Abstract
Harmful bloom-forming algae include some of the most prolific microbial producers of extracellular reactive oxygen species (ROS). However, the taxonomic diversity of ROS production, the underlying physiological mechanisms and ecophysiological roles of ROS cycling are not completely characterized among phytoplankton taxa that form harmful algal blooms (HABs). This study examines the extracellular production of the ROS superoxide and hydrogen peroxide by five marine HAB species: Chattonella marina, Heterosigma akashiwo, Karenia brevis, Pseudo-nitzschia sp. and Aureococcus anophagefferens. All species produced extracellular superoxide and hydrogen peroxide. Rates of ROS production per cell spanned several orders of magnitude and varied inversely with cell density, suggesting a potential signaling role for extracellular ROS. ROS production was also detected in the spent media of all cultures except K. brevis, indicating the presence of cell-free ROS-generating constituents, such as enzymes or metabolites, which could be further investigated as molecular targets for tracking ROS production in laboratory and field settings. Finally, ratios of superoxide to hydrogen peroxide production could not be accounted for by superoxide dismutation alone, except in the case of K. brevis, indicating a diversity of ROS production and degradation pathways that may ultimately help illuminate the functions of HAB-derived ROS.
Keywords: reactive oxygen species, superoxide, hydrogen peroxide, Pseudo-nitzschia, Karenia brevis, Aureococcus anophagefferens
INTRODUCTION
The reactive oxygen species (ROS) superoxide and hydrogen peroxide are intermediates in the reduction of oxygen to water. These ROS are powerful oxidants and reductants that shape ecological interactions and biogeochemistry in aquatic environments. For example, ROS contribute to the cycling of carbon (Pullin et al., 2004), transform vital trace metals such as iron (Rose, 2012) and regulate toxic elements like mercury (Siciliano et al., 2002). Recognition has been growing that extracellular ROS production by plankton communities contributes substantially to aquatic ROS fluxes (Zinser, 2018). Indeed, extracellular ROS production has been documented in several microbial groups including heterotrophic bacteria (Diaz et al., 2013), cyanobacteria (Rose et al., 2008; Hansel et al., 2016), diatoms (Kustka et al., 2005; Schneider et al., 2016), dinoflagellates (Saragosti et al., 2010; Zhang et al., 2016) and raphidophytes (Oda et al., 1997; Portune et al., 2010).
As a group, harmful bloom-forming algae exhibit the highest rates of extracellular ROS production observed among aquatic microorganisms. These ROS contribute to the toxic or noxious activity of several harmful algal bloom (HAB) taxa, such as raphidophytes (Oda et al., 1992, 1997; Yang et al., 1995; Kim et al., 1999b) and the dinoflagellates Margalefidinium polykrikoides (Kim et al., 1999a; Tang and Gobler, 2009b; Tang and Gobler, 2010) and Alexandrium spp. (Flores et al., 2012; Mardones et al., 2015). However, extracellular ROS production by these HAB species is not always linked to allelopathic or ichthyotoxic effects (Twiner et al., 2001; Marshall et al., 2003; Woo et al., 2006; Tang and Gobler, 2009a). In the harmful raphidophyte Chattonella marina, extracellular ROS have also been implicated in metal nutrient acquisition (Garg et al., 2007; Liu et al., 2007) and autocrine growth promotion (Oda et al., 1995), consistent with a range of other cell types (Saran, 2003; Buetler et al., 2004; Rose et al., 2005; Mittler et al., 2011; Roe and Barbeau, 2014). Thus, extracellular ROS production by HAB species may have a myriad of impacts on aquatic ecology and biogeochemistry, which are not completely understood. Here, we explore the rates, cell density-dependent regulation and mechanisms of extracellular ROS production by five species of harmful bloom-forming algae, including the key taxa Karenia brevis, Pseudo-nitzschia sp. and Aureococcus anophagefferens, for which extracellular ROS production either has not been reported or not been quantified previously.
MATERIALS AND METHODS
Algal strains, culturing conditions and sampling
C. marina ARC260, Heterosigma akashiwo ARC114, K. brevis ARC5, and Pseudo-nitzschia sp. ARC447 were obtained from the Algal Resources Collection at the University of North Carolina Wilmington (www.algalresourcescollection.com). A. anophagefferens CCMP1984 was obtained from the National Center for Marine Algae and Microbiota, Bigelow Laboratories, East Boothbay, Maine. All cultures were maintained in L1 media (Guillard and Hargraves, 1993) at 23°C on a 14 h:10 h light:dark cycle (340 μmol photons m−2 s−1), with the following exceptions: A. anophagefferens was grown at 18°C, and Pseudo-nitzschia sp. was cultured in f/25 (Guillard and Ryther, 1962) at 18°C. All media were prepared using filtered (0.2 μm) natural seawater from the South Atlantic Bight. Phytoplankton growth was monitored by measuring in vivo chlorophyll fluorescence with an AquaFluor handheld fluorometer (Turner Designs). C. marina and H. akashiwo were counted live using a Multisizer 4e coulter counter (Beckman Coulter). All other cultures were preserved in 2% Lugol’s solution and enumerated under the microscope using a hemocytometer counting chamber (Karlson et al., 2010). Cultures were sampled for ROS measurements within the first half of the 14-h daylight period during late-exponential growth, except A. anophagefferens, which was analyzed at the same point in the diel cycle but in early log phase. Cell abundances (cells mL−1) at the time of sampling were as follows: C. marina (1.4 × 104), H. akashiwo (1.5 × 105), K. brevis (5.3 × 103), Pseudo-nitzschia sp. (1.0 × 104), A. anophagefferens (2.1 × 105).
A dilution series of each culture was prepared using spent media (i.e. cell-free filtrate). For all species except A. anophagefferens, the cell-free filtrate was prepared by gravity filtering an aliquot of each culture (5 μm, 47 mm) through a polycarbonate membrane (EMD Millipore) and subsequently syringe-filtering (0.2 μm). To generate cell-free filtrates of A. anophagefferens, cultures were centrifuged (3000 × g, 10 min, 4°C), decanted, and then syringe-filtered (0.2 μm). For all species, the original, light-adapted culture was then diluted into the filtrate at a ratio of 1:10 and 1:100. After preparation, culture dilution series and the cell-free filtrate were immediately analyzed for extracellular ROS production under the conditions specified below for each ROS. After ROS measurements, microscopic inspection of the diluted and undiluted cultures confirmed that cell morphology and motility remained unaltered by sample handling and analysis.
