Abstract
Objective:
To determine if alcohol consumption is associated with incident overweight or obesity in normal-weight, postmenopausal women.
Design:
Prospective cohort study considering baseline alcohol consumption and subsequent weight change over 7 years.
Subjects:
15 920 normal-weight (BMI: 18.5–24.9 kg/m2), postmenopausal women enrolled in the Women’s Health Initiative clinical trial (WHI CT).
Measurements:
Body weight change and incident overweight and obesity (BMI, 25.0–29.9 and ≥ 30 kg/m2) over 7 years.
Results:
A third of the 13 822 women included in the analytical cohort reported no alcohol consumption. BMI differed little between abstainers (22.8 ± 1.58 kg/m2) and alcohol consumers in the upper quintile (22.7 ± 1.53 kg/m2). Among normal-weight women the risk of becoming overweight or obese over a 7-year follow-up period was 35 or 88% lower, respectively, for women in the upper quintile of alcohol intake relative to abstainers (HR, 0.65; 95% CI, 0.58–0.73 or HR, 0.12; 95% CI, 0.05–0.25, respectively). Risk for overweight and obesity was not significantly modified by age. Wine consumption showed the greatest protective association for risk of overweight (HR, 0.75; 95% CI, 0.68–0.84), followed by liquor (HR, 0.85; 95% CI, 0.78–0.93) and beer (HR, 0.90; 95% CI, 0.82–1.00).
Conclusion:
Postmenopausal women of normal-weight who report moderate alcohol intake have reduced risk of becoming overweight or obese over time. Perhaps weight control measures in this population should target behaviors other than reduction in alcohol for those consuming moderate amounts.
Keywords: alcohol, body weight, body mass index, overweight, obesity
INTRODUCTION
The prevalence of obesity among US adults has risen steadily over the past several decades, although an indication of stabilizing rates has recently emerged among older women (1–3). Alcohol contains 7 kilocalories (kcal) per gram and therefore can contribute significantly to the overall energy profile of the diet in frequent consumers. It is estimated that more than half of US adults consume alcohol on a regular basis (4), whereas 24.6% are lifelong abstainers (5). Evaluation of the relationship between alcohol intake and obesity risk has provided inconsistent results. The current 2010 Dietary Guidelines for Americans as well as a recent systematic review have called for additional study of this relationship in order to inform on future public health guidance (6,7) and to evaluate demonstrated gender differences in this relationship (8). In a study of middle-aged, female health professionals, alcohol intake was inversely associated with risk of overweight or obesity (9), possibly related to a concomitant reduction in energy intake from other sources (10). However, evidence has been inconsistent (11,12).
The Women’s Health Initiative Clinical Trial (WHI CT) (13) provides a rich dataset in which to test further the hypothesis that alcohol intake is associated with weight change in postmenopausal women. Importantly, the WHI CT provides an opportunity to assess whether the Women’s Health Study (WHS) (9) findings that alcohol intake was inversely associated weight gain can be replicated in a more representative and somewhat more multiethnic population of US women. Here we describe baseline alcohol intake in 15 920 postmenopausal women in WHI CT with normal BMI (18.5 to < 25 kg/m2) at study initiation and prospectively evaluate the relationship between alcohol intake and incidence of overweight/obesity over 7 years of follow-up. We hypothesized that women who reported greater intake of alcohol would demonstrate a trajectory characterized by stable or reduced body weight over time compared with abstainers of alcohol, thus replicating the WHS findings.
METHODS
Study Population
The study design and population sample for the overall WHI study, including the WHI CT, have been described previously (13). Briefly, the WHI CT enrolled women in one or more of three randomized, controlled clinical trials: 1) hormone therapy (HT; estrogen alone, estrogen plus progestin, or placebo); 2) diet modification (DM; low-fat and high-fruit/vegetable/grain diet or usual diet); and 3) calcium and vitamin D (800 mg/d calcium carbonate plus 400 IU/d vitamin D or placebo). Written informed consent was obtained from all study participants prior to study enrollment, and the trials were approved by the Institutional Review Boards of each of the 40 participating institutions. Main findings from the trials have been published previously (14–20). Of the 15 920 women included in the present analysis, 6 015 women were enrolled in the HT trial, 11 078 in DM, and 8 663 in calcium-vitamin D, understanding that women could concurrently enroll in multiple trials.
The analytical cohort for the present study included only those women in the WHI CT with normal BMI (18.5 to <25 kg/m2) at baseline and those with at least three additional BMI measures during the 7-year follow-up period (n = 16 274). Women were excluded if they reported implausible total energy intake (< 600 or > 5000 kcal/d; n = 241) or an average of more than one hospitalization per year (n = 117), yielding a final sample of 15 920 women. The final analytical cohort included 13 822 women due to missing data for covariates in the multivariate models. Although the WHI CT had follow-up data for up to 11 years, we restricted our analysis to 7 years because less than 50% of participants were scheduled for further weight assessments after this time point due to the multi-year enrollment of the cohort.
Alcohol Intake
Alcohol intake was assessed using the WHI semi-quantitative, validated food frequency questionnaire (FFQ), which also provided data for intake of other nutrients (21). Women were asked to report frequency of intake as one of nine possible responses, ranging from “never or less than once per month” to “6+ times per day”. Women were also asked to report a serving size of small, medium, or large using 12 oz beer, 6 oz wine, and 1.5 oz liquor as the referent amount for a medium serving of alcohol. Intake of alcohol and other nutrients was then estimated by multiplying frequency of intake by reported portion size. Consistent with published reports, diet records collected on a 6% sub-sample of WHI women showed a correlation between alcohol intake estimated from the FFQ and diet record of 0.86 (21, 22). Total nonalcohol energy intake was calculated by multiplying the amount of alcohol consumed (g) by 7 (kcal/g) and subtracting this value from total energy intake (kcal).
