Abstract
Introduction:
Preeclampsia represents a major public health burden worldwide, but predictive and diagnostic biomarkers are lacking. Metabolomics is emerging as a valuable approach to generating novel biomarkers whilst increasing the mechanistic understanding of this complex condition.
Objectives:
To summarize the published literature on the use of metabolomics as a tool to study preeclampsia.
Methods:
PubMed and Web of Science were searched for articles that performed metabolomic profiling of human biosamples using either Mass-spectrometry or Nuclear Magnetic Resonance based approaches and which included preeclampsia as a primary endpoint.
Results:
Twenty-eight studies investigating the metabolome of preeclampsia in a variety of biospecimens were identified. Individual metabolite and metabolite profiles were reported to have discriminatory ability to distinguish preeclamptic from normal pregnancies, both prior to and post diagnosis. Lipids and carnitines were among the most commonly reported metabolites. Further work and validation studies are required to demonstrate the utility of such metabolites as preeclampsia biomarkers.
Conclusion:
Metabolomic-based biomarkers of preeclampsia have yet to be integrated into routine clinical practice. However, metabolomic profiling is becoming increasingly popular in the study of preeclampsia and is likely to be a valuable tool to better understand the pathophysiology of this disorder and to better classify its subtypes, particularly when integrated with other omic data.
Keywords: metabolomics, preeclampsia, pregnancy, hypertension, carnitines, lipid dysregulation
1. Introduction
Current estimates suggest that preeclampsia affects 2% to 8% of all pregnancies and contributes to the more than half a million pregnancy-related deaths worldwide each year (Duley 2009). Preeclampsia is defined as a systolic blood pressure of ≥ 140 mmHg and/or a diastolic pressure of ≥ 90 mmHg in previously normotensive pregnant women after 20 weeks’ gestation, accompanied by proteinuria of ≥ 300 mg in a 24-hr urine collection (Tranquilli, Dekker, Magee, Roberts, Sibai, Steyn, Zeeman et al.). It is a heterogeneous disorder that is commonly classified by timing of onset; early-onset (EO-PE) diagnosed before 34 weeks gestation and late-onset (LO-PE) diagnosed after 34 weeks. These two subtypes are hypothesized to differ in their etiology and manifestations. EO-PE is thought to result from impaired trophoblastic invasion of the maternal spiral arteries and tends to have increased severity and higher incidence of fetal growth restriction. LO-PE appears to be more closely related to pre-existing maternal vascular disorders and other medical comorbidities (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012). However, emerging evidence suggests additional subtypes or endotypes (subtype defined by a distinct functional or pathobiological mechanism) may co-exist within these classifications (Leavey, Benton, Grynspan, Kingdom, Bainbridge, Cox 2016). Management and the study of preeclampsia are further complicated by its close relationship to a number of other pregnancy related disorders. In order to tolerate a genetically incompatible fetus, pregnant women undergo complex physiological changes. However in addition to allowing her to carry a child, these adaptions can render her insulin resistant, thrombophilic, immunosuppressed, hypervolemic and dislipidemic (Kaaja, Greer 2005). This state may manifest as preeclampsia, but can also lead to gestational hypertension, gestational diabetes, or cardiovascular disorder. Similarly preeclampsia and other pregnancy complications are inextricably linked with pre-term birth (Ananth, Ananth, Vintzileos 2006).
Currently, the pathology of preeclampsia , its differing subtypes and endotypes, and its relationships to other pregnancy complications are not fully understood, there are no validated predictive biomarkers of preeclampsia, few therapeutic options exist, and delivery remains the only definitive cure. Novel approaches are required for the study, prediction, diagnosis, classification, management and treatment of this condition (Navaratnam, Alfirevic, Baker, Gluud, Grüttner, Kublickiene, Zeeman et al. 2013).
Widespread alterations in metabolite levels have been demonstrated to precede the clinical onset of preeclampsia, and to continue with the progression of the disorder (Benton, Ly, Vukovic, Bainbridge ; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008). Consequently metabolomics, the study of all the small molecule metabolites in a biological sample, may be able to characterize these metabolic differences, inform on the biology underlying them and identify novel preeclampsia biomarkers. Metabolomics is also uniquely placed to identify different endotypes of this condition (Comhair, McDunn, Bennett, Fettig, Erzurum, Kalhan 2015), and to distinguish it from other pregnancy complications. This review aims to summarize the current literature in an effort to gain a better understanding of the potential role of metabolomics in the study and management of preeclampsia.
2. Methods
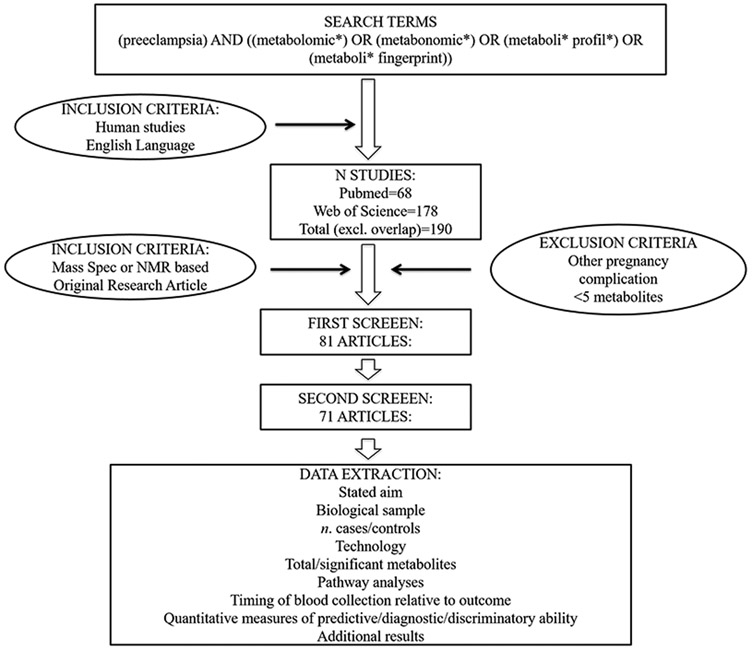
PubMed and Web of Science were searched for studies focusing on the metabolomics of preeclampsia using the following terms; (preeclampsia) AND ((metabolomic*) OR (metabonomic*) OR (metaboli* profil*) OR (metaboli* fingerprint)) (Figure 1). Studies were eligible for inclusion if the primary aim or outcome was the identification of metabolomic markers or profiles for the prediction, diagnosis, classification, or monitoring of preeclampsia, if they used human samples, and mass spectrometry or nuclear magnetic resonance (NMR) spectroscopy and if they were written in English. Exclusion criteria included, reporting on less than five metabolites. References of identified studies were reviewed for additional relevant literature, and authors were contacted if the articles could not be accessed. Searches were conducted independently by two of the authors.
Figure 1: Search Strategy.
3. Results
3.1. Selected Studies
Twenty-eight studies focusing on the metabolomics of preeclampsia were identified (Table 1). All were published between 2004 and 2017. Eleven studies compared metabolic profile of preeclampsia cases with controls before diagnosis, and 17 after diagnosis. Sixteen studies stated that these comparisons were in order to identify biomarkers of preeclampsia. Results were presented as either all preeclampsia, or stratified by EO-PE or LO-PE. The studies were not entirely independent; Kuc et al. (2014) and Koster et al. (2015) were based on the same population of 667 women; Turner et al. 2007 (Turner, Brewster, Simpson, Walker, Fisher 2007) and 2008 (Turner, Brewster, Simpson, Walker, Fisher 2008) on the same 21 women; Kenny et al. 2005 and 2008 sampled from the same prospective study; and the 2015 Bahado-Singh et al. used the same population as their 2012 study, with the addition of 30 cases and 60 controls for validation purposes. All studies utilized unaffected pregnancies as controls.
