incubated in a buffer containing (in mM) 125 KCl, 20 MOPS, 10 Tris, 0.5 EGTA, 2 MgCl2, and 2 KH2PO4, at pH 7.2, supplemented with 3 mM 2-oxoglutarate and 1.25 mM L-malate to measure the rate of oxygen consumption at complex I. Oxygen consumption was measured in the absence (state 2) and presence (state 3) of 1.25 mM ADP. Respiratory control index (RCI) was calculated as the ratio of state 3 to state 2 respiration.
mPTP opening
Swelling of de-energized mitochondria, as an indicator of mPTP opening, in the presence or absence of Ca2+ was monitored as a decrease in absorbance at 525 nm [15]. Briefly, freshly isolated mitochondria were incubated at 37°C in 0.1 mL of incubation buffer containing: 200 mM sucrose, 10 mM Tris-MOPS, 5 mM α-ketoglutarate, 2 mM malate, 1 mM Pi, and 10 μM EGTA-Tris, pH 7.4. Mitochondrial swelling was measured in the presence of CaCl2 at final (cumulative) concentrations of 100, 200, 300 and 1000 μM. To verify CyP-D dependence of mPTP opening, 0.5 μM SfA was directly added to the mitochondrial suspension before the addition of the above mentioned Ca2+ concentrations.
ROS production
Freshly isolated mitochondria were used for measurement of ROS levels as an indicator of ROS generation [14]. Mitochondrial H2O2 production was measured with 50 mM Amplex Red (Molecular Probes) at 37°C in the presence of CaCl2 at final (cumulative) concentrations of 100, 200, 300 and 1000 μM in the incubation buffer containing 25 mM sodium phosphate, 5 μM EGTA-Tris, pH 7.4, and 0.1 U/mL HRP. Fluorescence intensity was monitored in the presence or absence of Ca2+ at an excitation of 560 nm and emission at 590 nm.
Protein carbonylation
To detect the level of protein carbonyls, as a marker of protein oxidation, mitochondrial proteins were derivatized with dinitrophenylhydrazine (DNPH, Sigma-Aldrich) under acid denaturing conditions [15]. In order to correct for non-specific binding of antibodies, separate aliquots of the mitochondrial proteins that had been acid-denatured but not treated with DNPH were run in parallel.
Blue native PAGE
Mitochondrial respirasome levels were analyzed using blue native polyacrylamide gel electrophoresis (BN-PAGE) and normalized to total mitochondrial protein [14].
SDS-PAGE and western blotting
Equal amounts of mitochondrial protein were resolved by SDS-PAGE and transferred onto nitrocellulose membranes (GE Healthcare Bio-Sciences). The membranes were immunoblotted with acetylated lysine (Ac-K, Cell Signaling), SIRT3 (Cell Signaling), Estrogen Receptor (ER) α (Santa Cruz), ERβ (Abcam), or ATP5a (Abcam) antibodies followed by IRDye® (LI-COR Biosciences) secondary antibodies. Bands were visualized using ODYSSEY® CLx (LI-COR Biosciences) infrared scanner and analyzed with Image Studio Lite Software.
Statistical analysis
Data are presented as means±SEM. Data normality was evaluated using the Shapiro-Wilk test. Statistical significance was then evaluated using Prism Graph Pad (San Diego, CA) using an unpaired 2-tailed student’s t-test or a Mann-Whitney test. Differences were considered statistically significant when P<0.05.
Results
Cardiac function
To assess the possible divergent effects of CyP-D inhibition in cardioprotection against post-MI with and without reperfusion, SfA was administered to rats 25 min after CAL and cardiac function was assessed at 2- or 28-days post-MI (Fig. 1). Our rationale for choosing these time points is to determine cardiac and mitochondrial function during two distinct phases of post-MI remodeling. The first phase occurs within hours and peaks at 2-days post-MI. The second phase is reactive; myocardial remodeling develops within weeks of post-MI and entails scar formation to replace the nonviable myocardium [16].
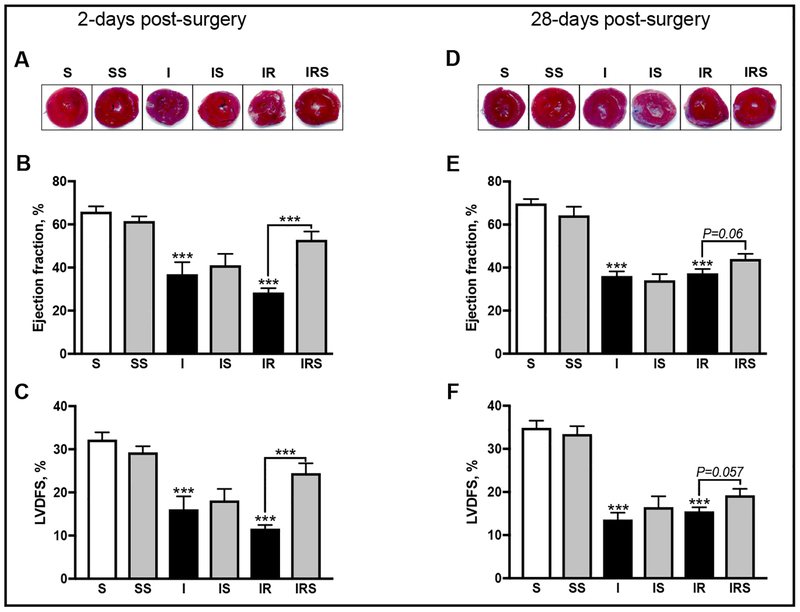
At 2-days post-surgery, SfA had no detrimental effects on the sham procedure, ejection fraction (EF) and left-ventricular dimension fraction shortening (LVDFS; Fig. 2B, C) were conserved. Animals that underwent non-reperfused MI developed severe cardiac dysfunction as observed by a 44% reduction of EF (P< 0.0003) and a 50% reduction of LVDFS (P<0.0003). SfA did not protect against the detrimental effects of non-reperfused MI (Fig. 2B, C). Similarly, animals that underwent reperfused MI developed impairm ent of cardiac function and revealed 5 7% (P< 0.0001) less EF compared to controls (Fig. 2B). Additionally, LVDFS was reduced by 64% (P < 0.0001) in reperfused hearts (Fig. 2C). However, SfA significantly improved (IR vs IRS) EF by 86% (P< 0.0001) and LVDFS by 111% (P < 0.0001; Fig. 2B, C).