Superoxide
Superoxide production rates were quantified based on the specific reaction between superoxide and the chemiluminescent probe methyl Cypridina luciferin analog (MCLA), to which cell membranes are impermeable. To avoid breakage of the relatively large, fragile cells examined in this study, superoxide production was quantified by adding MCLA to cell suspensions in a microplate assay (Godrant et al., 2009), rather than using a common flow injection analysis method that has been applied to measure extracellular superoxide production by smaller bacteria and phytoplankton cells immobilized on a filter (Diaz et al., 2013; Schneider et al., 2016; Zhang et al., 2016). Culture dilution series and cell-free filtrates were analyzed in replicate in a white 96-well plate with the addition of superoxide dismutase (SOD, 40 kU L−1, to account for the autooxidation of MCLA), each of three xanthine oxidase calibration standards (XO, 5, 10 and 50 mU L−1), or no addition. To complex metals that otherwise lower the lifetime and detectability of superoxide, diethylenetriaminepentaacetic acid (DTPA, 100 μmol L−1) was added to all wells, in addition to xanthine (X, 50 μmol L−1) and MCLA (5.7 μmol L−1). Chemiluminescence was measured at all wavelengths every 3.5 min for 1 h with a 1 s acquisition time per well using a SpectraMax M series multimode plate reader (Molecular Devices). This incubation period is in the acceptable range for this method given the concentrations of X and XO used (Godrant et al., 2009). Cultures remained in the dark (0 μmol photons m−2 s−1) within the plate reader during the 1 h analysis.
Superoxide production rates were calculated as follows. At each time point, each unamended and XO-amended sample was corrected for the baseline chemiluminescence measured in the presence of SOD, and then averaged across all time points. The average SOD-corrected signals from unamended samples were then converted to XO equivalents using the internal XO calibration curve for each sample. Finally, the XO standard (30 U L−1) was calibrated in the cell-free filtrate (50 μmol L−1 X, 100 μmol L−1 DTPA) in the presence of nitroblue tetrazolium (NBT, 100 μmol L−1), based on the rate of monoformazan production (MF, molar extinction coefficient = 12 800 L mol−1 cm−1, in a 1:2 molar ratio of MF:superoxide; (Bielski et al., 1980)) in the presence and absence of SOD (40 kU L−1). The superoxide production rate in the filtrate was subtracted from all diluted and undiluted culture samples. In subsequent experiments using an identical approach, background rates of superoxide production were determined in sterile (virgin) L1 and f/25 media, which were used to correct the superoxide production rates of cell-free filtrates. Corrected superoxide production rates in cell-free filtrates were normalized to the original cell density in the undiluted culture at the time of sampling. All chemicals were obtained from Millipore Sigma.
Hydrogen peroxide
Hydrogen peroxide production rates were measured from each culture at the same time as superoxide production rates. The hydrogen peroxide analysis was based on the reaction between hydrogen peroxide and the colorimetric probe Ampiflu™ Red (AR), which is catalyzed extracellularly by horseradish peroxidase (HRP). Culture dilution series and cell-free filtrates were analyzed in replicate in a clear 96-well plate with the addition of the hydrogen peroxide-degrading enzyme catalase (10 mg L−1; to account for the autooxidation of AR), each of four concentrations of hydrogen peroxide (10, 100, 700, 5000 nmol L−1), or no addition. Hydrogen peroxide standards were prepared from a primary stock solution made by diluting 2 μL of 30% hydrogen peroxide into 4 mL of ultrapure water, which was calibrated by measuring its absorbance at 240 nm and applying the molar extinction coefficient of hydrogen peroxide at this wavelength, 38.1 L mol−1 cm−1 (Miller and Kester, 1988). AR and HRP were added at final concentrations of 18 μmol L−1 and 0.4 kU L−1, respectively. Cultures were incubated under ambient low light conditions (~5 μmol photons m−2 s−1) for up to 4 h, and absorbance was measured at 530 nm and 700 nm once an hour using a SpectraMax M series multimode plate reader (Molecular Devices).
Hydrogen peroxide production rates were calculated as follows. First, absorbance at 700 nm was subtracted from the absorbance at 530 nm in every well at each time point. Next, at each time point, each unamended and hydrogen peroxide-amended sample was corrected for the baseline absorbance measured in the presence of catalase. Then, each catalase-corrected signal from the unamended samples was converted to hydrogen peroxide concentration using the internal hydrogen peroxide calibration curve for each sample. Finally, the increase in hydrogen peroxide in each unamended sample over time was quantified with simple linear regression (R typically > 0.95). The hydrogen peroxide production rate in the cell-free filtrate was subtracted from all diluted and undiluted culture samples. In subsequent experiments using an identical procedure, background rates of hydrogen peroxide production were determined in sterile (virgin) L1 and f/25 media, which were used to correct the hydrogen peroxide production rates of cell-free filtrates. Corrected hydrogen peroxide production rates in cell-free filtrates were normalized to the original cell density in the undiluted culture at the time of sampling. All chemicals were obtained from Millipore Sigma.