Table 1:
Twenty-eight Metabolic Studies of Preeclampsia Included in the Review
| Biologic media | Authors | Technology | Primary aim/outcome | Population | Comparison group |
|---|---|---|---|---|---|
| Serum | Bahado-Singh R.O., et al. (2013)(14) | NMR spectroscopy | Predictive first trimester biomarkers of LO-PE and a comparison of EO-PE and LO-PE | 30 LO-PE cases, 30 EO-PE cases, and 59 healthy pregnant controls, UK | Blood serum from healthy controls |
| Bahado-Singh R.O., et al. (2015)(15) | NMR spectroscopy | Predictive first trimester biomarkers of EO-PE; validation of Bahado-Singh et al. (2013)(14) | 50 EO-PE cases, 108 healthy pregnant controls, UK | Blood serum from healthy controls | |
| Kenny L.C., et al. (2008) | LC-MS | Diagnostic biomarkers of PE; validation of Kenny et al. (2005)(23) | 20 primiparous PE cases and 20 healthy pregnant controls, UK | Blood serum from matched healthy controls | |
| Odibo A.O., et al. (2011) | LC-MS | Predictive first trimester biomarkers of PE | 41 PE cases, 41 healthy pregnant controls, USA | Blood serum from healthy matched controls | |
| Koster M.P.H., et al. (2015) | LC-MS | Predictive first trimester biomarkers of EO-PE and LO-PE | 68 EO-PE cases, 99 LO-PE cases, 500 healthy pregnant controls, Netherlands | Blood serum from healthy, unmatched controls | |
| Kuc S., et al. (2014) | LC-MS | Predictive first trimester biomarkers of EO-PE and LO-PE | 68 EO-PE cases, 99 LO-PE cases, 500 healthy pregnant controls, Netherlands | Blood serum from healthy controls | |
| Chen T. et al (2017) | LC-MS | Diagnostic biomarkers of PE | 20 PE cases, 20 pregnant healthy controls, China | Blood serum from healthy controls | |
| Urine and serum | Austdal M., et al. (2014) | NMR spectroscopy | Comparison of metabolic profiles in urine and serum between PE cases and controls | 10 PE cases, 10 healthy pregnant controls, 10 healthy non-pregnant control, Norway | Blood serum and urine from healthy matched controls |
| Austdal M., et al. (2015a) | NMR spectroscopy | Predictive first trimester biomarkers of PE | 26 PE cases, and 552 healthy pregnant women, Norway | Blood and urine from women with healthy pregnancies | |
| Urine | Diaz S.O., et al. (2013) | NMR spectroscopy | Predictive second trimester biomarkers of PE | 9 PE cases and 84 healthy pregnant controls, Portugal | Urine from healthy controls |
| Plasma | Bahado-Singh R.O., et al. (2012) | NMR spectroscopy | Predictive first trimester biomarkers of EO-PE | 30 EO-PE cases requiring delivery before 34 weeks, 60 healthy pregnant controls, UK | Blood plasma from matched healthy controls |
| De Oliveira L., et al. (2012) | MALDI-MS, MALDI-MS/MS | Characterize lipid profile of EO-PE | 8 EO-PE cases, 8 healthy pregnant controls, Brazil | Blood plasma from matched healthy controls | |
| Kenny L.C., et al. (2005) | GC-MS | Diagnostic biomarkers of PE | 87 PE cases, 87 healthy pregnant controls, UK | Blood plasma from healthy, matched controls | |
| Turner E., et al. (2007) | NMR spectroscopy | Diagnostic biomarkers of PE | 11 PE cases, 11 healthy pregnant controls, UK | Blood plasma from healthy controls | |
| Turner E., et al. (2008) | NMR spectroscopy | Diagnostic aromatic amino acid biomarkers of PE | 11 PE cases, 11 healthy pregnant controls, UK | Blood plasma from healthy matched controls | |
| Kenny L.C., et al. (2010) | LC-MS | Predictive second trimester biomarkers of PE | 60 PE cases, 60 healthy pregnant controls, UK | Blood plasma from healthy, matched controls | |
| Schott S., et al. (2012) | NMR spectroscopy | Comparison of metabolic profiles in plasma between PE cases and controls | 10 PE cases, 10 healthy pregnant controls, Germany | Blood plasma from healthy controls | |
| Pinto J., et al. (2014) | HILIC LC-MS | Comparison of second trimester phospholipid profiles of EO-PE | 4 EO-PE cases women, 14 healthy pregnant women, Portugal | Blood plasma from healthy, matched controls | |
| Kelly R.S., et al. (2016) | HILIC LC-MS | Predictive first trimester biomarkers of PE | 47 PE cases; 62 healthy pregnant controls, USA | Blood plasma from healthy, matched controls | |
| Braekke K., et al. (2007) | LC-MS | Comparison of plasma metabolomic profiles of PE | Vein: 38 PE cases, 46 healthy pregnant controls Artery: 26 PE cases, 32 healthy pregnant controls, Norway |
Maternal and umbilical vein and artery blood plasma from healthy controls (delivery by C-section) | |
| Placenta and Intrauterine Tissue | Austdal M., et al. (2015, b) | HR-MAS MRS | Diagnostic biomarkers of PE and severe PE | 19 PE cases, 15 healthy pregnant controls, Norway | Placental tissue samples from healthy controls |
| Dunn W.B., et al. (2012) | GC-ToF-MS, UPLC-MS | Molecular, pathophysiological changes due to preeclampsia | 5 PE cases (vaginal birth); 5 PE cases (C-section); 6 healthy pregnant controls (vaginal birth); 5 healthy pregnant controls (C-section), UK | Placental tissue samples from healthy controls with vaginal or C-section births | |
| Sohlberg S. et al. (2014) | 31P-MRS | Characterize energy and membrane metabolism in the preeclamptic placenta | 5 EO-PE cases, 9 LO-PE cases, 7 healthy women in early pregnancy, 9 pregnant women in late pregnancy, Sweden | Placental scans of healthy matched controls | |
| Jain S. et al (2004) | ESI/MS | Characterize phospholipids in the preeclamptic placenta | 3 PE cases, 5 healthy pregnant controls, USA | Placental extracts from healthy controls | |
| Pearson T. et al (2010) | LC-MS | To determine whether levels of nonprostanoid eicosanoids, epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids (HETEs) are altered in preeclampsia |
Women undergoing cesarean section due to: PE (n=8), Intrauterine growth restriction (n=6), fetal distress/failed labor (n=6), self-selection (n=5) , UK | placenta plus matched myometrium from three other groups undergoing cesarean section | |
| Baig S. et al. (2013) | LC-MS | To determine whether Syncytiotrophoblast microvesicle lipid composition differs in pathologic and normal pregnancies | 6 PE cases, 9 cases recurrent miscarriage, 5 healthy pregnant controls, Singapore | Syncytiotrophoblast microvesicles from healthy matched controls | |
| Korkes et al. (2014) | MALDI TOF-TOF MS | To characterize the lipid profile in the placenta and plasma of patients with preeclampsia | 10 PE cases, 10 healthy pregnant controls, Brazil | Plasma and placental samples from healthy controls | |
| Breast Milk | Dangat K. et al (2016) | NMR spectroscopy | To compare the metabolic profile of breast milk in PE cases versus healthy controls | 29 PE cases, 31 pregnant healthy controls, India | Breast milk from healthy controls |
NMR-Nuclear Magnetic Resonance; LO-PE-Late-onset Preeclampsia; EO-PE-Early-onset preeclampsia; LC-MS-Liquid Chromatography; MALDI- Matrix-assisted laser desorption/ionization; C-section-Cesarean section; HR-MAS- High resolution magic angle spinning; MRS-Magnetic resonance spectroscopy; ToF – Time of flight; UPLC- Ultra high performance liquid chromatography; HILIC-Hydrophilic interaction chromatography; ESI-Electrospray Ionization
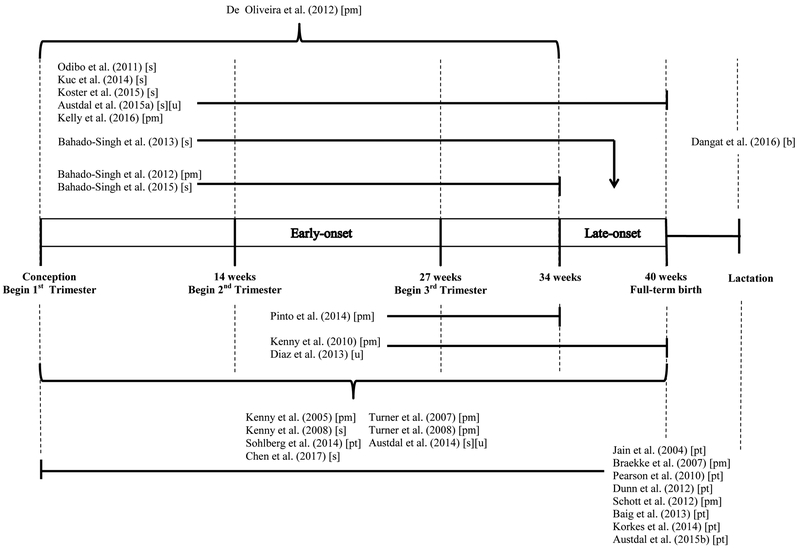
Blood plasma was the most commonly used biologic media (n = 10) (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012; De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010; Kenny, Dunn, Ellis, Myers, Baker, Kell 2005; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011; Pinto, Almeida, Martins, Duarte, Barros, Galhano, Pita et al. 2015; Schott, Hahn, Kurbacher, Moka 2012; Turner, Brewster, Simpson, Walker, Fisher 2007; Turner, Brewster, Simpson, Walker, Fisher 2008), followed by serum (n = 7) (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Chen, He, Tan, Xu 2017; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), and placental or intrauterine tissue samples (n = 7) (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Baig, Lim, Fernandis, Wenk, Kale, Su, Biswas et al. 2013; Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Jain, Jayasimhulu, Clark 2004; Korkes, Sass, Moron, Câmara, Bonetti, Cerdeira, Da Silva et al. 2014; Pearson, Zhang, Arya, Warren, Ortori, Fakis, Khan et al. 2010; Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.). The remaining studies used urine (n = 1) (Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013), urine and serum (n = 2) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015), or breast milk (n=1) (Dangat, Upadhyay, Kilari, Sharma, Kemse, Mehendale, Lalwani et al. 2016). Sixteen studies performed metabolic profiling with Mass Spectrometry (MS), while 12 used a form of nuclear magnetic resonance spectroscopy (NMR). Ten studies were targeted or semi-targeted to the measurement of specific groups of metabolites (Baig, Lim, Fernandis, Wenk, Kale, Su, Biswas et al. 2013; Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007; Jain, Jayasimhulu, Clark 2004; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011; Pearson, Zhang, Arya, Warren, Ortori, Fakis, Khan et al. 2010; Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014; Schott, Hahn, Kurbacher, Moka 2012; Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.). The number of metabolites measured differed according to the choice of technology and approach. The minimum number of metabolites for inclusion in the review was five (Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.), while as many as 8000 features, as defined by their mass (mz) and retention time (rt) were included in the untargeted studies (Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016). Of the prospective studies and nested case-control studies, seven analyzed first trimester metabolites (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011), while only three analyzed second trimester metabolites (Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010; Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014) (Figure 2).
Figure 2: Graphic representation of sample collection relative to the timing of onset of the preeclampsia cases studied onset of interest.
Studies are listed by time of sample collection, with an arrow indicating the onset of interest. Studies that look at both EO and LO biomarkers are shown here as observing the duration of the pregnancy.
Biological specimen collected: [s] serum; [u] urine; pm [plasma]; pt [placental and intrauterine tissue]; b [breast milk]
3.2. Comparison of metabolomics profiles prior to diagnosis
3.2.1. Preeclampsia
Seven studies compared metabolomic profiles of biological samples between controls and cases of any time of onset (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011). Lactate was consistently shown to be decreased in the blood of women who went on to develop preeclampsia, while histidine was consistently increased. Risk of the subsequent development of preeclampsia was also associated with an increase in triglycerides, lipids, phospholipids, and fatty acids in serum and in plasma (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010). Various carnitines were reported as significant (p < 0.05) in four studies spanning serum, plasma and urine (Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011), although with inconsistent directions of effect. Similarly, urinary measures of hippurate were associated with an increased risk of preeclampsia in the study of Diaz et al. (Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013), but a decreased risk in Austdal et al.’s 2015 study (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015).