Anthropometric Data
Height, weight, and waist circumference were measured using standardized procedures in the WHI clinics at baseline and annually in CT participants (n = 68 132). Height was measured using a wall-mounted stadiometer at end-inspiration without shoes, recorded to the nearest 0.1 cm. Weight was measured using a calibrated balance beam or digital scale, without shoes or heavy clothing, and recorded to the nearest 0.1 kg. BMI, calculated as weight (kg) divided by height (m) squared, was used to categorize women’s weight status, including underweight (< 18.5 kg/m2), normal weight (18.5– < 25 kg/m2), overweight (25– < 30 kg/m2), or obese (≥ 30 kg/m2), according to definitions provided by the World Health Organization (Fact Sheet No 311, updated March 2011). Waist circumference was measured at a horizontal plane, at the level of the natural waist and at end-inspiration, and recorded to the nearest cm. Incidence of overweight or obesity was defined as crossing from a normal weight (18.5– < 25 kg/m2) to a BMI category of overweight (≥ 25 kg/m2) or obese (≥ 30 kg/m2).
Statistical Analysis
Alcohol abstainers were defined as women with < 0.2 g/d intake to allow for trace alcohol consumed from food sources, and higher levels of intake were evenly divided into quartiles (quintiles 2–5). Participant characteristics were compared across quintiles of alcohol intake using a nonparametric test for trend (23) for continuous variables, Kruskal-Wallis test for ordinal variables (education), or chi-square test for categorical variables.
The association between alcohol intake and body weight change was tested using linear regression, with alcohol abstainers (quintile 1) as the reference group. Demographic, dietary, lifestyle, and clinical factors that were significantly associated with alcohol intake were selected as potential confounders. Thus, multivariate models were adjusted for the following: baseline age; race/ethnicity; education; baseline height and weight; physical activity; smoking (never, former, or current); clinical trial arms; history of diabetes treatment, cardiovascular disease, and cancer; percent of total energy intake as fat and carbohydrate; fruit and vegetable intake; nonalcohol energy; and total calcium (diet plus supplement). Potential effect modification by age was investigated via stratified analyses, dividing the population into two age groups, 50–59 and 60+ y, and tested using likelihood ratio tests.
Risk of incident overweight/obesity over 7 years across quintiles of alcohol intake was tested using Cox proportional hazards regression, yielding hazards ratios (HRs) and 95% confidence intervals (CIs), using alcohol abstainers (quintile 1) as the reference group. Time-to-overweight and time-to-obese were analyzed separately. Women were censored at the first year they had a BMI that qualified as overweight (≥ 25 kg/m2) or obese (≥ 30 kg/m2) or at their final weight measurement. Multivariate models were adjusted for potential confounders as listed above. Effect modification by age was investigated as described above. In addition to overall alcohol consumption, intake of beer, wine, or liquor (servings/d) was also tested for association with risk of overweight/obesity, using abstainers as the reference group. For individual alcohol sources, groups were combined (beer, quintiles 1–3; wine, quintiles 1–2; or liquor, quintiles 1–2) into a single category because a large proportion of participants consumed zero servings/d of the alcohol source in question.
Several supplemental analyses were conducted to see if the overall associations would persist. First, we restricted analyses to never-smokers (n = 7131). Second, we used quintile 2 as the reference group (excluding alcohol abstainers). Third, we restricted analyses to women with BMI measured at all 8 time points (baseline plus 7 years’ follow-up) and without any history or incidence of diabetes treatment, cancer, or cardiovascular disease/myocardial infarction (n = 5353). All statistical analyses were conducted using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
The 15 920 postmenopausal women had a mean age of 62.7 y, of which 87.6% were non-Hispanic white and one-third reported abstaining from alcohol. Compared with alcohol users, abstainers tended to be older, of minority race/ethnicity, and never-smokers (Table 1). Abstainers also were more likely to have a prior history of diabetes treatment or cardiovascular disease. Both total energy intake and nonalcohol energy intake were significantly higher in alcohol consumers than abstainers. Importantly, leisure-time physical activity also showed a positive relationship with alcohol intake. Though BMI differed little between abstainers (22.8 ± 1.58 kg/m2) and women in the upper quintile of alcohol intake (22.7 ± 1.53 kg/m2), these differences were statistically significant.
Table 1.
