Abstract
Heat stress (HS) jeopardizes animal productivity and health. The intestinal barrier is sensitive to HS and heat-induced hyperpermeability plays a key role in its pathophysiology. However, the biology of recovery following HS is less understood. Thus, study objectives were to determine the temporal pattern of metabolic, inflammatory, and intestinal histological parameters during HS recovery. Female pigs (n = 32; 19.5 ± 0.5 kg BW) were sacrificed following exposure to 1 of 4 environmental treatments: 1) constant thermoneutral (TN) conditions (TNC; 24.2 ± 0.5°C), 2) no TN recovery post HS (0D), 3) 3 d of TN recovery post HS (3D), and 4) 7 d of TN recovery post HS (7D). The HS protocol was cyclical (33.6 ± 1.8 to 37.4 ± 2.1°C) and lasted for 3 d for all HS treatments. During the 3 d of HS, rectal temperature, skin temperature, and respiration rates were increased (1.3°C, 4.8°C, and 77 breaths/min, respectively; P < 0.01) and ADFI was decreased (27%; P < 0.01) compared to TNC pigs. Skin temperature tended to be decreased 0.6°C in 3D pigs during days 1–3 of recovery (P = 0.06) and was decreased 1.6 and 0.7°C during days 1–3 and 4–7 of recovery, respectively, in 7D pigs (P ≤ 0.03) compared to TNC. Relative to TNC pigs, ADFI remained 14% decreased during days 1–3 of recovery in both 3D and 7D pigs, and 17% decreased during days 4–7 in 7D pigs (P ≤ 0.01). Plasma glucose was decreased (10%; P = 0.03) for 0D and 3D relative to TNC pigs. Circulating lipopolysaccharide-binding protein was increased in 3D and 7D vs. TNC pigs (110 and 147%, respectively; P = 0.01) and tended to increase linearly with increasing recovery time (P = 0.08). Circulating tumor necrosis factor alpha was decreased (15%) in 0D pigs and increased linearly with advancing recovery time (P < 0.01). Jejunum and ileum villus height were reduced 17 and 11% in 0D vs. TNC pigs and increased linearly with progressive recovery time (P < 0.01). Jejunum and ileum mucosal surface areas were reduced 17 and 9% in 0D pigs and remained decreased in the jejunum while the ileum recovered to TNC levels by day 3 of recovery. Relative to TNC pigs, goblet cell area was similar in jejunum and colon of 0D pigs but was reduced in the ileum of 0D pigs and in jejunum, ileum, and colon of 3D and 7D relative to TNC pigs (P < 0.01). In summary, HS has deleterious effects on intestinal morphology that seem to improve with recovery time. In contrast, feed consumption remained suppressed and inflammatory biomarkers indicative of leaky gut increased following the heat load.
Keywords: heat stress, inflammation, intestinal barrier, recovery
INTRODUCTION
Heat stress (HS) negatively affects multiple facets of animal agriculture, including growth, lactation, gestation, production efficiency, and product quality (Bhattacharya and Hussain 1974; Christon, 1988; Kadzere et al., 2002; Ross et al., 2017). In the American pig industry, HS-induced suboptimal growth and poor reproductive performance is thought to cost approximately $900 million annually (Baumgard and Rhoads, 2013) and this estimate does not take into consideration the negative and substantial consequences of in utero HS on future piglet performance (as reviewed in Johnson et al., 2015). Therefore, HS impedes efficient food production for human consumption and is a global economic and food security issue. Many negative production and metabolic consequences of HS are likely related to its deleterious effects on intestinal health (Baumgard and Rhoads, 2013). During HS, blood is directed from the splanchnic tissues to the periphery to maximize radiant heat dissipation (Crandall and González-Alonso, 2010) and this is a conserved response among species (Baumgard and Rhoads, 2013). The decreased blood flow leads to insufficient nutrient and oxygen supply to visceral tissue, which compromises intestinal barrier function (Hall et al., 1999). Hypoxia-induced epithelial damage allows luminal content to infiltrate the intestinal barrier, and if the antigen load exceeds the liver’s detoxification capacity it can cause endotoxemia and even bacteremia (Bouchama and Knochel, 2002; Leon and Bouchama, 2015). Inflammatory biomarkers and morphological changes to the intestine during HS are well-characterized (Lambert et al., 2002; Pearce et al., 2013c); however, the extent to which these negative effects persist following HS is less known. Our previous report demonstrates altered markers of metabolism during HS recovery (Mayorga et al., 2017), but the timing and extent of intestinal damage and how this may contribute to HS pathophysiology is unclear. Objectives of the current project were to investigate the effect of various recovery times following HS on gut morphology, as well as temporal patterns of physiological, metabolic, and inflammatory variables in growing pigs as we hypothesize that the altered aforementioned systems contribute to poor performance following HS. Characterizing the chronological post-insult effects of HS is important to holistically understanding the role of HS-induced intestinal dysfunction and will help to advance methods mitigating this pathophysiology.
MATERIALS AND METHODS
Animals and Experimental Design
Iowa State University Institutional Animal Care and Use Committee approved all procedures involving animals, which took place at the Iowa State University Swine Nutrition Farm. Thirty-two female pigs (virgin gilts; 19.5 ± 0.5 kg BW) were utilized for this experiment and were randomly assigned to 1 of 4 treatments: 1) constant thermoneutral (TN) conditions (TNC; n = 8), 2) 0-d TN recovery following HS (0D; n = 8), 3) 3-d TN recovery following HS (3D; n = 8), or 4) 7-d TN recovery following HS (7D; n = 8). Pigs were allotted to one of two environmentally controlled rooms (TN or HS room). Each room had 24 crates (43 × 121 cm) where pigs were housed individually. Each crate was equipped with a stainless steel feeder and a nipple drinker. Throughout the entire experiment, water and feed were provided ad libitum and pigs were provided a standard diet consisting mainly of ground corn and soybean meal formulated to meet or exceed the nutrient- and energy-predicted requirements (NRC, 2012; Table 1).
Table 1.
Ingredient and chemical composition of diet for growing pigs (as-fed basis)
| Item | % |
|---|---|
| Ingredient | |
| Corn | 62.70 |
| Soybean meal | 24.16 |
| DDGS1 | 10.00 |
| Vitamin–mineral premix2 | 2.25 |
| Monocalcium phosphate | 0.38 |
| Lysine HCl | 0.37 |
| dl-Methionine | 0.08 |
| l-Threonine | 0.06 |
| Calculated chemical composition | |
| CP | 18.92 |
| SID lysine | 1.20 |
| Crude fat | 4.56 |
| Crude fiber | 3.56 |
| Ash | 4.62 |
| Ca | 0.93 |
| Total P | 0.49 |
1Distilled dried grains with solubles.
2SCE 45-30 (Nutra Blend, LLC., Neosho, MO). Premix contains: 8.8% of Fe, 8.8% of Zn, 4.0% of Mn, 1.6% of Cu, 600 mg/kg of I, 133 mg/kg of Se, 1,360,776 IU/kg of vitamin A, 249,475 IU/kg of vitamin D3, 7,257 IU/kg of vitamin E, 4.5 mg/kg of vitamin B12, 363 mg/kg of Menadione, 680 mg/kg of Riboflavin, 2,721 mg/kg of D-Pantothenic acid, 3,425 mg/kg of Niacin, 308 mg/kg of Ethoxyquin.
