Abstract
Three ruminally fistulated Xuanhan steers weighting 312.5 (±23.85) kg were used to determine the kinetics of ruminal degradation of nutrients using in situ nylon bag technique, and a modified 3-step in vitro procedure was adopted to estimate intestinal digestibility of 16-h rumen undegradable protein (RUP) of maize cob (MC), distillers grains (DG), spent mushroom substrate (SMS), starch residue of sweet potato (SRSP), citrus pulp (CPP), and rice straw (RS). Samples were incubated for 0, 2, 6, 16, 24, 36, 48 and 72 h. Additional samples were incubated for 16 h in the rumen, and the residues from these bags were transferred to the nitrogen-free polyester bags for determination of intestinal digestibility in vitro. The highest DM disappearance at 6-h incubation was in SRSP (P < 0.01), and that at 36, 48, and 72 h was in CPP (P < 0.01). The lowest DM disappearance at 2- and 6-h incubation was in RS and SMS (P < 0.01), and that at 36, 48, and 72 h incubation was in RS, MC, and DG (P < 0.01). The lowest and greatest CP disappearance was in RS and DG, respectively, at all the incubation times (P < 0.01). There was no difference (P > 0.07) on CP disappearance between DG and MC at all the time points except for 16 and 24 h. NDF and ADF disappearance for SRSP was significantly higher (P < 0.01) than other roughages at all the time points except for ADF at 72 h. The lowest NDF and ADF disappearance was in DG at all the time points (P < 0.01) except 2 and 6 h. The effective degradability (ED) of DM was the highest in CPP (P < 0.01) and the lowest in MC and RS (P < 0.01). The highest and lowest ED of CP was in DG and in RS (P < 0.01), respectively. The ED of NDF was the highest in SRSP (P < 0.01), followed by CPP and RS, and the lowest in DG (P < 0.01). The ED of ADF was the highest in SRSP and CPP (P < 0.05), and the lowest in DG (P < 0.01). For MC, DG SMS, SRSP, CPP, and RS, the intestinal digestibility of RUP was 95.28%, 37.23%, 38.72%, 48.06%, 54.49%, and 37.88%, respectively, and the content of intestinal digestible crude protein (IDCP) was 23.65, 83.63, 35.63, 15.03, 25.60, and 12.03 g/kg, respectively. Distillers grain was considered to be of good quality for the greatest content of IDCP. Although not readily degraded in rumen, CP in MC may be digested well in small intestine.
Keywords: beef cattle, intestinal digestibility, roughages, rumen degradability
INTRODUCTION
Roughage was the main source of nutrients for ruminants, accounting for 40% to 80% of ruminant diets. The rumen degradable characteristics and intestinal digestibility of CP in roughage were important indexes in appraising nutritive values. When the amount of rumen undegradable protein (RUP) in feeds is known, diets can be formulated to maximize ruminal microbial protein synthesis without limiting available N, which is important because ruminal microbial protein provides a near optimal balance of essential AA for protein synthesis (Schingoethe, 1996). RUP is used by host animal in small intestine. Owing to the lack of empirical data, NRC (1989) suggested a constant intestinal digestion of RUP of 80% for all feeds, although it was recognized that the value differs among feeds. Obviously using the constant value of intestinal digestibility of RUP will lead to the wrong prediction of nutrient needs. Therefore, intestinal digestibility of RUP has become an important variable in recent protein evaluation systems for ruminants (Hvelplund and Nørgaard, 2003).
Many researchers have studied rumen degradability of protein supplements in both plant and animal feeds (Erasmus et al., 1994; Cozzi et al., 1995). Using nylon bag technique, Kamalak et al. (2005) examined the DM degradability of wheat straw, barley straw, lucerne hay, and maize silage. Gargallo et al. (2006) studied intestinal digestibility of 12 feedstuffs including blood meal, fish meal, green peas, lupin seed, whole cottonseed, corn gluten meal, alfalfa pellets, heat-processed SBM, sunflower seeds, barley-dried distillers grains, corn-dried distillers grains (DG), and corn gluten feed. Calsamiglia et al. (1995) studied intestinal digestibility of soybean meal, blood meal, hydrolyzed feather meal, corn gluten meal, and fish meal. However, there are limited data reported in the literature on intestinal digestibility of the RUP fraction of roughage. This study aimed to estimate rumen degradation characteristics and intestinal RUP digestibility of maize cob (MC), distillers grains (DG), spent mushroom substrate (SMS), starch residue of sweet potato (SRSP), citrus pulp (CPP), and rice straw (RS), using in situ nylon bag technique and modified 3-step in vitro procedure (TSP). The outcome of the study may enrich and improve the database of nutritive value of roughages in beef cattle, which in turn contributes to the improvement of ruminant production.
MATERIALS AND METHODS
This study was conducted at the Teaching and Experiment Station in Sichuan Agricultural University, Ya’an, Sichuan, China. All animals were managed in accordance with the guidelines of the Animal Care and Ethic Committee of Sichuan Agricultural University.