Baseline characteristics of WHI CT participants with normal BMI, by quintiles2 of alcohol intake (n = 15 920)
| Characteristic | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
|---|---|---|---|---|---|
| Participants, n (%) | 5304 (33.3) | 2654 (16.7) | 2655 (16.7) | 2653 (16.7) | 2654 (16.7) |
| Alcohol intake (g/d) | |||||
| Range | 0–<0.20 | 0.20–1.77 | 1.77–6.49 | 6.49–13.55 | 13.55–197 |
| Mean ± SD | 0.03 ± 0.03 | 0.92 ± 0.32 | 3.27 ± 1.32 | 9.12 ± 2.33 | 25.1 ± 13.9 |
| Median | 0.01 | 0.99 | 3.04 | 8.33 | 19.4 |
| Demographics | |||||
| Age (y), mean ± SD | 63.3 ± 7.37 | 62.8 ± 7.23 | 62.1 ± 7.24 | 62.2 ± 6.92 | 62.8 ± 7.06 |
| Race/ethnicity, n (%)3 | |||||
| White, non-Hispanic | 4188 (79.0) | 2309 (87.3) | 2400 (90.7) | 2489 (94.0) | 2526 (95.4) |
| Black or African-American | 378 (7.1) | 117 (4.4) | 97 (3.7) | 54 (2.0) | 48 (1.8) |
| Hispanic/Latina | 201 (3.8) | 89 (3.4) | 67 (2.5) | 49 (1.9) | 26 (1.0) |
| Asian or Pacific Islander | 444 (8.4) | 98 (3.7) | 49 (1.9) | 33 (1.3) | 25 (0.9) |
| American Indian or Alaska Native | 16 (0.30) | 6 (0.2) | 7 (0.3) | 5 (0.2) | 8 (0.3) |
| Other | 72 (1.4) | 26 (1.0) | 27 (1.1) | 17 (0.6) | 16 (0.6) |
| Education, n (%)3 | |||||
| Less than high school | 300 (5.7) | 77 (2.9) | 55 (2.1) | 34 (1.3) | 30 (1.1) |
| High school diploma or GED | 1033 (19.6) | 441 (16.7) | 372 (14.1) | 333 (12.6) | 309 (11.7) |
| Some college or associates degree | 2020 (38.3) | 980 (37.2) | 974 (36.9) | 957 (36.2) | 860 (32.7) |
| College degree | 597 (11.3) | 318 (12.1) | 341 (12.9) | 366 (13.9) | 434 (16.5) |
| Any post-graduate education | 1323 (25.1) | 822 (31.2) | 897 (34.0) | 951 (36.0) | 998 (37.9) |
| Anthropometrics, mean ± SD | |||||
| Height (cm) | 161.7 ± 6.48 | 162.1 ± 6.25 | 163.1 ± 6.21 | 163.4 ± 6.11 | 163.6 ± 6.14 |
| Weight (kg) | 59.7 ± 6.32 | 60.0 ± 6.03 | 60.8 ± 6.01 | 60.7 ± 5.92 | 60.8 ± 6.03 |
| BMI (kg/m2) | 22.8 ± 1.58 | 22.8 ± 1.55 | 22.8 ± 1.51 | 22.7 ± 1.54 | 22.7 ± 1.53 |
| Waist circumference (cm)3 | 75.1 ± 6.68 | 75.2 ± 6.47 | 74.9 ± 6.38 | 74.8 ± 6.21 | 75.7 ± 6.11 |
| Diet and energy, mean ± SD | |||||
| Total energy intake (kcal/d) | 1565 ± 607 | 1569 ± 596 | 1614 ± 587 | 1661 ± 576 | 1786 ± 581 |
| Nonalcohol energy intake (kcal/d) | 1565 ± 607 | 1562 ± 596 | 1591 ± 587 | 1597 ± 575 | 1610 ± 572 |
| Total fat (g/d) | 62.4 ± 30.6 | 62.2 ± 28.7 | 64.2 ± 28.8 | 64.7 ± 27.8 | 67.2 ± 29.6 |
| Carbohydrate (g/d) | 194.9 ± 74.1 | 193.7 ± 74.6 | 194.3 ± 72.4 | 193.2 ± 70.9 | 189.2 ± 70.0 |
| Fiber (g/d) | 15.4 ± 6.62 | 15.5 ± 6.29 | 15.5 ± 6.37 | 15.5 ± 6.09 | 15.2 ± 6.05 |
| Fruits and vegetables (servings/d) | 3.78 ± 2.00 | 3.86 ± 1.93 | 3.89 ± 1.90 | 3.98 ± 1.83 | 3.95 ± 1.85 |
| Calcium (diet + supplement) (mg/d) | 1125 ± 695 | 1159 ± 666 | 1182 ± 678 | 1211 ± 745 | 1186 ± 678 |
| Leisure-time physical activity (met-hr/wk)3 | 11.8 ± 13.3 | 13.6 ± 13.5 | 14.3 ± 14.1 | 16.1 ± 14.3 | 16.5 ± 15.1 |
| Behavior, n (%) | |||||
| Smoking status | |||||
| Never | 3261 (62.0) | 1520 (57.9) | 1308 (49.8) | 1173 (44.7) | 883 (33.6) |
| Past | 1525 (29.0) | 872 (33.2) | 1074 (40.9) | 1257 (47.9) | 1422 (54.2) |
| Current | 470 (8.9) | 232 (8.8) | 247 (9.4) | 195 (7.4) | 321 (12.2) |
| Multivitamin use | 1844 (34.8) | 976 (36.8) | 953 (35.9) | 943 (35.5) | 942 (35.5) |
| Medical history, n (%) | |||||
| Diabetes treatment3 | 145 (2.7) | 23 (0.9) | 23 (0.9) | 13 (0.5) | 16 (0.6) |
| Cardiovascular disease3 | 734 (15.6) | 324 (13.8) | 337 (14.4) | 268 (11.5) | 308 (12.9) |
| Cancer3 | 181 (3.4) | 100 (3.8) | 99 (3.8) | 97 (3.7) | 93 (3.5) |
Normal BMI was defined as 18.5– < 25 kg/m2.
