Abstract
Ergot alkaloids from endophyte-infected (Epichloë coenophiala) tall fescue (Lolium arundinaceum) induce vasoconstriction. Previous work has shown that serotonin receptor subtype, 5HT2A, is present in bovine ruminal (R) and mesenteric (M) vasculature, plays a role in vasoconstriction, and could be influenced by ergot alkaloids. To determine the influence of ergot alkaloids on 5HT2A, the vasoactivity of an agonist selective for 5HT2A, (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl) methylamine HCl (TCB-2), was evaluated using bovine ruminal and mesenteric arteries and veins (RA, RV, MA, MV) that were exposed to ergovaline (ERV) prior to or during the TCB-2 additions. Ruminal and mesenteric blood vessel segments were collected, cleaned, and cut into 2- to 3-mm cross-sections. Vessel segments were incubated in Krebs-Henseleit buffer containing 0, 0.01 or 1 µM ERV for 2 h prior to TCB-2 dose response or exposed to ERV concentrations simultaneously during TCB-2 dose response. For the dose response portion of the study, vessels were suspended in a multimyograph containing 5 mL of continuously oxygenated Krebs–Henseleit buffer and equilibrated to 1 g tension for 90 min. Vessels were exposed to increasing concentrations of TCB-2 every 15 min and contractile response data were normalized as a percentage of the maximum contractile response induced by 120 mM KCl reference. Analysis of variance was evaluated separately for each vessel and each ERV exposure experiment using the mixed models procedure of SAS for effects of TCB-2 and ERV concentrations. All blood vessels with previous ERV exposure had significantly lower contractile responses to TCB-2 (P < 0.01). All blood vessels with simultaneous exposure to 1 µM ERV had higher (P < 0.01) contractile responses at lower concentrations of TCB-2. Simultaneous ERV addition at 1 × 10−4 M TCB-2 did not affect contractility of RV, MA, MV (P > 0.05), but decreased contractility of RA (P < 0.01). These results indicate that ergopeptine alkaloid exposure influences contractility of bovine ruminal and mesenteric blood vessels through serotonin receptor subtype 5HT2A by acting as both an agonist and antagonist. Additional work is needed to determine if ergot alkaloids like ERV simply occupy receptor binding sites competitively, or influence receptor internalization to cause the observed divergent responses.
Keywords: bovine, serotonin, mesenteric artery and vein, ruminal artery and vein, vasoconstriction
INTRODUCTION
Epichlöe coenphialum is an endophyte that symbiotically exists within tall fescue grass (Lolium arundinaceum; Porter et al., 1979; Bush et al., 1982). Although the endophyte is beneficial to the plant by imparting tolerance to biotic and abiotic stressors, it also produces toxic ergot alkaloids that lead to fescue toxicosis in cattle (Lyons et al., 1986; Strickland et al., 2011). Ergot alkaloids have been shown to induce vasoconstriction in bovine core (Rhodes et al., 1991; Foote et al., 2011) and peripheral (Solomons et al., 1989; Aiken et al., 2007; Klotz et al., 2010) blood vessels. Ergot alkaloids decrease blood flow to ruminal epithelium which could decrease nutrient absorption rates (Foote et al., 2013). Furthermore, mesenteric vasculature from steers with previous exposure to endophyte-infected tall fescue displayed a diminished response to ergot alkaloids and serotonin (5-hydroxytrypatmine, 5-HT), in vitro (Egert et al., 2014a).
Structurally, ergot alkaloids are similar to biogenic amines, such as 5-HT, which allows them to act as agonists or antagonists (Berde, 1980) and bind to serotonergic receptor sites (Eckert et al., 1978). There are 14 different receptor subtypes of 5-HT (Hoyer et al., 1994; Klotz and Nicol, 2016) that could elicit different responses when exposed to various ergot alkaloids. Dyer (1993) showed that 5-HT2 receptors are involved in vasoconstriction by ergovaline (ERV) and Schöning et al. (2001) demonstrated that the vasoconstrictive effect of ERV is due to 5-HT2A activation in rat and guinea pig arteries. In addition, antagonism of 5-HT2A receptor suppresses vasoconstrictive effects of ERV in bovine vasculature (Klotz et al., 2013). Recently, ruminal and mesenteric blood vessels were shown to contract when exposed to the agonist (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl) methylamine HCl (TCB-2) selective for the serotonin receptor subtype, 5-HT2A (Snider et al., 2018). Therefore, the objective of this study was to characterize the vasoactivity of increasing concentrations of TCB-2 in ruminal and mesenteric blood vessels with prior or simultaneous ERV exposure to determine the relationship between ergot alkaloids and 5-HT2A receptor.
MATERIALS AND METHODS
No live animals were involved in this study, thus approval from the University of Kentucky Animal Care and Use Committee was not required.
Animals and Tissue Collection
Ruminal and mesenteric blood vessels were obtained from the gastrointestinal tracts of eight Holstein steers (BW = 659 ± 21 kg) that were slaughtered at the University of Kentucky Meats Laboratory. Steers were fed a corn silage–based diet that was devoid of ergot alkaloids for >30 d prior to slaughter. Ventral coronary and caudal grooves of the rumen were identified to remove the branch of the right ruminal artery (RA) and vein (RV) in the coronary groove with surrounding adipose and connective tissues as described by Klotz et al. (2011). The ilocecal fold and ileal flange of the small intestine were identified to remove branches of the mesenteric artery (MA) and vein (MV) with surrounding adipose and connective tissues (Klotz and Barnes, 2014). Vessel segments were immersed in oxygenated Krebs–Henseleit buffer (95% O2/5% CO2; pH = 7.4; 11.1 mM D-glucose; 1.2 mM MgSO4; 1.2 mM KH2PO4; 4.7 mM KCl; 118.1 mM NaCl; 3.4 mM CaCl2; 24.9 mM NaHCO3; Sigma Chemical Co., St. Louis, MO), stored on ice, and transported to the laboratory. Blood vessels were dissected and surrounding adipose and connective tissues were removed. Cleaned vessel segments were sliced into approximately 2-mm cross-sections using an adjustable acrylic tissue matrix (Braintree Scientific, Inc., Braintree, MA). Using a dissection microscope (Stemi 2000-C, Carl Zeiss Inc., Oberkochen, Germany) at 12.5× magnification, vascular dimensions for MA and RA cross-sections were recorded using Axiovision (version 20, Carl Zeiss Inc.). Ruminal vein and MV measurements were not recorded due to their pliant structure. In addition, vessel cross-sections were inspected under magnification for abnormalities (structural damage incurred during dissection and cleaning and branches) and abnormal sections were discarded and replaced with viable sections.
