Abstract
Farrowing pens with temporary crating have been developed as a compromise between conventional farrowing crates and pens to better accommodate the welfare of both sow and piglets during lactation. However, not much is known about the behavioral and physiological consequences of early removal of confinement on the sow and piglets during lactation. The aim of this study was to assess the effects on sow and piglet performance of temporary crating until 3-d postpartum at 2 times points, immediately after confinement removal and 25 d into lactation. Sows were crated from 5-d prepartum either to weaning (permanently crated—PC group; N =14) or to D3 (83.0 ± 1.3 h) postpartum (Temporarily crated - TC group N = 13). Sow postural changes, activity, cortisol and IgA concentrations, and piglet body weight gain and behavior were assessed on D4 and on D25 postpartum, whereas piglet mortality was assessed throughout lactation. Data were analyzed using PROC GLM and PROC GENMOD of SAS. On D4 postfarrowing, TC sows were more active (10.9% vs. 7.1%; SEM: 0.8; P = 0.002), rolled more frequently (21.3% vs. 14.4%; SEM: 1.6; P = 0.008), and had lower IgA concentrations (139.7 vs. 75.2 µg/mL; SEM: 20.3; P = 0.040) than PC sows. No effects of housing were found (P > 0.05) on standing-to-lying movement or cortisol concentrations. No differences for any variables were found (P > 0.05) on D25. Mortality, body weights, and activity levels at the udder or in the pen of pigs born to PC sows did not differ (P > 0.05) from those of piglets born to TC sows on D4 nor on D25. This study indicates that removal of confinement on the 4th-d postpartum may have had small short-term positive effects on sow behavior and stress levels (as measured by IgA), and that it did not impair piglets’ behavior and performance during lactation. Therefore, this work suggests that temporary crating limited to the first 3-d postpartum might be a feasible alternative to improve welfare under intensive production conditions.
Keywords: behavior, pig, productivity, stress hormones, temporary crating, welfare
INTRODUCTION
Temporary confinement provides lactating sows with the opportunity to move freely in the farrowing pen, once the crate has been opened a few days after parturition. This will allow less restrictive interactions with offspring and improve (physical) comfort, which is drastically compromised in crates (Johnson and Marchant-Forde, 2009; Baxter et al., 2011). Very little is known about the short- and long-term effects of temporary confinement on sow behavior and physiology. The removal of confinement may stimulate activity in the newly available space. Yet, relatively little research has been done to quantitatively assess the activity of temporarily crated sows once loose. Permanent confinement of sows leads to chronic stress at the end of lactation (Cronin et al., 1991; Jarvis et al., 2006; Yin et al., 2016). Moreover, prolonged stress may have deleterious effects on immunity (de Groot et al., 2001). Yet, it is not known whether temporary confinement reduces adrenal and immune reactivity of the sow in the long-term and whether opening the crate has any short-term effects on sow physiology.
Reducing the confinement period during lactation may improve sow welfare. However, piglets’ survival may be put at risk as the absence of confinement allows more sow postural changes which may increase crushing events (Marchant et al., 2000; Melišová et al., 2014). In most research on temporary confinement during lactation, sows were let loose after the 4th-d pp (Lambertz et al., 2015; Chidgey et al., 2016a). Not much is known about whether earlier removal of confinement may have detrimental effects on piglet performances and behavior (3rd-d pp; Killbride et al., 2012; Condous et al., 2016; Singh et al., 2017). Therefore, the aim of this study was to investigate whether confinement until the 3rd-d pp had short- (24 h postopening of the crate) and/or long-term (D25 pp) effects on sow activity and stress levels and to assess whether it influenced piglet performance and behavior.
MATERIALS AND METHODS
This study was carried out from July 2015 to July 2016 at the research farm of the institute of animal science in Prague, Czech Republic, and was conducted in accordance with Czech Central Committee for Protection of Animals number 60444/2011–17214.
Animals and Management
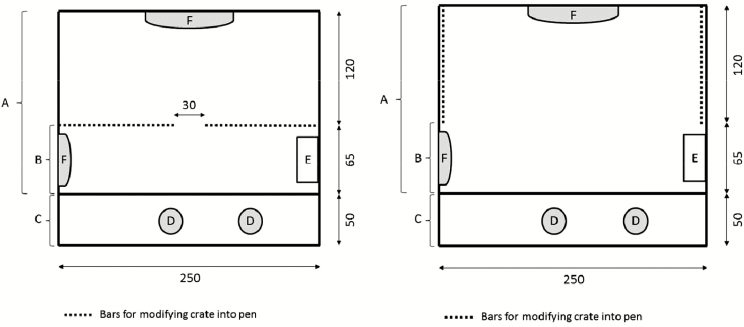
A total of 27 Large White × Landrace sows (parity: 2.5 ± 0.5, range: 1 to 12) inseminated with Large White × Pietrain boar semen were used. Sows were moved from a group-housing gestation unit to a farrowing unit 5 d before estimated parturition date and immediately crated. Sows were randomly allocated over 14 batches on the basis of parity to one of the following two treatments: sows permanently crated (PC; N = 14 sows and 192 piglets born; litter size: 13.7 ± 0.7 piglets) were confined in a crate from 5 d prefarrowing until weaning (approximately 28 d postfarrowing), whereas sows temporarily crated (TC; N = 13 sows and 172 piglets born, litter size: 13.5 ± 0.7 piglets) were confined in crates from 5 d prefarrowing to day 3 (83.0 ± 1.3 h; day 0 being the day of farrowing) postpartum on which sows were let loose (opening of the crate) until weaning. A batch consisted of 2 sows, one for each treatment, housed in adjacent pens of the same type of farrowing system (Figure 1), with one treatment let loose and the other one remained confined. One sow in the TC treatment was removed due to illness. The crate was always opened around 1000 h, after completion of a nursing. There was no equalization nor cross fostering of the litters. All sows were only familiar with permanent farrowing crating prior to the experiment. Farrowing was supervised through 24-h video recordings.