Fig. 2.
The effects of SfA on cardiac function during acute and chronic post-MI with and without reperfusion. Representative images of TTC analysis on infarct size at 2- (A) and 28-days (D) post-surgery. Left ventricular ejection fraction measured as ((LVESV-LVEDV)/LVEDV) * 100 where LVESV: left ventricular systolic volume; LVEDV: left ventricular end diastolic volume; at 2- (B) and 28-days (E) post-surgery. Left ventricular dimension fraction shortening (LVDFS%) as measured by ((LVDD-LVDS)/LVDD)*100, where LVDD: left ventricular dimension at diastole; LVDS: left ventricular dimension at systole at 2- (C) and 28-days (F) post-surgery. Data was collected using M-mode echocardiography by a technician blinded to the experimental groups. ***P<0.001 vs. S. n=7–11 animals per group.
At 28-days post-surgery, SfA did not affect EF or LVDFS in sham-operated animals. Nonreperfused post-MI resulted in severe impairment of cardiac function; EF was reduced by 48% (P< 0.0001) and LVDFS by 61% (P< 0.0001) compared to controls (Fig. 2E, F). SfA did not improve EF and LVDFS in non-reperfused MI hearts (Fig. 2E, F). Similarly, reperfused MI significantly deteriorated cardiac function as evidenced by a 46% (P< 0.0001) and 56% (P< 0.0001) reduction of EF and LVDFS, respectively. Although SfA increased EF by 18% (P<0.06) and LVDFS by 24% (P<0.057) in reperfused hearts (IR vs. IRS), this difference was not significant (Fig. 2E, F).
Thus, inhibition of CyP-D by SfA significantly improved cardiac function in reperfused hearts at 2-days post-surgery, but this effect was absent at 28-days post-MI. Also, SfA was not cardioprotective in non-reperfused MI hearts.
Mitochondrial respiratory function
To determine if there were any changes in mitochondrial content and respiratory function, CS activity and mitochondrial respiration rates were measured. At 2-days post-surgery, CS activity in non-reperfused and reperfused hearts was, respectively, 17% (P<0.007) and 23% (P<0.002) lower than sham. SfA did not affect CS activity in sham-operated rats. Interestingly, SfA was able to increase CS activity by 22% (P<0.039) in reperfused hearts but not in non-reperfused hearts (Fig. 3A). Mitochondrial RCI in non-reperfused MI was reduced by 17% (P<0.06) compared to sham, although the difference did not reach statistically significant levels. However, mitochondria isolated from reperfused hearts exhibited a 24% (P<0.004) lower RCI than sham hearts (Fig. 3B). SfA conferred no beneficial effects on RCI in reperfused MI. In conclusion, at 2-days post-surgery non-reperfused and reperfused MI reduced mitochondrial content; however, CyP-D inhibition was able to restore mitochondrial content in reperfused hearts; concurrent with its effects in cardioprotection (Fig. 3A). Although CyP-D inhibition preserved mitochondrial content after reperfusion, it did not affect mitochondrial respiration (Fig. 3B).
Fig. 3.
Citrate synthase activity and respiration rates of mitochondria in reperfused and nonreperfused post-MI hearts. Mitochondrial citrate synthase activity 2- (A) and 28-days (C) post-surgery. Respiratory control index (RCI) of complex I at 2- (B) and 28-days (D) post-surgery. Mitochondrial respiration was measured using a Clark-type oxygen sensitive probe. Respiration of mitochondria for complexI was stimulated by the addition of 3 mM 2-oxoglutarate and 1.25 mM L-malate (state 2] and in the presence of 1.25 mM ADP (state 3). RCI was calculated as the ratio of state 3 to state 2 respiration. *P<0.05 and **P<0.01 vs. S. n=7–11 animals per group.
At 28-days post-surgery, SfA increased CS activity by 37% (P<0.01) in sham-operated animals. Interestingly, although MI with or without reperfusion did not affect CS activity, SfA significantly increased its activity in non-reperfused and reperfused MI by 25% (P<0.039) and 28% (P<0.049), respectively (Fig. 3C). The RCI was the same in all groups at 28-days post-surgery (Fig. 3D]. In addition, SfA was able to increase mitochondrial content in all groups suggesting a possible role of CyP-D in mitochondrial biogenesis [17] (Fig. 3C). However, we observed no significant effect of SfA on mitochondrial respiration in post-MI hearts with or without reperfusion (Fig. 3D].
Mitochondrial swelling, ROS production, and respirasome levels
To determine if cardiac dysfunction was coupled to altered mitochondrial function, we examined mPTP formation, ROS production, and mitochondrial respirasome.