Pre-Myograph Incubations
A tall fescue seed extract was prepared and purified, as described by Ji et al. (2014) and Foote et al. (2012), and diluted to contain working concentrations of either 0.01 µM or 1 µM ERV. The ERV-containing tall fescue seed extract was incubated with blood vessels prior to addition of TCB-2 standards in the myographs (Jia et al., 2015). Blood vessel cross-sections were incubated for 2 h in a 6-well culture plate with 5 mL of Krebs–Henseleit buffer containing either 0 (control), 0.01, or 1 µM ERV. Prior to blood vessel addition, buffer solutions were preheated for 30 min in a CO2 incubator (95% O2/5% CO2; 37°C; Nu-8500, NUAIRE, Inc., Plymouth, MN). Blood vessel segments were randomly placed into each well and vascular dimensions were recorded for RA and MA immediately following the incubation period. For each steer and vessel, duplicate vessel segments were prepared for each treatment.
Standard Preparations
Stock solutions of the 5-HT2A receptor agonist, (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl) methylamine HCl, (TCB-2; Cat. No. 2592, Tocris Bioscience, Minneapolis, MN), were diluted to corresponding concentrations for final working concentrations in tissue wells of 5 × 10−9 to 1 × 10−4M. TCB-2 was prepared in dimethylsulfoxide (472301; Sigma Chemical Co.) for a total of 10 standard additions (5 × 10−9, 1 × 10−8, 5 × 10−8, 1 × 10−7, 5 × 10−7, 1 × 10−6, 5 × 10−6, 1 × 10−5, 5 × 10−4, and 1 × 10−4M). Standard additions of TCB-2 were combined with either 0 μM ERV (control), 0.01 µM ERV, or 1 µM ERV. Standard addition concentration ranges were prepared based on previous bovine vascular bioassay research using 5-HT agonists (Klotz et al., 2013; Snider et al., 2018). All additions were added to myograph chambers in order of increasing concentration.
Myograph Protocol
Blood vessels were mounted onto luminal supports in individual chambers of a multimyograph (DMT 610M, Danish Myo Technology, Atlanta, GA) with 5 mL Krebs–Henseleit buffer and constant gassing (95% O2/5% CO2; pH = 7.4; 37°C). The incubation buffer contained the same composition of the transport buffer with added 3 × 10−5M desipramine (D3900; Sigma Chemical Co.) to inactivate neuronal catecholamine reuptake and 1 × 10−6M propranolol (P0844; Sigma Chemical Co.) to block β-adrenergic receptors. An equilibration period was conducted under the conditions previously described for 90 min with buffer changes every 15 min to allow blood vessels to reach a resting tension of approximately 1 g. At completion of the 90-min equilibration period, 120 mM KCl (Sigma Chemical, Co.) was added to each chamber and incubated for 15 min. This served as a reference addition to confirm vessel responsiveness and viability. Myograph chambers were then emptied and refilled with incubation buffer to remove KCl and allow the vessels to return to an approximate 1-g tension. Once vessels had returned to resting tension, standard additions of TCB-2 were added for contractile response experiments. Additions were added in 15-min intervals in order of increasing agonist concentration. Each cycle consisted of a 9-min treatment incubation period, two 2.5-min buffer washes, a third buffer replacement, followed by a 1-min recovery period. At completion of the standard addition cycles, vessels were again exposed to 120 mM KCl to confirm viability for the experiment duration.
Isometric contractions in mesenteric and ruminal vessels to KCl, TCB-2 + 0 µM ERV, TCB-2 + 0.01 µM ERV, and TCB-2 + 1 µM ERV were digitized and recorded in grams of tension using PowerLab16/35 and Chart software (version 7.3, ADInstruments, Colorado Springs, CO). Baseline tension was recorded before addition of 120 mM KCl. For all contractile response data, the maximum tension (measured in g) during the 9-min incubation was recorded as the contractile response and corrected for baseline tension. Due to variation between animals and tissues, contractile response data were normalized as a percentage of the maximum grams of tension induced by the reference addition of KCl to compensate for differences in vessel responsiveness. Vessel contractile response data are reported as the percentage mean contractile response ± SEM of the maximum contractile response produced by the 120 mM KCl reference addition. Sigmoidal concentration response curves of ruminal and mesenteric vasculature to each treatment were calculated and plotted using nonlinear regression with fixed slope (GraphPad Prism 5, GraphPad Software Inc., La Jolla, CA). Data were plotted and calculated using a 3-parameter equation:
where y represents contractile response, x represents agonist concentration, top and bottom are the percentage of 120 mM KCl maximum contractile response at the plateaus, and EC50 is the molar concentration of the agonist producing 50% of the maximum response of KCl.
Statistical Analysis
All data were analyzed using the MIXED model of SAS (SAS 9.3, SAS Inst. Inc., Cary, NC). To assess if experimental conditions affected the reference compound response, contractile responses to KCl for each vessel type were analyzed for effects of ERV concentration and incubation and their interaction. Contractile responses to increasing TCB-2 concentrations and EC50 data for each agonist were analyzed as a completely randomized design for effects of TCB-2 and ERV concentrations and the interaction. The prior exposure and simultaneous exposure to ERV experiments were analyzed separately. The animal from which all four vessel types was collected was the experimental unit. An ERV × TCB-2 concentration interaction was analyzed with ERV and agonist concentration being the main effects. Mean separation was conducted for data if the probability of a greater F-statistic in the ANOVA was significant for the effect of agonist concentration. Individual mean differences were evaluated by using the LSD feature of SAS. Data were analyzed for deviations from normality and homogeneity. Results are considered significant if probabilities are P < 0.05, unless reported otherwise.