The study was divided into three experimental periods (P). During P1 (3 d), all pigs were housed in individual crates and subjected to TN conditions (24.2 ± 0.5°C and 57.2 ± 5.6% relative humidity) for collection of baseline body temperature indices and ADFI parameters. During P2 (3 d), 0D, 3D, and 7D pigs were exposed to cyclical HS conditions with temperatures ranging between 33.6 ± 1.8°C (1800–0700 h) and 37.4 ± 2.1°C (0700–1800 h) with 29.5 ± 5.3% relative humidity (a similar heat load as described in our previous reports; Pearce et al., 2013a, 2013b, 2013c, 2015; Sanz-Fernandez 2015a, 2015b). Pigs assigned to the TNC treatment remained in TN conditions during P2. Pigs assigned to the 0D treatment were sacrificed immediately following day 3 of HS (without access to TN conditions). During P3 (recovery period), pigs in 3D or 7D treatments were returned to TN conditions for 3 and 7 d (to represent the acute and chronic aspects of HS recovery), respectively. Pig entry into the experimental design was staggered so that an equal number of pigs from each treatment were euthanized on sacrifice days. All pigs were euthanized at the end of their respective environmental protocol via captive bolt followed by exsanguination.
Production and Temperature Parameters Collection and Analysis
Room temperature and humidity were recorded every 5 min by 4 data loggers (Lascar EL-USB-2-LCD, Erie, PA) located in different quadrants of each room and then condensed into averages by each 5-min time point. Average daily feed intake was measured at 0700 h as feed disappearance (difference in feeder weight during 1 24-h timeframe). Body weights were obtained prior to acclimation and sacrifice. Rectal temperature (Tr), respiration rate (RR), and skin temperature (Ts) were measured daily at 0600, 1200, and 1800 h. Rectal temperatures were measured using a digital thermometer (Digital Thermometer with Flex Tip, Jorgensen Labs Inc., Item #J0134F), Ts was measured on the rump using an infrared thermometer (IRT207: The Heat Seeker 8:1, General Tools, New York, NY), and RR was determined by counting flank movements for 15 s and then multiplied by 4 to obtain breaths/min. Rectal temperature, RR, and Ts were condensed into daily averages for analysis.
Plasma Collection and Analysis
At sacrifice, blood samples were collected during exsanguination into a tube containing EDTA as the anticoagulant (~10 mL; BD Vacutainer, Franklin Lakes, NJ). Plasma was harvested following centrifugation at 1,500 × g for 15 min at 4°C and subsequently frozen at −20°C until analysis. Plasma insulin, nonesterified fatty acids (NEFA), glucose, lipopolysaccharide-binding protein (LBP), and tumor necrosis factor alpha (TNFα) were determined using commercially available kits (insulin, Mercodia AB, Uppsala, Sweden; NEFA, Wako Chemicals USA, Richmond, VA; glucose, Wako Chemicals USA Inc., Richmond, VA; LBP, Hycult Biotech, Uden, Netherlands; TNFα, R&D Systems USA Inc., Minneapolis, MN).
Postmortem Tissue Collection and Analysis
Organs and tissues were harvested immediately after sacrifice. Liver, heart, and spleen weights were recorded. A liver sample (~50 g) was collected and snap-frozen in liquid nitrogen and stored at −80°C until further analysis. Liver moisture and fat content were determined using methods 950.46 and 960.39, respectively, with slight modifications (AOAC, 2000). Briefly, ~5.0 g of liver sample was dried in an oven for 18 h at 102°C, and reweighed after cooling to determine liver moisture percentage. The dried sample was then placed on a Soxhlet extractor and fat was removed using petroleum ether. The extracted sample was dried and reweighed to determine fat content. A jejunum segment was collected ~90 cm distal to the pyloric sphincter. An ileum segment was collected ~15 cm proximal to the ileocecal junction. A colon segment was collected ~35 cm proximal to the rectum. Intestinal segments were flushed with cold PBS to remove contents. A transversal section was collected from each sample, fixed in 10% neutral buffered formalin, and submitted to the Iowa State University Veterinary Diagnostic Laboratory for sectioning and staining. Periodic acid-Schiff (PAS) and hematoxylin-eosin staining were used for goblet cell area and morphology quantification, respectively. One slide per pig per tissue was generated for each stain. Using a microscope (Leica DMI3000 B Inverted Microscope, Bannockburn, IL) with an attached camera (QImaging 12-bit QICAM Fast 1394, Surrey, BC), 5 images per section of intestine were obtained at 40× magnification. All image processing and quantification was done using ImageJ 1.48v (National Institutes of Health, USA). Total mucosal area was determined after subtracting luminal area and the area of the PAS stain was measured using the ImageJ color deconvolution tool with H PAS vector. Goblet cell area was expressed as the percentage of the total mucosal area stained by PAS. Villus height was measured from the villus tip to the villus–crypt interface. Villus (v.) width was measured at mid-villus height. Crypt depth was measured from the villus–crypt opening to the laminae propria. Crypt (c.) width was measured at the villus–crypt interface level. A mucosal surface area estimate was obtained using the mucosal-to-serosal amplification ratio M, as previously reported by Kisielinski et al. (2002), where
Statistical Analysis
All data were statistically analyzed using PROC MIXED (SAS Institute Inc., Cary, NC). For production and thermal indices, the model included treatment, day, and treatment-by-day interaction as fixed effects. Within P2 and P3, each variable was analyzed by repeated measures with an autoregressive covariance structure and day as the repeated effect. Each specific variable’s P1 value (when available, Tr, ADFI, RR, etc.) served as a covariate. Due to the differences in the total length of the environmental treatments, body temperature indices, and ADFI were divided into 3 independently analyzed periods: P2, P3 (recovery) days 1–3, and P3 (recovery) days 4–7. Within each period, only the relevant treatments were included (i.e., P2 included TNC, 0D, 3D, and 7D; P3 [recovery] days 1–3 included TNC, 3D, and 7D; P3 [recovery] days 4–7 included TNC and 7D). For blood and postmortem data, the effect of treatment was analyzed using PROC MIXED. To test for linear and quadratic effects of recovery time on blood and postmortem parameters, PROC IML was utilized to generate contrast coefficients for unequally spaced HS recovery times for 0D, 3D, and 7D treatments. Preplanned contrasts were used to evaluate the effects of recovery period [TNC vs. HS (0D + 3D + 7D)] and the linear and quadratic effects of recovery time (0D, 3D, and 7D) using the CONTRAST statement of SAS. Results are reported as LSmeans and considered different when P ≤ 0.05 and a tendency was defined as 0.05 < P ≤ 0.10.