Quintile 1 was defined as alcohol abstainers (< 0.2 g/d); all other levels of intake were evenly distributed into 4 quartiles (quintiles 2–5).
Missing data: race/ethnicity (0.21%), education (0.62%), waist circumference (58.7%), physical activity (10.1%), smoking (1.01%), diabetes (0.05%), cardiovascular disease (11.1%), cancer (0.90%).
Over 7 years of follow-up, postmenopausal women showed a general pattern of weight gain, regardless of baseline alcohol consumption (Figure 1). After demonstrating a slight reduction in weight during the first year on study, women in all quintiles of alcohol consumption demonstrated a rise in weight. Women with the highest intake of alcohol (quintile 5) demonstrated the least weight gain over time, and women with lower intake (quintiles 1 and 2) had the most. In a series of seven multivariate linear regression models of body weight change at each year since baseline, a test for trend in the relationship between alcohol intake and weight was strongly significant for each year of follow-up (all P < 0.001).
Figure 1.
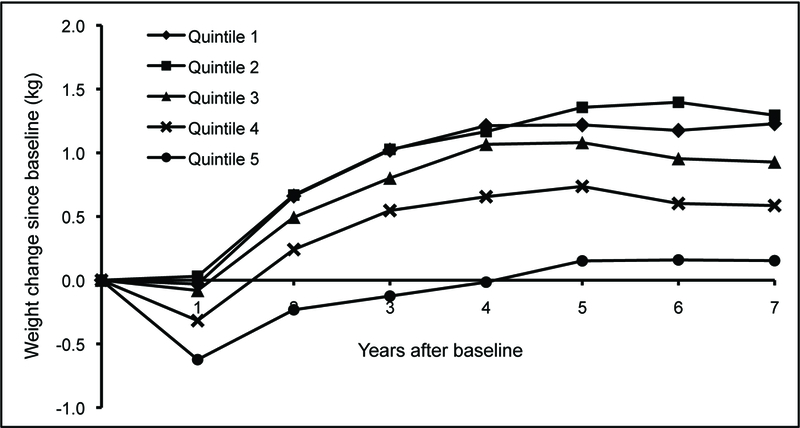
Adjusted mean body weight change (in kilograms) during 7-year follow-up for post-menopausal women in the WHI CT (n = 13 822) according to quintiles of baseline total alcohol intake. Quintile 1 represents 4624 alcohol abstainers, and the remaining cohort was divided evenly into quartiles to create quintiles 2–5. The range of alcohol intake in each quintile was as follows (g/d): quintile 1, 0–<0.2; quintile 2, 0.2–1.77; quintile 3, 1.77–6.49; quintile 4, 6.49–13.55; and quintile 5, 13.55–197
Since weight gain was demonstrated across all groups, we next investigated whether alcohol consumption was associated with higher or lower risk of becoming overweight or obese, given the sample included only women with normal BMI at baseline. The risk of incident overweight or obesity was significantly lower with increasing alcohol consumption (HR, 0.65; 95% CI, 0.58–0.73 and HR, 0.12; 95% CI, 0.05–0.25, respectively, for risk in the highest quintile relative to abstainers) over the 7-year study period (Table 2). A reduced incidence of overweight was also demonstrated for wine (HR, 0.75; 95% CI, 0.68–0.84), liquor (HR, 0.85; 95% CI, 0.78–0.93), and, to a lesser degree, beer (HR, 0.90; 95% CI, 0.82–1.00) consumption.
Table 2.
Hazard ratios (HR) and 95% confidence intervals (CI) for becoming overweight or obese in 7 years of follow-up, across quintiles of alcohol intake (n = 13 822)
| Alcohol intake (quintile) |
Total n (%) | Incident overweight, BMI ≥ 25 kg/m2 | Incident obesity, BMI ≥ 30 kg/m2 | ||
|---|---|---|---|---|---|
| n (%) | HR (95% CI)1 | n (%) | HR (95% CI)1 | ||
| Total2 | |||||
| 1 (abstainers) | 4624 (33.5) | 1711 (37.0) | 1.00 | 79 (1.7) | 1.00 |
| 2 | 2297 (16.6) | 829 (36.1) | 0.94 (0.86–1.02) | 32 (1.4) | 0.74 (0.49–1.12) |
| 3 | 2285 (16.5) | 812 (35.5) | 0.88 (0.81–0.96) | 27 (1.2) | 0.54 (0.34–0.84) |
| 4 | 2287 (16.6) | 719 (31.4) | 0.81 (0.74–0.90) | 21 (0.9) | 0.38 (0.22–0.64) |
| 5 | 2329 (16.9) | 706 (30.3) | 0.65 (0.58–0.73) | 13 (0.6) | 0.12 (0.05–0.25) |
| P for trend3 | < 0.001 | < 0.001 | |||
| Beer | |||||
| 1–3 (0 servings/d) | 10 454 (75.6) | 3691 (35.3) | 1.00 | 144 (1.4) | 1.00 |
| 4 | 1856 (13.4) | 601 (32.4) | 0.90 (0.83–0.98) | 20 (1.1) | 0.74 (0.46–1.19) |
| 5 | 1512 (10.9) | 485 (32.1) | 0.90 (0.82–1.00) | 8 (0.5) | 0.34 (0.17–0.71) |
| P for trend | 0.005 | 0.003 | |||
| Wine | |||||
| 1–2 (0 servings/d) | 5579 (40.4) | 2067 (37.1) | 1.00 | 93 (1.7) | 1.00 |
| 3 | 2810 (20.3) | 992 (35.3) | 0.92 (0.85–1.00) | 32 (1.1) | 0.65 (0.43–0.97) |
| 4 | 3245 (23.5) | 1043 (32.1) | 0.83 (0.76–0.90) | 29 (0.9) | 0.48 (0.31–0.75) |
| 5 | 2188 (15.8) | 675 (30.9) | 0.75 (0.68–0.84) | 18 (0.8) | 0.35 (0.19–0.65) |
| P for trend3 | < 0.001 | < 0.001 | |||
| Liquor | |||||
| 1–3 (0 servings/d) | 9537 (69.0) | 3341 (35.0) | 1.00 | 131 (1.4) | 1.00 |
| 4 | 1851 (13.4) | 664 (35.9) | 0.99 (0.91–1.08) | 21 (1.1) | 0.78 (0.49–1.24) |
| 5 | 2434 (17.6) | 772 (31.7) | 0.85 (0.78–0.93) | 20 (0.8) | 0.53 (0.32–0.88) |
| P for trend3 | 0.003 | 0.013 | |||
Multivariate models were adjusted for baseline age; race/ethnicity; education; height; weight; physical activity; smoking (never, former, or current); clinical trial arms; history of diabetes treatment, cardiovascular disease, and cancer; percent of total energy intake as fat and carbohydrate; fruit and vegetable intake; nonalcohol energy; and total calcium (diet plus supplement).