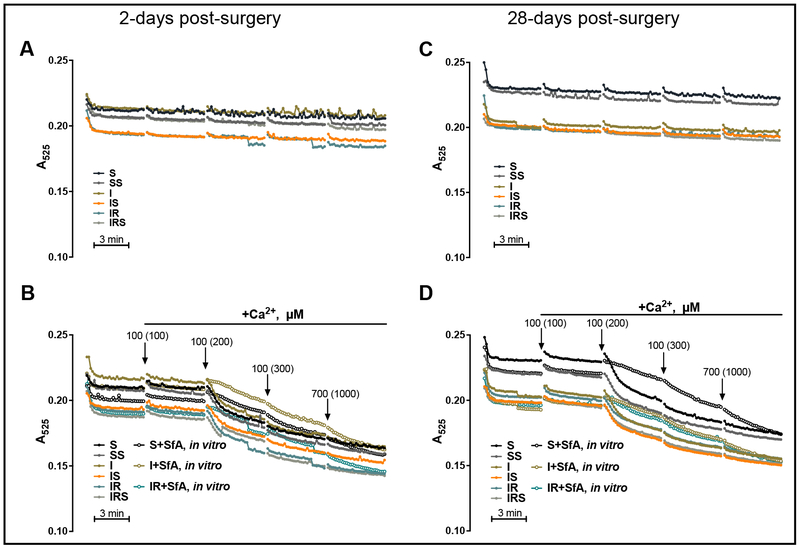
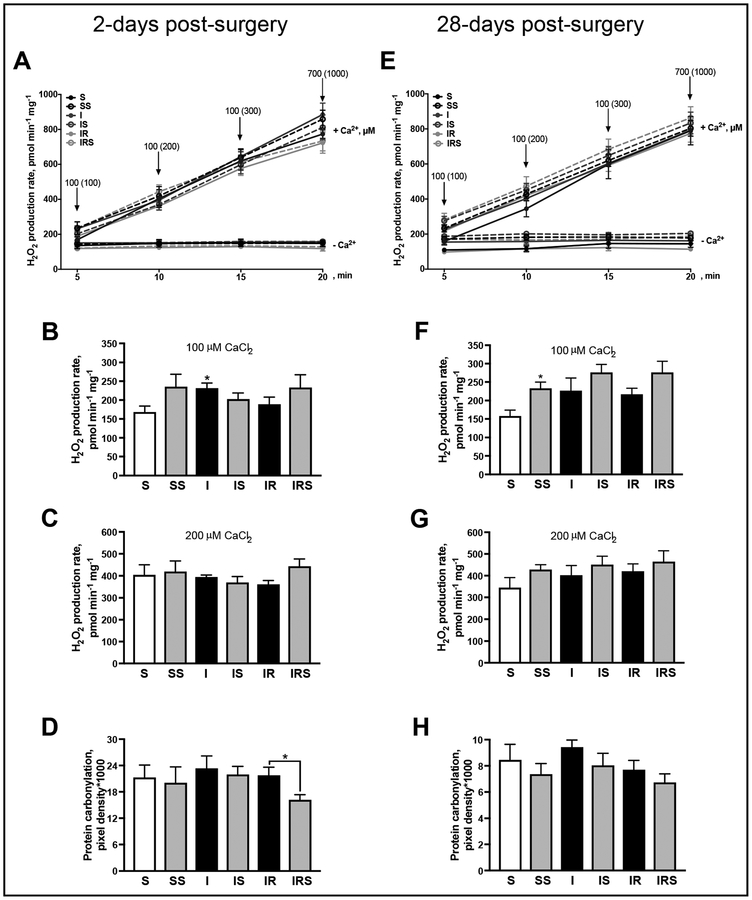
To determine the sensitivity of the mitochondria to mPTP, we measured Ca2+-induced mitochondrial swelling. Interestingly, no differences in mPTP sensitivity were observed between groups at 2- (Fig. 4A, B] and 28-days post-MI (Fig. 4C, D). Since mitochondrial Ca2+ overload has been shown to induce not only mPTP opening but also ROS production [14], we measured Ca2+-induced ROS production in mitochondria. At 2-days, Ca2+ gradually increased the rate of mitochondrial ROS production (Fig. 5A). At 100 μM of Ca2+, the rate of mitochondrial H2O2 production from non-reperfused MI hearts increased by 37% (P< 0.0286) compared to sham-operated hearts (Fig. 5B). The increased ROS production at 100 μM of Ca2+ is likely due to enhanced activity of TCA cycle and ETC chain. Indeed, high conductance mPTP and remarkable mitochondrial swelling does not occur in the presence of 100 μM of Ca2+. However, no significant differences between groups were observed in H2O2 production rate in the presence of 200 μM CaCl2 (Fig. 5C). To determine if the increased ROS production results in oxidation of mitochondrial proteins, we assessed protein carbonylation in mitochondria. Interestingly, the increase in ROS production after non-reperfused MI was not associated with an increase in protein carbonylation (Fig. 5D). SfA significantly decreased (by 26%, P < 0.049) protein carbonylation in reperfused hearts at 2-days post-MI (Fig. 5D).
Fig. 4.
Basal and Ca2+-induced swelling as an indicator of mPTP opening in sham or post-MI hearts. Measurement of mPTP was performed by monitoring the decrease in light scattering at 525 nm. Mitochondrial swelling in the absence of Ca2+ at 2- (A) and 28-days (C) post-surgery. Mitochondrial swelling in the presence of 100, 200, 300 and 1000 μM (cumulative or final concentration) of CaCl2 at 2- (B) and 28-days (D) post-surgery. S+SfA in vitro, I+SfA in vitro, and IR+SfA in vitro represent samples where 0.5 μM of SfA was added to the mitochondria in vitro prior to mPTP stimulation. Curves are presented as the average of raw absorbance values of all samples in the group over time. Arrows (B, D) indicate the addition of Ca2+, values in parenthesis represent final (cumulative) concentrations of Ca2+ in media at a given time point.
Fig. 5.
The basal and Ca2+-stimulated rate of ROS production in mitochondria isolated from reperfused and non-reperfused post-MI hearts. The basal and Ca2+-stimulated rate of ROS production 2- (A) and 28-days (E) post-surgery. The rate of ROS production after mitochondrial incubation with 100 μM (B, F) and 200 μM (C, G) of CaCl2 at 2- and 28-days post-surgery, respectively. Protein carbonyl levels, as a marker for protein oxidation, measured by DNPH derivatization in mitochondria at 2- (D) and 28-days (H) post-surgery. ROS production was assessed by measurement of resorufin, the Amplex Red oxidation product. Each arrow (A, E) indicates the addition of Ca2+, values in parenthesis represent final (cumulative) concentrations of Ca2+ in media at a given time point. *P<0.05 vs. S. n=7–11 per group.