Figure 1.
Farrowing pen equipped with movable bars, which allowed the modification from farrowing crate (left) to farrowing pen (right). A = sow area when farrowing crate is open; B = sow area when sow is crated; C = creep area for piglets; D = heating lamp; E = feed trough; F = piglet anticrushing bars. All measurements are in centimeters.
Sows were fed a standard lactation diet (17% CP, 13.75 MJ DE kg−1) twice a day, and water was available ad libitum from a nipple for the sow and another for the piglets. The sows received 1 bag of chopped straw each morning and evening until they had farrowed, and during the whole lactation. Pens were cleaned once a day. All piglets received an iron injection and males were surgically castrated during the first week of farrowing and ear tagged 3 d after birth. They received creep food from day 7 postfarrowing. There was no difference in the handling of the sows and litters in crates and those housed in pens.
Farrowing pens measured 5.88 m2 and were equipped with movable bars, which enabled them to be modified into crates and vice versa (Figure 1). The sow area (the part of the pen accessible to the sow) for crated sows measured 1.63 m2. When the sows were let loose, the sides of the crate were opened and placed along the sidewalls. In this configuration, the sow area measured 4.63 m2. There were no protection rails along the sidewalls or sloped wall when the sow was lying down. Solid concrete flooring was present in the whole pen. The creep area (the part of the pen accessible only to the piglets) measured 1.25 m2 and was noncovered, bedded with straw, and had 2 hanging heat lamps during the whole lactation.
Data Collection
Behavioral observations.
Behaviors of sows and their litters were continuously video recorded on days 3, 4, and 25 postpartum using an overhead CCTV camera for each pen (Panasonic CCTV, WV CP 470, Osaka, Japan) and software NUUO (IP Surveillance System, NVR/DVR/NVDR, Taipei, Taiwan) (Table 1). Sows were fed twice during observation hours, at 0800 and 1500 h. No observations were recorded from 10 min before to 10 min after feeding or while staff performed husbandry tasks (piglet processing, vaccinations, or any other veterinary procedure). From video recordings, sow and piglet (all piglets in every litter) activity were sampled by 1 observer using fixed interval scan sampling of 5 min over the 24-h period following the opening of the crate (D3 to D4) and D25. In contrast, posture changes were assessed using all occurrence sampling. Behaviors were analyzed by summing the occurrences of each piglet or sow behavioral parameters during those 2 periods.
Table 1.
Behaviors of sows and piglets
| Behaviors | Definitions |
|---|---|
| Sow activity | |
| Inactive | Sow sits or lies in lateral or sternal recumbency |
| Active | Sow is standing or moving in standing position |
| Sow postural changes | |
| Descending movements | Standing to lying |
| Lateral movements | Rolling: from a sternal to lateral recumbency and vice versa |
| Piglet activity | |
| Active at the udder | Piglet is teat seeking or massaging the udder |
| Active in the pen | Piglet is walking, standing or sitting in the pen (i.e., any area within the farrowing space that was not in the creep, or at the sow’s udder) |
Salivary cortisol and IgA concentrations.
Saliva samples were obtained (1 per animal per day) on D2 (control value, 24 h before opening of the crate), D4 (24 h after opening of the crate), and D25 postpartum by allowing the pig to chew on 2 cotton swabs (cotton swabs 150 × 4 mm WA 2PL; Heinz Herenz, Hamburg, Germany) until they were thoroughly moistened. Cotton swabs were attached at the end of a wooden rod, long enough that it was possible to obtain samples without entering the pen, thereby minimizing the disturbance of the animals. Saliva collection generally took less than 2 min and samples were taken on calm and lying sows, not during nursing bouts. Samples were always collected at the same time (around 0945 h) to avoid any confounding effect of circadian rhythm (Muneta et al., 2010, 2011). Saliva was extracted from the swabs by centrifugation at 1000 g for 15 min. Samples were stored at −20 °C until required for assay. Cortisol concentrations were measured by High Sensitive SALIVARY CORTISOL Enzyme Immunoassay Kit (Salimetrics, State College, PA 16803), with concentration ranging from 0.12 to 30 ng/mL, analytical sensitivity of 0.07 ng/mL, and a sample volume of 25 μL. IgA concentrations were determined using Pig IgA ELISA Kit (Bethyl Laboratories, Inc., TX 77356), with concentration ranging from 1.37 to 1000 ng/mL and a salivary sample with a dilution of 1:1000 by provided dilution buffer. Cortisol and IgA salivary concentrations were analyzed for each sow by using the difference in concentrations between D4 and D2, and D25 and D2, respectively.