RESULTS
Effects of Incubation
Because ergot alkaloids cause vasoconstriction, the vascular dimensions were assessed relative to prior ERV exposure. There were no differences in vascular internal or external diameters recorded for ruminal and mesenteric arteries (P > 0.05; Table 1) relative to ERV concentration. Vascular dimensions were not determined for vein preparations due to the thin walls and pliable nature of these vessels.
Table 1.
Means and SEM of vascular dimensions for bovine ruminal and mesenteric arteries incubated with ergovaline for 2 h prior to or not incubated prior to myograph experiments1
| Vessel and Dimension | Ergovaline, µM | ||||
| 0.0 | 0.01 | 1.0 | SEM | P-value | |
|---|---|---|---|---|---|
| Prior incubation with ergovaline | |||||
| Ruminal artery | |||||
| Outside diameter, mm | 2.55 | 2.47 | 2.39 | 0.06 | 0.23 |
| Inside diameter, mm | 1.30 | 1.28 | 1.19 | 0.04 | 0.20 |
| Mesenteric artery | |||||
| Outside diameter, mm | 2.39 | 2.35 | 2.22 | 0.07 | 0.22 |
| Inside diameter, mm | 1.31 | 1.29 | 1.16 | 0.08 | 0.39 |
| No prior incubation with ergovaline | |||||
| Ruminal artery | |||||
| Outside diameter, mm | 2.43 | 2.39 | 2.30 | 0.07 | 0.43 |
| Inside diameter, mm | 1.21 | 1.20 | 1.12 | 0.05 | 0.44 |
| Mesenteric artery | |||||
| Outside diameter, mm | 2.22 | 2.10 | 2.20 | 0.11 | 0.68 |
| Inside diameter, mm | 1.14 | 1.15 | 1.15 | 0.08 | 0.99 |
1Ergovaline was applied in the form of a tall fescue seed extract.
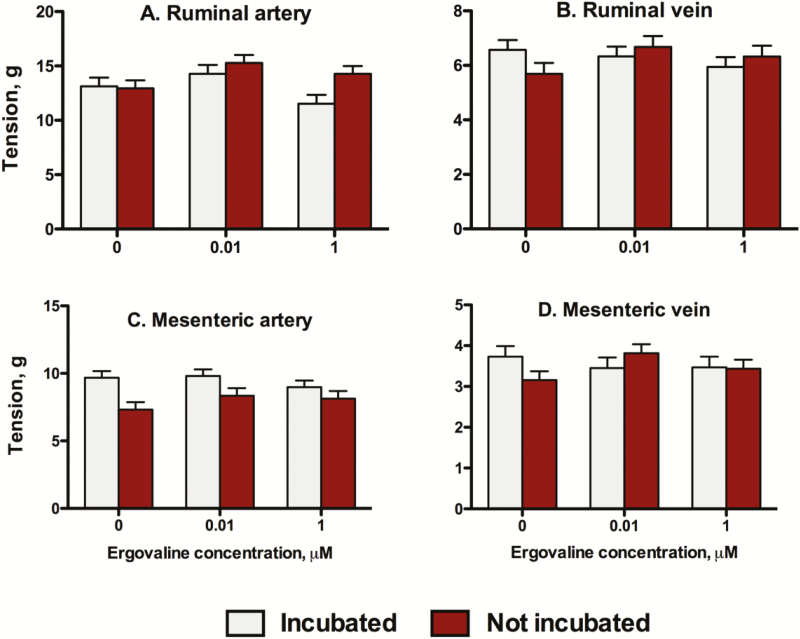
The ERV × incubation interaction was not significant (P > 0.05) in RA (Fig. 1A), RV (Fig. 1B), MA (Fig. 1C), or MV (Fig. 1D) for contractile responses to 120 mM KCl reference dose. However, there was a main effect of ERV concentration in RA with arteries incubated with 0.01 µM ERV producing larger KCl responses than the 0 or 1 µM ERV treated arteries (P = 0.026). There was also a main effect of incubation in MA with arteries that were incubated producing larger KCl responses that those that were not incubated (P < 0.001).
Figure 1.
Mean tension (g) of (A) ruminal artery (RA), (B) ruminal vein (RV), (C) mesenteric artery (MA), and (D) mesenteric vein (MV) exposed to the reference dose of 120 mM KCl using blood vessel cross-sections that were previously exposed to 0, 0.01, or 1 µM ergovaline for 2 h (incubated) and compared to those blood vessels that were not incubated before the myograph experiment.
Previous Exposure to ERV
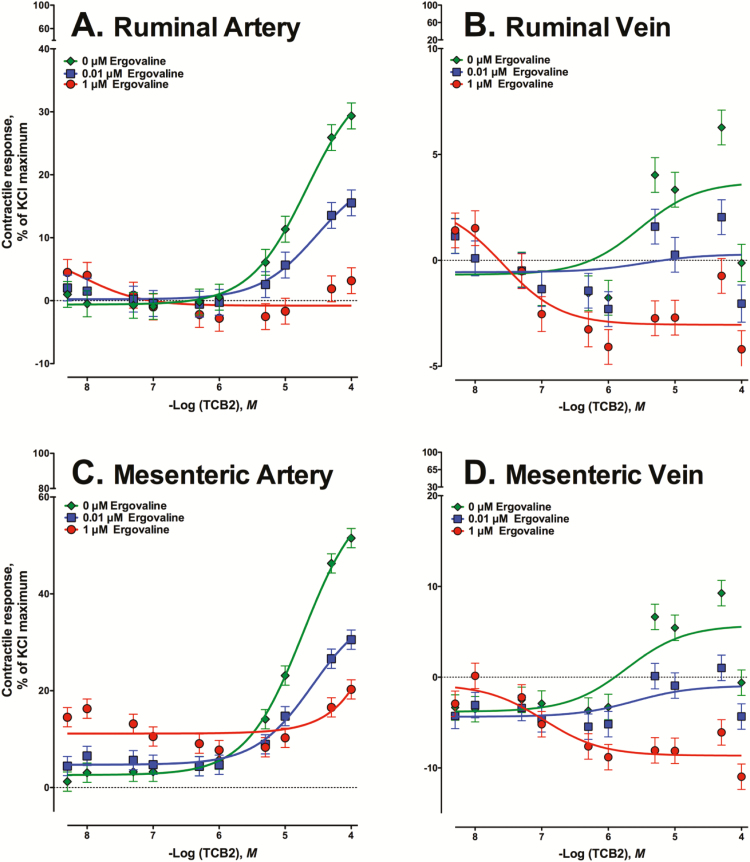
For the contractile response to increasing concentrations of TCB-2, an ERV × TCB-2 concentration interaction was observed in all ruminal and mesenteric blood vessels with previous exposure to ERV (P < 0.001; Fig. 2). Maximum contractile responses to TCB-2 + 0 µM ERV were observed at 5 × 10−5M TCB-2 for RA (Fig. 2A), RV (Fig. 2B), MA (Fig. 2C), and at 5 × 10−5M TCB-2 for MV (P < 0.001; Fig. 2D). All blood vessels with previous ERV exposure had significantly lower contractile responses to TCB-2 compared to the 0 µM ERV control (P < 0.01). Previous exposure to 0.01 µM ERV decreased the contractility of arteries (Fig. 2A and C) at 1 × 10−4M TCB-2 (P < 0.001), but did not affect contractility of either vein tested (P > 0.05; Fig. 2B and 2D) in comparison to control.