For total alcohol intake, quintile 1 was defined as alcohol abstainers (< 0.2 g/d), and higher levels of intake were evenly divided into quartiles (quintiles 2–5). The range of alcohol intake in each quartile was as follows (g/d): quintile 1, 0–<0.2; quintile 2, 0.2–1.77; quintile 3, 1.77–6.49; quintile 4, 6.49–13.55; and quintile 5, 13.55–197.
Test for trend (using quintile number as an ordinal variable).
The inverse relationship between alcohol intake and risk of overweight did not significantly differ for women age ≥ 60 y (HR, 0.61; 95% CI, 0.52–0.71) from those age 50–59 y (HR, 0.74; 95% CI, 0.61–0.89; likelihood ratio test P = 0.165) (Table 3). The same was true for risk of obesity (younger women versus older women; HR, 0.09; 95% CI, 0.03–0.27 versus HR, 0.16; 95% CI, 0.05–0.46, respectively; likelihood ratio test P = 0.838). The overall associations were unchanged when analysis was restricted to never smokers (Table 4). Less than 10% of this cohort was identified as current smokers, but the effect of alcohol on overweight/obesity risk was similar in this group, although the estimates were less precise (data not shown). No substantial differences were shown after restricting the analysis to women with BMI measured at all 8 time points and without any history or incidence of diabetes treatment, cancer, or cardiovascular disease/myocardial infarction (data not shown).
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for becoming overweight or obese in 7 years of follow-up, across quintiles of total alcohol intake, stratified by age at baseline
| Age | Alcohol (quintile)1 |
Total n (%) | Incident overweight, BMI ≥ 25 kg/m2 | Incident obesity, BMI ≥ 30 kg/m2 | ||
|---|---|---|---|---|---|---|
| n (%) | HR (95% CI)2 | n (%) | HR (95% CI)2 | |||
| 50–59 y | ||||||
| 1 | 1498 (31.4) | 609 (40.7) | 1.00 | 38 (2.5) | 1.00 | |
| 2 | 797 (16.7) | 315 (39.5) | 0.89 (0.78–1.03) | 14 (1.8) | 0.59 (0.32–1.10) | |
| 3 | 863 (18.1) | 345 (40.0) | 0.93 (0.81–1.06) | 17 (2.0) | 0.57 (0.31–1.02) | |
| 4 | 839 (17.6) | 273 (32.5) | 0.79 (0.67–0.92) | 9 (1.1) | 0.29 (0.14–0.63) | |
| 5 | 779 (16.3) | 280 (35.9) | 0.74 (0.61–0.89) | 6 (0.8) | 0.09 (0.03–0.27) | |
| P for trend3 | 0.001 | < 0.001 | ||||
| 60+ y | ||||||
| 1 | 3126 (34.6) | 1102 (35.3) | 1.00 | 41 (1.3) | 1.00 | |
| 2 | 1500 (16.6) | 514 (34.3) | 0.96 (0.86–1.07) | 18 (1.2) | 0.92 (0.52–1.61) | |
| 3 | 1422 (15.7) | 467 (32.8) | 0.86 (0.77–0.96) | 10 (0.7) | 0.48 (0.24–0.98) | |
| 4 | 1448 (16.0) | 446 (30.8) | 0.83 (0.74–0.94) | 12 (0.8) | 0.51 (0.25–1.04) | |
| 5 | 1550 (17.1) | 426 (27.5) | 0.61 (0.52–0.71) | 7 (0.5) | 0.16 (0.05–0.46) | |
| P for trend3 | < 0.001 | 0.001 | ||||
Quintile 1 was defined as alcohol abstainers (< 0.2 g/d), and higher levels of intake were evenly divided into quartiles (quintiles 2–5). The range of alcohol intake in each quartile was as follows (g/d): quintile 1, 0–<0.2; quintile 2, 0.2–1.77; quintile 3, 1.77–6.49; quintile 4, 6.49–13.55; and quintile 5, 13.55–197.