Piglet mortality and body weight gain.
Piglet mortality rate was assessed at the end of farrowing (after expulsion of the placenta) and every day until D25 postpartum. Piglets were individually marked by an animal marking crayon (Raidex, Germany) and were weighed at the same time (around 0930 h) on D3 (30 min before opening), D4 (24 h after opening of the crate), and D25. The 2 variables were analyzed by using, for each litter, the difference in individual piglet weight and mortality, respectively, from D3 to D4 (short-term effect) and D4 to D25 (long-term effect).
Statistical Analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). The fixed effects included in all models were 1 class variable, housing (temporary or permanent crating), and 2 continuous variables, parity and litter size. The identity of the batch was considered as a random effect in the models. The analysis of piglet mortality was done at a litter level using a generalized linear model with a binomial link (PROC GENMOD). The other response variables were analyzed by generalized linear models (PROC GLM). Results were considered statistically significant when P ≤ 0.05 and as tending to differ when 0.05 < P ≤ 0.10. Only significant results of parity and litter size effects are presented below.
RESULTS
Sow Hormone Stress Levels, Activity, and Postural Changes
Short-term effects of removal of confinement.
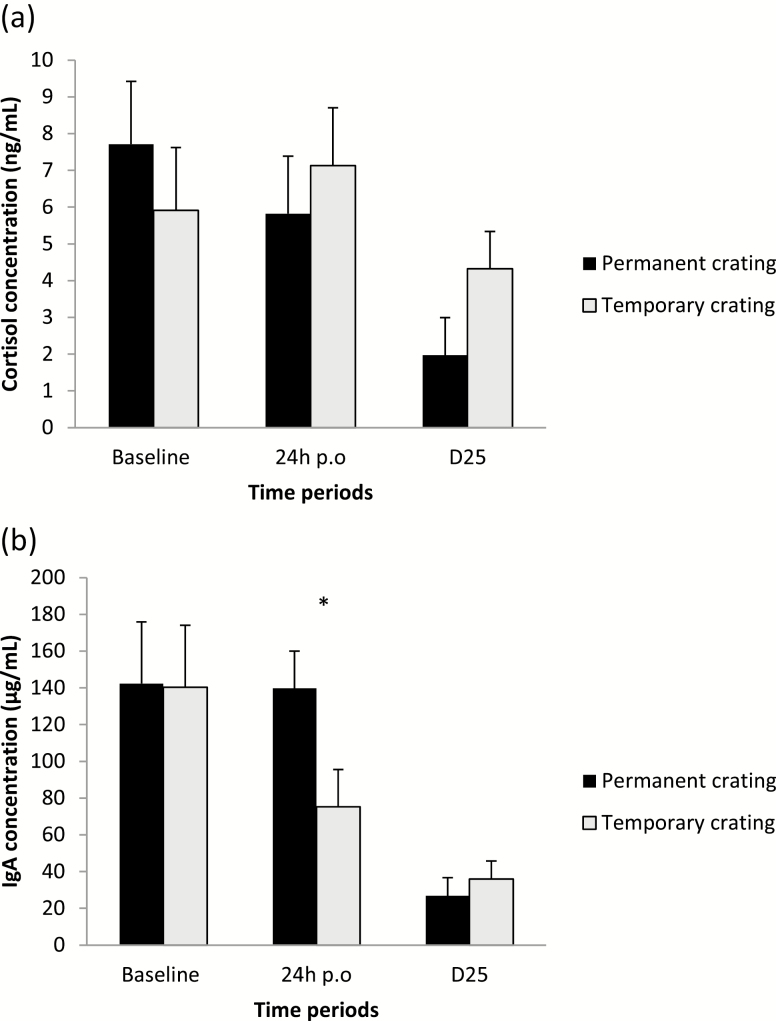
Housing conditions had an influence on the sow activity, IgA concentrations, and the frequency of rolling behavior (P = 0.002, P = 0.040, and P = 0.008, respectively), with TC sows being more active, having lower IgA concentrations and rolling more frequently than PC sows (Figure 2; Table 2). No effects were found on standing-to-lying movements or cortisol concentrations (P = 0.449 and P = 0.360, respectively). Litter size influenced rolling frequency (P = 0.009), with sows with bigger litters rolling more. Parity had an effect on sow activity (P = 0.04) with activity levels decreasing with increased parity.