Figure 2.
Mean contractile responses of (A) ruminal artery (RA), (B) ruminal vein (RV), (C) mesenteric artery (MA), and (D) mesenteric vein (MV) to increasing concentrations of TCB-2 (a selective agonist for receptor 5-HT2A) using vessels that were exposed to 0, 0.01, or 1 µM ergovaline for 2 h before TCB-2 additions.
Prior exposure to 1 µM ERV increased the potency of TCB-2 and the stimulation of 5HT2A-induced contractile response (Table 2). All blood vessels with prior exposure to 1 µM ERV had higher EC50 values compared to 0 or 0.01 µM ERV for the response to TCB-2 (P < 0.006; Table 2). Previous incubation of RV with 0.01 µM ERV increased the EC50 compared to control, but EC50 for RV incubated with 1 µM ERV was greater than both 0 and 0.01 µM ERV (P < 0.001).
Table 2.
The –log EC50 means and SEM of bovine ruminal and mesenteric arteries and veins exposed to ergovaline for 2 h prior to or simultaneously with increasing concentrations of a selective agonist for receptor 5-HT2A (TCB-2)1,2
| Ergovaline exposure | Ergovaline, µM3 | ||||
|---|---|---|---|---|---|
| 0.0 | 0.01 | 1.0 | SEM | P-value | |
| Prior exposure | |||||
| Ruminal artery | 4.63b | 4.48b | 7.91a | 0.10 | <0.001 |
| Ruminal vein | 5.42c | 6.05b | 7.51a | 0.22 | <0.001 |
| Mesenteric artery | 4.70b | 4.55b | 6.71a | 0.45 | 0.004 |
| Mesenteric vein | 5.69b | 5.82b | 6.67a | 0.20 | 0.006 |
| Simultaneous exposure | |||||
| Ruminal artery | 4.57b | 4.42b | 6.52a | 0.19 | <0.001 |
| Ruminal vein | 5.79b | 5.38b | 6.71a | 0.22 | 0.001 |
| Mesenteric artery | 4.70b | 4.65b | 6.41a | 0.15 | <0.001 |
| Mesenteric vein | 5.76 | 5.64 | 6.04 | 0.24 | 0.47 |
1The effective concentration at which 50% of the contractile response is achieved (EC50).
2TCB-2 is a selective agonist for serotonin (5HT) receptor subtype 5HT2A.
3Ergovaline was applied in the form of a tall fescue seed extract.
abcMeans with different superscripts within a row differ P <0.05.
Simultaneous Exposure of Blood vessels to ERV and TCB-2
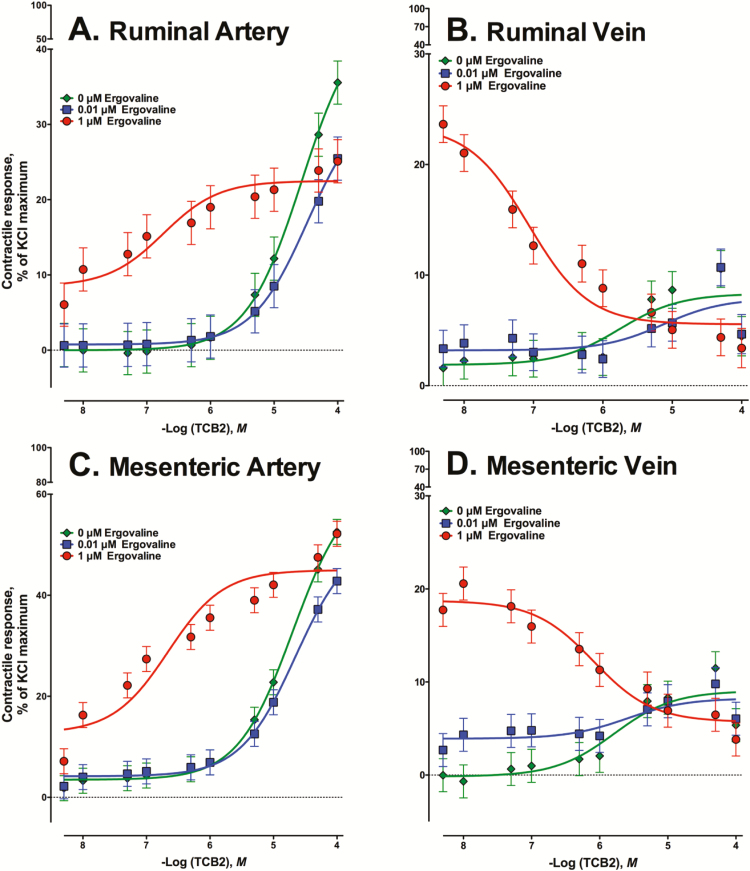
An ERV × TCB-2 concentration interaction was observed in all ruminal and mesenteric blood vessels with simultaneous exposure to ERV during the TCB-2 concentration response experiment (P < 0.001; Fig. 3). Maximum contractile responses to TCB-2 + 0 µM ERV were observed at 5 × 10−5M TCB-2 for RA (Fig. 3A) and RV (Fig. 3B) and at 1 × 10−4M TCB-2 for MA (P < 0.001; Fig. 3C). Although the magnitude of the maximum contractile response for RA to TCB-2 + 0 µM ERV at 1 × 10−4M TCB-2 was greater than 5 × 10−5M TCB-2, they did not differ (P > 0.05). All blood vessels with simultaneous exposure to 1 µM ERV had higher (P < 0.01) contractile responses than control at lower concentrations of TCB-2. Simultaneous 0.01 µM ERV addition at 1 × 10−4M TCB-2 decreased the contractility of arteries (P < 0.001; Fig. 3A and C), but did not affect contractility of veins (P > 0.05; Fig. 3B and D) compared to control.