Among the studies that developed predictive models, the most accurate was Kenny et al. (2010), which, after validation, used 34 metabolites measured in plasma to generate a PLS-DA model with R2 and Q2 values of 0.57 and 0.53, respectively. Further testing with ROC curve analysis determined the area under the curve (AUC) was 0.95, . The lowest accuracy was produced by a combination of triglycerides, 3-hydroxybutyrate, pyruvate, phosphatidylcholine, and lactose measured in serum, yielding an accuracy of 64.6%, 63.8% sensitivity, and 65.4% specificity (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015). Austdal et al. (2015) (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015) compared the ability of metabolomic biomarkers to improve upon traditionally used biomarkers of preeclampsia; a combination of mean arterial pressure (MAP), maternal age, and uterine artery pulsatility index (UtAPI) resulted in a model with an AUC of 0.738, which increased to 0.807 when including the ratio of hippurate to creatinine in urine. Similarly, Kelly et al. (2016) (Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016) reported that the inclusion of a summary score based on 72 plasma metabolites significantly outperformed a baseline model including maternal age and race, study site, and gestational age (AUC: 0.573 versus AUC: 0.753).
3.2.2. Early-onset preeclampsia
Five studies investigated EO-PE (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014; Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014), three of which were targeted to specific metabolite classes; amines and oxylipins (Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), acylcarnitines (Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015) and phospholipids (Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014),. Bahado-Singh et al. validated a number the serum metabolites from their 2013 study (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013) in a 2015 follow up study (Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015), including increased levels of propylene glycol and 3-hydroxyisovalerate and decreased levels of formate, choline, 3-hydroxyisovalerate, succinate, phenylalanine, glycerol, glycine, glucose, isopropanol, and acetate.
In a separate plasma-based study Bahado-Singh et al. (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012) concluded that the addition of three metabolites; 3-hydroxyisovalerate,citrate and glycerol, improved the predictive ability of a clinical panel including parity, uterine artery pulsatility index (UtAPI), fetal crown rump length, and maternal medical history, reporting an AUC of 0.98. Interestingly, only one of these metabolites; 3-hydroxyisovalerate was among those validated in the serum based studies noted above. Conversely, Kuc et al. 2014 (Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014) and Koster et al. 2015 (Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015) noted little to no improvement in the prediction of EO-PE with the inclusion of taurine and asparagine or acylcarnitines, compared to maternal characteristics (MAP, pregnancy-associated plasma protein A and placental growth factor) alone.
3.2.3. Late-onset Preeclampsia
Three studies also reported on LO-PE, and all were serum based (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014). Again, carnitine levels were increased in women who went on to develop LO-PE (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015). However, Koster et al. 2015 (Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015) observed that the inclusion of stearoylcarnitine in a predictive risk model provided only an incremental improvement over the clinical risk evaluation based on maternal clinical characteristics and mean MAP. Similarly Kuc et al. 2014 (Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014) determined that these clinical indices were more effective predictors of LO-PE risk than metabolomic profiling. However, Bahado-Singh et al. 2013 reported a model with an AUC of 0.96, 76.7% sensitivity and 100% specificity, using a combination of valine, pyruvate, 3-hydroxybutyrate, 1-methylhistidine, glycerol, trimethylamine (a downstream metabolite of carnitine) and maternal characteristics (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013).
3.2.4. Overlap between the predictive metabolites
Despite the potential differences in the pathophysiology of EO-PE as compared to LO-PE, a large number of metabolites appeared to be associated with both subtypes. Among those, the levels of carnitines, glucose, pyruvate, 3-hydroxyisovalerate, glycine and their related metabolites were consistently associated with both late and early onset preeclampsia as compared to normal pregnancy in blood and urine, and were also identified in studies that did not stratify by the timing of onset. This may reflect the fact that these two subtypes likely share some underlying biological mechanisms, or more crucially support the hypothesis that classification by age at onset cannot capture all the nuanced subtypes and endotypes of preeclampsia (Leavey, Benton, Grynspan, Kingdom, Bainbridge, Cox 2016). Conversely, glycerol, isopropanol, and trimethylamine were linked to both EO-PE and LO-PE; but with differing directions of effect, which is in agreement with Bahado-Singh et al.’s (2013) finding that serum levels of glycerol, trimethylamine, and succinate were significantly different when comparing early- and late-onset preeclamptic pregnancies (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013).
3.3. Comparison of metabolomic profiles post-diagnosis
3.3.1. Preeclampsia
Seventeen studies compared metabolomic profiles between cases and controls either at or post diagnosis (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007; Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008; Kenny, Dunn, Ellis, Myers, Baker, Kell 2005; Schott, Hahn, Kurbacher, Moka 2012; Turner, Brewster, Simpson, Walker, Fisher 2007; Turner, Brewster, Simpson, Walker, Fisher 2008). Phospholipids, lipoproteins (n = 5) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Korkes, Sass, Moron, Câmara, Bonetti, Cerdeira, Da Silva et al. 2014; Schott, Hahn, Kurbacher, Moka 2012; Turner, Brewster, Simpson, Walker, Fisher 2007), creatinine (n = 2) (Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008), histidine (n = 2,) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Turner, Brewster, Simpson, Walker, Fisher 2008), glutamate-related metabolites (n = 2) (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008), glycine-related metabolites (n = 2) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012), and alanine-related metabolites (n = 3) (Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008; Turner, Brewster, Simpson, Walker, Fisher 2008) were all significant (p < 0.05) in at least two studies. Increased creatinine, alanine, and phenylalanine levels were consistently associated with preeclampsia, while glycine, glutamate, and glutamine levels were inversely associated. Opposite directions of effect were reported for histidine in plasma as opposed to serum (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Turner, Brewster, Simpson, Walker, Fisher 2008). Interestingly, amino acid concentrations have previously been shown to correlate poorly across serum and plasma, relative to other metabolite classes (Yu, Kastenmüller, He, Belcredi, Möller, Prehn, Mendes et al. 2011). The association with lipids was similarly inconclusive; Schott et al. (2012) (Schott, Hahn, Kurbacher, Moka 2012) reported no significant change in the total amount of phospholipids between the two groups, which was supported by Jain et al. (Jain, Jayasimhulu, Clark 2004) but both proposed there may be an alteration in the overall lipid profile.
The studies searching for biomarkers reported impressive AUCs. The most accurate diagnostic model was developed in placental tissue; it included glycerophosphocholine (GPC), phosphocholine (PCho), aspartate, ascorbate, ethanolamine (EtAm), taurine, glutamate, and glycine, and produced an AUC of 0.927, with a sensitivity of 87%, and a specificity of 98% (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015). While phosphatidylcholine (14:0/00) measured in serum had an AUC of 0.935 (Chen, He, Tan, Xu 2017). Another study reported an AUC, sensitivity and specificity of 0.900, 90% and 100% respectively using a combination of urinary choline, creatine, and glycine (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014)., Kenny et al.(2005) achieved 100% sensitivity and 98% specificity with a combination of three unnamed metabolite peaks in plasma (Kenny, Dunn, Ellis, Myers, Baker, Kell 2005).Finally, Dangat et al. (Dangat, Upadhyay, Kilari, Sharma, Kemse, Mehendale, Lalwani et al. 2016) determined using PLS-DA that a combination of seven metabolites in breast milk could discriminate cases from controls even up to six months after delivery (R2=0.93, Q2=0.61).
3.3.2. Early-onset Preeclampsia
Only two studies focused on early-onset preeclampsia, both of which were targeted to the study of lipid metabolism (De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012; Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.). Sohlberg et al. (Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.) were able to detect increases in phosphodiesters (PDE) and phosphomonoesters (PME) in the placental tissue of EO-PE cases. De Oliveira et al. (De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012) did not quantify their results, but stated that good resolution could be achieved using partial least squares discriminant analysis (PLS-DA) and plasma lipid profiles.
3.3.3. Other comparisons
To date, only one study, by Braekke et al. (Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007), has compared =metabolomic profiles of early- and late-onset PE cases. Unlike Bahado-Singh et al.’s (2013) predictive study in serum referenced above, Braekke et al. reported that early- and late-onset PE could not be distinguished based on a plasma metabolomic profile, and furthermore the profile could not distinguish cases by severity. However, it should be noted these studies utilized samples conducted at different time points and are not necessarily comparable. Austdal et al. (2015b) (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015), who conducted their study in placenta, were able to classify individuals by severity using the first principal component of 25 metabolites, including aspartate, phosphocholine, glycerophosphocholine, glutamate, ascorbate, glutamine and taurine. Interestingly, taurine was also identified to be predictive of EO-PE in Kuc et al.’s study (Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), the more severe manifestation of disease, potentially due to its role in placenta trophoblast development and survival (Desforges, Parsons, Westwood, Sibley, Greenwood 2013).
3.4. Similarities between metabolomics profiles prior and post-diagnosis
Identifying metabolites that ae altered in preeclamptic women both before and at diagnosis provides important information on the clinical continuum and how early before the onset of clinical symptoms the disease may be manifesting.. P-cresol sulfate (n = 2 studies) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013), stearoylcarnitine (n = 2) (Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015), glycine and related metabolites (n = 3) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012), and lactate and related metabolites (n = 3) (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Turner, Brewster, Simpson, Walker, Fisher 2007) were decreased both before and during the clinical manifestation of preeclampsia. Similarly, creatinine (n = 4) (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008), alanine (n = 3) (Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011), histidine-related metabolites (n = 4) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Turner, Brewster, Simpson, Walker, Fisher 2008), dimethylamine (n = 3) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016), and vitamin D derivatives (n = 2) (Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010) were increased. The findings of Pinto et al. (Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014) also suggest that alterations in lipid metabolism associated with EO-PE can be detected as early as the second trimester and persist for the duration of the pregnancy. The consistency of metabolites found in studies using biological samples extracted both pre- and post-diagnosis suggests that the metabolic alterations detected at diagnosis may have been present in the earlier stages of the affected pregnancy.