Multivariate models were adjusted for baseline age; race/ethnicity; education; height; weight; physical activity; smoking (never, former, or current); clinical trial arms; history of diabetes treatment, cardiovascular disease, and cancer; percent of total energy intake as fat and carbohydrate; fruit and vegetable intake; nonalcohol energy; and total calcium (diet plus supplement).
Test for trend (using quintile number as an ordinal variable).
Table 4.
Hazard ratios (HR) and 95% confidence intervals (CI) for becoming overweight or obese in 7 years of follow-up, across quintiles of total alcohol intake, restricted to never-smokers
| Alcohol (quintile)1 |
Total n (%) | Incident overweight, BMI ≥ 25 kg/m2 | Incident obesity, BMI ≥ 30 kg/m2 | ||
|---|---|---|---|---|---|
| n (%) | HR (95% CI)2 | n (%) | HR (95% CI)2 | ||
| 1 | 2870 (40.3) | 1010 (35.2) | 1.00 | 38 (1.3) | 1.00 |
| 2 | 1318 (18.5) | 467 (35.4) | 0.95 (0.85–1.07) | 16 (1.2) | 0.85 (0.47–1.54) |
| 3 | 1129 (15.8) | 375 (33.2) | 0.85 (0.75–0.97) | 12 (1.1) | 0.68 (0.35–1.33) |
| 4 | 1027 (14.4) | 325 (31.7) | 0.85 (0.74–0.97) | 8 (0.8) | 0.46 (0.20–1.04) |
| 5 | 787 (11.0) | 224 (28.5) | 0.66 (0.55–0.79) | 3 (0.4) | 0.16 (0.04–0.61) |
| P for trend3 | < 0.001 | 0.005 | |||
Quintile 1 was defined as alcohol abstainers (< 0.2 g/d), and higher levels of intake were evenly divided into quartiles (quintiles 2–5). The range of alcohol intake in each quartile was as follows (g/d): quintile 1, 0–<0.2; quintile 2, 0.2–1.77; quintile 3, 1.77–6.49; quintile 4, 6.49–13.55; and quintile 5, 13.55–197.
Multivariate models were adjusted for baseline age; race/ethnicity; education; height; weight; physical activity; smoking (never, former, or current); clinical trial arms; history of diabetes treatment, cardiovascular disease, and cancer; percent of total energy intake as fat and carbohydrate; fruit and vegetable intake; nonalcohol energy; and total calcium (diet plus supplement).
Test for trend (using quintile number as an ordinal variable).
DISCUSSION
Despite high rates of obesity and alcohol use in the US, current epidemiological evidence evaluating the relationship between them remains inconsistent. Our results suggest that moderate alcohol intake is not associated with incident overweight or obesity in postmenopausal women. Current US Dietary Guidelines (2010) support our findings (6). However, in a recent review of 67 studies investigating this association, half reported a positive association, approximately 20% reported null results, and the remaining 30% reported an inverse association (12). An understanding of energy balance would suggest that increasing intake of alcohol, a relatively energy-dense dietary item, would contribute to weight gain in most individuals. Yet, in studies testing these associations in women, an inverse association is more commonly shown (24–27).
The only study included in the review (12) with prospective evaluation of weight change in a similar sample size of women was the WHS, which also showed an inverse association between alcohol intake and weight gain (9). In their study of over 19 000 adult women, those who consumed > 30 g alcohol daily (well above the 13.5 g/d reported here) had a 27% lower risk of incident overweight or obesity than abstainers (8). Similar findings were observed in an evaluation of women in the first National Health and Nutrition Examination Survey (NHANES), wherein drinkers were more likely to be weight stable over a 10-year follow-up (27). In a secondary analysis evaluating the role of dietary behaviors and weight change, alcohol intake was inversely associated with weight gain in both the Nurses’ Health Study (NHS) I and NHS II (28). Risk for weight gain was also reduced in alcohol consumers compared with abstainers in a UK study of 49 324 younger women (age 27–44 y), particularly for intakes between 15 and 29.9 g/d (HR, 0.86; 95% CI, 0.76–0.78) (29). Our results corroborate these published findings, suggesting an overall 35% lower risk of becoming overweight and 88% lower risk of becoming obese for postmenopausal women who consume a median intake of 19.4 g alcohol per day.
Our results suggest that the lower weight gain associated with greater alcohol intake is independent of total energy intake, in that women who reported the greatest intake of alcohol also reported greater total and nonalcohol energy intake, similar to findings from NHANES (1999–2006) (30). Evidence from short-term, controlled feeding studies also indicates that there is usually no compensation for energy intake, and alcohol use tends to increase involuntary intake (10). In contrast, findings from the WHS (9) and NHANES I (31) showed greater total energy intake in alcohol consumers but lower nonalcohol energy intake, suggesting some compensation for energy intake from alcohol among women in the upper quintiles of consumption. A few studies have suggested that carbohydrate intake is inversely associated with alcohol intake (24, 32), as was shown here (P = 0.032).