Figure 3.
Mean contractile responses of (A) ruminal artery (RA), (B) ruminal vein (RV), (C) mesenteric artery (MA), and (D) mesenteric vein (MV) to increasing concentrations of TCB-2 (a selective agonist for receptor 5-HT2A) using vessels that were exposed to 0, 0.01, or 1 µM ergovaline simultaneously with TCB-2 additions. In the simultaneous exposure, vessels received the same concentration of ergovaline as TCB-2 concentrations increased.
Like blood vessels exposed to 1 µM ERV prior to the TCB-2 dose response experiment, simultaneous exposure of 1 µM ERV with increasing TCB-2 concentrations altered the potency of TCB-2 (Table 2). For EC50 values, RA, RV, and MA with simultaneous exposure to 1 µM ERV had higher EC50 values compared to 0 or 0.01 µM ERV (P < 0.001) and 0.01 µM ERV addition did not affect EC50 values in any blood vessel compared to control (P > 0.05; Table 2). Inclusion of ERV did not affect the EC50 values of the MV to increasing TCB-2 even though the slopes of the response curves were opposite for 1 µM ERV compared to 0 and 0.01 µM ERV (P = 0.47).
DISCUSSION
Previous researchers (Julien et al., 1974; Williams et al., 1975; Garner and Cornell, 1978) have reported that ergot alkaloid exposure produced morphological changes in livestock vasculature and thickening of small peripheral blood vessels. In addition, Klotz et al. (2012) and Egert et al. (2014a) reported a smaller internal diameter of blood vessels from steers with exposure to ergot alkaloids. Morphological changes associated with ERV exposure were not observed in the present study (Table 1) or by Jia et al. (2015) and could be attributed to the relatively short duration of ERV exposure in the pre-myograph incubation period compared to previous experiments with dietary exposure of ergot alkaloids. Because there were no differences in vascular dimensions or vascular lengths, there were no preexisting morphological differences that could have contributed to observed treatment differences.
There was also concern that the 2-h incubation period and exposure to ERV during the incubation period could influence treatment differences by altering the response to the KCl reference addition. When compared, vessel contractility to the KCl reference did not differ thus, vessel responsiveness and viability were deemed as uncompromised by prior exposure to ERV through the 2-h incubation period. However, because vessels from simultaneous and previous exposure experiments were treated differently (vessels exposed to ERV and TCB-2 simultaneously did not undergo a 2-h incubation period), they were analyzed as 2 separate experiments.
Serotonin can act as a vasoconstrictor or a vasodilator depending on the animal species, blood vessel type, or endothelial cell layer (Ni and Watts, 2006). Serotonin has been shown to be vasoactive in bovine ruminal artery and vein (Klotz et al., 2011) and mesenteric artery and vein (Egert et al., 2014a). The serotonin receptor subtype, 5-HT2A, is the primary receptor involved in vasoconstrictive responses (Schöning et al., 2001). Using the antagonist ketanserin, Klotz et al. (2013) identified the presence of 5-HT2A receptors in bovine lateral saphenous veins. Recently, Snider et al. (2018) determined that serotonin receptor 5-HT2A is involved in vasoconstriction in bovine ruminal and mesenteric blood vessels and suggested that ergot alkaloids could further influence contractility of vessels through 5-HT2A. In the current study, ergot alkaloid, specifically ERV-containing tall fescue seed extract exposure influenced contractility of bovine ruminal and mesenteric blood vessels through serotonin receptor 5-HT2A by acting as both an agonist and antagonist.
Berde (1980) stated that structural differences between ergot alkaloids and biogenic amines may explain why ergot compounds act as agonists or antagonists at biogenic amine receptor sites. Moreover, ergot alkaloids may assume the dual role of a partial agonist and antagonist. Specifically, ergotamine was shown to act as a noncompetitive dualist at serotonin receptors in human temporal arteries (Müller-Schweinitzer and Weidmann, 1977). Similar responses were observed in this study where previous exposure to ERV diminished contractile responses to TCB-2, whereas simultaneous exposure to 1 µM ERV increased contractility of blood vessels at low concentrations of TCB-2. A possible explanation for this dual nature could be attributed to slow receptor association (Unett et al., 2013) and disassociation rates (Klotz et al., 2007). In contrast, the potency of ergot alkaloids for activation are highly time-dependent and receptor signaling produced from ergot alkaloids can persist for many hours without loss of potency (Pesqueira et al., 2014). In fact, bioaccumulation could be a possible explanation for the sustained agonistic response (Klotz et al., 2009) and irreversible receptor binding of ERV (Schöning et al., 2001). Persistent signaling from internalized or sequestered receptors may provide the mechanistic basis for the agonistic response of ERV with serotonin receptor 5-HT2A (Tan et al., 2004; Unett et al., 2013) while removing the receptor from subsequent interaction with ligands.