4. Discussion
4.1. Comparisons with other conditions
As noted in the introduction, preeclampsia is closely linked to a number of other adverse disorders of pregnancy, complicating the definition of preeclampsia endotypes. In assessing the utility of metabolomics both for understanding underlying biology and identifying biomarkers, it is important to determine whether the observed changes in the metabolome are specific to preeclampsia. Austdal et al. (2014) (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014) attempted to define a metabolome of uncomplicated pregnancy and determined that a number of the aforementioned ‘preeclampsia metabolites,’ including alanine, choline, and lactate, also differ in pregnant women compared to non-pregnant controls. Further, it has been demonstrated that the metabolome changes throughout pregnancy; a previous comparison of amniotic fluid and maternal plasma identified 21 metabolites that were significantly different between second and third trimester samples from healthy pregnancies (Orczyk-Pawilowicz, Jawien, Deja, Hirnle, Zabek, Mlynarz 2016). Again these included several ‘preeclampsia metabolites’ such as carnitine, creatinine, pyruvate, glucose, alanine, and phenylalanine. Crucially, these did not include lipid mediators, which may therefore be more sensitive biomarkers of the inflammatory process in preeclamptic pregnancies
Several of the included studies in this review also considered other adverse pregnancy outcomes. Austdal et al. (2015) (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015) identified predictive biomarkers of gestational hypertension, which included dimethylamine (DMA), phenylacetylglutamine (PAG), and alanine in the urine, and triglycerides, high density lipoprotein (HDL) cholesterol, lactate, N-acetyl glycoproteins, phosphatidylcholine, and glucose in serum; all of which have been implicated in preeclampsia. Similarly, Diaz et al. (2013) (Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013) analyzed the urinary metabolomic profiles of women with gestational diabetes mellitus (GDM) and was able to identify several metabolic trends common to both GDM and preeclampsia, including decreases in lactose, glycine, creatinine, and an increase in glucose (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013). Diaz et al. (Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013) also demonstrated considerable overlap between metabolites associated with preterm birth (PD) (delivery < 37 weeks of gestation) and preeclampsia including an increase in 3-hydroxybutyrate (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013), citrate (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013), and histidine (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Turner, Brewster, Simpson, Walker, Fisher 2008). Biag et al. (Baig, Lim, Fernandis, Wenk, Kale, Su, Biswas et al. 2013) reported that dysregulation of lipid metabolism was evident in the placental syncytiotrophoblast microvesicles of women with preeclampsia and those with recurrent miscarriage relative to controls, and specifically that levels of sphingomyelin and phosphatidylserine were increased while phosphatidylinositol. Similarly Pearson et al. (Pearson, Zhang, Arya, Warren, Ortori, Fakis, Khan et al. 2010) observed dysregulation of vasoactive metabolites in women undergoing cesarean due to preeclampsia or a growth restricted pregnancy, relative to elective cesareans. These findings are not unexpected given the strong correlation between these disorders and preeclampsia, and further work is required to disentangle the shared and distinct metabolic dysregulation underlying these conditions, and particularly the role that lipid metabolism may be playing.
Obesity is an established risk factor for preeclampsia (Poston, Caleyachetty, Cnattingius, Corvalán, Uauy, Herring, Gillman 2016). Accordingly, there is significant overlap in the metabolites highlighted for these two conditions. Hippurate has previously been associated with obesity in a number of metabolomics studies, which may relate to compositional differences in the gut microbiome associated with BMI (Lees, Swann, Wilson, Nicholson, Holmes 2013). Other preeclampsia metabolites including triglycerides, lipids, phospholipids, and fatty acids (Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010) comprise a well-known metabolomic signature of immune dysfunction and systemic low-grade inflammation (Ruiz-Núñez, Dijck-Brouwer, Muskiet 2016) which is a key feature of obesity induced dyslipidemia. Carnitines and fatty acid metabolism are also associated with obesity (Wahl, Yu, Kleber, Singmann, Holzapfel, He, Mittelstrass et al. 2012) through the exertion of immune regulatory functions supported predominantly by carnitine-dependent energy production from fatty acids (Famularo, de Simone, Trinchieri, Mosca 2004; Ruiz-Núñez, Dijck-Brouwer, Muskiet 2016).
Alterations to carnitine and fatty acid metabolism has been demonstrated in a wide range of other idiopathic inflammatory disorders and in cardiovascular disease (Famularo, de Simone, Trinchieri, Mosca 2004; Ruiz-Núñez, Dijck-Brouwer, Muskiet 2016). Similar to obesity, the literature suggests very high concordance between the cardiovascular and preeclampsia associated metabolites. Intriguingly there is also evidence that both obesity and cardiovascular disease share a similar genetic architecture to preeclampsia (Sitras, Fenton, Acharya 2015). Carnitines have further been associated with blood pressure and hypertension unrelated to pregnancy (Miguel-Carrasco, Mate, Monserrat, Arias, Aramburu, Vázquez 2008), as have 3-hydroxyisovalerate (Jennings, MacGregor, Pallister, Spector, Cassidy 2016), hippurate (Lees, Swann, Wilson, Nicholson, Holmes 2013) and glycine (Currie, Schulze, Zechner, Walther, Farese Jr 2013), among others. Again these relationships are likely due to various roles of these metabolites in oxidative stress, inflammation and immune dysregulation.
Interestingly, there are also a number of similarities between the metabolites identified in the preeclampsia studies and those identified in studies of asthma including acetate, adenosine, alanine, hippurate, succinate, threonine and trans-aconitate, as well as pathways relating to hypoxia response, oxidative stress, immunity, inflammation and lipid metabolism (Kelly, Dahlin, McGeachie, Qiu, Sordillo, Wan, Wu et al. 2016). These relationships may help explain the recently reported association between preeclampsia and risk of asthma in the offspring (Stokholm, Sevelsted, Anderson, Bisgaard 2016) and further suggests shared pathophysiology between the two disorders.
4.2. Methodological Considerations
Two differing technologies were utilized by the identified studies; Mass-spec and NMR. Each has advantages and disadvantages NMR spectroscopy is quantitative and requires less intensive sample preparation making it more reproducible between studies, while MS is inherently more sensitive allowing for the detection of a much larger number of metabolites (Emwas 2015). The complement of metabolites measured by these two methods are not necessarily comparable. In this review this is further complicated by the hypothesis–driven targeted approach taken in a number of the studies, limiting the potential for replication and validation. Interestingly, no study employed both technologies on the same sample, yet combining the two may provide the greatest opportunity to develop robust and accurate metabolomic profiles of preeclampsia. The chosen technology also dictated the reporting of results to an extent. The level of quantitative detail varied between the studies, and a number of the non-biomarker studies (De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012; Jain, Jayasimhulu, Clark 2004; Korkes, Sass, Moron, Câmara, Bonetti, Cerdeira, Da Silva et al. 2014; Pearson, Zhang, Arya, Warren, Ortori, Fakis, Khan et al. 2010; Schott, Hahn, Kurbacher, Moka 2012; Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al. ; Turner, Brewster, Simpson, Walker, Fisher 2007; Turner, Brewster, Simpson, Walker, Fisher 2008) did not report effect sizes for individual or groups of metabolites, but rather commented on general trends observed in the results. This was particularly common among the NMR studies which often reported spectral regions rather than actual metabolites. This limits the utility of these findings in understanding biology, although they do serve as further supportive evidence for the overarching conclusion that the preeclamptic metabolome, as measured in a variety of biological samples, differs from that of a healthy pregnancy.
As the placenta is the interface between mother and baby, metabolomic profiling of the placenta and related intrauterine tissues may provide some of the most biologically informative findings, and some of the strongest findings were reported for placenta based studies (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015). Unfortunately, due to its relative inaccessibility for the duration of the pregnancy, most studies can only sample placental and intrauterine tissue following delivery. Only one study included ultrasound information about the placenta in the third trimester, but even then, observation was limited to anterior placentas due to technical limitations (Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.). Furthermore, because metabolites identified in the later stages of pregnancy cannot be assumed to correspond to those detected in earlier trimesters, these metabolites may only generate hypotheses for future investigations into their predictive and diagnostic merit. In contrast, blood and urine are considerably more accessible and have greater potential for clinical translation. The discriminatory ability was largely comparable between these different bio specimens in the included studies. It should be noted that it has been reported that measures of metabolites in plasma may be more reproducible that serum due to the less intensive sample preparation requirements (Yu, Kastenmüller, He, Belcredi, Möller, Prehn, Mendes et al. 2011). However, it is unclear based on these studies which specimen type is most ideal for identifying metabolomic biomarkers of preeclampsia and how specimen choice influences findings.
Regardless of the bio-specimen used, he metabolome is dynamic and reflects both the endogenous and exogenous metabolites present in the body at a given point in time. As such, it is highly susceptible to the influence of age, diet, BMI, comorbidities and other unknown factors. Matching was used in a number of studies to address potential confounding factors. However, regardless of matched status, significant epidemiological differences between cases and controls were noted in many studies for factors that may have influenced the findings such as race (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011), maternal weight or BMI (Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016; Turner, Brewster, Simpson, Walker, Fisher 2007; Turner, Brewster, Simpson, Walker, Fisher 2008), fetal birth weight (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007; Kenny, Dunn, Ellis, Myers, Baker, Kell 2005; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011; Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.), history of hypertension (Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), parity (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), UtAPI (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Sohlberg, Wikström, Olovsson, Lindgren, Axelsson, Mulic-Lutvica, Weis et al.), MAP (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014), gestational age (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011), smoking status (Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), fetal growth restriction (FGR) (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015), and/or maternal age (Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015). In particular, differences in maternal BMI were shown to influence findings in some studies (Turner, Brewster, Simpson, Walker, Fisher 2007; Turner, Brewster, Simpson, Walker, Fisher 2008). Race may also have an important effect due to the higher risk of preeclampsia that has been reported among Black and Hispanic women (Tanaka, Jaamaa, Kaiser, Hills, Soim, Zhu, Shcherbatykh et al. 2007). Five studies described the racial breakdown by percentage (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011), and two studies did not report the cohort’s ethnicity, but mentioned that it was adjusted for in the final analysis (Kenny, Dunn, Ellis, Myers, Baker, Kell 2005; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014). Many studies included majority Caucasian populations (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010; Korkes, Sass, Moron, Câmara, Bonetti, Cerdeira, Da Silva et al. 2014; Turner, Brewster, Simpson, Walker, Fisher 2007), which may have implications for the wider generalizability of the findings. Nevertheless the existence of additional, unexplored confounding factors including diet, vitamin supplementation, medication use, exercise, stress, or other medical disorders cannot be ruled out.