Statistical analysis
Statistical analyses were conducted in JMP Pro (version 13.0). Potential monotonic relationships between cell density and cell-normalized ROS production rates were determined by calculating Spearman’s rank correlation coefficient. Average ratios of superoxide to hydrogen peroxide production were analyzed using a one-sample t-test of the population mean.
RESULTS
Superoxide
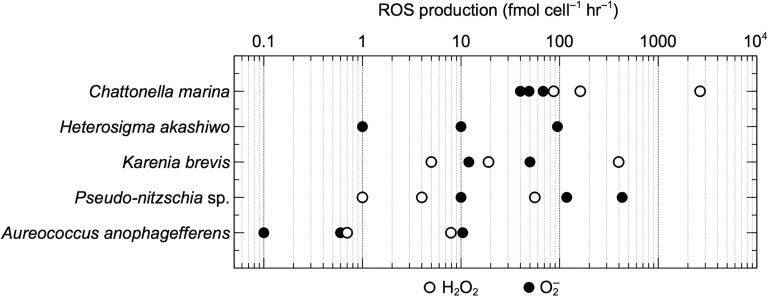
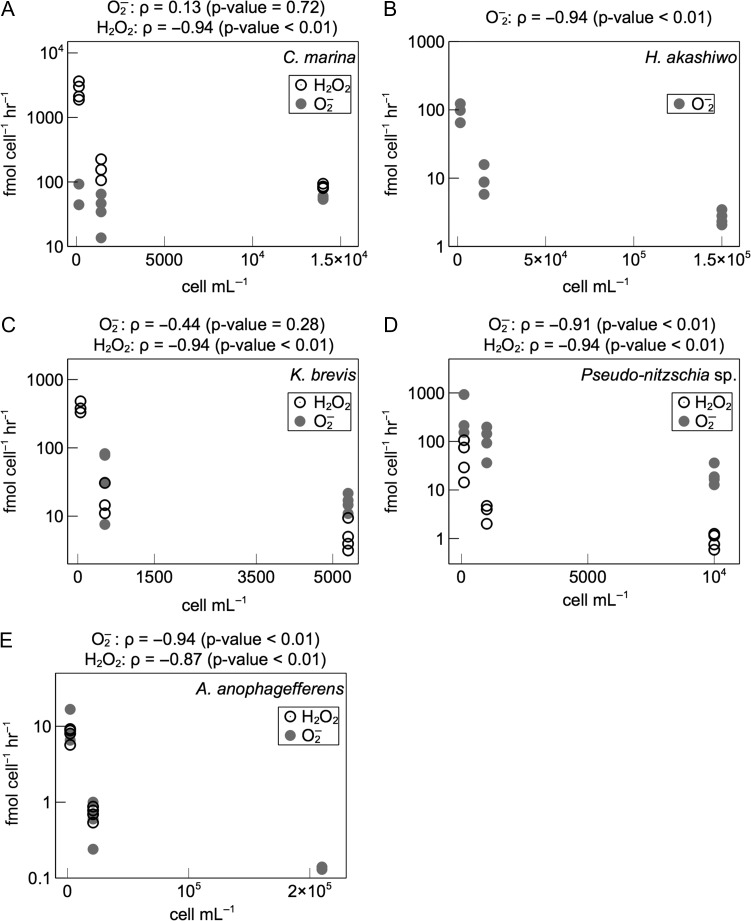
Rates of extracellular superoxide production measured over a broad range of cell densities of C. marina, H. akashiwo, K. brevis, Pseudo-nitzschia sp. and A. anophagefferens were corrected for superoxide production rates in cell-free filtrates. These corrected rates therefore reflect cell-associated superoxide production. Cell-associated superoxide production rates exhibited substantial inter- and intraspecific variability (Fig. 1; Table I). Maximum rates (average ± SE) were observed in Pseudo-nitzschia sp. (431 ± 248 fmol cell−1 h−1; n = 3), followed by H. akashiwo (95 ± 15 fmol cell−1 h−1; n = 3), C. marina (68 ± 24 fmol cell−1 h−1; n = 2), K. brevis (50 ± 18 fmol cell−1 h−1; n = 4), and finally A. anophagefferens (10 ± 3 fmol cell−1 h−1; n = 3). Cell-associated superoxide levels remained below detection in only one case, which was K. brevis at the lowest cell density (~50 cells mL−1). In all species except C. marina, cell-normalized superoxide production rates increased with decreasing cell density, which was statistically significant (P < 0.01) in all species except K. brevis (Fig. 2). With 10-fold and 100-fold decreases in cell density, cell-normalized superoxide production increased by ~4–12 times and ~45 times, respectively, by Pseudo-nitzschia sp.; ~10 times and ~100 times, respectively, by H. akashiwo; and ~15 times and ~200 times, respectively, by A. anophagefferens. With a 10-fold decrease in cell density, cell-normalized superoxide production increased by ~4 times in K. brevis.
Fig. 1.
Cell-associated rates of extracellular superoxide (O2−) and hydrogen peroxide (H2O2) production by the organisms examined in this study.
Table I:
Extracellular superoxide (O2−) and hydrogen peroxide (H2O2) production by the organisms examined in this study
| Species | Strain | Dilution level | cells mL−1 | ROS Production | |
|---|---|---|---|---|---|
| fmol cell−1 h−1 | |||||
| O2− | H2O2 | ||||
| Chattonella marina | ARC260 | 1:1 | 14 000 | 49 ± 1 (n = 4) | 87 ± 3 (n = 4) |
| 1:10 | 1400 | 40 ± 11 (n = 4) | 162 ± 35 (n = 3) | ||
| 1:100 | 140 | 68 ± 24 (n = 2) | 2658 ± 408 (n = 4) | ||
| Filtrate | 0 | 5 ± 1 (n = 4) | 12 ± 1 (n = 4) | ||
| Heterosigma akashiwo | ARC114 | 1:1 | 150 000 | 0.9 ± 0.3 (n = 4) | BD |
| 1:10 | 15 000 | 10 ± 2 (n = 4) | BD | ||
| 1:100 | 1500 | 95 ± 15 (n = 3) | BD | ||
| Filtrate | 0 | 1.5 ± 0.2 (n = 4) | 1.2 ± 0.1 (n = 4) | ||
| Karenia brevis | ARC5 | 1:1 | 5300 | 12 ± 2 (n = 4) | 5 ± 1 (n = 4) |
| 1:10 | 530 | 50 ± 18 (n = 4) | 19 ± 6 (n = 3) | ||
| 1:100 | 53 | BD | 397 ± 45 (n = 3) | ||
| Filtrate | 0 | BD | BD | ||
| Pseudo-nitzschia sp. | ARC447 | 1:1 | 10 000 | 10 ± 9 (n = 2) | 0.9 ± 0.2 (n = 4) |
| 1:10 | 1000 | 118 ± 35 (n = 4) | 4 ± 1 (n = 3) | ||
| 1:100 | 100 | 431 ± 248 (n = 3) | 56 ± 21 (n = 4) | ||
| Filtrate | 0 | 24 ± 2 (n = 4) | BD | ||
| Aureococcus anophagefferens | CCMP1984 | 1:1 | 210 000 | 0.1 ± 0.1 (n = 4) | BD |
| 1:10 | 21 000 | 0.6 ± 0.2 (n = 4) | 0.7 ± 0.1 (n = 4) | ||
| 1:100 | 2100 | 10 ± 3 (n = 3) | 8 ± 1 (n = 4) | ||
| Filtrate | 0 | 0.1 ± 0.0 (n = 4) | 0.1 ± 0.0 (n = 4) | ||
The ROS production rates in cell-free filtrates were normalized to the original cell density in the undiluted (1:1) culture at the time of sampling. Mean ROS production rates ± standard error of the mean are provided. BD, below detection.
Fig. 2.
Cell density dependence of extracellular superoxide (O2−) and hydrogen peroxide (H2O2) production. Spearman’s rank correlation coefficient (ρ) and associated P-value describe the degree of monotonicity in the relationship between cell-normalized superoxide production rates and cell density.
Superoxide production rates in cell-free filtrates were corrected for the rates of superoxide production in sterile media. After normalizing to the original (undiluted) cell density, the corrected superoxide production rates (average ± SE) in the cell-free filtrates were 24 ± 2 fmol cell−1 h−1 (Pseudo-nitzschia sp.; n = 4), 5 ± 1 fmol cell−1 h−1 (C. marina; n = 4), 1.5 ± 0.2 fmol cell−1 h−1 (H. akashiwo; n = 4), and 0.1 ± 0.0 fmol cell−1 h−1 (A. anophagefferens; n = 4). Superoxide production in sterile media was sufficient to explain the generation of hydrogen peroxide in cell-free filtrates of K. brevis.