Figure 2.
Cortisol (a) and IgA (b) concentrations 24 h before opening (baseline) and 24 h postopening (24 h p.o.) of the crate and on D25 in permanent and crated sows. (*P < 0.05).
Table 2.
Short-1 and long-term2 effects of housing conditions on sow activity and postural changes
| Permanent crating (N = 14) | Temporary crating (N = 13) | SEM | P value | |
|---|---|---|---|---|
| Short-term effect3 | ||||
| Active time (%) | 7.1 | 10.9 | 0.8 | 0.002* |
| Standing to lying (%) | 4.9 | 5.6 | 0.6 | 0.449 |
| Rolling (%) | 14.4 | 21.3 | 1.6 | 0.008* |
| Long-term effect3 | ||||
| Active time (%) | 12.6 | 11.1 | 1.6 | 0.524 |
| Standing to lying (%) | 5.5 | 6.5 | 0.7 | 0.393 |
| Rolling (%) | 15.1 | 22.9 | 2.7 | 0.073 |
124-h period after opening of the crate.
224-h period on D25 postpartum.
3Percentage of fixed interval scan sampling of 5 min during which the animal was seen displaying the behavior over the given period. * P < 0.05.
Long-term effects of removal of confinement.
Housing conditions had no effects (P > 0.05) on sow activity levels, standing-to-lying movements, or on cortisol and IgA concentrations. Rolling tended (P = 0.073) to be more frequent in TC sows than in PC sows.
Piglet Mortality, Body Weight Gain, and Activity
Short-term effects of removal of confinement.
Because of the low incidence of piglet death over the first 24 h postopening, statistical analysis was not appropriate, and mortality data are presented in descriptive form (Table 3). Body weights and activity levels at the udder or in the pen of pigs born to PC sows did not differ from those of piglets born to TC sows (P = 0.423, P = 0.436, and P = 0.195, respectively). Litter size influenced body weight gain (P < 0.001) with decreasing weight gain in larger litters. Parity influenced activity at the udder (P = 0.016) with higher parity associated with increased piglet activity.
Table 3.
Short-1 and long-term2 effects of housing conditions on piglet mortality, weight gain, and activity
| Permanent crating (N = 14) | Temporary crating (N = 13) | SEM | P value | |
|---|---|---|---|---|
| Short-term effect | ||||
| Mortality3 (%) | 1.6 | 1.7 | 0.1 | . |
| Weight gain (g) | 167.9 | 178.7 | 9.0 | 0.423 |
| Active at udder4 (%) | 19.1 | 18.2 | 0.8 | 0.436 |
| Active in pen4 (%) | 12.6 | 14.3 | 0.9 | 0.195 |
| Long-term effect | ||||
| Mortality (%) | 8.9 | 8.1 | 2.4 | 0.902 |
| Weight gain (g) | 4091.1 | 4359.0 | 166.8 | 0.269 |
| Active at udder4 (%) | 16.5 | 16.8 | 1.3 | 0.871 |
| Active in pen4 (%) | 15.6 | 18.3 | 1.4 | 0.208 |
124-h period after opening of the crate.
2D25 postpartum (24 h) for activity and period from D4 to D25 for mortality and weight gain.
3Descriptive statistics only.
4Percentage of fixed interval scan sampling of 5 min during which the animal was seen displaying the behavior over the given period.
Long-term effects of removal of confinement.
Housing systems had no effects on the probability of piglet death from D4 to D25 (P = 0.902), body weight (P = 0.269), or activity levels at the udder or in the pen (P = 0.877 and P = 0.103, respectively). Litter size and parity influenced body weight gain (P = 0.030 and P = 0.041, respectively) with decreasing weight gain in larger litters and in higher parity.
Nonudder related activity levels in the pen were lower (P = 0.007) for piglets from larger litters and tended to be lower for piglets from higher parity sows (P = 0.062).
DISCUSSION
Sow Stress Levels and Behavior
Short-term effects of removal of confinement.
The removal of confinement did not influence salivary cortisol concentrations 24 h after opening of the crate which suggests similar adrenal reactivity in crated and loose sows a day after opening of the crate. Research on cortisol levels of sows in early lactation is rather inconclusive as to whether confinement is a chronic stressor at that stage. Although some studies found no differences in salivary cortisol concentrations between permanently loose housed and crated sows during the first week of lactation (Cronin et al., 1991; Biensen et al., 1996), other found either greater (Oliviero et al., 2008) or lower cortisol levels (Hales et al., 2016) in crated sows.