In this analysis, weight gain was attenuated in the upper quintiles of alcohol intake, controlling for energy intake. One explanation may be the estimated 20% increase in metabolism-associated or diet-associated thermogenesis induced by alcohol, a level well above the usual thermogenic response with intake of other macronutrients (33). Further, a greater proportion of alcohol is metabolized through the microsomal ethanol oxidizing system in heavy, regular alcohol consumers than in individuals with low-to-moderate consumption, thus contributing to a reduced energy gain via ATP production during periods of heavy consumption (34). In fact, there is some indication that alcohol intake increases resting energy expenditure (35). Individuals with higher BMI metabolize alcohol more efficiently and thus may not benefit from the thermogenic rise observed in lean subjects (36,37). Calories from alcohol are estimated to contribute 3–9% of total energy consumption among those who drink alcohol (38). However, dose of alcohol consumed may explain why intake is not associated with weight gain, particularly in women who, as in this study, report low intake (mean and median of 6.4 and 1.8 g/day, respectively).
The present study has several strengths. The robust dataset of the WHI CT allowed an opportunity to evaluate these important associations with scientific rigor, controlling for several relevant confounders and applying repeat clinic measures of body weight. Limitations of this study also deserve comment. First, alcohol use/intake is known to be underreported, and no objective measures of exposure are available. Second, we used dietary data from the FFQ, and it has been demonstrated that significant measurement error exists in relation to FFQs, particularly for energy intake. The error estimates for this FFQ have been reported previously and suggest error is positively correlated with BMI and varies by race/ethnicity (39). Third, while the number of women who progressed from normal weight to overweight was quite large, the sample size to evaluate progression to obesity was much smaller and limited our ability to draw inference. In addition, the dataset did not afford an opportunity to evaluate the relationship between body weight change and patterns of alcohol consumption such as binge versus regular intake or alcohol consumption over adulthood, which are additional factors that may inform of this association. In fact, evidence, albeit limited, has supported the notion that drinking frequency is positively associated with central adiposity; however, in our sample waist circumference was not associated with intake. Further, despite having evaluated the associations in adjusted models, residual confounding may bias these results. Additionally, new and compelling evidence suggests obesity may be more prevalent in women with familial alcoholism despite the avoidance of alcohol (40), since the dopamine-modulated neurobiological adaptation response may play similar roles in alcoholism and overeating. Finally, we did not have complete weight data for all subjects at all time points, and women with co-morbidities such as diabetes, cancer, or cardiovascular disease were not excluded in order to maximize our sample size. Thus, our results may be biased by the fact that obese individuals, those with co-morbidities that could alter alcohol intake, or those with high alcohol intake may be less likely to attend clinic visits for weight measurement. However, our findings remain similar in a final sensitivity analysis wherein the sample was further restricted to women with BMI measured at all 8 time points and those without any history or incidence of diabetes treatment, cancer, or cardiovascular disease/myocardial infarction (data not shown).
In conclusion, our analyses from a large-scale, prospective cohort suggest that moderate alcohol intake in normal-weight, postmenopausal women is associated with significantly reduced risk of becoming overweight or obese. These results support emerging evidence from other large cohorts, including results from the WHS, and extend the findings to an older sample of women. Clinically these data suggest that moderate alcohol intake does not promote weight gain after menopause in women of normal BMI. They may also suggest that moderate intake will not compromise a diet plan targeting healthy weight maintenance after menopause as suggested by current Dietary Guidelines, but this would need to be evaluated further (6).
ACKNOWLEDGEMENTS
Financial Support:
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Conflict of interest:
None of the authors have any financial arrangements to disclose or conflicts of interest relevant to this research.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 3: 235–241. [DOI] [PubMed] [Google Scholar]
- 2.Heart National, Lung, and Blood Institute (NHLBI). Clinical Guidelines, 1998. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. (Accessed September 20, 2011).
- 3.Center for Disease Control and Prevention (CDC). U.S. Obesity trends http://www.cdc.gov/obesity/data/trends.html. (Accessed September 20, 2011).
- 4.Nelson DE, Naimi TS, Brewer RD, Neson HA. State alcohol-use estimates among youth and adults, 1993–2005. AJPM 2009; 3: 218–224. [DOI] [PubMed] [Google Scholar]
- 5.Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005–2007. Vital Health Stat 10 2010; 245: 1–132. [PubMed] [Google Scholar]
- 6.U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: US Government Printing Office, December, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayon-Orea C, Martinez-Gonzalez MA, Bes-Rastrollo M. Alcohol consumption and body weight: a systematic review. Nutr Rev 2011; 8: 419–431. [DOI] [PubMed] [Google Scholar]
- 8.Liangpunsakul S Relationship among alcohol intake, body fat and physical activity: a population-based study. Ann Epidemiol 2010; 9: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Lee IM, Manson JE, Buring JE, Sesso HD. Alcohol consumption, weight gain, and risk of becoming overweight in middle-aged and older women. Arch Intern Med 2010; 5: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeomans MR. Alcohol, appetite and energy balance: is alcohol intake a risk factor for obesity? Physiol Behav 2010; 1: 82–89. [DOI] [PubMed] [Google Scholar]