However, an antagonistic, or diminished response to ergot alkaloids or selective agonists could also be explained by bioaccumulation of alkaloids that prevents receptor dissociation and therefore, reduces the number of available receptors for alkaloid binding and subsequent vasoconstriction. Diminished vessel contractility associated with previous exposure to ERV has been noted in several studies in lateral saphenous veins (Klotz et al., 2012, 2013, 2016, 2018) and mesenteric vasculature (Egert et al., 2014a; Jia et al., 2015). Alterations in vascular responses to selective agonists for 5-HT2A and 5-HT7 in lateral saphenous veins from cattle grazing endophyte-infected tall fescue were noted by Klotz et al. (2012). Antagonism of the 5-HT2A receptor with ketanserin suppressed the contractile response to ERV, ergotamine, and ergocornine (Klotz et al., 2013). Additionally, steers from the same study that grazed endophyte-infected tall fescue pastures were more sensitive to lower concentrations of ERV, ergotamine, and ergocornine. Results from Klotz et al. (2016) show that previous dietary exposure to ERV diminished the contractile response of lateral saphenous veins when exposed to TCB-2. However, veins from steers biopsied at different times after removal from toxic tall fescue pasture demonstrated an increased contractile response which provides evidence that ergot alkaloid-induced vascular changes in cattle are reversible.
More recently, the observed decrease in 5HT2A-derived vasoactivity due to previous ergot alkaloid exposure was found to be independent of pasture levels of ERV in lateral saphenous veins (Klotz et al., 2018). Similarly, the level of previous ERV exposure did not affect the degree of decreased vasoactivity in ruminal or mesenteric veins in this study. However, increasing the level of previous ERV exposure did decrease the degree of vasoactivity in ruminal and mesenteric arteries. Previous myograph contractility experiments using bovine vasculature have varied in response to agonist treatments and it is unclear why different ERV levels induce a dose-dependent response in arteries, but not veins. Snider et al. (2018) speculated that larger contractile responses in arteries may be explained by differential expression of the 5-HT2A receptor, as this has been observed in rats (Kato et al., 1999; Watts, 2002).
In mesenteric vasculature, dietary exposure to ergot alkaloids diminishes contractility of mesenteric arteries and veins (Egert et al., 2014a). Furthermore, Jia et al. (2015) incubated bovine mesenteric blood vessels in vitro in a medium with ERV-containing tall fescue extract which was used to achieve an ERV pretreatment like the current study. Results from the current study support the findings of the previous authors and provides strong evidence that previous exposure to ERV reduces the contractility of mesenteric vasculature through serotonin receptor 5-HT2A.
Only 50–60% of dietary ergot alkaloids fed in the diet are recovered in the abomasum which suggests that 40–50% of dietary ergot alkaloids are either metabolized or absorbed in the foregut (Westendorf et al., 1993). Klotz et al. (2011) was the first to use bovine ruminal artery and vein in an in vitro contractility bioassay to evaluate the contractile response to serotonin. Ergot alkaloids were shown to induce vasoconstriction in ruminal blood vessels (Foote et al., 2011) and it was later determined that the tall fescue seed extract (used in the current study) is similar to pure ERV in ruminal vessels (Foote et al., 2012). Furthermore, Foote et al. (2013) demonstrated that ergot alkaloids from endophyte-infected tall fescue decrease ruminal epithelial blood flow and VFA absorption. However, acute exposure to ergot alkaloids does not alter absorptive or barrier function of the rumen epithelium (Foote et al., 2014).
To our knowledge, this is the first study to demonstrate that previous exposure to ERV diminishes the contractile response of bovine ruminal arteries and veins in vitro. However, the mechanisms by which previous ERV exposure reduces vessel contractility through serotonin receptor 5-HT2A appear to be similar, regardless of vessel type. Steers exposed to tall fescue seed have suppressed gene expression of serotonin receptor 5-HT2A in smooth muscle of the foregut, midgut, and hindgut (Klotz et al., 2014). Observations by Foote et al. (2013) and Koontz et al. (2013) demonstrated that exposure to ergot alkaloids increased ruminal DM mass in steers controlled for DM intake which may suggest that ERV could alter rumen motility (Egert et al., 2014b) through serotonin receptor 5-HT2A or 5-HT4 (Poole et al., 2009; Klotz et al., 2014).
Maximum contractile responses to TCB-2 + 0 uM ERV in ruminal and mesenteric blood vessels were observed at similar TCB-2 concentrations as reported previously by Snider et al. (2018). For EC50 values, the units are logarithmic and therefore, the larger the EC50 value, the lower the TCB-2 concentration needed to achieve 50% of the total response and the more sensitive a vessel is to ERV. In general, as ERV concentration increases from 0.01 µM to 1 µM ERV, EC50 values increase, and therefore bovine vasculature becomes more sensitive, regardless of vessel type or method of exposure. However, there were differences observed in the type of response in arteries compared to veins at the 1 µM ERV treatment with arterial preparations increasing and venous preparations decreasing with each addition. It is also worth noting that although vessels exposed to 1 µM ERV become very sensitive to TCB-2, the contractile response to TCB-2 was all but abolished by ERV exposure. No direct comparisons between blood vessel types were conducted in this study. However, the numerical values for blood vessels with no ERV exposure are similar to EC50 values reported by Snider et al. (2018), supporting the observation that RV and MV are more sensitive to TCB-2 than RA and MA. Lower EC50 values in the RA and MA support the larger contractile responses observed in those vessels compared to RV and MV. This evidence could correspond with a larger number of 5-HT2A receptors in arteries compared to veins.
CONCLUSIONS
Ergovaline, a suspected causative agent of fescue toxicosis in ruminants, induces vasoconstriction in bovine ruminal and mesenteric blood vessels through serotonin receptor 5-HT2A. Previous exposure to ERV significantly diminished the contractile response to TCB-2 in all blood vessels and simultaneous exposure to ERV increased the contractile response at concentrations below the low concentration vasoactive threshold of TCB-2 in all blood vessels. This study provides strong evidence that ERV initially acts as an agonist and then as an antagonist post-binding in bovine vasculature through serotonin receptor 5-HT2A. This is an indication that membrane bound G-protein-coupled receptors, such as 5HT2A, that interact with ergot alkaloids, like ERV, are functionally altered. Future studies should focus on defining the fate of the ERV-receptor complex to better understand the mechanisms and to mitigate alkaloid binding to 5-HT2A. This will aid in understanding the fate of ERV and other ergopeptine alkaloids in vivo.
Footnotes
Mention of trade name, proprietary product of specified equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be available. The authors acknowledge Adam J. Barnes of the Forage-Animal Production Research Unit and the University of Kentucky Meats Lab for their hard work and collaboration towards the completion of this experiment.