Hydrogen peroxide
Like superoxide, rates of extracellular hydrogen peroxide production measured over a broad range of cell densities of C. marina, K. brevis, Pseudo-nitzschia sp. and A. anophagefferens were corrected for hydrogen peroxide production rates in cell-free filtrates. These corrected rates therefore reflect cell-associated hydrogen peroxide production. Cell-associated hydrogen peroxide production rates exhibited a wide range of variation within and between species (Fig. 1; Table I). Hydrogen peroxide production remained below detection in undiluted A. anophagefferens and in all H. akashiwo samples, except the H. akashiwo cell-free filtrate. Maximum rates of hydrogen peroxide production (average ± SE) revealed C. marina as the most prolific hydrogen peroxide producer (2658 ± 408 fmol cell−1 h−1; n = 4), followed by K. brevis (397 ± 45 fmol cell−1 h−1; n = 3), Pseudo-nitzschia sp. (56 ± 21 fmol cell−1 h−1; n = 4), and finally, A. anophagefferens (8 ± 1 fmol cell−1 h−1; n = 4). Cell-normalized hydrogen peroxide production rates increased with decreasing cell density, which was statistically significant (P < 0.01) in all species (Fig. 2). With 10-fold and 100-fold decreases in cell density, cell-normalized hydrogen peroxide production increased by ~4–16 times and ~60 times, respectively, by Pseudo-nitzschia sp.; ~2–16 times and ~31 times, respectively, by C. marina; and ~3–21 times and ~74 times, respectively, by K. brevis. Similarly, a 10-fold decrease in cell density resulted in an ~11-fold increase in cell-normalized hydrogen peroxide production by A. anophagefferens.
Hydrogen peroxide production rates in cell-free filtrates were corrected for the rates of hydrogen peroxide production in sterile media. After normalizing to the original (undiluted) cell density, the corrected rates of hydrogen peroxide production (average ± SE) in the cell-free filtrates were 12 ± 1 fmol cell−1 h−1 (C. marina; n = 4), 1.2 ± 0.1 fmol cell−1 h−1 (H. akashiwo; n = 4), and 0.1 ± 0.0 fmol cell−1 h−1 (A. anophagefferens; n = 4). Hydrogen peroxide production in sterile media was sufficient to explain the generation of hydrogen peroxide in cell-free filtrates of K. brevis and Pseudo-nitzschia sp.
Comparison of superoxide and hydrogen peroxide production rates
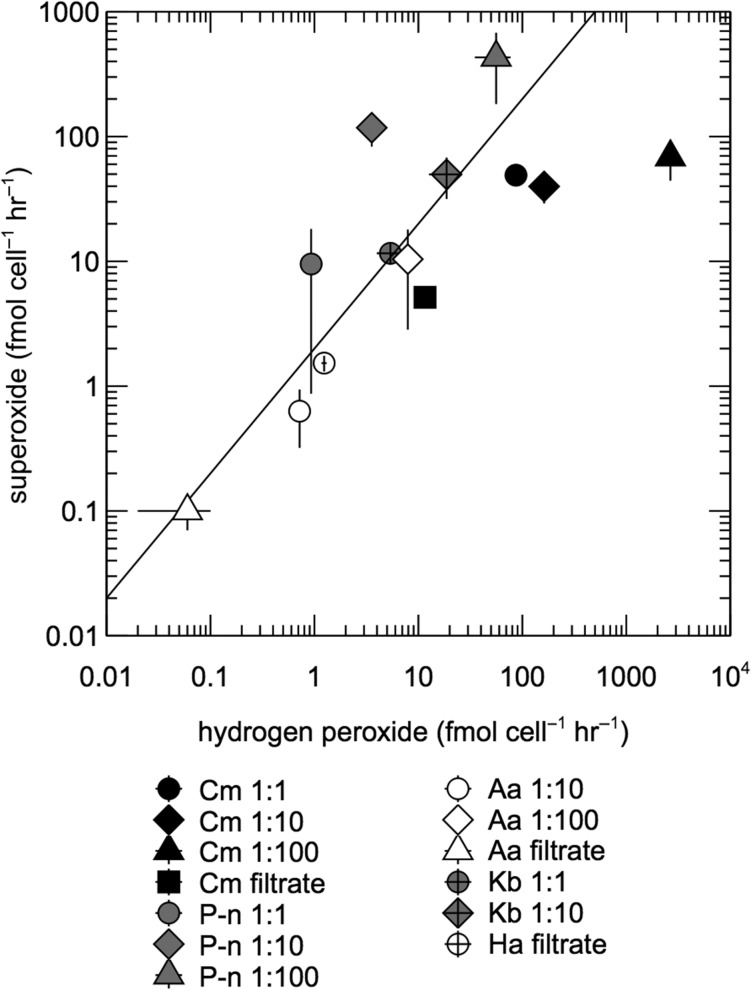
A range of superoxide to hydrogen peroxide production ratios (PO2−:PH2O2) were observed in the organisms tested here (Fig. 3), which reveal underlying dynamics of each ROS. For example, the complete reduction of superoxide to hydrogen peroxide yields PO2−:PH2O2 = 1. The self-reaction of two moles of superoxide via dismutation produces one mole of hydrogen peroxide (PO2−:PH2O2 = 2), while the complete oxidation of superoxide gives rise to no hydrogen peroxide, leading to PO2−:PH2O2 > 2. K. brevis produced ROS at a ratio consistent with superoxide dismutation (PO2−:PH2O2 = 2–3). However, A. anophagefferens (PO2−:PH2O2 = ~1; P < 0.01), H. akashiwo (PO2−:PH2O2 = ~1; P«0.01) and C. marina (PO2−:PH2O2 = 0.03–0.6; P«0.01) produced more hydrogen peroxide than expected from superoxide dismutation alone. On average, Pseudo-nitzschia sp. produced less hydrogen peroxide expected from superoxide dismutation (PO2−:PH2O2 = 8–30), which was statistically significant at the 1:10 and 1:100 dilution levels (P < 0.05), but not in the undiluted case (P = 0.07), due to the high level of variability in superoxide production rates measured in the undiluted culture (relative standard deviation >100%).
Fig. 3.
Comparison of superoxide and hydrogen peroxide production rates in diluted cell suspensions and cell-free filtrates. C. marina (Cm), Pseudo-nitzschia sp. (P-n), A. anophagefferens (Aa), K. brevis (Kb), and H. akashiwo (Ha). Error bars represent the standard error of the mean of biological replicates. The diagonal line represents the 2:1 molar ratio of superoxide to hydrogen peroxide production expected from the dismutation of superoxide.
DISCUSSION
In this study, the rates, cell density-dependent regulation and mechanisms of extracellular ROS production were explored in five marine species of harmful bloom-forming algae, including the key taxa K. brevis, Pseudo-nitzschia sp. and A. anophagefferens. Previous work has documented the ability of K. brevis to generate extracellular superoxide based on relative chemiluminescence (Marshall et al., 2005a; Mooney et al., 2011). Yet no other prior reports of extracellular ROS production by K. brevis, Pseudo-nitzschia sp., or A. anophagefferens could be found. Therefore, this study broadens the diversity of harmful algal species known to generate extracellular hydrogen peroxide and superoxide.