- 11.Pajari M, Pietilainen KH, Kapiro J, Rose RJ, Saarni SE. The effect of alcohol consumption on later obesity in early adulthood – a population based longitudinal study. Alcohol Alcohol 2010; 2: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suter PM. Is alcohol consumption a risk factor for weight gain and obesity? Crit Rev Clin Lab Sciences 2005; 3: 197–227. [DOI] [PubMed] [Google Scholar]
- 13.Rossouw J, Anderson G, Oberman A. Baseline Monograph – Foreword. Ann Epidemiol 2003; 13: S1–S4. [Google Scholar]
- 14.Rossouw J, Anderson G, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML et al. The Writing Group for the WHI Investigators. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: Principal results of the Women’s Health Initiative randomized controlled trial. JAMA 2002; 3: 321–333. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black HR et al. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomized Controlled Trial. JAMA 2004; 14: 1701–1712. [DOI] [PubMed] [Google Scholar]
- 16.Beresford S, Johnson K, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR et al. Low-Fat Dietary Pattern and Risk of Colorectal Cancer: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006; 6: 643–654. [DOI] [PubMed] [Google Scholar]
- 17.Howard B, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wasertheil-Smoller S et al. Low-Fat Dietary Pattern and Risk of Cardiovascular Disease: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006; 6: 655–666. [DOI] [PubMed] [Google Scholar]
- 18.Prentice R, Caan B, Chlebowski R, Patterson R, Kuller LH, Ockene JK et al. Low-Fat Dietary Pattern and Risk of Invasive Breast Cancer: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006; 6: 629–642. [DOI] [PubMed] [Google Scholar]
- 19.Wactawski-Wende J, Kotchen J, Anderson G, Assaf AR, Brunner RL, O’Sullivan MJ et al. Calcium plus Vitamin D Supplementation and the Risk of Colorectal Cancer. N Engl J Med 2006; 7: 684–696. [DOI] [PubMed] [Google Scholar]
- 20.Jackson R, LaCroix A, Gass M, Wallace RB, Robbins J, Lewis CE et al. Calcium plus Vitamin D Supplementation and the Risk of Fractures. N Engl J Med 2006; 7: 669–683. [DOI] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement Characteristics of the Women’s Health Initiative Food Frequency Questionnaire. Ann Epidemiol 1999; 3: 178–187. [DOI] [PubMed] [Google Scholar]
- 22.Ward H, Luben RN, Wareham NJ, Khaw K. CHD risk in relation to alcohol intake from categorical and open-ended dietary instruments. Pub Health Nutr 2010; 3: 402–409. [DOI] [PubMed] [Google Scholar]
- 23.Cuzick J A Wilcoxon-type test for trend. Stat Med 1985; 4: 87–90. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Giovannucci E, Rimm EB, Stampfer MJ, Rosner B, Speizer FE et al. Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr 1991; 1: 49–55. [DOI] [PubMed] [Google Scholar]
- 25.Lahti-Koski M, Pietinen P, Heliovaara M, Vartiainen E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982-FINRISK Studies. Am J Clin Nutr 2002; 5: 809–817. [DOI] [PubMed] [Google Scholar]
- 26.Barry D, Petry NM. Associations between body mass index and substance use disorders differ by gender: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addict Behav 2009; 1: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Serdula MK, Williamson DF, Mokdad AH, Byers T. A prospective study of alcohol intake and change in body weight among US adults. Am J Epidemiol 1994; 10: 912–920. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long term weight gain in women and men. N Engl J Med 2011; 364: 2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wannamethee SG, Field AE, Colditz A, Rimm EB. Alcohol intake and 8-year weight gain in women: a prospective study. Obes Res 2004; 9: 1386–1396. [DOI] [PubMed] [Google Scholar]
- 30.Breslow R, Guenther PM, Juan W, Graubard BI. Alcoholic beverage consumption, nutrient intakes, and diet quality in the US adult population. J Am Diet Assoc 2010; 4: 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruchow HW, Sobocinski KA, Barboriak JJ, Scheller JG. Alcohol consumption, nutrient intake and relative body weight among US adults. Am J Clin Nutr 1985; 2: 289–295 [DOI] [PubMed] [Google Scholar]
- 32.Ruf T, Nagel G, Altenburg HP, Miller AB, Thorand B. Food and nutrient intake, anthropometric measurements and smoking according to alcohol consumption in the EPIC Heidelberg Study. Ann Nutr Metabol 2005; 1: 16–25. [DOI] [PubMed] [Google Scholar]
- 33.Westerterp KR. Diet induced thermogenesis. Nutrition & Metabol 2004; 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suter PM. The pardox of the alcohol-pardox-another step towards the resolution of the ‘alcohol energy wastage’ controversy. Eur J Clin Invest 2000. 9: 749–750. [DOI] [PubMed] [Google Scholar]
- 35.Klesges RC, Mealer CZ, Klesges LM. Effects of alcohol intake on resting energy expenditure in young women social drinkers. Am J Clin Nutr 1994; 4: 805–809. [DOI] [PubMed] [Google Scholar]
- 36.Suter PM, Schutz Y, Jequier R. The effect of ethanol on fat storage in healthy subjects. N Engl J Med 1992; 15: 983–987. [DOI] [PubMed] [Google Scholar]
- 37.Clevidence BA, Taylor PR, Campbell WS, Judd JT. Lean and heavy women may not use energy from alcohol with equal efficiency. J Nutr 1995; 10: 2536–2540. [DOI] [PubMed] [Google Scholar]
- 38.Westerterp-Plantenga MS, Verwegen CR. The appetizing effect of an aperitif in overweight and normal-weight humans. Am J Clin Nutr 1999; 2: 205–212. [DOI] [PubMed] [Google Scholar]
- 39.Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Van Horn L et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am J Epidemiol 2008; 10: 1247–1259. [DOI] [PubMed] [Google Scholar]
- 40.Grucza RA, Krueger RF, Racette SB, Norberg KE, Hipp PR, Bierut LJ. The emerging link between alcoholism risk and obesity in the United States. Arch Gen Psychiatry 2010; 12: 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]