In conclusion, although the mitochondrial ROS production rate was higher in nonreperfused hearts at 100 μM of CaCl2, this increase was not observed basally (not shown) nor with the subsequent addition of CaCl2 As a consequence, no difference in protein carbonylation was observed between S and I groups although SfA was able to reduce protein carbonylation in reperfused hearts.
Next, we examined mitochondrial Ca2+-induced ROS production at 28-days post-surgery. First, Ca2+addition to a mitochondrial suspension significantly increased H2O2 production (Fig. 5E). SfA addition to the control group induced a 47% (P < 0.0283) increase in H2O2 production at 100 μM Ca2+ (Fig. 5F). Interestingly, neither non-reperfused nor reperfused MI significantly increased mitochondrial ROS production rate when compared to sham. Subsequent additions of Ca2+ did not result in significant changes in H2O2 production rate between groups (Fig. 5G). Similarly, no differences were observed in the level of mitochondrial protein oxidation (Fig. 5H). These data suggest that at 28-days post-surgery the H2O2 production rate does not differ between groups resulting in no differences in protein oxidation levels (Fig. 5H).
The mitochondrial respirasome is a mitochondrial supercomplex containing the ETC complexes I, III and IV. The respirasome has been suggested to increase the effectiveness of ETC and ATP production thereby reducing electron leakage and ROS production [18], although its physiological role is unclear. We recently demonstrated that in an ex vivo model of cardiac IR in male rats, respirasome integrity was affected in an reperfusion time-dependent manner and this was prevented by SfA treatment [19]. We examined whether the structural integrity of the respirasome was affected by reperfused and non-reperfused MI in female rats. At 2-days post-surgery, there was a 17% (P<0.014) decrease in respirasome levels only in the IRS group compared to IR (Fig. 6A, B). No significant difference was observed after non-reperfused MI. At 28-days post-surgery, reperfusion alone was able to increase by 14% (P<0.02) respirasome content (Fig. 6C, D). These data suggest that mitochondrial respirasome integrity is minimally affected in reperfused and non-reperfused MI.
Fig. 6.
Analysis and quantification of respirasome levels in mitochondria. Representative BN-PAGE of left ventricle mitochondria at 2- (A) and 28-days (C) post-surgery. Respirasome levels at 2- (B) and 28-days (D) post-surgery, expressed as percent change of sham (S). Mitochondrial proteins were digested using digitonin and separated using BN-PAGE. *P<0.05 vs. S. n=6–7 per group.
SIRT3 and mitochondrial protein acetylation
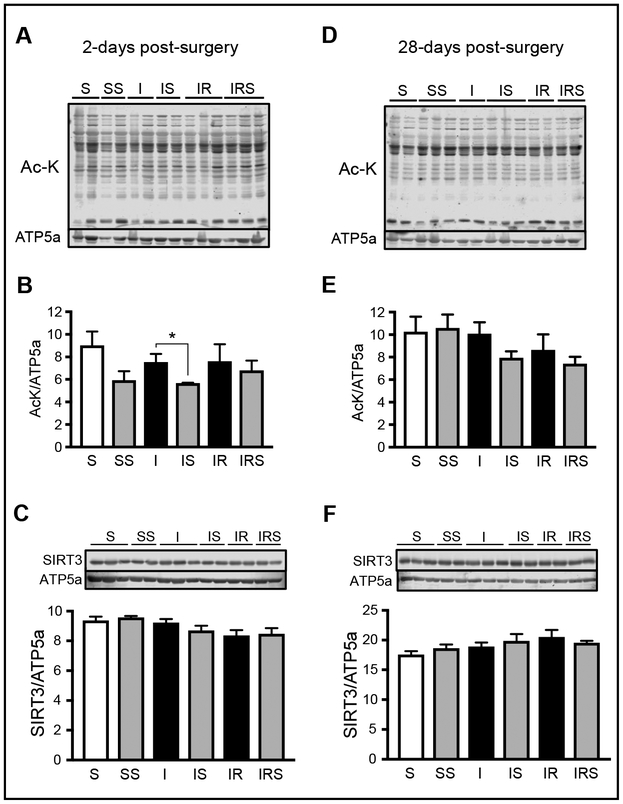
Acetylation is an im portant regulator of mitochondrial metabolism [20]. We have previously shown an increase in acetylation of mitochondrial proteins in post-MI HF in male rats [11]. Acetylation of CyP-D has been shown to be associated with increased mPTP formation [21]. In addition to CyP-D, many other mitochondrial proteins involved in energy metabolism have been found acetylated due to downregulation of sirtuins in aging and aging-related diseases such as HF [11, 22]. Sirtuins are NAD+-dependent deacetylases and SIRT3 is the main mitochondrial deacetylase. In post-MI HF, a decrease in mitochondrial metabolism would result in a decrease NAD+/NADH, therefore, reducing SIRT3 activity and increasing mitochondrial acetylation. Therefore, we determined whether the impaired cardiac function observed in non-reperfused or reperfused MI was associated with increased protein acetylation.
At 2-days post-surgery, SfA administration to sham-operated animals resulted in a 34% (P< 0.065) decrease in protein acetylation, although this difference did not reach statistical significance. Also, SfA was able to reduce protein acetylation after non-reperfused MI by 25% (P<0.04; Fig. 7A, B); however, no difference was observed between IR and IRS groups. Next, we examined if there was a decrease in SIRT3 expression in post-MI hearts. We were unable to detect any differences in SIRT3 expression between all six groups (Fig. 7C). Thus, these findings suggest that the difference in acetylation levels in post-MI with SfA could be due to reduced SIRT3 activity but not expression. At 28-days post-surgery, we found no difference in protein acetylation (Fig. 7D, E) nor SIRT3 expression (Fig. 7F) in mitochondria. Overall, it seems that conservation of SIRT3 expression between groups prevented any significant changes in protein acetylation.