Interestingly, IgA levels of temporarily crated sows decreased (−47%) after opening of the crate, whereas those of crated sows did not vary. The valence of this response remains unclear. To the best of our knowledge, no other study has assessed the short- or long-term effect of removal of confinement postpartum on sow IgA levels. Only 2 studies (Muneta et al., 2010; Escribano et al., 2015) assessed pig salivary IgA levels in response to various stressors (snitch restraint, isolation, and mixing). They both reported an increase of IgA levels after exposure to these negative conditions. Based on these findings, the decrease found in the present study could indicate that loose sows were experiencing lower stress levels than crated sows 24 h after opening of the crate. However, contradicting this interpretation, studies on other species have reported a decrease in IgA after exposure to similar stressors (dogs: Svobodová et al. (2014); rats: Guhad and Hau (1996)).
The different hormone responses in the present study may tentatively be explained by the difference in kinetics and sensibility of the response to the removal of confinement between those stress biomarkers. Cortisol is known to quickly respond (within 30 min) to different types of stressor in pigs (Muneta et al., 2010; Escribano et al, 2015). On the other hand, limited research in pigs shows that IgA has an immediate response (within 30 min) after acute physical stress (Muneta et al., 2010) but a delayed response (1–3 days) after exposure to social stress (Escribano et al., 2015). Therefore, it may be speculated that, in the present study, there was a delayed response in IgA levels and a change in cortisol levels soon after opening the crate which was only short lasting, and thus not recorded as saliva was sampled 24 h after removal of confinement.
As expected, opening the crate, thus increasing the space allowance, made temporarily confined sows spend more time standing and be more active than permanently crated sows over the first 24 h after removal of confinement. However, although significant, the increase in activity is small (+3.8%) and should be interpreted with caution. This increase may have reflected a slightly greater motivation for exploration of the new space available to the temporarily crated sows. Our finding contrasts those of Lambertz et al. (2015) and Chidgey et al. (2016b) who found no short-term differences in the amount of time spent active between temporary confined sow (until D5 or until D7 and D14, respectively) and permanently crated sows. The smaller size of the pen and late opening of the crate (D7 or D14, Lambert et al., 2015) and/or the sampling period (restricted to 4 daytime periods, Chidgey et al., 2016b) may explain the differences with the present study.
Removal of confinement was found to increase (+6.9 %) the frequency of rolling events over the first 24 h postopening. Similarly, Hales et al. (2016) found an increase in the number of rolling events in loose sows compared with crated sows during the first few days postfarrowing. Greater frequency of rolling events has been shown to indicate greater restlessness or discomfort during or outside of nursing bouts (Weary et al., 1996; Harris and Gonyou, 1998; Damm et al., 2005; Bozděchová et al., 2014). Even though it cannot be completely ruled out, it may not have been the case in the present study as other indicators such as increased standing-to-lying postural changes or stress levels were absent. Thus, it seems more likely that the increase in rolling was simply linked to the increase in space allowing sows to roll more easily, whereas rolling was more prevented in crates (Damm et al., 2005; Danholt et al., 2011).
Long-term effects of removal of confinement.
The removal of confinement had no effects on salivary cortisol and IgA levels on D25, which may indicate that adrenal and immune reactivity levels were similar at the end of lactation in both housing conditions. The cortisol findings of the present study are in contradiction with the scarce literature on the effect of farrowing environment on cortisol concentration at the end of lactation. Comparing pens and crates, Cronin et al. (1991), Jarvis et al. (2006), and Yin et al. (2016) found higher plasma cortisol levels in crated sows compared with sows housed in pens only at the end of lactation (days 28, 29, and 35 postfarrowing, respectively), indicative of chronic stress. Differences between studies may be explained by the different sampling methods (salivary vs. blood), types of pens, parity (gilt vs. multiparous), and probably the sampling periods. In the present study, the saliva samples on D25 were collected after the peak of milk output (D21) and possibly before the point at which sows may start reducing milk output and contacts with piglets (sow-offspring conflict). Like in the other studies, higher cortisol concentrations in permanently confined sows could have speculatively been observed with later sampling as loose sows may have had more control over their investment compared with crated sows (Pajor et al., 2000, 2002).
The effect of opening of the crate on IgA concentrations of temporarily crated sows seen on D4 was no longer observed at the end of lactation. This may support the assumption of a transient novelty effect of the removal of confinement. However, due to the only 2 saliva samples being collected in the present study (on D4 and D25), it is unfortunately impossible to accurately know how long the decrease of IgA in temporary crated sows lasted for. Further research should investigate in more detail the temporal dynamics of salivary IgA and cortisol responses to confinement to provide a better picture of the sow stress levels during the entirety of lactation. Moreover, other indicators such as heart rate variability may be valuable to assess the effect of temporary confinement on sow physiology.
The similar activity levels found in both treatments at the end of lactation suggest that increasing the space available did not steadily motivate sows to be more active. In agreement with the findings of Lambertz et al. (2015), sows spent a low amount of time (approximately 10%) active in late lactation regardless of the farrowing system in which they were housed. The overall low activity levels may be related to sows preserving their energy levels and avoiding a catabolic state as a result of the metabolic burden of rearing a litter (Valros et al., 2003). Although it did not increase activity levels, the removal of confinement was assumed to allow a wider range of behaviors and motion. Hence, it may have enabled temporarily crated sows to interact more with their environment, which is paramount to good animal welfare as it may decrease boredom (Burn, 2017). Unfortunately, in the present study, the activity levels were roughly assessed, considering only the time spent active and standing and not providing detailed information on the behaviors of the sows during the active bouts. Thus, it would be valuable that further refined research focuses on the quality of activity (e.g., total distance walked, qualitative and quantitative assessment of interactions with environment) in order to assess the long-term potential benefits of removal of temporary confinement such as leg health or improved affective states.