RESULTS
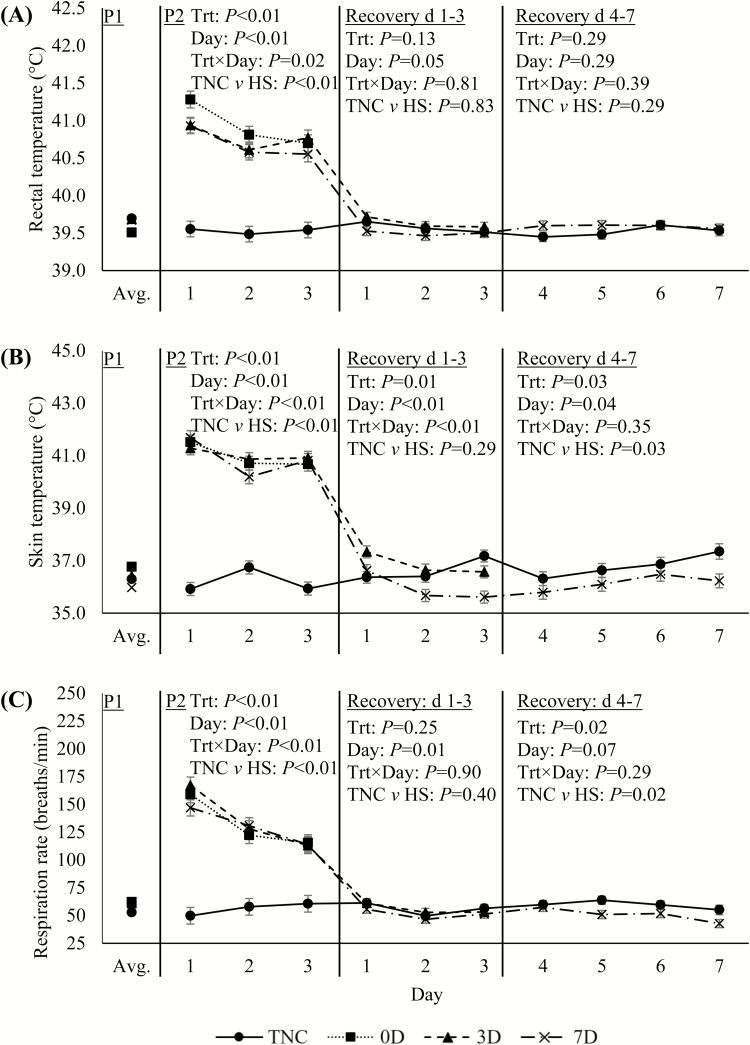
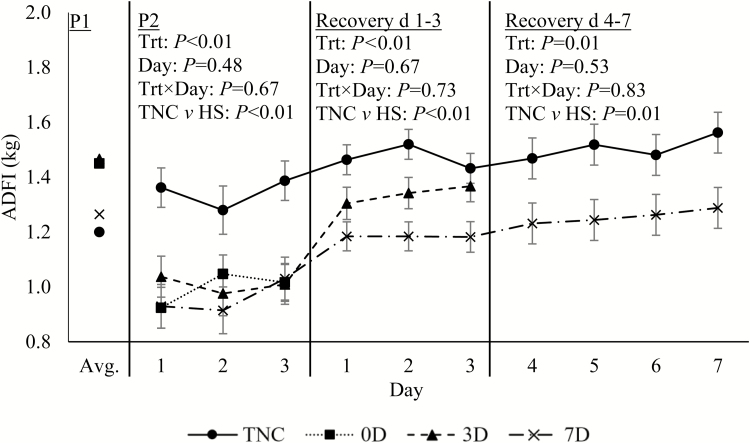
As expected, during the 3-d heat exposure, Tr, Ts, and RR were markedly increased (1.3°C, 4.8°C, and 77 breaths/min, respectively; P < 0.01; Fig. 1A–C) relative to TNC pigs; and all decreased sharply when returned to TN conditions at the end of P2. Between days 1 and 3 of recovery, Tr and RR decreased slightly over time for all treatments (P ≤ 0.05; Fig. 1A and C). On day 3 of recovery, Ts tended to be decreased in 3D (0.6°C; P = 0.06) and was decreased in 7D pigs (1.6°C; P < 0.01) relative to TNC animals (Fig. 1B). During days 4–7 of recovery, Ts continued to be decreased in 7D relative to TNC pigs (0.7°C; P = 0.03; Fig. 1B). Similarly, RR was decreased slightly during days 4–7 of the recovery period in 7D relative to TNC pigs (9 breaths/min; P = 0.02; Fig. 1C). Predictably, P2 ADFI was decreased in HS relative to TNC pigs (27%; P < 0.01; Fig. 2). Additionally, ADFI remained depressed in HS relative to TNC pigs (14%; P < 0.01; Fig. 2), during the first 3 d of recovery; and in 7D relative to TNC pigs (17%; P = 0.01; Fig. 2), during days 4–7 of recovery.
Figure 1.
Effects of treatment (Trt): constant thermoneutral conditions (TNC; n = 8), no recovery following HS (0D; n = 8), 3-d thermoneutral recovery following HS (3D; n = 8), or 7-d thermoneutral recovery following HS (7D; n = 8) on pig (A) rectal temperature, (B) skin temperature, and (C) respiration rate during period (P) 1 (24.2°C and 57.2% RH), P2 (thermoneutral [24.2°C and 57.2% RH] or cyclical HS [33.6 ± 1.8°C to 37.4 ± 2.1°C]), days 1–3 of the thermoneutral recovery period, and days 4–7 of the thermoneutral recovery period. Results are expressed as LSmeans ± SEM. The P1 average was used as a covariate. HS = heat stress; RH = relative humidity.
Figure 2.
Effects of treatment (Trt): constant thermoneutral conditions (TNC; n = 8), no recovery following HS (0D; n = 8), 3-d thermoneutral recovery following HS (3D; n = 8), or 7-d thermoneutral recovery following HS (7D; n = 8) on pig ADFI during period (P) 1 (24.2°C and 57.2% RH), P2 (thermoneutral [24.2°C and 57.2% RH] or cyclical HS [33.6 ± 1.8°C to 37.4 ± 2.1°C]), days 1–3 of the thermoneutral recovery period, and days 4–7 of the thermoneutral recovery period in growing pigs. Results are expressed as LSmeans ± SEM. The P1 average was used as a covariate. HS = heat stress; RH = relative humidity.
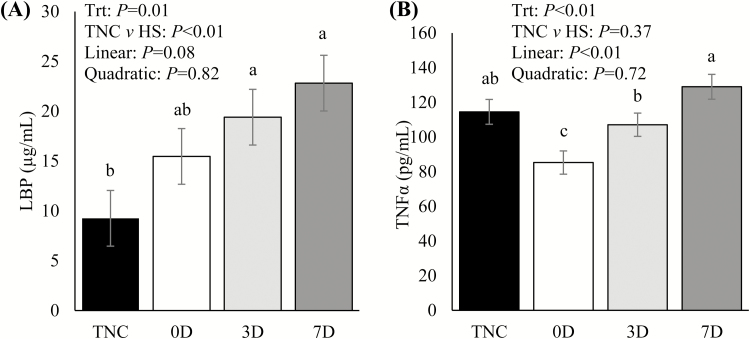
Relative to TNC pigs, circulating glucose at sacrifice was decreased in 0D and 3D pigs (10%; P = 0.03; Table 2), but was not different in 7D pigs (P = 0.17; Table 2). There were no treatment differences in circulating insulin, the insulin-to-ADFI ratio, the insulin-to-glucose ratio, or NEFA (P ≥ 0.22; Table 2). However, a weak tendency for a linear decrease in the insulin-to-ADFI ratio was observed with recovery time (P = 0.11; Table 2). Circulating LBP concentrations were increased in 3D and 7D compared to TNC pigs (110 and 147%, respectively; P = 0.01; Fig. 3A) and tended to increase linearly with increasing recovery time (P = 0.08; Fig. 3A). Relative to TNC pigs, circulating TNFα was decreased in 0D pigs (29%; P < 0.01; Fig. 3B), while in 3D and 7D pigs it did not differ. Circulating TNFα increased linearly with increasing recovery time (P < 0.01; Fig. 3B).