LITERATURE CITED
- Aiken G. E., Kirch B. H., Strickland J. R., Bush L. P., Looper M. L., and Schrick F. N.. 2007. Hemodynamic responses of the caudal artery to toxic tall fescue in beef heifers. J. Anim. Sci. 85:2337–2345. doi: 10.2527/jas.2006-821 [DOI] [PubMed] [Google Scholar]
- Berde B. 1980. Ergot compounds: A synopsis. In: Goldstein M., Lieberman A., Calne D. B., and Thorner M. O., editors. Ergot Compounds and Brain Function: Neuroendocrine and Neuropsychiatric Aspects. Raven Press, New York, NY: p. 4–23. [Google Scholar]
- Bush L. P., Cornelius P. L., Buckner R. C., Varney D. R., Chapman R. A., Burriss P. B. II, Kennedy C.W., Jones T. A., and Saunders M. J.. 1982. Association of N-acetylloline and N-formylloline with epichloe typhina in tall fescue. Crop Sci. 22:941–943. [Google Scholar]
- Dyer D. C. 1993. Evidence that ergovaline acts on serotonin receptors. Life Sci. 53:PL223–228. [DOI] [PubMed] [Google Scholar]
- Eckert H., Kiechel J. R., Rosenthaler J., Schmidt R., and Schreier E.. 1978. Biopharmaceutical aspects. In: Berde B, Schild HO, editors. ‘Ergot alkaloids and related compounds’. Springer-Verlag, Berlin: p. 719–803. [Google Scholar]
- Egert A. M., Kim D. H., Schrick F. N., Harmon D. L., and Klotz J. L.. 2014a. Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature. J. Anim. Sci. 92:1768–1779. doi:10.2527/jas.2013-7141 [DOI] [PubMed] [Google Scholar]
- Egert A. M., Klotz J. L., McLeod K. R., and Harmon D. L.. 2014b. Development of a methodology to measure the effect of ergot alkaloids on forestomach motility using real-time wireless telemetry. Front. Chem. 2:90. doi: 10.3389/fchem.2014.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote A. P., Harmon D. L., Brown K. R., Strickland J. R., McLeod K. R., Bush L. P., and Klotz J. L.. 2012. Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline. J. Anim. Sci. 90:1603–1609. doi: 10.2527/jas.2011-4513 [DOI] [PubMed] [Google Scholar]
- Foote A. P., Harmon D. L., Strickland J. R., Bush L. P., and Klotz J. L.. 2011. Effect of ergot alkaloids on contractility of bovine right ruminal artery and vein. J. Anim. Sci. 89:2944–2949. doi:10.2527/jas.2010-3626 [DOI] [PubMed] [Google Scholar]
- Foote A. P., Kristensen N. B., Klotz J. L., Kim D. H., Koontz A. F., McLeod K. R., Bush L. P., Schrick F. N., and Harmon D. L.. 2013. Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen. J. Anim. Sci. 91:5366–5378. doi: 10.2527/jas.2013-6517 [DOI] [PubMed] [Google Scholar]
- Foote A. P., Penner G. B., Walpole M. E., Klotz J. L., Brown K. R., Bush L. P., and Harmon D. L.. 2014. Acute exposure to ergot alkaloids from endophyte-infected tall fescue does not alter absorptive or barrier function of the isolated bovine ruminal epithelium. Animal. 8:1106–1112. doi: 10.1017/S1751731114001141 [DOI] [PubMed] [Google Scholar]
- Garner G. B., and Cornell C. N.. 1978. Fescue foot in cattle In: Wylie T. D. and Morehouse L. G., editors, Mycotoxic fungi, mycotoxins, and mycotoxicoses. 2nd ed. Marcel Dekker, New York: p. 45–62. [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., and Humphrey P. P.. 1994. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 46:157–203. [PubMed] [Google Scholar]
- Ji H., Fannin F., Klotz J., and Bush L.. 2014. Tall fescue seed extraction and partial purification of ergot alkaloids. Front. Chem. 2:110. doi: 10.3389/fchem.2014.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Harmon D. L., Flythe M. D., and Klotz J. L.. 2015. Interaction of isoflavones and endophyte-infected tall fescue seed extract on vasoactivity of bovine mesenteric vasculature. Front. Nutr. 2:32. doi: 10.3389/fnut.2015.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien W. E., Martz F. A., Williams M., and Garner G. B.. 1974. Feed intake in hereford calves infused intraperitoneally with toxic fescue extract. J. Dairy Sci. 57:1385–1387. doi: 10.3168/jds.S0022-0302(74)85071-X [DOI] [PubMed] [Google Scholar]
- Kato S., Kumamoto H., Hirano M., Akiyama H., and Kaneko N.. 1999. Expression of 5-HT2A and 5-HT1B receptor mRNA in blood vessels. Mol. Cell. Biochem. 199:57–61. [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Bussard J. R., Foote A. P., Harmon D. L., Goff B. M., Schrick F. N., and Strickland J. R.. 2016. Vasoactivity and vasoconstriction changes in cattle related to time off toxic endophyte-infected tall fescue. Toxins. 8:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Egert-McLean A. M., Shrick F. N., Chattopadhyay N., and Harmon D. L.. 2018. Effects of grazing different ergovaline concentrations on vasoactivity of bovine lateral saphenous vein. J. Anim. Sci. 96(7):3022–3030. doi:10.1093/jas/sky163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Johnson J. M., Brown K. R., Bush L. P., and Strickland J. R.. 2013. Antagonism of lateral saphenous vein serotonin receptors from steers grazing endophyte-free, wild-type, or novel endophyte-infected tall fescue. J. Anim. Sci. 91:4492–4500. doi: 10.2527/jas.2012-5896 [DOI] [PubMed] [Google Scholar]