Piglet Performance and Behavior
Similar mortality rates found in both treatments in the present study indicate that piglets were not at a higher risk of dying in temporary crating pens compared with permanent crates in the short and long term. Therefore, it is possible to achieve an acceptable overall mortality (from birth to D25: 12.5%) in regard to production standards with this system. The absence of short-term differences in piglet survival suggests that sows were as careful in pens as in crates, even though TC sows were slightly more active and changed postures more frequently. Our mortality results are consistent with the short-term and long-term findings of many other studies in which sows were loose for a few (2 to 4) days after farrowing (Stabenow and Manteuffel, 2002; Mouesten et al., 2013; Hales et al., 2015; Chidgey et al., 2016a; Condous et al., 2016; Singh et al., 2017). Confinement until D3 pp did not influence piglet short- and long-term weight gains, which is in line with other studies comparing similar crating conditions (Moustsen et al., 2013; Condous et al., 2016; Singh et al., 2017). Even though it remains to be tested, the similar weight gains in the present study may indicate that nursing and suckling behaviors may not have been disturbed shortly after removal of confinement, and possibly at the end of lactation. However, the latter case is very speculative as creep food intake may have played a role (Oostindjer et al., 2010). This would contrast the hypothesis (better nursing behavior in PC sows) of Chidgey et al., (2015) for recording a higher weaning weight gain (+0.1 kg) in piglets of sows housed in temporary crating (for 4 d) compared with those of sows housed in permanent crating.
Along with mortality and weight gain, piglet activity (walking, standing, and massaging) was not modified by removal of confinement during lactation, providing additional evidence supporting that temporary crating until the 3rd-d postpartum was not detrimental to the piglets during lactation, even when sow activity and postures were moderately more frequent. Although contrasting results of other studies showing that piglet activity level increases with increased sow activity level and posture changes during the first week of lactation (Chidgey et al., 2016b), our results were in line with those reported by Singh et al. (2017), who assessed general activity in the pen on D4, 11, and 18 pp between piglets of sows in crates or temporary crating (until D3 pp). Further investigation could look at other specific behaviors indicative of positive welfare such as play, which has been shown to be increased in enriched farrowing pens with increased space and straw available for piglets and sow (Chaloupkova et al., 2007).
CONCLUSION
The results of this study suggest that loose-housing of sows after a short postnatal period of confinement in a crate may have small positive effects on the sow’s welfare behavior in the short term only (as reflected by activity and IgA levels). More detailed work is needed to assess the potential benefits of temporary confinement on sow welfare throughout lactation. This study also indicates that confining the sow during farrowing and until day 3 postpartum is sufficient to ensure a similar preweaning piglet survival, growth, and behavior compared with sows which were permanently crated throughout lactation. From a piglet welfare standpoint, short temporary confinement of the sow may then be considered as a safe alternative housing option to permanent confinement.
Conflict of interest statement. None declared.
Footnotes
Research supported by grant numbers NAZV QJ1610390 and MZE-RO0718 from the Ministry of Agriculture, Czech Republic. The authors would like to acknowledge Zuzanna Kocourková for her help with data collection.