Table 2.
Effect of recovery length from heat stress1 on circulating metabolites
| Parameter | Treatment2 | Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TNC | 0D | 3D | 7D | SEM | P | Linear3 | Quadratic3 | TNC vs. HS4 | |
| Glucose, mg/dL | 129a | 117b | 116b | 123ab | 3 | 0.03 | 0.19 | 0.37 | 0.01 |
| Insulin, µg/L | 0.17 | 0.18 | 0.17 | 0.16 | 0.03 | 0.96 | 0.61 | 0.88 | 0.93 |
| Insulin:ADFI | 11.1 | 16.6 | 11.9 | 11.4 | 2.0 | 0.22 | 0.11 | 0.34 | 0.37 |
| Insulin:glucose | 13.0 | 15.2 | 15.2 | 13.1 | 2.1 | 0.80 | 0.46 | 0.73 | 0.56 |
| NEFA, µEq/L | 66 | 62 | 69 | 63 | 5 | 0.74 | 0.96 | 0.29 | 0.73 |
NEFA = nonesterified fatty acid.
a,bMeans with different letters differ (P ≤ 0.05).
1Heat stress protocol: 3 d of cyclical heat stress (33.6 ± 1.8°C to 37.4 ± 2.1°C).
2TNC = thermoneutral control; 0D = no thermoneutral recovery; 3D = 3 d of thermoneutral recovery; 7D = 7 d of thermoneutral recovery.
3Assessed using 0D, 3D, and 7D treatments.
40D, 3D, and 7D treatments.
Figure 3.
Circulating (A) lipopolysaccharide-binding protein (LBP) and (B) tumor necrosis factor alpha (TNFα) immediately prior to sacrifice for pigs assigned to different environmental treatments (Trt): constant thermoneutral conditions (TNC), no TN recovery following 3-d HS (0D), 3-d TN recovery following 3-d HS (3D), or 7-d TN recovery following 3-d HS (7D). Results are expressed as LSmeans ± SEM. a–cMeans with different letters differ (P ≤ 0.05). HS = heat stress.
Compared to TNC counterparts, 3D pigs had increased liver weight as a percentage of BW (9%; P = 0.04; Table 3), which contributed to the quadratic effect observed (P = 0.01; Table 3). Liver moisture content tended to increase linearly with increasing recovery time (P = 0.07; Table 3). Moreover, treatments tended to differ (P = 0.07) for both spleen weight and spleen as a percentage of BW, mostly due to a ~15% decrease in 0D vs. TNC pigs (Table 3). No other treatment differences were detected for liver weight, liver fat, liver moisture, heart weight, and heart as a percentage of BW (Table 3).
Table 3.
Effect of recovery length from heat stress1 on organ measurements
| Parameter | Treatment2 | Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TNC | 0D | 3D | 7D | SEM | P | Linear3 | Quadratic3 | TNC vs. HS4 | |
| Liver | |||||||||
| Weight, g | 764 | 729 | 806 | 739 | 26 | 0.17 | 0.94 | 0.03 | 0.84 |
| % of BW | 2.38b | 2.33b | 2.59a | 2.40b | 0.07 | 0.04 | 0.63 | 0.01 | 0.41 |
| Fat, % | 1.28 | 1.46 | 1.22 | 1.30 | 0.09 | 0.28 | 0.26 | 0.12 | 0.64 |
| Moisture, % | 72.4 | 72.2 | 72.6 | 72.8 | 0.2 | 0.29 | 0.07 | 0.65 | 0.69 |
| Spleen | |||||||||
| Weight, g | 76x | 62y | 74xy | 77x | 4 | 0.07 | 0.03 | 0.30 | 0.30 |
| % of BW | 0.24x | 0.20y | 0.24xy | 0.25x | 0.01 | 0.07 | 0.02 | 0.33 | 0.55 |
| Heart | |||||||||
| Weight, g | 165 | 158 | 163 | 153 | 5 | 0.39 | 0.46 | 0.28 | 0.25 |
| % of BW | 0.51 | 0.50 | 0.52 | 0.50 | 0.02 | 0.62 | 0.77 | 0.22 | 0.71 |
a,bMeans with different letters differ (P ≤ 0.05).
x,yMeans with different letters tended to differ (P ≤ 0.10).
1Heat stress protocol: 3 d of cyclical heat stress (33.6 ± 1.8°C to 37.4 ± 2.1°C).
2TNC = thermoneutral control; 0D = no thermoneutral recovery; 3D = 3 d of thermoneutral recovery; 7D = 7 d of thermoneutral recovery.
3Assessed using 0D, 3D, and 7D treatments.
40D, 3D, and 7D treatments.
Jejunum villus height and crypt depth were decreased in 0D and 3D vs. TNC pigs (~15 and ~11%, respectively; P < 0.01) and increased linearly with progressive recovery time so that 7D were not different from TNC pigs (Table 4). Jejunum goblet cell area and mucosal surface area were reduced in HS vs. TNC pigs (21 and 15% respectively; P < 0.01; Table 4). Recovery time had a quadratic effect on jejunum goblet cell area, where 3D pigs had the lowest goblet cell area of all treatments (44% decrease relative to TNC; P < 0.01; Table 3). In the ileum, villus height, villus height–to–crypt depth ratio, and mucosal surface area were decreased in 0D vs. TNC pigs (11, 14, and 9%, respectively; P ≤ 0.05; Table 4), and increased linearly with increasing recovery time so that 3D and 7D treatments did not differ from TNC (P ≤ 0.03; Table 4). Ileum villus width was increased in 0D relative to TNC animals (16%; P = 0.01) and tended to decrease linearly with increasing recovery time so that 3D and 7D treatments were not different from TNC (P = 0.06; Table 4). Ileum goblet cell area was decreased in all HS treatments relative to TNC (30%; P < 0.01; Table 4). Colon goblet cell area was decreased in 3D and 7D pigs relative to TNC pigs (P < 0.01; 41 and 20%, respectively) and was further decreased in 3D vs. 7D pigs (27%; P < 0.01; Table 4).
Table 4.