Rates of ROS production by intact cells
Consistent with literature observations, C. marina was the most prolific ROS producer observed in this study, followed by intermediate-level ROS producers H. akashiwo, K. brevis and Pseudo-nitzschia sp., and finally A. anophagefferens. Although ROS production by A. anophagefferens was much lower than the other species examined, superoxide production rates by A. anophagefferens were comparable to Synechococcus sp., which has been shown to represent a substantial potential source of ROS in the marine environment (Rose et al., 2008). Cell size is directly related to ROS production and thus drives substantial interspecific differences in extracellular ROS production rates (Oda et al., 1997; Marshall et al., 2005a; Diaz et al., 2013). Indeed, A. anophagefferens (a ~2–5 μm cell) is much smaller than any other species examined in this study, and the relatively lower ROS production rates by this organism are to be expected. A. anophagefferens was also the only species in this study that was analyzed in early exponential phase, as opposed to late-exponential phase. Although the growth phase dependence of extracellular ROS production by A. anophagefferens is not known, biomass-normalized rates of extracellular ROS production decline with age in batch cultures of several marine phytoplankton, including C. marina (Oda et al., 1995; Kawano et al., 1996; Garg et al., 2007), Chattonella antiqua (Portune et al., 2010), H. akashiwo (Skeen et al., 2004; Portune et al., 2010) and M. polykrikoides (Kim et al., 1999a). If the same is true for A. anophagefferens, then the rates measured in this study may be at the upper end of the range for this species, which is further consistent with the large interspecific differences in ROS production observed here.
Production rates of extracellular superoxide and hydrogen peroxide reported in this study are lower than previously documented rates of extracellular ROS production by HAB taxa (Diaz and Plummer, 2018), even compared to observations generated using the same or similar techniques on the same species (Dorantes-Aranda et al., 2013; Mardones et al., 2015). Because superoxide and hydrogen peroxide were measured by independent methods herein, the relatively low production rates measured for these ROS corroborate each other. Indeed, it is simpler to presume that the relatively low rates for both ROS reflect a shared biological explanation, rather than separate technical shortcomings of each method. The discrepancies between literature values and the rates measured in this study may be related to a number of factors. For example, ROS production can be affected by growth phase (Kawano et al., 1996; Kim et al., 1999a; Skeen et al., 2004; Garg et al., 2007; Portune et al., 2010), cell density (Yang et al., 1995; Twiner and Trick, 2000; Kim et al., 2002; Marshall et al., 2005b; Dorantes-Aranda et al., 2015) and light exposure (Kim et al., 1999a; Dorantes-Aranda et al., 2013). Substantial intraspecific variability in extracellular ROS production has also been documented (Ishimatsu et al., 1996; Oda et al., 1997; Portune et al., 2010; Dorantes-Aranda et al., 2013; Mardones et al., 2015), with extracellular superoxide production by different strains of C. marina varying up to 6-fold (Band-Schmidt et al., 2012). Consistent with this intraspecific variability, C. marina ARC260 produced extracellular superoxide at an average rate of 52 fmol cell−1 hr−1 in this study, which was ~6–9 times lower than another strain, C. marina CMDE01, during the same late-exponential growth stage in a previous investigation (Garg et al., 2007).
Cultures were not axenic. However, assuming bacterial abundance did not exceed 106 cell mL−1, which is consistent with the lack of visible bacterial growth in the cultures, the contribution of bacterial associates would have been less than 0.2% (median) or 8% (maximum) of total ROS fluxes measured, based on previously determined rates of bacterial extracellular ROS production (Gonzàlez-Flecha and Demple, 1995; Diaz et al., 2013). Because ROS measurements were conducted in the dark or under low light conditions below the compensation irradiances of the cultures (Wilson and Collier, 1955; Aldrich, 1962; Eng-Wilmot et al., 1977; Yamaguchi et al., 1991; Milligan and Cosper, 1997; El-Sabaawi and Harrison, 2006; Shikata et al., 2008; Smayda, 2008), another potential concern is that oxygen concentrations could decrease due to respiration and potentially become rate-limiting on the production of extracellular ROS. However, ROS data did not appear to be limited by oxygen consumption because hydrogen peroxide concentrations increased linearly throughout incubations (R > 0.95), and superoxide chemiluminescence signals did not attenuate significantly over time. Moreover, the sustained production of superoxide for 1 h in the dark indicates the presence of non-photosynthetic pathways for superoxide generation. In agreement with these results, extracellular superoxide production by C. marina and H. akashiwo could not be quenched by the photosynthetic inhibitor dichlorophenyldimethylurea (DCMU) in a previous study (Oda et al., 1998).
The effect of cell density on ROS production
Cell-normalized superoxide and hydrogen peroxide production by all species varied inversely with 10- and 100-fold changes in cell density, except superoxide production by C. marina and K. brevis. Inverse relationships between cell density and cell-normalized ROS production have been observed previously in harmful algae (Twiner and Trick, 2000; Kim et al., 2002; Marshall et al., 2005b) and the colonial marine cyanobacterium Trichodesmium spp. (Hansel et al., 2016). Such results suggest a cell density-dependent signaling role for ROS production in a variety of phytoplankton species, as outlined in a prior study. (Hansel et al., 2016).
A number of potential artifacts are possible with the use of MCLA in cell suspensions, however, which could lead to the underestimation of superoxide at high cell densities. For example, MCLA chemiluminescence may be absorbed by chlorophyll and quenched via binding of the probe to cell membranes (Godrant et al., 2009). These potential artifacts cannot be ruled out here, and thus, the current results must be interpreted with caution. However, using the same technique and approximately similar cell densities of C. marina, Dorantes-Aranda et al. (2013) reported much higher rates of superoxide production by this species. Thus, cell density may not be the predominant factor determining MCLA chemiluminescence in cell suspensions of C. marina. Moreover, because superoxide and hydrogen peroxide dynamics are coupled (e.g. superoxide gives rise to hydrogen peroxide via dismutation), the inverse relationship between cell-normalized hydrogen peroxide production and cell density supports the conclusion that the cell density dependence of superoxide production has a physiological basis.
Cell-free ROS production
In addition to cell-associated ROS production, the results from this study demonstrate ROS generation in the cell-free filtrate of several species, which could not be accounted for with sterile media controls. For example, C. marina, H. akashiwo and A. anophagefferens exhibited superoxide and hydrogen peroxide production in the cell-free filtrate, while Pseudo-nitzschia sp. cell-free filtrates only generated superoxide. Any cell-free ROS production by K. brevis, if present, remained below detection. These results point to the production of ROS by cell-free constituents (e.g. enzymes or metabolites) in the spent media, consistent with previous observations from C. marina (Kim et al., 2000; Li et al., 2015), the diatom Phaeodactylum tricornutum (Schneider et al., 2016), and a marine bacterium belonging to the Roseobacter clade of Alphaproteobacteria (Andeer et al., 2015).
Comparison of superoxide and hydrogen peroxide production
The underlying dynamics of superoxide and hydrogen peroxide can be inferred from the ratio of superoxide to hydrogen peroxide production. Here, comparisons of superoxide and hydrogen peroxide production were drawn from cultures of varying cell density, as well as cell-free filtrates. These comparisons were limited when superoxide and/or hydrogen peroxide levels were below detection (Table I). For hydrogen peroxide measurements, cultures were incubated in low ambient light (~5 μmol photons m−2 s−1), while superoxide measurements were conducted in the dark. Although photosynthesis may play a role in extracellular hydrogen peroxide production (Diaz and Plummer, 2018), the ambient light was likely below the compensation point for all species (Wilson and Collier, 1955; Aldrich, 1962; Eng-Wilmot et al., 1977; Yamaguchi et al., 1991; Milligan and Cosper, 1997; El-Sabaawi and Harrison, 2006; Shikata et al., 2008; Smayda, 2008) and therefore would have probably supported only minimal rates of photosynthesis, which is comparable to the conditions during superoxide analysis.