LITERATURE CITED
- Baxter E. M., Lawrence A. B., and Edwards S. A.. 2011. Alternative farrowing systems: design criteria for farrowing systems based on the biological needs of sows and piglets. Animal 5:580–600. doi: 10.1017/S1751731110002272 [DOI] [PubMed] [Google Scholar]
- Biensen N. J., von Borell E. H., and Ford S. P.. 1996. Effects of space allocation and temperature on periparturient maternal behaviors, steroid concentrations, and piglet growth rates. J. Anim. Sci. 74:2641–2648. [DOI] [PubMed] [Google Scholar]
- Bozděchová B., Illmann G., Andersen I. L., Haman J., and Bhrlenbruch R.. 2014. Litter competition during nursings and its effect on sow response on Day 2 postpartum. Appl. Anim. Behav. Sci. 150:9–16. doi:10.1016/ j.applanim.2013.10.006 [Google Scholar]
- Burn C. C. 2017. Bestial boredom: a biological perspective on animal boredom and suggestions or its scientific investigation. Anim. Behav. 130:141–151. doi:10.1016/ j.anbehav.2017.06.006 [Google Scholar]
- Chaloupková H., Illmann G., Neuhauserová K., Tománek M., and Valis L.. 2007. Preweaning housing effects on behavior and physiological measures in pigs during the suckling and fattening periods. J. Anim. Sci. 85:1741–1749. doi: 10.2527/jas.2006-504 [DOI] [PubMed] [Google Scholar]
- Chidgey K. L., Morel P. C. H., Stafford K. J., and Barugh I. W.. 2015. Sow and piglet productivity and sow reproductive performance in farrowing pens with temporary crating and farrowing crates on a commercial New Zealand pig farm. Livest. Sci. 173:87–94. doi:10.1016/j.livsci.2015.01.003 [Google Scholar]
- Chidgey K. L., Morel P. C. H., Stafford K. J., and Barugh I. W.. 2016a. The performance and behavior of gilts and their piglets is influenced by whether gilts were born and reared in farrowing crates or farrowing pens. Livest. Sci. 193: 51–57. doi:10.1016/j.livsci.2016.09.011 [Google Scholar]
- Chidgey K. L., Morel P. C. H., Stafford K. J., and Barugh I. W.. 2016b. Observations of sows and piglets housed in farrowing pens with temporary crating or farrowing crates on a commercial farm. Appl. Anim. Behav. Sci. 176:12–18. doi:10.1016/j.applanim.2016.01.004 [Google Scholar]
- Condous P. C., Plush K. J., Tilbrook A. J., and van Wettere W. H.. 2016. Reducing sow confinement during farrowing and in early lactation increases piglet mortality. J. Anim. Sci. 94:3022–3029. doi: 10.2527/jas.2015-0145 [DOI] [PubMed] [Google Scholar]
- Cronin G. M., Barnett J. L., Hodge F. M., Smith J. A., and McCallum T. H.. 1991. The welfare of pigs in two farrowing/lactation environments: cortisol responses of sows. Appl. Anim. Behav. Sci. 32:117–127. doi:10.1016/S0168-1591(05)80036-X [Google Scholar]
- Damm B. I., Forkman B., and Pedersen L. J.. 2005. Lying down and rolling behaviour in sows in relation to piglet crushing. Appl. Anim. Behav. Sci. 90:3–20. doi:10.1016/ j.applanim.2004.08.008 [Google Scholar]
- Danholt L., Moustsen V. A., Nielsen M. B. F., and Kristensen A. R.. 2011. Rolling behaviour of sows in relation to piglet crushing on sloped versus level floor pens. Livest. Sci. 141:59–68. doi:10.1016/j.livsci.2011.05.005 [Google Scholar]
- de Groot J., Ruis M. A., Scholten J. W., Koolhaas J. M., and Boersma W. J.. 2001. Long-term effects of social stress on antiviral immunity in pigs. Physiol. Behav. 73:145–158. doi:10.1016/S0168-1591(05)80036-X [DOI] [PubMed] [Google Scholar]
- Escribano D., Tvarijonaviciute A., Tecles F., and Cerón J. J.. 2015. Serum paraoxonase type-1 activity in pigs: assay validation and evolution after an induced experimental inflammation. Vet. Immunol. Immunopathol. 163:210–215. doi: 10.1016/j.vetimm.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Guhad F. A., and Hau J.. 1996. Salivary iga as a marker of social stress in rats. Neurosci. Lett. 216:137–140. doi:10.1016/0304-3940(96)13037-8 [DOI] [PubMed] [Google Scholar]
- Hales J., Moustsen V. A., Nielsen M. B., and Hansen C. F.. 2015. Temporary confinement of loose-housed hyperprolific sows reduces piglet mortality. J. Anim. Sci. 93:4079–4088. doi: 10.2527/jas.2015-8973 [DOI] [PubMed] [Google Scholar]
- Hales J., Moustsen V. A., Nielsen M. B. F., and Hansen C. F.. 2016. The effect of temporary confinement of hyperprolific sows in Sow Welfare and Piglet protection pens on sow behaviour and salivary cortisol concentrations. Appl. Anim. Behav. Sci. 183:19–27. doi:10.1016/ j.applanim.2016.07.008 [Google Scholar]
- Harris M. J., and Gonyou H. W.. 1998. Increasing available space in a farrowing crate does not facilitate postural changes or maternal responses in gilts. Appl. Anim. Behav. Sci. 59:285–296. doi:10.1016/S0168-1591(98)00142-7 [Google Scholar]