Effect of recovery length from heat stress1 on intestinal morphology parameters
| Parameter | Treatment2 | Contrasts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TNC | 0D | 3D | 7D | SEM | P | Linear3 | Quadratic3 | TNC vs. HS4 | |
| Jejunum | |||||||||
| Villus height, µm | 579a | 481c | 509bc | 544ab | 14 | <0.01 | <0.01 | 0.97 | <0.01 |
| Villus width, µm | 177 | 189 | 178 | 198 | 7 | 0.11 | 0.28 | 0.09 | 0.17 |
| Crypt depth, µm | 466a | 415b | 412b | 449a | 11 | <0.01 | 0.02 | 0.20 | <0.01 |
| Villus height:crypt depth | 1.31 | 1.22 | 1.29 | 1.27 | 0.05 | 0.54 | 0.46 | 0.37 | 0.36 |
| Goblet cell area, %5 | 3.9a | 3.8ab | 2.2c | 3.3b | 0.2 | <0.01 | 0.19 | <0.01 | <0.01 |
| Mucosal surface area6 | 3.6a | 3.0b | 3.2b | 3.2b | 0.1 | <0.01 | 0.28 | 0.12 | <0.01 |
| Ileum | |||||||||
| Villus height, µm | 364a | 323b | 370a | 385a | 9 | <0.01 | <0.01 | 0.08 | 0.66 |
| Villus width, µm | 187b | 216a | 181b | 193b | 8 | 0.01 | 0.06 | 0.01 | 0.31 |
| Crypt depth, µm | 350 | 362 | 357 | 376 | 14 | 0.65 | 0.47 | 0.56 | 0.37 |
| Villus height:crypt depth | 1.15a | 0.99b | 1.14a | 1.14a | 0.05 | 0.05 | 0.03 | 0.15 | 0.29 |
| Goblet cell area, %5 | 6.4a | 4.6b | 4.7b | 4.2b | 0.3 | <0.01 | 0.30 | 0.43 | <0.01 |
| Mucosal surface area6 | 2.3a | 2.1b | 2.4a | 2.4a | <0.1 | <0.01 | <0.01 | 0.01 | 0.23 |
| Colon | |||||||||
| Goblet cell area, %5 | 12.1a | 12.1a | 7.1c | 9.7b | 0.5 | <0.01 | <0.01 | <0.01 | <0.01 |
a,bMeans with different letters differ (P ≤ 0.05).
1Heat stress protocol: 3 d of cyclical heat stress (33.6 ± 1.8°C to 37.4 ± 2.1°C).
2TNC = thermoneutral control; 0D = no thermoneutral recovery; 3D = 3 d of thermoneutral recovery; 7D = 7 d of thermoneutral recovery.
3Assessed using 0D, 3D, and 7D treatments.
40D, 3D, and 7D treatments.
5Goblet cell area expressed as percentage of total epithelial area.
6Mucosal surface area is expressed as an M-index as described by Kisielinski et al. (2002).
DISCUSSION
Despite improvements in farm management (e.g., barn construction, cooling strategies, diet, etc.), livestock production remains suboptimal during the warm summer months. The mechanisms governing the negative effects of HS on productivity have been intensely studied and convincing evidence indicates that a compromised intestinal barrier is in large part responsible for the unique pathophysiology of thermal stress (Baumgard and Rhoads, 2013; Leon and Bouchama, 2015). However, the biology of recovery from HS has received considerably less attention. An improved understanding of any lasting negative effects of HS and identifying strategies aimed at accelerating recuperation following a heat insult could potentially help ameliorate seasonal production losses. In the current study, our objectives were to investigate the temporal pattern of physiological, metabolic, inflammatory, and gut morphology variables at different stages of recovery following a 3-d cyclical HS event in pigs.
Pigs exposed to the HS protocol had markedly increased body thermal indices (1.3°C, 4.8°C, and 77 breaths/min for Tr, Ts, and RR, respectively; Fig. 1), indicating a substantial heat load was successfully implemented. Rectal temperature of HS pigs returned to TNC levels following HS, and while this is in contrast to several rodent models which become hypothermic following an acute and severe thermal stimulus (Wilkinson et al., 1988; Leon et al., 2005), it agrees with our previous reports in pigs (Mayorga et al., 2017). Despite no Tr differences, Ts was decreased by day 3 of recovery in HS animals relative to TNC pigs and remained decreased through 7 d of recovery (Fig. 1B) and this partially agrees with our recent report (Mayorga et al., 2017). Using just body temperature indices as a proxy of thermogenesis and thermolysis is difficult as feed intake and the ostensible thermic effect of feed intake was decreased in previously heat-stressed pigs. Regardless, rationale for why Ts but not Tr decrease below TNC levels during recovery from HS are not entirely clear, but suggest that peripheral vasoconstriction was employed to prioritize core euthermia. The long-term consequences of dysregulated thermal homeostasis on animal productivity and health following HS are unknown.
Despite a similar BW and age, there were numerical differences in P1 feed consumption, but these initial differences were covariately adjusted for within the statistical model. During HS, the magnitude of reduced feed intake was comparable to our previous experiments (Sanz-Fernandez et al., 2015a; Mayorga et al., 2017; Seibert et al., 2018). Reducing feed intake during HS is a highly conserved response and presumably represents an attempt to minimize metabolic heat production (Quiniou et al., 2000). Similar to our earlier observation (Mayorga et al., 2017), feed intake remained depressed (~15%) in pigs previously exposed to HS and the hypophagia lasted the entire 7 d of recovery. Reasons why the pattern of feed intake differed between the 3D and 7D pigs during the recovery phase is not known, as both experienced an identical heat load during P2. Regardless, utilizing a pair-feeding model, Mayorga et al. (2017) observed that feed intake during recovery was increased in pair-fed animals, however, remained decreased in HS animals relative to their respective pre-insult levels. Determining underlying mechanisms leading to decreased feed intake were beyond the scope of this investigation; however, speculatively, the post-HS inflammatory state (discussed below) is a likely contributor, as immune stimulation-induced anorexia is a highly conserved response (Scrimshaw, 1991). Regardless of why and how, the slow return to eutrophia has critical repercussions to on-farm economic variables.
Heat stress is a hypercatabolic state (e.g. increase in catabolic hormones and loss of BW), yet we and others have previously demonstrated increased insulin (an acute and potent anabolic signal) and decreased adipose tissue mobilization during HS (Baumgard and Rhoads, 2013; Pearce et al., 2013a; Sanz-Fernandez et al., 2015b). In the current study, circulating glucose was decreased in 0D pigs, and this agrees with previous reports (Becker et al., 1992; Sanz-Fernandez et al., 2015a, 2015b). Interestingly, mild hypoglycemia remained following 3 d of recovery in TN conditions. However, circulating glucose returned to TNC levels following 7 d of recovery. Exact reasons for the aforementioned temporal pattern in blood glucose are not clear, but are likely explained by a combination of reduced intestinally absorbed glucose (stemming from reduced feed intake) and leukocyte glucose utilization. We have recently demonstrated that immunoactivation copiously utilizes glucose (Kvidera et al., 2017a, 2017b) and HS-induced leaky gut is the assumed origin of inflammation (Baumgard and Rhoads, 2013; Leon and Bouchama, 2015).