Additional biases and inconsistences in results may arise from technical factors related to sample collection and laboratory procedures. Such factors can add to the inherent ‘noisiness” of metabolomic data. . Only seven studies reported on quality control procedures (Braekke, Ueland, Harsem, Karlsen, Blomhoff, Staff 2007; Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016; Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010; Korkes, Sass, Moron, Câmara, Bonetti, Cerdeira, Da Silva et al. 2014; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), and only seven corrected for multiple comparisons (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014; Turner, Brewster, Simpson, Walker, Fisher 2007; Turner, Brewster, Simpson, Walker, Fisher 2008). Furthermore, only two reported power calculations (Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Odibo, Goetzinger, Odibo, Cahill, Macones, Nelson, Dietzen 2011). Thirteen studies sought to validate their results through additional testing, including internal cross validation (Austdal, Skråstad, Gundersen, Austgulen, Iversen, Bathen 2014; Austdal, Tangerås, Skråstad, Salvesen, Austgulen, Iversen, Bathen 2015; Austdal, Thomsen, Tangerås, Skei, Mathew, Bjørge, Austgulen et al. 2015; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2012; Bahado-Singh, Akolekar, Mandal, Dong, Xia, Kruger, Wishart et al. 2013; Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Chen, He, Tan, Xu 2017; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013), partitioning of data into a training and test set (Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Kenny, Dunn, Ellis, Myers, Baker, Kell 2005; Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), assessing a signature developed in first trimester samples in third trimester samples (Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016), or verification in an independent cohort (Kenny, Broadhurst, Dunn, Brown, North, McCowan, Roberts et al. 2010). Additionally, two of the studies were designed to validate previously published results (Bahado-Singh, Syngelaki, Akolekar, Mandal, Bjondahl, Han, Dong et al. 2015; Kenny, Broadhurst, Brown, Dunn, Redman, Kell, Baker 2008). Validation is particularly important for the correct interpretation of PLS-DA which tends to over-fit data. Two studies failed to present evidence of PLS-DA validation (De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012; Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014), although De Oliveira et al. (De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012) does state that the use of PLS-DA was not to assess predictive ability, but rather to confirm significant metabolites. Furthermore, Pinto et al. (Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014) only report Q2 values, which provides an estimate of prediction, but no indication of significance, and caution should be taken with the interpretation of such studies. It should also be noted that the human metabolome remains incompletely characterized. Only two studies reported specific un-annotated metabolites (Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Kelly, Croteau-Chonka, Dahlin, Mirzakhani, Wu, Wan, McGeachie et al. 2016). If these un-annotated metabolites were associated with disease, their omission could introduce bias to the biological interpretation of the findings.
Finally, it should be noted that a number of these studies are very limited by sample size with seven studies including less than ten preeclampsia cases in any one study group (Baig, Lim, Fernandis, Wenk, Kale, Su, Biswas et al. 2013; De Oliveira, Câmara, Bonetti, Turco, Bertolla, Moron, Sass et al. 2012; Diaz, Barros, Goodfellow, Duarte, Galhano, Pita, Almeida et al. 2013; Dunn, Brown, Worton, Davies, Jones, Kell, Heazell 2012; Jain, Jayasimhulu, Clark 2004; Pearson, Zhang, Arya, Warren, Ortori, Fakis, Khan et al. 2010; Pinto, Maciel, Melo, Domingues, Galhano, Pita, Almeida et al. 2014). Such studies are likely underpowered, although biologically relevant results in keeping with some of the larger studies were reported. Nevertheless, more weight should be given to the larger well-designed studies. The largest studies contained a total of 167 cases (Koster, Vreeken, Harms, Dane, Kuc, Schielen, Hankemeier et al. 2015; Kuc, Koster, Pennings, Hankemeier, Berger, Harms, Dane et al. 2014), and interestingly although these studies identified serum steroylcarnitine and taurine as potential predictive biomarkers they concluded at present metabolomics-based assays are not suitable for preeclampsia. Larger studies such as the ongoing multi-center IMPROvED (improved pregnancy outcomes via early detection) (Navaratnam, Alfirevic, Baker, Gluud, Grüttner, Kublickiene, Zeeman et al. 2013) study, which aims to recruit 5000 pregnant women in order to develop predictive metabolomic and proteomic signatures of preeclampsia, are required.
5. Conclusion
Metabolomics is a rapidly emerging field in preeclampsia that has the potential to address some of the biggest challenges in the management of this disorder; the development of biomarkers, the identification of endotypes and an increased understanding of pathogenesis. The published literature to date suggests metabolomics may be able to fulfill this potential with the identification of biologically meaningful metabolites and metabolomics profiles that can distinguish preeclamptic from normal pregnancies in a variety of biosamples both prior and post diagnosis.
The findings were reasonably consistent between studies, with lipids and carnitines among the most commonly highlighted metabolites. The results support the existence of a preeclampsia metabolome that is characterized largely by dysregulated fatty acid metabolism, which can be measured in a variety of biological samples, including blood, urine, placental tissue and breast milk. Even more intriguingly, these results suggest that the plasma metabolome may differ between early and late onset cases which is in agreement with the investigations implicating discriminant pathogenic pathways within these subtypes. But also, that there are likely further as yet unknown subtypes, or endotypes within these classifications. In fact metabolomics is likely to play a key role in defining such endotypes. Similar work has already been performed utilizing transcriptomic profiling in preeclampsia (Leavey, Benton, Grynspan, Kingdom, Bainbridge, Cox 2016).
In addition to metabolomics, multiple other omic studies have been conducted to try and better understand the molecular mechanisms underlying preeclampsia and to search for novel biomarkers. For example, a placental meta-signature that can identify preeclampsia cases has been developed. This was based on differential gene-expression and included genes relating to the pathways of angiogenesis, immunomodulation, vascular function and trophoblast invasion, many of which were also highlighted in this review (Kleinrouweler, van Uitert, Moerland, Ris-Stalpers, van der Post, Afink 2013). Similarly, almost 120 miRNAs have been reported to be dysregulated in preeclampsia (Jairajpuri, Almawi 2016). The most consistent being miR-210 which is associated with hypoxia, another process highlighted by the metabolomics studies. Oxidative stress has further been identified as a key process from proteomic studies of preeclampsia (Law, Han, Tong, Baker 2015). Multiple other processes identified in this review including dysregulation of lipids, steroidogenesis, membrane function and energy metabolism have also been reported for a number of different omics. Therefore, the most information and the most global understanding of preeclampsia subtypes can likely be garnered by integrating metabolomics with other hierarchical omic levels, and such work should form a key feature of the study of preeclampsia as the field moves forward.
To date, clinical translation of metabolomic biomarkers of preeclampsia has yet to be realized. . Additional studies addressing the clinical complexities of preeclampsia, as well as the technical complexities of metabolomic profiling, are required before clinical translation can become a reality. For example appropriate subtyping of preeclampsia, the identification of the ideal biological sample, the role of potential confounders and the best way to harness MS or NMR or both. Furthermore, the issue of parsimony must be taken into account and profiles containing large numbers of metabolites may prove to be unfeasible in a clinical setting. Larger scale studies in diverse populations with comprehensive phenotyping, including multiple biological media, covering a broad and detailed measure of the metabolome and utilizing sophisticated statistical methods that allow for the aforementioned complexities are required. Until such studies are performed, the currently published literature suggests the most important role metabolomic studies of preeclampsia can play will be as tools to begin to better understand preeclampsia pathophysiology, and to better define the subtypes comprising this condition.
Table 2:
Assessment of Proposed Metabolomic Biomarkers of Preeclampsia
| Authors | No. of metabolites | Biomarkers | ROC AUC | Sensitivity | Specificity | Additional results | Conclusions | Validation |
|---|---|---|---|---|---|---|---|---|
| PE vs. control prior to diagnosis | ||||||||
| Onset not specified or both early and late onset combined | ||||||||
| Kenny L.C., et al. (2010) | 457 (of which 45 were identified to be a unique molecular entity) | 14 metabolite model: 5-Hydroxytryptophan, monosaccharide(s), decanoylcarnitine, methylglutaric acid and/or adipic acid, oleic acid, docosahexaenoic acid, butyrolactone and/or oxolan-3-one, acetoacetic acid, hexadecenoyleicosatetraenoyl- snglycerol, di-(octadecadienoyl)- snglycerol, sphingosine 1-phosphate, sphinganine 1-phosphate, Vitamin D3 derivatives, 2-oxovaleric acid and/or oxo-methylbutanoic acid | 0.92 | R2 = 0.43 | Q2 = 0.39 | . | Plasma based metabolic profiles of early pregnancy plasma can predict PE | Validated in independent cohort |