ROS measurements from each species generally reflected a consistent relationship between superoxide and hydrogen peroxide production across all levels of cell density and the cell-free filtrate. In K. brevis, the disproportionation of superoxide (e.g. by the enzyme superoxide dismutase and/or uncatalyzed dismutation) can completely explain values of PO2−:PH2O2, suggesting that extracellular hydrogen peroxide is only produced through a superoxide intermediate and that self-reaction is the predominant fate for superoxide. Comparable results were recently obtained for the diatom Thalassiosira oceanica (Schneider et al., 2016), which also produced superoxide and hydrogen peroxide in a ratio consistent with superoxide dismutation. In contrast, low PO2−:PH2O2 ratios suggest that additional sources of extracellular hydrogen peroxide besides superoxide dismutation must exist in cultures of H. akashiwo, A. anophagefferens, and C. marina, similar to previous results from Thalassiosira pseudonana (Schneider et al., 2016). These sources may include the complete reduction of superoxide to hydrogen peroxide, direct enzymatic reduction of oxygen to hydrogen peroxide and the passive diffusion of hydrogen peroxide out of the cell. Indeed, extracellular superoxide and hydrogen peroxide production by C. marina appear to be decoupled according to previous research, with intracellular sources accounting for the majority of extracellular hydrogen peroxide and cell-surface oxidoreductase activity generating most of the extracellular superoxide (Oda et al., 1997; Kim et al., 2007). Finally, Pseudo-nitzschia sp. generated less hydrogen peroxide than expected from the dismutation of superoxide, similar to recent reports of Thalassiosira weissflogii (Schneider et al., 2016). Although these results from Pseudo-nitzschia sp. were not always statistically significant, they suggest the presence of constituents that can oxidize superoxide and therefore prevent its reduction to hydrogen peroxide.
CONCLUSIONS
All HAB species examined in this study produced extracellular superoxide and hydrogen peroxide, and cell-associated ROS production rates ranged several orders of magnitude within and between organisms. The potential role(s) of these extracellular ROS in the ecophysiology of K. brevis, Pseudo-nitzschia sp. and A. anophagefferens should now be considered. For example, the inverse relationship between cell-normalized ROS production and cell density suggests a population-dependent signaling role for these ROS, as previously suggested (Marshall et al., 2005b; Hansel et al., 2016), which transcends the common assumption that ROS are predominantly cytotoxic agents. Indeed, toxicity and signaling are not necessarily mutually exclusive functions, which is consistent with ROS playing a diversity of roles in HABs (Diaz and Plummer, 2018). Furthermore, an understanding of the substrates with which ROS react will improve knowledge of their biogeochemical and ecological functions. For example, superoxide has previously been implicated in reactions with polyunsaturated fatty acids (PUFAs), which yield toxic lipid oxidation products (Arzul et al., 1998; Kim et al., 1999a; Jenkinson and Arzul, 2001; Marshall et al., 2003, 2005b; Mardones et al., 2015). Similarly, results from this study revealed that a wide variety of reactions are likely involved in the production and degradation of extracellular superoxide and hydrogen peroxide in the species examined. For example, superoxide rapidly reacts with itself to produce hydrogen peroxide, which may fully explain ROS dynamics in K. brevis, but other, yet unknown oxidants are likely present in Pseudo-nitzschia sp., while additional reductants may exist in A. anophagefferens and H. akashiwo. The production of ROS observed in spent media points to cell-free constituents (e.g. enzymes and/or metabolites) that can be developed as molecular targets for tracking extracellular ROS production. Such tools may be especially useful in the field, where ROS may be rapidly cycled and difficult to detect by direct chemical methods.
ACKNOWLEDGEMENTS
The authors wish to thank James G. Sanders for feedback on the manuscript.
FUNDING
This work was supported by a Junior Faculty Seed Grant from the University of Georgia Research Foundation (J.M.D.) and a National Science Foundation Graduate Research Fellowship (S.P.).
AUTHOR CONTRIBUTIONS
J.M.D. conceived and carried out the experiments. All authors contributed to the optimization of methods used in this study, as well as preparation of the manuscript.
REFERENCES
- Aldrich D. V. (1962) Photoautotrophy in Gymnodinium breve Davis. Science, 137, 988. [DOI] [PubMed] [Google Scholar]
- Andeer P. F., Learman D. R., McIlvin M., Dunn J. A. and Hansel C. M. (2015) Extracellular haem peroxidases mediate Mn(II) oxidation in a marine Roseobacter bacterium via superoxide production. Environ. Microbiol., 17, 3925–3936. [DOI] [PubMed] [Google Scholar]
- Arzul G., Bodennec G., Gentien P., Bornens P. and Crassous M.-P. (1998) The effect of dissolved oxygen on the haemolytic property of Gymnodinium ichthyotoxins In Reguera B., Blanco J., Fernandez M. L. and Wyatt T. (eds), Harmful Algae. Xunta de Calicia and Intergovernmental Oceanographic Commision of UNESCO, Vigo, Spain, pp. 611–614. [Google Scholar]
- Band-Schmidt C. J., Martinez-Lopez A., Bustillos-Guzman J. J., Carreon-Palau L., Morquecho L., Olguin-Monroy N. O., Zenteno-Savin T., Mendoza-Flores A. et al. (2012) Morphology, biochemistry, and growth of raphidophyte strains from the Gulf of California. Hydrobiologia, 693, 81–97. [Google Scholar]
- Bielski B. H. J., Shiue G. G. and Bajuk S. (1980) Reduction of nitro blue tetrazolium by CO2− and O2− radicals. J. Phys. Chem., 84, 830–833. [Google Scholar]
- Buetler T. M., Krauskopf A. and Ruegg U. T. (2004) Role of superoxide as a signaling molecule. News Physiol. Sci., 19, 120–123. [DOI] [PubMed] [Google Scholar]
- Diaz J. M., Hansel C. M., Voelker B. M., Mendes C. M., Andeer P. F. and Zhang T. (2013) Widespread production of extracellular superoxide by heterotrophic bacteria. Science, 340, 1223–1226. [DOI] [PubMed] [Google Scholar]
- Diaz J. M. and Plummer S. (2018) Production of extracellular reactive oxygen species by phytoplankton: past and future directions. J. Plankton Res., 1–12. doi:10.1093/plankt/fby039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorantes-Aranda J. J., Nichols P. D., Waite T. D. and Hallegraeff G. M. (2013) Strain variability in fatty acid composition of Chattonella marina (Raphidophyceae) and its relation to differing ichthyotoxicity toward rainbow trout gill cells. J. Phycol., 49, 427–438. [DOI] [PubMed] [Google Scholar]