Despite marked reductions in nutrient intake, circulating NEFA, insulin, and the insulin-to-glucose ratio were similar in pigs from all environmental protocols (Table 2). This is in contrast to our previous report, which observed increased circulating insulin during HS recovery (Mayorga et al., 2017). This discrepancy is likely due to increased frequency of blood sampling by Mayorga et al. (2017). In the current study, post-hoc analysis revealed the insulin:ADFI tended to be increased (~48%) for 0D pigs compared to both TN and 7D pigs. The increased ratio demonstrates 0D pigs were presumably secreting more insulin per unit of feed consumed and this agrees with previous studies in pigs (Pearce et al., 2013a; Sanz-Fernandez et al., 2015b) and other species (Baumgard and Rhoads, 2013). Increased circulating insulin during HS is difficult to explain from an energetics standpoint, but insulin secretion is stimulated either directly or indirectly by lipopolysaccharide (LPS; Baumgard et al., 2016). We and others have demonstrated that HS-induced leaky gut increases circulating LPS (Hall et al., 2001; Lambert et al., 2002; Pearce et al., 2013b) and i.v. infusing LPS acutely and markedly increases circulating insulin in both pigs and ruminants (Kvidera et al., 2017a, 2017b). Furthermore, insulin regulates glucose uptake by activated immune cells and therefore immune cell development and function (Walrand, 2004). Thus, HS-induced hyperinsulinemia appears to be a strategy to ensure optimal fuel uptake by LPS-activated leukocytes.
The gut mucosal barrier is responsible for nutrient and water absorption while simultaneously preventing endotoxin and bacterial translocation. Intact intestinal morphological structure is important for nutrient utilization and absorption, as longer villi are indicators of a healthy gut (Langhout et al. 1999; Yasar and Forbes 1999; Hou et al., 2012; Yang et al., 2014). Furthermore, there is a positive correlation between longer villi height and pig production performance (Zijlstra et al., 1996; Mekbungwan and Yamauchi, 2004). Peripheral vasodilation is a conserved HS response, however, it comes at the expense of blood delivery to splanchnic tissues. Reduced nutrient and oxygen supply causes intestinal epithelial hypoxia, which alters intestinal morphology, impairs intestinal nutrient absorptive potential, and increases intestinal permeability (Lambert et al., 2002; Yan et al., 2006). Consequently, intestinal morphological changes such as shortened villi can be cautiously used as a proxy of increased permeability and endotoxin (i.e., LPS) infiltration (Hall et al., 2001; Xu et al., 2003; Yu et al., 2010; Hou et al., 2012; Ashraf et al., 2013). Accordingly, the present results demonstrate decreased villus height in both jejunum and ileum for the 0D pigs in comparison with their TN counterparts. However, ileum villus height recovered in the 3D and jejunum villi returned to TNC levels in the 7D pigs. Reasons why different segments of the small intestine responded (i.e., “healed”) differently to HS are not clear, but the current results complement a recent report indicating there are regional differences within the small intestine in response to HS in poultry (Varasteh et al., 2015). Furthermore, the temporal pattern of intestinal morphological changes in our study agrees with a previous report suggesting approximately 6 d are necessary for intestinal recovery from HS (Liu et al., 2009). The relatively quick recovery is not surprising as the half-life of intestinal epithelial cells is approximately 3 d (Karam, 1999) and are rapidly repaired following injury (Blikslager et al., 2007; Grootjans et al., 2011). In fact, intestinal epithelial cells have been reported to return to their baseline integrity by ~ 3 h after ischemia or HS injury (Grootjans et al., 2011; Oliver et al., 2012). Heat stress also decreases enterocyte proliferation, which contributes to shorter villi and a reduced mucosal surface area (Sandikci et al., 2004). In the present experiment, HS pigs without a TN recovery had decreased jejunum and ileum mucosal surface area. The decreased mucosal surface area remained evident in the jejunum throughout the recovery period, but the ileum mucosal surface area recovered to TN levels within 3D. It appears the gastrointestinal tract recuperates quickly (particularly the ileum) following heat damage, and continued or residual intestinal barrier dysfunction after 3 d of recovery is likely not responsible for inadequate appetite during recovery.
In addition to intestinal epithelial cell integrity, goblet cells are involved in protecting and maintaining a healthy intestinal mucosa (Masuda et al., 2003; Deng et al., 2012) by creating a mucus barrier (Matovelo et al., 1989; Smirnova et al., 2003). In the current study, pigs not allowed to recover from HS had similar goblet cell area to TNC pigs in both jejunum and colon; however, goblet cell area decreased in both regions with 3 and 7 d of recovery. In the ileum, goblet cell area was decreased independent of recovery length. Unfortunately, the effects of HS on intestinal goblet cell dynamics is inconsistent, as some report both increased and decreased goblet cell parameters in response to HS (Deng et al., 2012; Ashraf et al., 2013; Pearce et al., 2015), and these discordant reports may be due to differences in species, intestinal segment, or length and severity of heating protocols.
Lipopolysaccharide infiltration into portal and systemic circulation is recognized by immune cells, eliciting the transcription and production of inflammatory cytokines and hepatic acute phase protein (APP) synthesis (Ceciliani et al., 2012). Acute phase proteins play a major role during inflammation (Gabay and Kushner, 1999; Petersen et al., 2004) and are involved in pathogen opsonization, toxic substance removal, and regulating the immune system response to inflammation (Ceciliani et al., 2012). Lipopolysaccharide-binding protein is an important APP produced in response to endotoxin infiltration and is partially responsible for recognizing and clearing LPS via the Toll-like receptor 4 (Vreugdenhil et al., 2003; Lu et al., 2008; Schumann, 2011). In the current study, circulating LBP increased (67%) in 0D vs. TNC pigs, and tended to linearly increase with advancing recovery time. By day 7 post HS, LBP was increased 148% relative to TNC pigs. Interestingly, TNFα was decreased (29%) in 0D relative to TNC pigs, increased linearly with recovery and returned to TNC levels by 3 d post-HS; a pattern corroborating previous reports indicating suppressed circulating cytokines during HS in pigs (Pearce et al., 2013c, 2015). Collectively, it appears the systemic inflammatory response occurs following the thermal injury (Bouchama and Knochel, 2002; Leon, 2007) and circulating heat shock proteins (HSP) may be responsible for this unique pattern. During a heat load, HSPs are increased within hours and act as molecular chaperones or protein stabilizers (Horowitz, 2002), but they also inhibit proinflammatory cytokines synthesis (Chen et al., 2006). Therefore, the linear increase in both LBP and TNFα with extended recovery may be due to gradual removal of HSP following HS resolution and consequent abolition of their anti-inflammatory signaling.
CONCLUSION
Our experiment investigated the temporal recovery pattern of HS-induced changes in metabolism, intestinal morphology, and systemic inflammation. Interestingly, thermal homeostasis remained altered (primarily characterized by reduced Ts) during the entire recovery. Furthermore, feed intake remained reduced during HS recovery. Heat stress negatively affected intestinal villi morphology, and the ileum appeared to recover more quickly than the jejunum. In pigs not allowed to recover from HS (0D), circulating TNFα was suppressed and LBP was increased, and both circulating inflammatory parameters increased linearly with advancing recovery time. These data suggest HS has profound and lasting effects on pre- and post-absorptive systems. In particular, the lack of full-appetite recovery (likely caused by post-HS inflammation) from HS is concerning as it has large implications to important on-farm economic variables. Furthermore, it appears intestinal repair and inflammation remain active for at least a week following an intense heat load and both have obvious energetic costs, which deleteriously affects productivity as well as the likelihood of impaired absorptive capacity. Thus, identifying strategies aimed at accelerating recuperation following HS could potentially help ameliorate seasonal production losses.