| 34 metabolite model | 0.95 | R2 = 0.57 | Q2 = 0.53 | |||||
| Odibo A.O., et al. (2011) | 40 acylcarnitines and 32 amino acids | Hydroxyhexanoylcarnitine | 0.78 | First-trimester metabolomics serum profiling could be used in screening for PE | No | |||
| Phenylalanine | 0.8 | |||||||
| Glutamate | 0.79 | |||||||
| Alanine | 0.78 | |||||||
| Hydroxyhexanocylcarnitine, alanine, phenylalanine, glutamate | 0.82 | |||||||
| Alanine, phenylalanine, glutamate | 0.81 | |||||||
| Diaz S.O., et al. (2013) | 64,000 signals/data points | All variables (25591 signals b | CR = 91 | 23% | 93% | Full variable-selected models consistently performed better than those using a limited group | Second trimester maternal urine carries putatively predictive metabolites for PE | Cross validation |
| VIP>1 (9374 signals)b | CR = 93 | 65% | 93% | |||||
| VIP/VIPcvSE > 1 (21363 signals )b | CR = 92 | 33% | 93% | |||||
| b/bcvSE (9521 signals )b | CR = 93 | 55% | 93% | |||||
| Intersection of the last three methods (5793 signals)b | CR = 94 | 75% | 94% | |||||
| Kuc S., et al. (2014) | 58 amines, 46 oxylipids | EO-PE vs. control: | No oxylipids were found to be significant. | Taurine in serum may have a role in PE pathophysiology and first trimester screening for EO-PE | Data split into training and test set | |||
| Taurine + prior risk + MAP | 0.78 | 55% | FPR = 10% | |||||
| LO-PE vs. control: No metabolite models were validated | ||||||||
| Koster M., et al. (2015) | 24 acylcarnitines | EO-PE vs. control: | Acylcarnitines do not seem to aid in the prediction of preeclampsia | Metabolomics-based assays in serum are not yet suitable for clinical use. | Data split into training and test set | |||
| Stearoylcarnitine + prior risk, MAP | 0.747 | |||||||
| Taurineb + stearoylcarnitine + prior risk, MAP, PAPPA, PlGF | 0.784 | |||||||
| LO-PE vs. control: | ||||||||
| Stearoylcarnitine + prior risk, MAP | 0.692 | |||||||
| Steraoylcarnitine + prior risk, MAP, PAPPA, PlGF | 0.7 | |||||||
| Austdal M., et al. (2015, a) | 54 urinary metabolites | Urine | Addition of metabolites to a model including maternal characteristics contributes additional predictive power | Hippurate excretion precedes preeclampsia. | Cross validation | |||
| Hippurate:creatinine | 0.694 | 19.20% | ||||||
| Hippurate:creatinine + maternal MAP, age, UtAPI | 0.807 | 53.80% | ||||||
| Gly, 4-DEA, DMA, Hipp, Lac, Cre, ProlB | 51.30% | 74.20% | ||||||
| 30 serum metabolites | Serum | |||||||
| Triglycerides, 3-HB, pyruvate, PtdCho, Lac | 15% | 65.40% | ||||||
| Kelly et al. (2016) | 8099 metabolite features | Summary score based on 72 metabolite features including Cohibin A, 16-alpha-Hydroxypregnenolone, phosphocholines, 13'-Carboxy-gamma-tocopherol, Dimethamine Quinoline Montecristin, and (mono-, di- and tri-) glycerides | 0.752 (0.658, 0.846) | - | - | Integration of the metabolomic with transcriptomic data identified lipid imbalance, and dysfunction of the immune and circulatory system as key drivers of preeclampsia | It is possible to identify predictive metabolomics signatures of preeclampsia, but the most information can be obtained through integrative omics | Validation of predictive profiles generated in first trimester samples in third trimester samples |
| Early onset | ||||||||
| Bahado-Singh R.O., et al. (2012) | 42 metabolites | Logistic Regression: | There is a profound change in first-trimester urine metabolites when comparing EO-PE patients to healthy controls | Cross validation | ||||
| Citrateb, glycerol, hydroxyisovalerate, methionine + mat. weight, medical disorders | 0.904 | 75.90% | FPR = 4.9% | |||||
| + UtAPI + CRL ( − methionine) | 0.98 | 82.60% | FPR = 1.6% | |||||
| Genetic Programming: | ||||||||
| Glutamine, pyruvate, propylene glycol, trimethylamine, hydroxybutyrateb + mat. weight, medical disorders | 0.84 | 50% | FPR = 5% | |||||
| + UtAPI, 3-hydroxyisovalerate ( − glutamine, hydroxybutyrate) |
0.84 | 60% | FPR = 3% | |||||
| Pinto J., et al. (2014) | Phospholipids | nr (preeclampsia) | Q2=0.57 | Maternal phospholipid metabolism is highly sensitive to pre-diagnostic stages of preeclampsia. | No | |||
| nr (pre-diagnostic preeclampsia) | Q2 = 0.82 | |||||||
| Bahado-Singh R.O., et al. (2015) | 50 metabolites (38 verified) | 2-HBb, 3-hydroxyisovalerate, acetone, citrate, glycerol | 0.835 | 75% | 74.40% | First-trimester serum metabolomic biomarkers can predict future development of EO-PE | Data split into training and test set - Validated Bahado-Singh et al. (2013) | |
| glycerol, 3-hydroxyisovalerate, arginine + UtAPI | 0.916 | 90% | 88.40% | |||||
| Late onset | ||||||||
| Bahado-Singh R.O., et al. (2013) | 40 metabolites | Logistic Regression: | Strong separation of EO- and LO-PE was also achieved | There is a significant difference in first-trimester serum metabolites between LO-PE and controls as well as between EO-PE and LO-PE cases | Cross validation | |||
| Glycerol, carnitine + race | 0.79 | 40% | 94.10% | |||||
| + maternal weight | 0.796 | 40% | 95% | |||||
| + 1-MH | 0.783 | 56.70% | 95% | |||||
| Genetic Programming: | ||||||||
| Valine, pyruvate, 3-HB, 1-MH, glycerol, trimethylamine + maternal weight, race, and medical disorders | 0.96 | 76.70% | 100% | |||||
| 1-MH, glycerol, acetoacetateb, maternal weight and race | 0.885 | 60% | 96.50% | |||||
| PE vs. control post diagnosis | - | - | - | - | - | - | - | |
| Kenny L.C., et al. (2005) | nr | Rule 1: peaks 403b, 415, 427 | 2 FP, 0 FN | Machine learning methods are superior to classical statistics for the identification of preeclampsia Low levels of 415 tended to be associated with more severe cases of PE. | Plasma based metabolomics is a plausible screening tool for preeclampsia | Data split into training and test set | ||
| Rule 2: peaks 403b, 415 | 100% | 100% | ||||||
| Rule 1 + Rule 2 | 100% | 98% | ||||||
| Chen T. et al (2017) | nr | PC(14:0/00) | 0.935 (0.831, 1) | - | - | Results indicate perturbation of iNOS signaling, nitric oxide signaling in the cardiovascular system, and mitochondrial dysfunction are responsible for the pathogenesis of preeclampsia | Cross validation | |
| Proline betaine | 0.923 (0.826,0.990) | - | - | |||||
| Proline | 0.928 (0.820, 1) | - | - | |||||
| Austdal M., et al. (2014) | nr | Urine: cholineb, creatine, glycine | 0.9 | 90% | 100% | lipid levels were increased in women with PE | Metabolic profiles of urine and serum can clearly differentiate between normal and PE pregnancies. | Cross validation |
| Serum: lipids | 0.86 | 80% | 100% | |||||
| Serum: lipoproteins | 0.7 | 100% | 80% | |||||
| Austdal M., et al. (2015, b) | 25 metabolites | GPCb, PChob, Aspb, EtAmb, Tau, Glu, Asc, Glyb | 0.927 | 87% | 98% | Significant differences observed between cases and controls and between severe and non-severe PE cases. | Placental metabolic profiles can clearly differentiate between normal and PE pregnancies | Cross validation |
| severe PE vs. non-severe PEc | FGR is not reflected in placental metabolites | |||||||
| Cho, Lys, Ala, Glu, Myo, Tau, Asp. Gln, 3-HB | 0.832 | 77% | 88% | |||||
| Dangat K. et al (2016) | 200 spectral bins | oligosaccharides, lactose, acetate, acetone, glutamate, glutamine and glycerophosphocholine at day 3 lactation | day 3 lactation | R2=0.94 | Q2=0.61 | Various milk metabolites are altered in women with PE. It is likely that the altered vasculature in PE may influence mammary gland function and lactation | No | |
| oligosaccharides, lactose, acetate, acetone, glutamate, glutamine and glycerophosphocholine at month 6 lactation | month 6 lactation | R2 = 0.93 | Q2 = 0.61 | |||||
for validated studies, only the results from the validation set have been reported here
denotes metabolites not otherwise found to be significant by the study in question after employing the most stringent p-value
additional significant metabolites reported
Abbreviations: MAP, mean arterial pressure; PAPPA, pregnancy-associated plasma protein A; PlGF, placental growth factor; CRL, crown rump length; UtAPI, uterine arterial pulsatility index; 1-MH, 1-methylhistidine; 3-HB- 3-hydroxybutyrate; Gly-glycine; Hipp-hippurate; DMA-dimethylamine, 4-DEA, 4-deoxythreonic acid; ProlB- proline betaine; Lac-lactate; Cre- creatine, PtdCho,-phosphatidylcholine; GPC-glycerophosphocholine; PCho-phosphocholine; Myo- myo-inositol; Tau- taurine; Gln- glutamine; Glu- glutamate; Lys- lysine; Ala- alanine; Asp-asparagine; Asc- ascorbate; EtAm-ethanolamine; nr-not relevant; FGR - Fetal growth restriction; IUGR- inter-uterine growth restriction; nr – not reported; CR- Classification rate
Table 3:
Results from metabolomics Studies that did not Assess Biomarker Utility
| Authors | No. of metabolites | Results | Validated? | Conclusions |
|---|---|---|---|---|
| PE vs. control post diagnosis | ||||
| Jain S. et al (2004) | nr | plasmenyl phosphatidylethanolamine is decreased, but free fatty acids are increased in preeclamptic placenta | no | The majority of phospholipid species do not differ between preeclamptic and healthy placentas |
| Braekke K., et al. (2007) | 7 | Median concentrations of tCys, choline, and betaine were significantly greater in PE patients compared to controls | No | Cysteine, choline, and betaine were elevated in maternal and fetal plasma of preeclamptic compared to healthy pregnancies |
| . No significant difference was found between EO and LO patients, nor between severe and non-severe cases | ||||
| Turner E., et al. (2007) | Spectral signals between δ6 and 9.5 | Glucose levels are not an indicator of health of pregnancy. | No | Differing lipid levels in the plasma distinguish women with preeclampsia from those with normal pregnancies |
| The spectral region between 1.29 and 1.42 ppm contributed most to the separation, indicating decreased levels of lipids (mainly VLDL), lactate, fucose, and threonine in PE as compared to controls. | ||||
| The region between 0.87 and 0.96 ppm was also influential, containing lipids (including VLDL), cholesterol, isoleucine, and leucine. 2.22 to 2.26 ppm also emphasizes the importance of lipids. | ||||