- Dorantes-Aranda J. J., Seger A., Mardones J. I., Nichols P. D. and Hallegraeff G. M. (2015) Progress in understanding algal bloom-mediated fish kills: the role of superoxide radicals, phycotoxins and fatty acids. PLoS One, 10, e0133549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sabaawi R. and Harrison P. J. (2006) Interactive effects of irradiance and temperature on the photosynthetic physiology of the pennate diatom Pseudo-nitzschia granii (Bacillariophyceae) from the northeast subarctic Pacific. J. Phycol., 42, 778–785. [Google Scholar]
- Eng-Wilmot D. L., Hitchcock W. S. and Martin D. F. (1977) Effect of temperature on the proliferation of Gymnodinium breve and Gomphosphaeria aponina. Mar. Biol., 41, 71–77. [Google Scholar]
- Flores H. S., Wikfors G. H. and Dam H. G. (2012) Reactive oxygen species are linked to the toxicity of the dinoflagellate Alexandrium spp. to protists. Aquat. Microb. Ecol., 66, 199–209. [Google Scholar]
- Garg S., Rose A. L., Godrant A. and Waite T. D. (2007) Iron uptake by the ichthyotoxic Chattonella marina (Raphidophyceae): impact of superoxide generation. J. Phycol., 43, 978–991. [Google Scholar]
- Godrant A., Rose A. L., Sarthou G. and Waite T. D. (2009) New method for the determination of extracellular production of superoxide by marine phytoplankton using the chemiluminescence probes MCLA and red-CLA. Limnol. Oceanogr. Methods, 7, 682–692. [Google Scholar]
- Gonzàlez-Flecha B. and Demple B. (1995) Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J. Biol. Chem., 270, 13681–13687. [DOI] [PubMed] [Google Scholar]
- Guillard R. R. L. and Hargraves P. E. (1993) Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia, 32, 234–236. [Google Scholar]
- Guillard R. R. L. and Ryther J. H. (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol., 8, 229–239. [DOI] [PubMed] [Google Scholar]
- Hansel C. M., Buchwald C., Diaz J. M., Ossolinski J. E., Dyhrman S. T. and Van Mooy B. A. S. (2016) Dynamics of extracellular superoxide production by Trichodesmium colonies from the Sargasso Sea. Limnol. Oceanogr., 61, 1188–1200. [Google Scholar]
- Ishimatsu A., Oda T., Yoshida M. and Ozaki M. (1996) Oxygen radicals are probably involved in the mortality of yellowtail by Chattonella marina. Fish. Sci., 62, 836–837. [Google Scholar]
- Jenkinson I. R. and Arzul G. (2001) Mitigation by cysteine compounds of rheotoxicity, cytotoxicity and fish mortality caused by the dinoflagellates, Gymnodinium mikimotoi and G. cf. maguelonnense In Hallegraeff G., Bolch C. J. S., Blackburn S. I. and Lewis R. (eds), Harmful Algal Blooms 2000. UNESCO, Paris, pp. 461–464. [Google Scholar]
- Karlson B., Cusak C. and Bresnan E. (2010) Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis, UNESCO, Paris, France. [Google Scholar]
- Kawano I., Oda T., Ishimatsu A. and Muramatsu T. (1996) Inhibitory effect of the iron chelator Desferrioxamine (Desferal) on the generation of activated oxygen species by Chattonella marina. Mar. Biol., 126, 765–771. [Google Scholar]
- Kim C. S., Lee S. G., Lee C. K., Kim H. G. and Jung J. (1999. a) Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J. Plankton Res., 21, 2105–2115. [Google Scholar]
- Kim D., Nakamura A., Okamoto T., Komatsu N., Oda T., Iida T., Ishimatsu A. and Muramatsu T. (2000) Mechanism of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: Possible involvement of NAD(P)H oxidase. Biochimica Et Biophysica Acta-General Subjects, 1524, 220–227. [DOI] [PubMed] [Google Scholar]
- Kim D., Nakamura A., Okamoto T., Komatsu N., Oda T., Ishimatsu A. and Muramatsu T. (1999. b) Toxic potential of the raphidophyte Olisthodiscus luteus: mediation by reactive oxygen species. J. Plankton Res., 21, 1017–1027. [Google Scholar]
- Kim D., Nakashima T., Matsuyama Y., Niwano Y., Yamaguchi K. and Oda T. (2007) Presence of the distinct systems responsible for superoxide anion and hydrogen peroxide generation in red tide phytoplankton Chattonella marina and Chattonella ovata. J. Plankton Res., 29, 241–247. [Google Scholar]
- Kim D., Oda T., Muramatsu T., Kim D., Matsuyama Y. and Honjo T. (2002) Possible factors responsible for the toxicity of Cochlodinium polykrikoides, a red tide phytoplankton. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 132, 415–423. [DOI] [PubMed] [Google Scholar]
- Kustka A. B., Shaked Y., Milligan A. J., King D. W. and Morel F. M. M. (2005) Extracellular production of superoxide by marine diatoms: Contrasting effects on iron redox chemistry and bioavailability. Limnol. Oceanogr., 50, 1172–1180. [Google Scholar]
- Li X., Liu T., Wang K. and Waite T. D. (2015) Light-induced extracellular electron transport by the marine raphidophyte Chattonella marina. Environ. Sci. Technol., 49, 1392–1399. [DOI] [PubMed] [Google Scholar]
- Liu W., Au D. W. T., Anderson D. M., Lam P. K. S. and Wu R. S. S. (2007) Effects of nutrients, salinity, pH and light:dark cycle on the production of reactive oxygen species in the alga Chattonella marina. J. Exp. Mar. Bio. Ecol., 346, 76–86. [Google Scholar]
- Mardones J. I., Dorantes-Aranda J. J., Nichols P. D. and Hallegraeff G. M. (2015) Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: synergistic action of ROS and PUFA. Harmful Algae, 49, 40–49. [Google Scholar]
- Marshall J. A., de Salas M., Oda T. and Hallegraeff G. (2005. a) Superoxide production by marine microalgae: I. survey of 37 species from 6 classes. Mar. Biol., 147, 533–540. [Google Scholar]
- Marshall J. A., Nichols P. D., Hamilton B., Lewis R. J. and Hallegraeff G. M. (2003) Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): the synergistic role of reactive oxygen species and free fatty acids. Harmful Algae, 2, 273–281. [Google Scholar]
- Marshall J. A., Ross T., Pyecroft S. and Hallegraeff G. (2005. b) Superoxide production by marine microalgae: II. towards understanding ecological consequences and possible functions. Mar. Biol., 147, 541–549. [Google Scholar]
- Miller W. L. and Kester D. R. (1988) Hydrogen peroxide measurement in seawater by (para-hydroxylphenyl)acetic acid dimerization. Anal. Chem., 60, 2711–2715. [Google Scholar]