- Klotz J. L. and Barnes A. J.. 2014. Isolating and using sections of bovine mesenteric artery and vein as a bioassay to test for vasoactivity in the small intestine. J. Vis. Exp. 92:e52020. doi:10.3791/52020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Brown K. R., Xue Y., Matthews J. C., Boling J. A., Burris W. R., Bush L. P., and Strickland J. R.. 2012. Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 90:682–693. doi: 10.2527/jas.2011-4323 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Bush, L. P., Smith, D. L., Shafer, W. D., Smith, L. L., Arrington, B. C., and Strickland, J. R.. 2007. Ergovaline-induced vasoconstriction in an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 85:2330–2336. doi:10.2527/jas.2006-803 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Bush L. P., and Strickland J. R.. 2011. A vascular contractility bioassay using bovine right ruminal artery and vein. J. Anim. Sci. 89:1944–1951. doi: 10.2527/jas.2010-3532 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Kim D. H., Foote A. P., and Harmon D. L.. 2014. Effects of ergot alkaloid exposure on serotonin receptor mRNA in smooth muscle of the bovine gastrointestinal tract. J. Anim. Sci. 92:890–891. [Google Scholar]
- Klotz J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. 2009. Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro. J. Anim. Sci. 87:2437–2447. doi: 10.2527/jas.2008-1692 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. 2010. Contractile response of fescue-naive bovine lateral saphenous veins to increasing concentrations of tall fescue alkaloids. J. Anim. Sci. 88:408–415. doi: 10.2527/jas.2009-2243 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., and Nicol A. M.. 2016. Ergovaline, an endophytic alkaloid. 1. Animal physiology and metabolism. Anim. Prod. Sci. 56:1761–1774. doi:10.1071/an14962 [Google Scholar]
- Koontz A. F., Kim D. H., Foote A. P., Bush L. P., Klotz J. L., McLeod K. R., and Harmon D. L.. 2013. Alteration of fasting heat production during fescue toxicosis in Holstein steers. J. Anim. Sci. 91:3881–3888. doi: 10.2527/jas.2013-6232 [DOI] [PubMed] [Google Scholar]
- Lyons P. C., Plattner R. D., and Bacon C. W.. 1986. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science. 232:487–489. [DOI] [PubMed] [Google Scholar]
- Müller-Schweinitzer E., and Weidmann H.. 1977. Regional differences in the responsiveness of isolated arteries from cattle, dog and man. Agents Actions. 7:383–389. [DOI] [PubMed] [Google Scholar]
- Ni W., and Watts S. W.. 2006. 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clin. Exp. Pharmacol. Physiol. 33:575–583. doi: 10.1111/j.1440-1681.2006.04410.x [DOI] [PubMed] [Google Scholar]
- Pesqueira A., Harmon D. L., Branco A. F., and Klotz J. L.. 2014. Bovine lateral saphenous veins exposed to ergopeptine alkaloids do not relax. J. Anim. Sci. 92:1213–1218. doi: 10.2527/jas.2013-7142 [DOI] [PubMed] [Google Scholar]
- Poole D. P., Littler R. A., Smith B. L., and McLeay L. M.. 2009. Effects and mechanisms of action of the ergopeptides ergotamine and ergovaline and the effects of peramine on reticulum motility of sheep. Am. J. Vet. Res. 70:270–276. doi: 10.2460/ajvr.70.2.270 [DOI] [PubMed] [Google Scholar]
- Porter J. K., Bacon C. W., and Robbins J. D.. 1979. Ergosine, ergosinine, and chanoclavine I from epichloë typhina. J. Agric. Food Chem. 27:595–598. [DOI] [PubMed] [Google Scholar]
- Rhodes M. T., Paterson J. A., Kerley M. S., Garner H. E., and Laughlin M. H.. 1991. Reduced blood flow to peripheral and core body tissues in sheep and cattle induced by endophyte-infected tall fescue. J. Anim. Sci. 69:2033–2043. [DOI] [PubMed] [Google Scholar]
- Schöning C., Flieger M., and Pertz H. H.. 2001. Complex interaction of ergovaline with 5-HT2A, 5-HT1B/1D, and alpha1 receptors in isolated arteries of rat and guinea pig. J. Anim. Sci. 79:2202–2209. [DOI] [PubMed] [Google Scholar]
- Snider M. A., Harmon D. L., and Klotz J. L.. 2018. Pharmacologic assessment of bovine ruminal and mesenteric vascular serotonin receptor populations. J. Anim. Sci. 96:1570–1578. doi: 10.1093/jas/sky038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomons R. N., Oliver J. W., and Linnabary R. D.. 1989. Reactivity of dorsal pedal vein of cattle to selected alkaloids associated with Acremonium coenophialum-infected fescue grass. Am. J. Vet. Res. 50:235–238. [PubMed] [Google Scholar]
- Strickland J. R., Looper M. L., Matthews J. C., Rosenkrans C. F. Jr, Flythe M. D., and Brown K. R.. 2011. Board-invited review: St. Anthony’s fire in livestock: causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi: 10.2527/jas.2010-3478 [DOI] [PubMed] [Google Scholar]
- Tan C. M., Brady A. E., Nickols H. H., Wang Q., and Limbird L. E.. 2004. Membrane trafficking of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558 [DOI] [PubMed] [Google Scholar]
- Unett D. J., Gatlin J., Anthony T. L., Buzard D. J., Chang S., Chen C., Chen X., Dang H. T., Frazer J., Le M. K., et al. 2013. Kinetics of 5-HT2B receptor signaling: profound agonist-dependent effects on signaling onset and duration. J. Pharmacol. Exp. Ther. 347:645–659. doi: 10.1124/jpet.113.207670 [DOI] [PubMed] [Google Scholar]
- Watts S. W. 2002. Serotonin-induced contraction in mesenteric resistance arteries: signaling and changes in deoxycorticosterone acetate-salt hypertension. Hypertension. 39:825–829. [DOI] [PubMed] [Google Scholar]
- Westendorf M. L., Mitchell G. E. Jr, Tucker R. E., Bush L. P., Petroski R. J., and Powell R. G.. 1993. In vitro and in vivo ruminal and physiological responses to endophyte-infected tall fescue. J. Dairy Sci. 76:555–563. doi: 10.3168/jds.S0022-0302(93)77375-0 [DOI] [PubMed] [Google Scholar]
- Williams M., Shaffer S. R., Garner G. B., Yates S. G., Tookey H. L., Kintner L. D., Nelson S. L., and McGinity J. T.. 1975. Induction of fescue foot syndrome in cattle by fractionated extracts of toxic fescue hay. Am. J. Vet. Res. 36:1353–1357. [PubMed] [Google Scholar]