Footnotes
This work was supported by Agriculture and Food Research Initiative Competitive Grants no. 2011-67003-30007 and 2014-67015-21627 from the USDA National Institute of Food and Agriculture and the Iowa Pork Producers Association.
LITERATURE CITED
- AOAC. 2000. Official methods of analysis. 17th ed. Assoc. Off. Anal. Chem. Int., Gaithersburg, MD. [Google Scholar]
- Ashraf S., Zaneb H., Yousaf M. S., Ijaz A., Sohail M. U., Muti S., Usman M. M., Ijaz S., and Rehman H.. 2013. Effect of dietary supplementation of prebiotics and probiotics on intestinal microarchitecture in broilers reared under cyclic heat stress. J. Anim. Physiol. Anim. Nutr. (Berl). 97(Suppl 1):68–73. doi: 10.1111/jpn.12041 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., Hausman G. J., and Sanz Fernandez M. V.. 2016. Insulin: pancreatic secretion and adipocyte regulation. Domest. Anim. Endocrinol. 54:76–84. doi:10.1016/j/domaniend.5015.07.001 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Becker B. A., Knight C. D., Buonomo F. C., Jesse G. W., Hedrick H. B., and Baile C. A.. 1992. Effect of a hot environment on performance, carcass characteristics, and blood hormones and metabolites of pigs treated with porcine somatotropin. J. Anim. Sci. 70:2732–2740. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A. N., and Hussain F.. 1974. Intake and utilization of nutrients in sheep fed different levels of roughage under heat stress. J. Anim. Sci. 38:877–886. [DOI] [PubMed] [Google Scholar]
- Blikslager A. T., Moeser A. J., Gookin J. L., Jones S. L., and Odle J.. 2007. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 87:545–564. doi: 10.1152/physrev.00012.2006 [DOI] [PubMed] [Google Scholar]
- Bouchama A., and Knochel J. P.. 2002. Heat stroke. N. Engl. J. Med. 346:1978–1988. doi: 10.1056/NEJMra011089 [DOI] [PubMed] [Google Scholar]
- Ceciliani F., Ceron J. J., Eckersall P. D., and Sauerwein H.. 2012. Acute phase proteins in ruminants. J. Proteomics. 75:4207–4231. doi: 10.1016/j.jprot.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Chen H., Wu Y., Zhang Y., Jin L., Luo L., Xue B., Lu C., Zhang X., and Yin Z.. 2006. Hsp70 inhibits lipopolysaccharide-induced NF-kappab activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 580:3145–3152. doi: 10.1016/j.febslet.2006.04.066 [DOI] [PubMed] [Google Scholar]
- Christon R. 1988. The effect of tropical ambient temperature on growth and metabolism in pigs. J. Anim. Sci. 66:3112–3123. [DOI] [PubMed] [Google Scholar]
- Crandall C. G., and González-Alonso J.. 2010. Cardiovascular function in the heat-stressed human. Acta Physiol. (Oxf). 199:407–423. doi: 10.1111/j.1748-1716.2010.02119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Dong X. F., Tong J. M., and Zhang Q.. 2012. The probiotic bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 91:575–582. doi: 10.3382/ps.2010-01293 [DOI] [PubMed] [Google Scholar]
- Gabay C., and Kushner I.. 1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340:448–454. doi: 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- Grootjans J., Thuijls G., Derikx J. P., van Dam R. M., Dejong C. H., and Buurman W. A.. 2011. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J. Pathol. 224:411–419. doi: 10.1002/path.2882 [DOI] [PubMed] [Google Scholar]
- Hall D. M., Baumgardner K. R., Oberley T. D., and Gisolfi C. V.. 1999. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 276(5 Pt 1):G1195–G1203. [DOI] [PubMed] [Google Scholar]
- Hall D. M., Buettner G. R., Oberley L. W., Xu L., Matthes R. D., and Gisolfi C. V.. 2001. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509 [DOI] [PubMed] [Google Scholar]
- Horowitz M. 2002. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 131:475–483. [DOI] [PubMed] [Google Scholar]
- Hou Y., Wang L., Zhang W., Yang Z., Ding B., Zhu H., Liu Y., Qiu Y., Yin Y., and Wu G.. 2012. Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids. 43:1233–1242. doi: 10.1007/s00726-011-1191-9 [DOI] [PubMed] [Google Scholar]
- Kadzere C. T., Murphy M. R., Silanikove N., and Maltz E.. 2002. Heat stress in lactating dairy cows: a review. Lives. Prod. Sci. 77:59–91. [Google Scholar]
- Karam S. M. 1999. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 4:D286–D298. [DOI] [PubMed] [Google Scholar]
- Kisielinski K., Willis S., Prescher A., Klosterhalfen B., and Schumpelick V.. 2002. A simple new method to calculate small intestine absorptive surface in the rat. Clin. Exp. Med. 2:131–135. doi:10.1007/s102380200018 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Fernandez M. V., and Baumgard L. H.. 2017a. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:2360–2374. doi: 10.3168/jds.2016-12001 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Horst E. A., Mayorga E. J., Sanz-Fernandez M. V., Abuajamieh M., and Baumgard L. H.. 2017b. Estimating glucose requirements of an activated immune system in growing pigs. J. Anim. Sci. 95:5020–5029. doi: 10.2527/jas2017.1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., Abuajamieh M., Sanz-Fernandez M. V., Seibert J. T., Stoakes S. K., Nteeba J., Keating A. F., Ross J. W., Rhoads R. P. and Baumgard L. H.. 2015. Thermal stress alters post-absorptive metabolism during pre- and postnatal development. In: Veerasamy S., Gaughan J., Baumgard L. and Prasad C. editors. Chapter 5. In climate change impact on livestock: adaptation and mitigation. Springer Verlag, Berlin Heidelberg: p. 61–79. [Google Scholar]
- Lambert G. P., Gisolfi C. V., Berg D. J., Moseley P. L., Oberley L. W., and Kregel K. C.. 2002. Selected contribution: hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. (1985). 92:1750–1761; discussion 1749. doi: 10.1152/japplphysiol.00787.2001 [DOI] [PubMed] [Google Scholar]
- Langhout D. J., Schutte J. B., Van Leeuwen P., Wiebenga J., and Tamminga S.. 1999. Effect of dietary high- and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Br. Poult. Sci. 40:340–347. doi: 10.1080/00071669987421 [DOI] [PubMed] [Google Scholar]
- Leon L. R. 2007. Heat stroke and cytokines. Prog. Brain. Res. 162:481–524. doi:10.1016/S0079-6123(06)62024-4 [DOI] [PubMed] [Google Scholar]
- Leon L. R., and Bouchama A.. 2015. Heat stroke. Compr. Physiol. 5:611–647. doi: 10.1002/cphy.c140017 [DOI] [PubMed] [Google Scholar]
- Leon L. R., DuBose D. A., and Mason C. W.. 2005. Heat stress induces a biphasic thermoregulatory response in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288:R197–R204. doi: 10.1152/ajpregu.00046.2004 [DOI] [PubMed] [Google Scholar]
- Liu F., Yin J., Du M., Yan P., Xu J., Zhu X., and Yu J.. 2009. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling. J. Anim. Sci. 87:1941–1949. doi: 10.2527/jas.2008-1624 [DOI] [PubMed] [Google Scholar]