Fig. 7.
Acetylated lysine (Ac-K; A, B, D, E) and SIRT3 (C, F) levels in cardiac mitochondria. A, D, Representative immunoblots of mitochondrial proteins acetylated on lysine residues at 2- (A) and 28-days (D) post-surgery. B, E, Quantitative data of mitochondrial protein acetylation. Levels of lysine-acetylated proteins were normalized to ATP synthase subunit alpha (ATP5a), a mitochondrial housekeeping protein. C, F, Protein levels of SIRT3. Representative immunoblots of SIRT3 expression (upper panels) were obtained by western blotting. Protein expression levels (bottom panels) were normalized to ATP5a. *P<0.05; n=6–7 per group.
Discussion
Recent failure of CIRCUS clinical trials [9] stipulates a necessity for additional studies on the role of the mPTP as a therapeutic target in MI/IR injury. Previous studies on ex-vivo models of IR revealed beneficial effects of CsA at the onset of reperfusion [10, 23]. It should be noted that a myriad of mPTP-independent factors that are involved in post-MI remodeling with the progression of MI to HF, such as inflammation [24] and nitric oxide [25], can affect the efficiency of mPTP inhibitors in chronic MI/IR injury. Moreover, long-term inhibition of CyP-D may compromise the physiological role it plays in mitochondria [26, 27]. Also, the lack of studies in females may potentially limit the translational power of findings obtained in males to clinical studies [28]. In this study we sought to: (i) understand the divergent effects of SfA in reperfused and non-reperfused post-MI, (ii) evaluate two distinct phases (acute and chronic) of post-MI, (iii) study the role of cardiac mitochondria in reperfusion injury, and (iv) include the use of females in pre-clinical studies of mPTP inhibition as a target for protection against reperfusion injury.
We first evaluated the effects of SfA in an in vivo model of non-reperfused vs. reperfused post-MI. SfA conferred no cardioprotection in non-reperfused hearts at 2- and 28-days (Fig. 2). However, it significantly improved EF and LVDFS by 86% and 111%, respectively, in reperfused hearts at 2-days post-MI. Interestingly, SfA was only effective at 2-days but not at 28-days post-MI; EF and LVDFS were recovered by 18% and 24%, respectively, and the difference was not significant. These data suggest the presence of different molecular mechanisms during early and late reperfusion. During early post-MI, cell death is primarily mediated via apoptotic pathways [29]. Furthermore, the reintroduction of oxygen to a previously ischemic tissue results in a significant increase in ROS production that induces tissue damage, mPTP formation, and activation of an inflammatory response. However, during the chronic phases of reperfusion (28-days post-MI), ROS primarily mediates generation of growth factors, remodeling mechanisms, and scar tissue formation [30].
In the CIRCUS trial, patients with an elevated S-T segment MI (STEMI) were given a single bolus of CsA before coronary intervention [9]. This clinical trial evaluated the effects of CsA 1-year post-intervention, a period where the therapeutic benefits of mPTP inhibition were no longer observed. The outcome of the clinical trial and our results support the notion that inhibition of the mPTP at early reperfusion could prevent mitochondrial swelling, ROS burst, and recover respiratory function of mitochondria and ATP synthesis. At late phases of post-MI, characterized by myocardial remodeling and scar tissue formation, mPTP inhibition has no cardioprotection due to the involvement of mPTP-independent factors.
CsA is an immunosuppressant drug that inhibits calcineurin and CyP-D activity. SfA is a more specific inhibitor than CsA for CyP-D. Unlike CsA, SfA does not inhibit calcineurin activity and binds to Cyp-D at a site different from that for CsA [10]. Although CsA and SfA have proven to exert cardioprotective effects on IR [10, 23], inhibition of CyP-D, which possesses important physiological functions in mitochondria, was recently questioned [27]. CyP-D, a mitochondrial isoform of cyclophilins, has several functions in the mitochondria under physiological conditions. It is involved in the regulation of synthasome assembly [31] and ATP synthase activity [32], mitochondrial dynamics [17], mitoflashes and mitochondrial metabolism [33, 34] as well as the redox status of the cell [35]. Therefore, the lack of cardioprotection observed in clinical trials [9] along with the reduced efficacy at 28-days post-surgery observed in our experiments could be explained by the inhibition of the physiological functions of CyP-D. However, further studies are needed for understanding the mPTP-mediated mechanisms of reperfusion injury during acute and chronic post-MI. The therapeutic limitations in the use of CyP-D inhibitors are associated with the suppression of its physiological functions. This emphasizes the importance of developing new compounds that could prevent mPTP opening without inhibiting CyP-D [27].
Mitochondria play a central role in IR injury and prevention of mitochondrial dysfunction has been shown to be cardioprotective [23]. At 2-days post-MI, we observed a marked decrease in mitochondrial CS activity in reperfused and non-reperfused hearts (Fig. 3A). The activity of CS, a matrix-localized enzyme of the TCA cycle, is a marker of mitochondrial mass, and CS activity is widely used to estimate mitochondrial content under physiological and pathological conditions. Mitochondrial mass (and CS activity) in our studies could be affected by oxidative stress, in addition to artefacts associated with the isolation procedure. Ischemia-reperfusion injury can induce mitochondrial fission through recruitm ent of dynamin-regulated protein-1 (Drp-1) from the cytoplasm to mitochondria [36]. Fission increases the number of small-sized mitochondria which cannot be isolated from the homogenate using standard isolation techniques. Loss of fragmented and dysfunctional mitochondria during the isolating procedure results in decreased CS activity [37]. Also, damaged mitochondria could be eliminated by mitophagy, or mitochondrial autophagy, thereby reducing mitochondria content [38, 39]. However, at 28-days post-MI, animals treated with SfA display a significant increase in CS activity (Fig. 3C). A recent study demonstrated that CyP-D could modulate phosphorylation and activation/translocation of Drp-1 to the mitochondria, therefore, promoting mitochondrial fission [17]. In our study, SfA could protect cardiac mitochondria from excessive fission through inhibition of CyP-D-mediated Drp-1 phosphorylation thereby preserving mitochondria content.