- Milligan A. J. and Cosper E. M. (1997) Growth and photosynthesis of the ‘brown tide’ microalga Aureococcus anophagefferens in subsaturating constant and fluctuating irradiance. Mar. Ecol. Prog. Ser., 153, 67–75. [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V. B., Vandepoele K., Gollery M., Shulaev V. et al. (2011) ROS signaling: the new wave? Trends Plant Sci., 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Mooney B. D., Dorantes-Aranda J. J., Place A. R. and Hallegraeff G. M. (2011) Ichthyotoxicity of gymnodinioid dinoflagellates: PUFA and superoxide effects in sheepshead minnow larvae and rainbow trout gill cells. Mar. Ecol. Prog. Ser., 426, 213–224. [Google Scholar]
- Oda T., Ishimatsu A., Shimada M., Takeshita S. and Muramatsu T. (1992) Oxygen-radical-mediated toxic effects of the red tide flagellate Chattonella marina on Vibrio alginolyticus. Mar. Biol., 112, 505–509. [Google Scholar]
- Oda T., Moritomi J., Kawano I., Hamaguchi S., Ishimatsu A. and Muramatsu T. (1995) Catalase-induced and superoxide dismutase-induced morphological changes and growth inhibition in the red tide phytoplankton Chattonella marina. Biosci. Biotechnol. Biochem., 59, 2044–2048. [Google Scholar]
- Oda T., Nakamura A., Okamoto T., Ishimatsu A. and Muramatsu T. (1998) Lectin-induced enhancement of superoxide anion production by red tide phytoplankton. Mar. Biol., 131, 383–390. [Google Scholar]
- Oda T., Nakamura A., Shikayama M., Kawano I., Ishimatsu A. and Muramatsu T. (1997) Generation of reactive oxygen species by raphidophycean phytoplankton. Biosci. Biotechnol. Biochem., 61, 1658–1662. [DOI] [PubMed] [Google Scholar]
- Portune K. J., Cary S. C. and Warner M. E. (2010) Antioxidant enzyme response and reactive oxygen species production in marine raphidophytes. J. Phycol., 46, 1161–1171. [Google Scholar]
- Pullin M. J., Bertilsson S., Goldstone J. V. and Voelker B. M. (2004) Effects of sunlight and hydroxyl radical on dissolved organic matter: Bacterial growth efficiency and production of carboxylic acids and other substrates. Limnol. Oceanogr., 49, 2011–2022. [Google Scholar]
- Roe K. L. and Barbeau K. A. (2014) Uptake mechanisms for inorganic iron and ferric citrate in Trichodesmium erythraeum IMS101. Metallomics, 6, 2042–2051. [DOI] [PubMed] [Google Scholar]
- Rose A. L. (2012) The influence of extracellular superoxide on iron redox chemistry and bioavailability to aquatic microorganisms. Front. Microbiol., 3, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A. L., Salmon T. P., Lukondeh T., Neilan B. A. and Waite T. D. (2005) Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ. Sci. Technol., 39, 3708–3715. [DOI] [PubMed] [Google Scholar]
- Rose A. L., Webb E. A., Waite T. D. and Moffett J. W. (2008) Measurement and implications of nonphotochemically generated superoxide in the equatorial Pacific Ocean. Environ. Sci. Technol., 42, 2387–2393. [DOI] [PubMed] [Google Scholar]
- Saragosti E., Tchernov D., Katsir A. and Shaked Y. (2010) Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium. PLoS One, 5, e12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran M. (2003) To what end does nature produce superoxide? NADPH oxidase as an autocrine modifier of membrane phospholipids generating paracrine lipid messengers. Free Radic. Res., 37, 1045–1059. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Roe K. L., Hansel C. M. and Voelker B. M. (2016) Species-level variability in extracellular production rates of reactive oxygen species by diatoms. Front. Chem., 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata T., Nagasoe S., Matsubara T., Yoshikawa S., Yamasaki Y., Shimasaki Y., Oshima Y., Jenkinson I. R. et al. (2008) Factors influencing the initiation of blooms of the raphidophyte Heterosigma akashiwo and the diatom Skeletonema costatum in a port in Japan. Limnol. Oceanogr., 53, 2503–2518. [Google Scholar]
- Siciliano S. D., O’Driscoll N. J. and Lean D. R. S. (2002) Microbial reduction and oxidation of mercury in freshwater lakes. Environ. Sci. Technol., 36, 3064–3068. [DOI] [PubMed] [Google Scholar]
- Skeen A. R., Tomas C. R. and Cooper W. J. (2004) The production of hydrogen peroxide by Heterosigma akashiwo under varying N:P ratios In Steidinger K. A., Landsberg J. H., Tomas C. R. and Vargo G. A. (eds), Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO, St. Petersburg, FL, USA, pp. 77–79. [Google Scholar]
- Smayda T. J. (2008) Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae, 8, 140–151. [Google Scholar]
- Tang Y. Z. and Gobler C. J. (2009. a) Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae, 8, 454–462. [Google Scholar]
- Tang Y. Z. and Gobler C. J. (2009. b) Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar. Biol., 156, 2601–2611. [Google Scholar]
- Tang Y. Z. and Gobler C. J. (2010) Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Mar. Ecol. Prog. Ser., 406, 19–31. [Google Scholar]
- Twiner M. J., Dixon S. J. and Trick C. G. (2001) Toxic effects of Heterosigma akashiwo do not appear to be mediated by hydrogen peroxide. Limnol. Oceanogr., 46, 1400–1405. [Google Scholar]
- Twiner M. J. and Trick C. G. (2000) Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. J. Plankton Res., 22, 1961–1975. [Google Scholar]
- Wilson W. B. and Collier A. (1955) Preliminary notes on the culturing of Gymnodinium breve Davis. Science, 121, 394–395. [DOI] [PubMed] [Google Scholar]
- Woo S. P. S., Liu W. H., Au D. W. T., Anderson D. M. and Wu R. S. S. (2006) Antioxidant responses and lipid peroxidation in gills and erythrocytes of fish (Rhabdosarga sarba) upon exposure to Chattonella marina and hydrogen peroxide: Implications on the cause of fish kills. J. Exp. Mar. Bio. Ecol., 336, 230–241. [Google Scholar]
- Yamaguchi M., Imai I. and Honjo T. (1991) Effects of temperature, salinity and irradiance on the growth rates of the noxious red tide flagellates Chattonella antiquia and C. marina (Raphidophyceae). Nippon Suisan Gakkaishi, 57, 1277–1284. [Google Scholar]
- Yang C. Z., Albright L. J. and Yousif A. N. (1995) Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis. Aquat. Organ., 23, 101–108. [Google Scholar]
- Zhang T., Diaz J. M., Brighi C., Parsons R. J., McNally S., Apprill A. and Hansel C. M. (2016) Dark production of extracellular superoxide by the coral Porites astreoides and representative symbionts. Front. Mar. Sci., 3, 232. [Google Scholar]
- Zinser E. R. (2018) The microbial contribution to reactive oxygen species dynamics in marine ecosystems. Environ. Microbiol. Rep., 10, 412–427. [DOI] [PubMed] [Google Scholar]