| Turner E., et al. (2008) | Spectral signals between δ6 and 9.5 | Histidine, tyrosine, and phenylalanine were all found to be significantly higher in PE patients when compared to normotensive controls | No | 1H NMR in plasma is capable of differentiating between patients with and without preeclampsia |
| Kenny L.C., et al. (2008) | 45 metabolites | 8 discriminatory metabolites were validated; Alanine, 2-hydroxy-3-methyl-butanoic acid, 2-Ethyl-3-hydroxypropionic acid, 2-Oxoglutaric acid, Glutamic acid, Xylitol/ribitol, uric acid, Creatinine | Validation of Kenny et al. (2005) | Small-molecular-weight metabolites measured in serum can effectively detect preeclampsia |
| Pearson T. et al (2010) | 8 | Placentae of preeclamptic women contained significantly (P< 0.05) larger amounts of 5-HETE, 12-HETE and 15-HETE |
no | Increased production of 5-HETE, 12-HETE and 15-HETE metabolites in preeclamptic placentae indicates an important role for this family of eicosanoids in the cause of this disease. vasoconstrictive or proinflammatory actions |
| Dunn W.B., et al. (2012)(25) | 4825 metabolite features | Acyl glycerides, fatty acids and related metabolites, amino acid-related metabolites, Vitamin D-related metabolites, isoprenoids and steroids were decreased in PE cases; phospholipids were increased | No | Metabolomics of placenta has potential to aid in the understanding of pregnancy disorders |
| De Oliveira L., et al. (2012) | 1290 signals | 12 variables were identified with a PLS-DA VIP value > 2 and a p-value < 0.05. 1-acyl-lysophosphatidylcholine was found to be higher in PE patients than controls | No | Lipid fingerprinting in plasma is a useful method for future identification of metabolic biomarkers of early onset preeclampsia |
| Schott S., et al. (2012)(24) | 16 metabolites | 2-acyl-lysophosphatidylcholine and phosphatidylinositol were found to be lower in PE patients when compared to healthy controls. | No | Preeclampsia is associated with malfunction in membrane and/or phospholipid metabolism, although the causal relationship is unclear. |
| Total amount of phospholipids was not significantly different. | ||||
| HDL cholesterol levels were lower in PE cases, with a trend towards higher VLDL 2 and LDL 2. | ||||
| Baig S. et al. (2013) | ~200 | phosphatidylserine was significantly higher and ganglioside mannoside 3 (a sphingolipid) significantly lower in the STBM from PE patients compared to normal controls | No | Results suggest lipid metabolism is altered in the STBM of PE patients, which may relate to dysregulation of the immune response, coagulation, oxidative stress, and apoptosis. |
| Sohlberg S., et al. (2014) | 5 phosphorous metabolites (reported as 6 ratios) | EO-PE had a higher median PDE spectral intensity fraction than women with an early normal pregnancy. EO-PE also had a higher PDE/PME spectral intensity ratio. | No | Placental energy and membrane metabolism is affected in EO-PE compared to early normal pregnancies. They are not affected in LO-PE compared to late normal pregnancies |
| No significant difference in fractions of metabolites between LO-PE and controls | ||||
| Korkes et al. (2014) | 200 lipid signals | Plasma: PS (p=0.0001), PC (p=0.0001) and FLV (p=0.0001) were increased in PE cases relative to controls. Placenta:PS (p, 0.0001) and Macrolides/polyketides-PK04 (p,0.0001) were increased in PE cases relative to controls. | No | Lipid profiles differ in both the placenta and blood in healthy compared to preeclamptic pregnancies |
EO-PE-Early-onset preeclampsia; LO-PE-Late-onset preeclampsia; HDL-high density lipo-protein; VLDL-very low density lipo-protein; LDL-low density lipo-protein; PDE- phosphodiesters; PME- phosphomonoesters; PLS-DA – partial least squares discriminant analysis; VIP – Variable Importance in the projection
Table 4;
Summary Metabolomic Biomarkers Associated with Preeclampsia in twenty-eight studies
| Biological Sample | Comparison of PE Cases and Controls Prior to Diagnosis | Comparison of PE Cases and Controls Prior post Diagnosis | Comparisons of PE Endotypes | ||||
|---|---|---|---|---|---|---|---|
| 1st Trimester | 2nd Trimester | ||||||
| early onset | Late onset | onset not specified | early onset | onset not specified | |||
| Serum | 2-HB; 3-hydroxyisovalerate; acetone; citrate; glycerol; 3-hydroxyisovalerate; arginine; Stearoylcarnitine; taurine* | glycerol; carnitine; valine; pyruvate; 3-HB; 1-MH; glycerol; trimethylamine; acetoacetate; Stearoylcarnitine propylene glycol; 3-hydroxyisovalerate; formate, choline;, succinate; phenylalanine; glycine, glucose, isopropanol; acetate | hydroxyhexanoylcarnitine; Phenylalanine; Glutamate; Alanine; Triglycerides, 3-HB, pyruvate, PtdCho, lactate; carnitine | Alanine; 2-hydroxy-3-methyl-butanoic acid; 2-Ethyl-3-hydroxypropionic acid; 2-Oxoglutaric acid; Glutamic acid; Xylitol/ribitol; uric acid; Creatinine; PC(14:0/00); Proline betaine; Proline; lipids; lipoproteins |
Glycerol; trimethylamine; succinate |
||
| Plasma | Cohibin A; 16-alpha-Hydroxypregnenolone; phosphocholines; 13'-Carboxy-gamma-tocopherol; Dimethamine Quinoline Montecristin; (mono-, di- and tri-) glycerides |
Phospholipids |
5-Hydroxytryptophan, monosaccharide(s), decanoylcarnitine, methylglutaric acid and/or adipic acid, oleic acid, docosahexaenoic acid, butyrolactone and/or oxolan-3-one, acetoacetic acid, hexadecenoyleicosatetraenoyl- snglycerol, di-(octadecadienoyl)- snglycerol, sphingosine 1-phosphate, sphinganine 1-phosphate, Vitamin D3 derivatives, 2-oxovaleric acid and/or oxo-methylbutanoic acid; carnitines | lactate; fucose; threonine; cholesterol; isoleucine; leucine; histidine; tyrosine; phenylalanine; 1-acyl-lysophosphatidylcholine; 2-acyl-lysophosphatidylcholine and phosphatidylinositol; fluvoxamine; choline; betaine |
|||
| Urine | Citrate; glycerol; hydroxyisovalerate; methionine; glutamine; pyruvate; propylene glycol; trimethylamine; hydroxybutyrate; hydroxyisovalerate; | Hippurate; creatinine; glycine; 4-DEA, DMA; hippurate; lactate; creatine; ProlB | Carnitines |
choline; creatine; glycine | |||
| Placenta and Interuterine Tissue | GPC, PCho, Aspartate, EtAm, Taurine; Glucose;, Ascididmen, Glycine;; 5-HETE; 12-HETE; 15-HETE; phosphatidylserine; ganglioside mannoside 3; Macrolides/polyketides-PK04 |
Choline, Lysine, Alanine, Glucose, Myo-inositol, Taurine, Aspartate. Glycine, 3-HB; Acyl glycerides, fatty acids; amino acid-related metabolites, Vitamin D-related metabolites, isoprenoids; phospholipids |
|||||
| Breast Milk | Oligosaccharides; lactose; acetate; acetone; glutamate; glutamine; glycerophosphocholine | ||||||
Acknowledgments
Funding Information:
This manuscript is supported by an R01 grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health to Brigham and Women’s Hospital, NHLBI 1R01HL123915–01 (JALS, STW, RSK, RTG).BC is supported by a European Respiratory Society, Fellowship LTRF 2016. NP is supported by the National Institutes of Health. KJG is supported by an NICHD postdoctoral fellowship 1 F32 HD86948–01.
Footnotes
Conflict of Interest: RTG, RSK, BC, NP, AW, SW, KJG and HM declare that they have no conflicts of interest. JALS is a consultant to Metabolon, Inc.
Ethical standards: This review was conducted in accordance with ethical standards
References
- Ananth CV, Ananth CV, Vintzileos AM (2006). Epidemiology of preterm birth and its clinical subtypes. The Journal of Maternal-Fetal & Neonatal Medicine 19, 773–782 doi:10.1080/14767050600965882 [DOI] [PubMed] [Google Scholar]
- Austdal M, Skråstad RB, Gundersen AS, Austgulen R, Iversen A-C, Bathen TF (2014). Metabolomic Biomarkers in Serum and Urine in Women with Preeclampsia. PLoS ONE 9, e91923 doi:10.1371/journal.pone.0091923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austdal M, et al. (2015). First Trimester Urine and Serum Metabolomics for Prediction of Preeclampsia and Gestational Hypertension: A Prospective Screening Study. International Journal of Molecular Sciences 16, 21520–21538 doi:10.3390/ijms160921520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austdal M, et al. (2015). Metabolic profiles of placenta in preeclampsia using HR-MAS MRS metabolomics. Placenta 36, 1455–1462 doi:http://dx.doi.org/10.1016/j.placenta.2015.10.019 [DOI] [PubMed] [Google Scholar]
- Bahado-Singh RO, et al. (2012). Metabolomics and first-trimester prediction of early-onset preeclampsia. The Journal of Maternal-Fetal & Neonatal Medicine 25, 1840–1847 doi:10.3109/14767058.2012.680254 [DOI] [PubMed] [Google Scholar]
- Bahado-Singh RO, et al. (2013). First-trimester metabolomic detection of late-onset preeclampsia. American Journal of Obstetrics and Gynecology 208, 58.e1–58.e7 doi:http://dx.doi.org/10.1016/j.ajog.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Bahado-Singh RO, et al. (2015). Validation of metabolomic models for prediction of early-onset preeclampsia. Am J Obstet Gynecol 213, 530 e1–530 e10 doi:10.1016/j.ajog.2015.06.044 [DOI] [PubMed] [Google Scholar]
- Baig S, et al. (2013). Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta 34, 436–42 doi:10.1016/j.placenta.2013.02.004 [DOI] [PubMed] [Google Scholar]
- Benton SJ, Ly C, Vukovic S, Bainbridge SA Andrée Gruslin award lecture: Metabolomics as an important modality to better understand preeclampsia. Placenta, doi:http://dx.doi.org/10.1016/j.placenta.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Braekke K, Ueland PM, Harsem NK, Karlsen A, Blomhoff R, Staff AC (2007). Homocysteine, Cysteine, and Related Metabolites in Maternal and Fetal Plasma in Preeclampsia. Pediatr Res 62, 319–324 doi:10.1203/PDR.0b013e318123fba2 [DOI] [PubMed] [Google Scholar]
- Chen T, He P, Tan Y, Xu D (2017). Biomarker identification and pathway analysis of preeclampsia based on serum metabolomics. Biochem Biophys Res Commun 485, 119–125 doi:10.1016/j.bbrc.2017.02.032 [DOI] [PubMed] [Google Scholar]
- Comhair SAA, McDunn J, Bennett C, Fettig J, Erzurum SC, Kalhan SC (2015). Metabolomic Endotype of Asthma. The Journal of Immunology 195, 643–650 doi:10.4049/jimmunol.1500736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie E, Schulze A, Zechner R, Walther Tobias C., Farese Robert V. Jr (2013). Cellular Fatty Acid Metabolism and Cancer. Cell Metabolism 18, 153–161 doi:http://dx.doi.org/10.1016/j.cmet.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangat K, et al. (2016). Altered breast milk components in preeclampsia; An in-vitro proton NMR spectroscopy study. Clin Chim Acta 463, 75–83 doi:10.1016/j.cca.2016.10.015 [DOI] [PubMed] [Google Scholar]