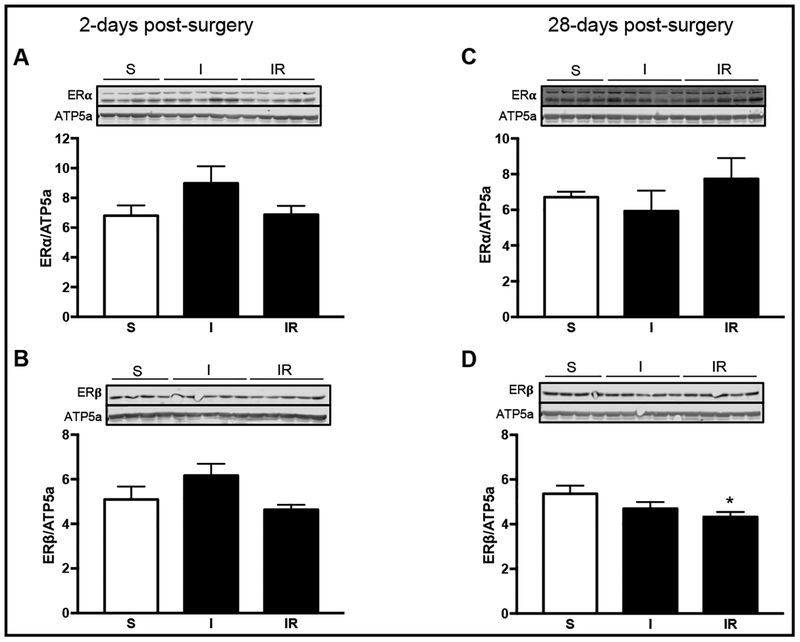
Studies have shown that estrogen can regulate mitochondrial function and that estrogen receptors are present in the mitochondria [40]. We were able to detect ERβ and ERα in the mitochondrial fraction (Fig. 8). In heart mitochondria, oophorectomy resulted in decreased glutamate-malate respiration [41], which was able to be restored in similar experiments through estrogen treatm ent [42]. Our results demonstrate that mitochondrial RCI was reduced by 17% (P<0.06) in non-reperfused and 24% (P< 0.004) in reperfused MI at 2-days post-surgery. However, no differences in RCI were observed at 28-days post-MI (Fig. 3B, D). It is possible that estrogen could have an important role in maintaining mitochondrial function and metabolism. However, it is not clear if the activation of mitochondrial ERs is involved in the regulation of mitochondrial respiratory function.
Fig. 8.
Mitochondrial protein expression of estrogen receptor (ER) ERα and ERβ. Left ventricle mitochondria protein expression of ERα at 2- (A) and 28-days (C) post-surgery. Protein levels of ERβ at 2- (B) and 28-days (D) post-surgery normalized to ATP5a. *P<0.05 vs. sham (S); n=4–5 per group.
Induction of mPT stimulates mitochondria-mediated cell death during IR. Therefore, we measured mPTP formation and ROS production. Interestingly, we found no differences in mPTP between groups (Fig. 4). In addition to preservation of mitochondrial respiration, estrogen inhibited mPTP in mitoplasts from rat liver using patch-clamp [43]. A variety of studies have shown that estrogen protects the mitochondria from mPTP [41, 42], cytochrome c release, and apoptosis [44]. However, it should be noted that our studies did not include comparable groups containing male rats which is the limitation of the study. In addition to prevention of mPTP opening, there were minimal effects of post-MI on ROS production (Fig. 5). Several groups have demonstrated that estrogen protects the mitochondria by preventing ROS accumulation. These effects could be mediated through its antioxidant abilities independent of its hormonal actions [45] or through preservation of antioxidant capacity [41, 42]. Therefore, we suggest that preserved mitochondrial metabolism, despite cardiac dysfunction, might be due to local effects of estrogen on the mitochondria throughout post-MI progression.
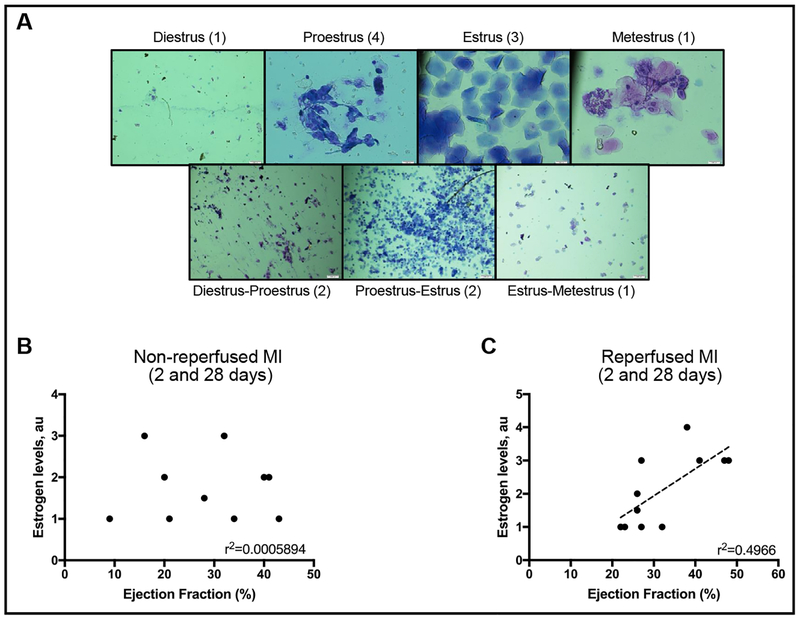
Similar to the effects of estrogen on mitochondrial function, it has also been shown to have a role in cardioprotection. Estrogen treatment to ovariectomized mice for two weeks before ex-vivo IR was able to improve cardiac recovery and decrease infarction size [46]. We observed a positive correlation between EF and estrogen levels (Fig. 9). Female rats that were in cycles of high estrogen at the moment of surgery positively correlated with higher cardiac function (EF) when compared to rats that were in cycles with low estrogen. This correlation was absent in non-reperfused hearts suggesting that the presence of estrogen in the blood at the moment of reperfusion significantly improved cardiovascular outcome.