- Lu Y. C., Yeh W. C., and Ohashi P. S.. 2008. LPS/TLR4 signal transduction pathway. Cytokine. 42:145–151. doi: 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Masuda K., Ikeda H., Kasai K., Fukuzawa Y., Nishimaki H., Takeo T., and Itoh G.. 2003. Diversity of restitution after deoxycholic acid-induced small intestinal mucosal injury in the rat. Dig. Dis. Sci. 48:2108–2115. doi:10.1023/A:1026295014525 [DOI] [PubMed] [Google Scholar]
- Matovelo J. A., Sund R. B., and Landsverk T.. 1989. Morphological and functional recovery following exposure to deoxycholic acid. A study in the rat small intestine in vivo. Apmis. 97:798–810. [DOI] [PubMed] [Google Scholar]
- Mayorga E. J., Kvidera S. K., Horst E. A., Al-Qaisi M., Dickson M. J., Seibert J. T., Lei S., Rambo Z. J., Wilson M. E., and Baumgard L. H.. 2017. Effects of zinc amino acid complex on biomarkers of gut integrity and metabolism during heat stress and a following recovery period in growing pigs. J. Anim. Sci. 95(E-Suppl. 2):325. (Abstr.) doi: 10.2527/asasmw.2017.325 [DOI] [Google Scholar]
- Mekbungwan A., and Yamauchi K.. 2004. Growth performance and histological intestinal alterations in piglets fed dietary raw and heated pigeon pea seed meal. Histol. Histopathol. 19:381–389. doi: 10.14670/HH-19.381 [DOI] [PubMed] [Google Scholar]
- National Research Council 2012. Nutrient requirements of swine. 11th, Revised ed The National Academies Press, Washington, DC. [Google Scholar]
- Oliver S. R., Phillips N. A., Novosad V. L., Bakos M. P., Talbert E. E., and Clanton T. L.. 2012. Hyperthermia induces injury to the intestinal mucosa in the mouse: evidence for an oxidative stress mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302:R845–R853. doi: 10.1152/ajpregu.00595.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H.. 2013a. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91:2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Boddicker R. L., Johnson J. S., Weber T. E., Ross J. W., Rhoads R. P., Baumgard L. H., and Gabler N. K.. 2013b. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. Plos One. 8:e70215. doi: 10.1371/journal.pone.0070215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Weber T. E., Rhoads R. P., Patience J. F., Baumgard L. H., and Gabler N. K.. 2013c. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi: 10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Sanz Fernandez M. V., Torrison J., Wilson M. E., Baumgard L. H., and Gabler N. K.. 2015. Dietary organic zinc attenuates heat stress-induced changes in pig intestinal integrity and metabolism. J. Anim. Sci. 93:4702–4713. doi: 10.2527/jas.2015-9018 [DOI] [PubMed] [Google Scholar]
- Petersen H. H., Nielsen J. P., and Heegaard P. M.. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 35:163–187. doi: 10.1051/vetres:2004002 [DOI] [PubMed] [Google Scholar]
- Quiniou N., Dubois S., and Noblet J.. 2000. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 63:245–253. doi: 10.1016/S0301-6226(99)00135-9 [DOI] [Google Scholar]
- Ross J. W., Hale B. J., Seibert J. T., Romoser M. R., Adur M. K., Keating A. F., and Baumgard L. H.. 2017. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 84:934–945. doi: 10.1002/mrd.22859 [DOI] [PubMed] [Google Scholar]
- Sandikci M., Eren U., Onol A. G., and Kum S.. 2004. The effect of heat stress and the use of Saccharomyces cerevisiae or (and) bacitracin zinc against heat stress on the intestinal mucosa in quails. Revue. Vet Med. 155:552–556. [Google Scholar]
- Sanz-Fernandez M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Isom S. C.,. et al. 2015a. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi:10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Fernandez M. V., Stoakes S. K., Abuajamieh M., Seibert J. T., Johnson J. S., Horst E. A., Rhoads R. P., and Baumgard L. H.. 2015b. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 3:e12478. doi:10.14814/phy2.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann R. R. 2011. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem. Soc. Trans. 39:989–993. doi: 10.1042/BST0390989 [DOI] [PubMed] [Google Scholar]
- Scrimshaw N. S. 1991. Rhoades lecture. Effect of infection on nutrient requirements. JPEN. J. Parenter. Enteral Nutr. 15:589–600. doi: 10.1177/0148607191015006589 [DOI] [PubMed] [Google Scholar]
- Seibert J. T., Graves K. L., Hale B. J., Keating A. F., Baumgard L. H., and Ross J. W.. 2018. Characterizing the acute heat stress response in gilts: I. Thermoregulatory and production variables. J. Anim. Sci. 96:941–949. doi: 10.1093/jas/skx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova M. G., Guo L., Birchall J. P., and Pearson J. P.. 2003. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell. Immunol. 221:42–49. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., and Fink-Gremmels J.. 2015. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. Plos One. 10:e0138975. doi: 10.1371/journal.pone.0138975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil A. C., Rousseau C. H., Hartung T., Greve J. W., van ‘t Veer C., and Buurman W. A.. 2003. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immunol. 170:1399–1405. [DOI] [PubMed] [Google Scholar]
- Walrand S., Guillet C., Boirie Y., and Vasson M. P.. 2004. In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions. J. Leukoc. Biol. 76:1104–1110. doi: 10.1189/jlb.0104050 [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., Burholt D. R., and Shrivastava P. N.. 1988. Hypothermia following whole-body heating of mice: effect of heating time and temperature. Int. J. Hyperthermia. 4:171–182. [DOI] [PubMed] [Google Scholar]
- Xu Z. R., Hu C. H., Xia M. S., Zhan X. A., and Wang M. Q.. 2003. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 82:1030–1036. doi: 10.1093/ps/82.6.1030 [DOI] [PubMed] [Google Scholar]
- Yan Y. E., Zhao Y. Q., Wang H., and Fan M.. 2006. Pathophysiological factors underlying heatstroke. Med. Hypotheses. 67:609–617. doi: 10.1016/j.mehy.2005.12.048 [DOI] [PubMed] [Google Scholar]
- Yang K. M., Jiang Z. Y., Zheng C. T., Wang L., and Yang X. F.. 2014. Effect of lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 92:1496–1503. doi: 10.2527/jas.2013-6619 [DOI] [PubMed] [Google Scholar]
- Yasar S., and Forbes J. M.. 1999. Performance and gastro-intestinal response of broiler chickens fed on cereal grain-based foods soaked in water. Br. Poult. Sci. 40:65–76. doi: 10.1080/00071669987854 [DOI] [PubMed] [Google Scholar]
- Yu J., Yin P., Liu F., Cheng G., Guo K., Lu A., Zhu X., Luan W., and Xu J.. 2010. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 156:119–128. doi: 10.1016/j.cbpa.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Zijlstra R. T., Whang K. Y., Easter R. A., and Odle J.. 1996. Effect of feeding a milk replacer to early-weaned pigs on growth, body composition, and small intestinal morphology, compared with suckled littermates. J. Anim. Sci. 74:2948–2959. [DOI] [PubMed] [Google Scholar]