- De Oliveira L, et al. (2012). Lipid fingerprinting in women with early-onset preeclampsia: A first look. Clinical Biochemistry 45, 852–855 doi:http://dx.doi.org/10.1016/j.clinbiochem.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Desforges M, Parsons L, Westwood M, Sibley CP, Greenwood SL (2013). Taurine transport in human placental trophoblast is important for regulation of cell differentiation and survival. Cell Death & Disease 4, e559 doi:10.1038/cddis.2013.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz SO, et al. (2013). Second Trimester Maternal Urine for the Diagnosis of Trisomy 21 and Prediction of Poor Pregnancy Outcomes. Journal of proteome research 12, 2946–2957 doi:10.1021/pr4002355 [DOI] [PubMed] [Google Scholar]
- Duley L (2009). The Global Impact of Pre-eclampsia and Eclampsia. Seminars in Perinatology 33, 130–137 doi:http://dx.doi.org/10.1053/j.semperi.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Dunn WB, et al. (2012). The metabolome of human placental tissue: investigation of first trimester tissue and changes related to preeclampsia in late pregnancy. Metabolomics 8, 579–597 doi:10.1007/s11306-011-0348-6 [Google Scholar]
- Emwas AH (2015). The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol 1277, 161–93 doi:10.1007/978-1-4939-2377-9_13 [DOI] [PubMed] [Google Scholar]
- Famularo G, de Simone C, Trinchieri V, Mosca L (2004). Carnitines and Its Congeners: A Metabolic Pathway to the Regulation of Immune Response and Inflammation. Annals of the New York Academy of Sciences 1033, 132–138 doi:10.1196/annals.1320.012 [DOI] [PubMed] [Google Scholar]
- Jain S, Jayasimhulu K, Clark JF (2004). Metabolomic analysis of molecular species of phospholipids from normotensive and preeclamptic human placenta electrospray ionization mass spectrometry. Front Biosci 9, 3167–75 [DOI] [PubMed] [Google Scholar]
- Jairajpuri DS, Almawi WY (2016). MicroRNA expression pattern in pre-eclampsia (Review). Mol Med Rep 13, 2351–8 doi:10.3892/mmr.2016.4846 [DOI] [PubMed] [Google Scholar]
- Jennings A, MacGregor A, Pallister T, Spector T, Cassidy A (2016). Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: A twin study(). International Journal of Cardiology 223, 992–998 doi:10.1016/j.ijcard.2016.08.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaja RJ, Greer IA (2005). Manifestations of chronic disease during pregnancy. JAMA 294, 2751–7 doi:10.1001/jama.294.21.2751 [DOI] [PubMed] [Google Scholar]
- Kelly RS, et al. (2016). Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics 13, 7 doi:10.1007/s11306-016-1149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RS, et al. (2016). Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest, doi:http://dx.doi.org/10.1016/j.chest.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny LC, et al. (2008). Detection and Identification of Novel Metabolomic Biomarkers in Preeclampsia. Reproductive Sciences 15, 591–597 doi:10.1177/1933719108316908 [DOI] [PubMed] [Google Scholar]
- Kenny LC, et al. (2010). Robust Early Pregnancy Prediction of Later Preeclampsia Using Metabolomic Biomarkers. Hypertension 56, 741–749 doi:10.1161/hypertensionaha.110.157297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny LC, Dunn WB, Ellis DI, Myers J, Baker PN, Kell DB (2005). Novel biomarkers for pre-eclampsia detected using metabolomics and machine learning. Metabolomics 1, 227–234 doi:10.1007/s11306-005-0003-1 [Google Scholar]
- Kleinrouweler CE, van Uitert M, Moerland PD, Ris-Stalpers C, van der Post JAM, Afink GB (2013). Differentially Expressed Genes in the Pre-Eclamptic Placenta: A Systematic Review and Meta-Analysis. PLoS ONE 8, e68991 doi:10.1371/journal.pone.0068991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkes HA, et al. (2014). Lipidomic Assessment of Plasma and Placenta of Women with Early-Onset Preeclampsia. PLoS ONE 9, e110747 doi:10.1371/journal.pone.0110747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MPH, et al. (2015). First-Trimester Serum Acylcarnitine Levels to Predict Preeclampsia: A Metabolomics Approach. Disease Markers 2015, 8 doi:10.1155/2015/857108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuc S, et al. (2014). Metabolomics Profiling for Identification of Novel Potential Markers in Early Prediction of Preeclampsia. PLoS ONE 9, e98540 doi:10.1371/journal.pone.0098540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law KP, Han T-L, Tong C, Baker PN (2015). Mass Spectrometry-Based Proteomics for Pre-Eclampsia and Preterm Birth. International Journal of Molecular Sciences 16, 10952–10985 doi:10.3390/ijms160510952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavey K, Benton SJ, Grynspan D, Kingdom JC, Bainbridge SA, Cox BJ (2016). Unsupervised Placental Gene Expression Profiling Identifies Clinically Relevant Subclasses of Human Preeclampsia. Hypertension 68, 137–147 doi:10.1161/hypertensionaha.116.07293 [DOI] [PubMed] [Google Scholar]
- Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E (2013). Hippurate: The Natural History of a Mammalian–Microbial Cometabolite. Journal of proteome research 12, 1527–1546 doi:10.1021/pr300900b [DOI] [PubMed] [Google Scholar]
- Miguel-Carrasco JL, Mate A, Monserrat MT, Arias JL, Aramburu O, Vázquez CM (2008). The Role of Inflammatory Markers in the Cardioprotective Effect of L-Carnitine in L-NAME-Induced Hypertension. American Journal of Hypertension 21, 1231–1237 doi:10.1038/ajh.2008.271 [DOI] [PubMed] [Google Scholar]
- Navaratnam K, et al. (2013). A multi-centre phase IIa clinical study of predictive testing for preeclampsia: improved pregnancy outcomes via early detection (IMPROvED). BMC Pregnancy and Childbirth 13, 226 doi:10.1186/1471-2393-13-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odibo AO, et al. (2011). First-trimester prediction of preeclampsia using metabolomic biomarkers: a discovery phase study. Prenatal Diagnosis 31, 990–994 doi:10.1002/pd.2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orczyk-Pawilowicz M, Jawien E, Deja S, Hirnle L, Zabek A, Mlynarz P (2016). Metabolomics of Human Amniotic Fluid and Maternal Plasma during Normal Pregnancy. PLoS ONE 11, e0152740 doi:10.1371/journal.pone.0152740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, et al. (2010). Measurement of vasoactive metabolites (hydroxyeicosatetraenoic and epoxyeicosatrienoic acids) in uterine tissues of normal and compromised human pregnancy. J Hypertens 28, 2429–37 doi:10.1097/HJH.0b013e32833e86aa [DOI] [PubMed] [Google Scholar]
- Pinto J, et al. (2015). Prediction of Gestational Diabetes through NMR Metabolomics of Maternal Blood. Journal of proteome research 14, 2696–2706 doi:10.1021/acs.jproteome.5b00260 [DOI] [PubMed] [Google Scholar]
- Pinto J, et al. (2014). Maternal plasma phospholipids are altered in trisomy 21 cases and prior to preeclampsia and preterm outcomes. Rapid Communications in Mass Spectrometry 28, 1635–1638 doi:10.1002/rcm.6941 [DOI] [PubMed] [Google Scholar]
- Poston L, et al. (2016). Preconceptional and maternal obesity: epidemiology and health consequences. The Lancet Diabetes & Endocrinology 4, 1025–1036 doi:http://dx.doi.org/10.1016/S2213-8587(16)30217-0 [DOI] [PubMed] [Google Scholar]
- Ruiz-Núñez B, Dijck-Brouwer DAJ, Muskiet FAJ (2016). The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. The Journal of Nutritional Biochemistry 36, 1–20 doi:http://dx.doi.org/10.1016/j.jnutbio.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Schott S, Hahn J, Kurbacher C, Moka D (2012). (31)P and (1)H Nuclear Magnetic Resonance Spectroscopy of Blood Plasma in Female Patients with Preeclampsia. International Journal of Biomedical Science : IJBS 8, 258–263 [PMC free article] [PubMed] [Google Scholar]
- Sitras V, Fenton C, Acharya G (2015). Gene expression profile in cardiovascular disease and preeclampsia: A meta-analysis of the transcriptome based on raw data from human studies deposited in Gene Expression Omnibus. Placenta 36, 170–178 doi:http://dx.doi.org/10.1016/j.placenta.2014.11.017 [DOI] [PubMed] [Google Scholar]
- Sohlberg S, et al. In vivo 31P-MR spectroscopy in normal pregnancy, early and late preeclampsia: A study of placental metabolism. Placenta 35, 318–323 doi:10.1016/j.placenta.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Stokholm J, Sevelsted A, Anderson UD, Bisgaard H (2016). Preeclampsia Associates with Asthma, Allergy and Eczema in Childhood. American journal of respiratory and critical care medicine, doi:10.1164/rccm.201604-0806OC [DOI] [PubMed] [Google Scholar]
- Tanaka M, et al. (2007). Racial Disparity in Hypertensive Disorders of Pregnancy in New York State: A 10-Year Longitudinal Population-Based Study. American Journal of Public Health 97, 163–170 doi:10.2105/AJPH.2005.068577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranquilli AL, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health 4, 97–104 doi:10.1016/j.preghy.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Turner E, Brewster JA, Simpson NAB, Walker JJ, Fisher J (2007). Plasma From Women with Preeclampsia Has a Low Lipid and Ketone Body Content—A Nuclear Magnetic Resonance Study. Hypertension in Pregnancy 26, 329–342 doi:10.1080/10641950701436073 [DOI] [PubMed] [Google Scholar]
- Turner E, Brewster JA, Simpson NAB, Walker JJ, Fisher J (2008). Aromatic Amino Acid Biomarkers of Preeclampsia—A Nuclear Magnetic Resonance Investigation. Hypertension in Pregnancy 27, 225–235 doi:10.1080/10641950801955725 [DOI] [PubMed] [Google Scholar]
- Wahl S, et al. (2012). Childhood Obesity Is Associated with Changes in the Serum Metabolite Profile. Obesity Facts 5, 660–670 [DOI] [PubMed] [Google Scholar]
- Yu Z, et al. (2011). Differences between Human Plasma and Serum Metabolite Profiles. PLoS ONE 6, e21230 doi:10.1371/journal.pone.0021230 [DOI] [PMC free article] [PubMed] [Google Scholar]