Fig. 9.
The possible contribution of reperfusion and estrogen levels on the day of surgery to cardiac recovery. (A)Representative slides of vaginal smears and the menstrual cycle assigned. (B) EF and estrogen correlation on non-reperfused hearts (I group) from 2-and 28-days post-surgery. (C) EF and estrogen correlation on reperfused hearts (IR group) from 2- and 28-days post-surgery. Estrogen levels were estimated from the menstrual cycle [50] at the time of surgery, using a vaginal swab. Menstrual cycles with the highest estrogen levels were assigned a value 4 (proestrus), and those with lower estrogen levels were given a value of 1 (diestrus, metestrus, and estrus-metestrus transition).
In addition to mediating different aspects of cardiac and mitochondrial metabolism and function, estrogen and ER are not the only factors that could enhance the differences between males and females. Sex-differences in response to therapeutic drugs, such as CsA, should be taken into consideration. CsA has been shown to have sexually-dimorphic effects in rats [47]. Furthermore, CsA metabolites in blood of males were higher than females due to differences in hepatic clearance [48] requiring in some studies to double the dose in females to match the observed results in males [49]. These results highlight the importance of evaluating physiological and pharmacological differences between males and females in pre-clinical studies.
Conclusion
This study demonstrates that the cardioprotective effects of CyP-D inhibition occur in early reperfusion of post-MI where mPTP opening is evident [7]. Therefore, a multifactorial treatment would be necessary to decrease the development of HF in patients after reperfusion therapy; rather than solely using mPTP inhibition. Different from our previous studies in male rats [11, 12], we demonstrate that cardiac dysfunction is not linked to mitochondrial dysfunction in female rats perhaps due to a protective role of estrogen. We therefore emphasize the importance of considering sexually-dimorphic characteristics in pre-clinical trials.
Acknowledgements
The authors would like to thank Dr. Sehwan Jang for his valuable advice on techniques used in this study. This study was supported by the National Heart, Lung, and Blood Institute (Grant SC 1H L118669), and National Institute of General Medical Sciences (Grants SC1GM 128210 and R 25G M 061838) of the National Institutes of Health. RPR is supported by the 2017 William Townsend Porter Predoctoral Fellowship from the American Physiological Society.
Abbreviations
- Ac-K
acetylated lysine
- CS
citrate synthase
- CsA
cyclosporine A
- CyP-D
cyclophilin D
- CIRCUS
Cyclosporine Improve Clinical Outcome in ST Elevation Myocardial Infarction Patients
- ER
estrogen receptor
- EF
ejection fraction
- ETC
electron transport chain
- HF
heart failure
- IR
ischemia-reperfusion
- LVDFS
left-ventricular dimension fraction shortening
- MI
myocardial infarction
- mPTP
mitochondrial permeability transition pore
- RCI
respiratory control index
- ROS
reactive oxygen species
- SfA
sanglifehrin A
Footnotes
Disclosure Statement
The authors confirm that there are no conflicts of interest.
References
- 1.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B: Deaths: Final data for 2014 Natl Vital Stat Rep 2016;65:1–122. [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L et al. : American Heart Association Council on Epidemiologyand Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics-2018 update: a report from the american heart association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Ong S-B, Samangouei P, Kalkhoran SB, Hausenloy DJ: The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol 2015;78:23–34. [DOI] [PubMed] [Google Scholar]
- 4.Giordano FJ: Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 2005;115:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris DA, Das AM: Control of mitochondrial ATP synthesis in the heart. Biochem J 1991;280:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neubauer S: The failing heart--an engine out of fuel. N Engl J Med 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 7.Walters AM, Porter GA, Brookes PS: Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ Res 2012;111:1222–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P: Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem 2005;280:18558–18561. [DOI] [PubMed] [Google Scholar]
- 9.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y et al. : Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–1031. [DOI] [PubMed] [Google Scholar]
- 10.Clarke SJ, McStay GP, Halestrap AP: Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 2002;277:34793–34799. [DOI] [PubMed] [Google Scholar]
- 11.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S: Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol Biochem 2012;29:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barreto-Torres G, Javadov S: Possible role of interaction between PPARα and cyclophilin D in cardioprotection of AMPK against in vivo ischemia-reperfusion in rats. PPAR Res 2016;2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Zucker I, Beery AK: Males still dominate animal studies. Nature 2010;465:690–690. [DOI] [PubMed] [Google Scholar]
- 14.Jang S, Javadov S: Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch Biochem Biophys 2017;630:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parodi-Rullán RM, Chapa-Dubocq X, Rullán PJ, Jang S, Javadov S: High sensitivity of SIRT3 deficient hearts to ischemia-reperfusion is associated with mitochondrial abnormalities. Front Pharmacol 2017;8:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaman AKMT, Sobel BE: Causes, consequences, and amelioration of late adverse ventricular remodeling after myocardial infarction. Coron Artery Dis 2012;23:221–222. [DOI] [PubMed] [Google Scholar]
- 17.Xiao A, Gan X, Chen R, Ren Y, Yu H, You C: The cyclophilin D/Drp1 axis regulates mitochondrial fission contributing to oxidative stress-induced mitochondrial dysfunctions in SH-SY5Y cells. Biochem Biophys Res Commun 2017;483:765–771. [DOI] [PubMed] [Google Scholar]