- Jarvis S., D’Eath R. B., Robson S. K., and Lawrence A. B.. 2006. The effect of confinement during lactation on the hypothalamic-pituitary-adrenal axis and behaviour of primiparous sows. Physiol. Behav. 87:345–352. doi: 10.1016/j.physbeh.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Johnson A. K., and Marchant-Forde R. M.. 2009. Welfare of pigs in the farrowing environment. In Marchant-Forde J. N. editor, The welfare of pigs. Springer Science + Business Media B.V, Dordrecht, The Netherlands, pp. 141–188. [Google Scholar]
- Kilbride A. L., Mendl M., Statham P., Held S., Harris M., Cooper S., and Green L. E.. 2012. A cohort study of preweaning piglet mortality and farrowing accommodation on 112 commercial pig farms in England. Prev. Vet. Med. 104:281–291. doi: 10.1016/j.prevetmed.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Lambertz C., Petig M., Elkmann A., and Gauly M.. 2015. Confinement of sows for different periods during lactation: effects on behaviour and lesions of sows and performance of piglets. Animal 9:1373–1378. doi: 10.1017/S1751731115000889 [DOI] [PubMed] [Google Scholar]
- Marchant J. N., Rudd A. R., Mendl M. T., Broom D. M., Meredith M. J., Corning S., and Simmins P. H.. 2000. Timing and causes of piglet mortality in alternative and conventional farrowing systems. Vet. Rec. 147:209–214. [DOI] [PubMed] [Google Scholar]
- Melišová M., Illmann G., Chaloupková H., and Bozděchová B.. 2014. Sow postural changes, responsiveness to piglet screams, and their impact on piglet mortality in pens and crates. J. Anim. Sci. 92:3064–3072. doi: 10.2527/jas.2013-7340 [DOI] [PubMed] [Google Scholar]
- Moustsen V. A., Hales J., Lahrmann H. P., Weber P. M., and Hansen C. F.. 2013. Confinement of lactating sows in crates for 4 days after farrowing reduces piglet mortality. Animal 7:648–654. doi: 10.1017/S1751731112002170 [DOI] [PubMed] [Google Scholar]
- Muneta Y., Minagawa Y., Nakane T., Shibahara T., Yoshikawa T., and Omata Y.. 2011. Interleukin-18 expression in pig salivary glands and salivary content changes during acute immobilization stress. Stress 14:549–556. doi: 10.3109/10253890.2011.565392 [DOI] [PubMed] [Google Scholar]
- Muneta Y., Yoshikawa T., Minagawa Y., Shibahara T., Maeda R., and Omata Y.. 2010. Salivary iga as a useful non-invasive marker for restraint stress in pigs. J. Vet. Med. Sci. 72:1295–1300. doi:10.1292/jvms.10-0009 [DOI] [PubMed] [Google Scholar]
- Oliviero C., Heinonen M., Valros A., Hälli O., and Peltoniemi O. A.. 2008. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim. Reprod. Sci. 105:365–377. doi: 10.1016/j.anireprosci.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Oostindjer M., Bolhuis J. E., Mendl M., Held S., Gerrits W., van den Brand H., and Kemp B.. 2010. Effects of environmental enrichment and loose housing of lactating sows on piglet performance before and after weaning. J. Anim. Sci. 88:3554–3562. doi: 10.2527/jas.2010-2940 [DOI] [PubMed] [Google Scholar]
- Pajor E. A., Kramer D. L. and Fraser D.. 2000. Regulation of contact with offspring by domestic sows: temporal patterns and individual variation. Ethol. 106:37–51. doi:10.1046/j.14390310.2000.00494.x [Google Scholar]
- Pajor E. A., Weary D. M., Caceres C., Fraser D. and Kramer D. L.. 2002. Alternative housing for sows and litters: part 3. Effects of piglet diet quality and sow controlled housing on performance and behaviour. Appl. Anim. Behav. Sci. 76:267–277. doi:10.1016/S0168-1591(02)00010-2 [Google Scholar]
- Singh C., Verdon M., Cronin G. M., and Hemsworth P. H.. 2017. The behaviour and welfare of sows and piglets in farrowing crates or lactation pens. Animal 11:1210–1221. doi: 10.1017/S1751731116002573 [DOI] [PubMed] [Google Scholar]
- Stabenow B., and Manteuffel G.. 2002. A better welfare for nursing sows without increased piglet loss applying peri-parturition short term crating. Arch. Tierz. Dummerstorf. 45:53–60. [Google Scholar]
- Svobodová I., Chaloupková H., Končel R., Bartoš L., Hradecká L., and Jebavý L.. 2014. Cortisol and secretory immunoglobulin A response to stress in german shepherd dogs. PLoS ONE 9:e90820. doi: 10.1371/journal.pone.0090820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valros A., Rundgren M., Špinka M., Saloniemi H., Rydhmer L., Hultén F., Uvnäs-Moberg K., Tománek M., Krejcí P., and Algers B.. 2003. Metabolic state of the sow, nursing behaviour and milk production. Livest. Prod. Sci. 79:155–167. doi:10.1016/S0301-6226(02)00154-9 [Google Scholar]
- Weary D. M., Pajor E. A., Fraser D., and Honkanen A. M.. 1996. Sow body movements that crush piglets: a comparison between two types of farrowing accommodation. Appl. Anim. Behav. Sci. 49:149–158. doi:10.1016/0168-1591(96)01042-8 [Google Scholar]
- Yin G., Liu H., Li X., Quan D. and Bao J.. 2016. Effect of farrowing environment on behaviour and physiology of primiparous sows with 35-day lactation. Intern. J. Appl. Res. Vet. Med. 14:159–169. [Google Scholar]