- 18.Lobo-Jarne T, Ugalde C: Respiratory chain supercomplexes: Structures, function and biogenesis. Semin Cell Dev Biol 2018;76:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang S, Lewis TS, Powers C, Khuchua Z, Baines CP, Wipf P, Javadov S: Elucidating mitochondrial electron transport chain supercomplexes in the heart during ischemia-reperfusion. Antioxid Redox Signal 2017;27:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baeza J, Smallegan MJ, Denu JM: Mechanisms and dynamics of protein acetylation in mitochondria. Trends Biochem Sci 2016;41:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA: Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging 2010;2:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vadvalkar SS, Baily CN, Matsuzaki S, West M, Tesiram YA, Humphries KM: Metabolic inflexibility and protein lysine acetylation in heart mitochondria of a chronic model of type 1 diabetes. Biochem J 2013;449:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths EJ, Halestrap AP: Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol 1993;25:1461–1469. [DOI] [PubMed] [Google Scholar]
- 24.Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ: Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther 2018;186:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P, Zweier JL: Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem 1996;271:29223–29230. [DOI] [PubMed] [Google Scholar]
- 26.Brenner C, Moulin M: Physiological roles of the permeability transition pore. Circ Res 2012;111:1237–1247. [DOI] [PubMed] [Google Scholar]
- 27.Javadov S, Jang S, Parodi-Rullan R, Khuchua Z, Kuznetsov AV: Mitochondrial permeability transition in cardiac ischemia-reperfusion: whether cyclophilin D is a viable target for cardioprotection? Cell Mol Life Sci 2017;74:2795–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton JA, Collins FS: Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P: Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 1996;74:86–107. [PubMed] [Google Scholar]
- 30.Kalogeris T, Bao Y, Korthuis RJ: Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2014;2:702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beutner G, Alanzalon RE, Porter GA: Cyclophilin D regulates the dynamic assembly of mitochondrial ATP synthase into synthasomes. Sci Rep 2017;7:14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G: Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem 2009;284:33982–33988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang W, Gao H, Lu F, Ma Q, Fang H, Sun T, Xu J, Ding Y, Lin Y, Wang Y, Wang X, Cheng H, Zheng M: Cyclophilin D regulates mitochondrial flashes and metabolism in cardiac myocytes. J Mol Cell Cardiol 2016;91:63–71. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TT, Wong R, Menazza S, Sun J, Chen Y, Wang G, Gucek M, Steenbergen C, Sack MN, Murphy E: Cyclophilin D modulates mitochondrial acetylome. Circ Res 2013;113:1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz K-J, Knoops B: Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys 2009;491:39–45. [DOI] [PubMed] [Google Scholar]
- 36.Ong S-B, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ: Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010;121:2012–2022. [DOI] [PubMed] [Google Scholar]
- 37.Frisell WR, Patwardhan MV, Mackenzie CG: Quantitative studies on the soluble compartments of light and heavy mitochondria from rat liver. J Biol Chem 1965;240:1829–1835. [PubMed] [Google Scholar]
- 38.Chen-Scarabelli C, Agrawal PR, Saravolatz L, Abuniat C, Scarabelli G, Stephanou A, Loomba L, Narula J, Scarabelli TM, Knight R: The role and modulation of autophagy in experimental models of myocardial ischemia-reperfusion injury. J Geriatr Cardiol 2014;11:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bravo-San Pedro JM, Kroemer G, Galluzzi L: Autophagy and mitophagy in cardiovascular disease. Circ Res 2017;120:1812–1824. [DOI] [PubMed] [Google Scholar]
- 40.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW: Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA 2004;101:4130–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavón N, Cabrera-Orefice A, Gallardo-Pérez JC, Uribe-Alvarez C, Rivero-Segura NA, Vazquez-Martínez ER, Cerbón M, Martínez-Abundis E, Torres-Narvaez JC, Martínez-Memije R, Roldán-Gómez FJ, Uribe-Carvajal S: In female rat heart mitochondria, oophorectomy results in loss of oxidative phosphorylation. J Endocrinol 2017;232:221–235. [DOI] [PubMed] [Google Scholar]
- 42.Rattanasopa C, Phungphong S, Wattanapermpool J, Bupha-Intr T: Significant role of estrogen in maintaining cardiac mitochondrial functions. J Steroid Biochem Mol Biol 2015;147:1–9. [DOI] [PubMed] [Google Scholar]
- 43.Thiede A, Gellerich FN, Schönfeld P, Siemen D: Complex effects of 17β-estradiol on mitochondrial function. Biochim Biophys Acta 2012;1817:1747–1753. [DOI] [PubMed] [Google Scholar]
- 44.Morkuniene R, Arandarcikaite O, Borutaite V: Estradiol prevents release of cytochrome c from mitochondria and inhibits ischemia-induced apoptosis in perfused heart. Exp Gerontol 2006;41:704–708. [DOI] [PubMed] [Google Scholar]
- 45.Moosmann B, Behl C: The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci USA 1999;96:8867–8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menazza S, Sun J, Appachi S, Chambliss KL, Kim SH, Aponte A, Khan S, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW, Murphy E: Non-nuclear estrogen receptor alpha activation in endothelium reduces cardiac ischemia-reperfusion injury in mice. J Mol Cell Cardiol 2017;107:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Bassossy HM, Eid BG: Cyclosporine A exhibits gender-specific nephrotoxicity in rats: Effect on renal tissue inflammation. Biochem Biophys Res Commun 2018;495:468–472. [DOI] [PubMed] [Google Scholar]
- 48.Jäger W, Xu H, Wlcek K, Schüler C, Rubel F, Erben RG: Gender- and dose-related effects of cyclosporin A on hepatic and bone metabolism. Bone 2012;50:140–148. [DOI] [PubMed] [Google Scholar]
- 49.Muller V, Szabo AJ, Erdely A, Tain Y-L, Baylis C: Sex differences in response to cyclosporine immunosuppression in experimental kidney transplantation. Clin Exp Pharmacol Physiol 2008;35:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emanuele MA, Wezeman F, Emanuele NV: Alcohol’s effects on female reproductive function. Alcohol Res Health 2002;26:274–281. [PMC free article] [PubMed] [Google Scholar]