Animals and Diets
Three ruminally fistulated Xuanhan steers with an average BW of 312.5 (±23.85) kg were used to evaluate ruminal degradability of nutrients. Animals were fed twice daily at 8:00 and 16:00 h, and access to water ad libitum. The composition and nutrient level of the basal diet were summarized in Table 1.
Table 1.
Ingredient and chemical composition of the basal diet fed during the in situ experiment (DM basis)
| Ingredient | Amount, % of DM |
|---|---|
| Ingredients | |
| Corn | 35.53 |
| Wheat bran | 5.13 |
| Alfalfa meal | 25.48 |
| Distilled grain | 7.06 |
| Rice straw | 25.02 |
| NaCl | 0.5 |
| NaHCO3 | 0.58 |
| Premix1 | 0. 70 |
| Total | 100.00 |
| Chemical composition2 | |
| DM | 88.14 |
| CP | 10.40 |
| Fat | 2.80 |
| Ca | 0.53 |
| P | 0.33 |
| NDF | 38.77 |
| ADF | 21.30 |
1Supplied the following per kg of diet: 2200 IU, vitamin A; 2751 IU, vitamin D3; 11 IU, vitamin E; 50 mg, Fe; 25 mg, Cu; 20 mg, Mn; 30 mg, Zn; 0.50 mg, I; 0.10 mg, Se.
2Values for the contents of dry matter, crude protein, neutral detergent fiber; acid detergent fiber; Ca and P were analyzed.
Samples Preparation
MC,DG,SMS,SRSP, CPP, and RS were collected commercially in Sichuan province. The samples were dried at 55 °C for 48 h in a forced-air oven and then milled through a 1-mm sieve for chemical analysis and 3-mm sieve for in situ degradation. DM was determined by drying the samples at 105 °C overnight. Nitrogen (N) content was measured by the Kjeldahl method (AOAC, 1990). CP was calculated as N × 6.25. NDF and ADF contents were determined by the method of Van Soest et al. (1991).
In Situ Nylon Bag Experiment
The in situ DM, CP NDF, and ADF degradation in the 6 roughages were determined according to the procedure described by Mehrez and Ørskov (1977). Five-gram samples dried and milled through a 3-mm sieve were weighed into nylon bags (45-m pore size, 8- × 16-cm bag size) in triplicate and incubated for 2, 6, 16, 24, 36, 48, and 72 h in the rumen of 3 ruminally fistulated steers. After removal of the bags at each time point, each bag was washed in running tap water until the outlet water becomes clear. Bags were then dried to a constant weight at 55 °C for 48 h and weighted. The residues were ground through 1-mm sieve for laboratory analysis.
A Modified 3-Step In Vitro Procedure
The 16-h rumen undegradable samples were obtained from the in situ nylon bag experiment and were then digested using the pepsin and pancreatin digestion steps of the modified TSP described by Gargallo et al. (2006). The 16-h RUR samples were ground to pass a 1-mm screen. One gram of RUP sample was weighed into nitrogen-free polyester bags (3.5 × 5.5 cm, pore size 50 ± 15 cm). The samples were then incubated in a pepsin/HCI solution (pH = 1.9) for 1 h in an incubator (Ankom Technology) and then incubated in a pancreatin/KH2PO4 solution at 38 °C for 24 h. After 24-h incubation, the liquid was drained from bottles, and nylon bags were washed in running tap water until the outlet water becomes clear. Bags were dried in a forced hot air oven at 55 °C for 48 h. The weight of samples and bags was recorded, and bags were then opened and samples for laboratory analysis. Digestibility of 16-h RUP was calculated based on the RUP remaining in the bags and that disappearing from the bags.
Calculation and Statistical Analyses
The degradation data were fitted to the following exponential equation:
| (1) |
where y is the nutrient disappearance in rumen at time t, a is the the rapidly soluble fraction, b is the the slowly soluble fraction, and c is the the constant rate of degradation of b (%/h).
Effective degradability (ED) of nutrients was calculated applying the following equation of Ørskov and McDonald (1979):
| (2) |
where a, b, and c are the same as in equation (1) and k is the rumen outflow rate. The ED of the nutritions was calculated using the outflow rate of 0.031/h at the maintenance level, according to Ørskov et al. (1988).
Pepsin-pancreatin digestion of CP in the 16-h undegraded residuals was calculated as follows:
The intestinal digestible crude protein (IDCP) content of the original feeds can be calculated from ED (IDCPED) and 16-h RUP (IDCP16-h RUP) as follows:
The coefficient of ruminal degraded protein to microbial protein (MCP) was 0.85 (NRC, 2001), and intestinal digestibility of MCP was 0.775 (Storm, 1983).
Analysis of variance (ANOVA) was carried out for chemical composition disappearance and estimated parameters using General Linear Model (GLM) procedure of SAS. Significant differences between the intestinal digestible of samples were identified using Tukey’s Multiple Range Test (Pearson et al. (1966)). Mean differences were considered significant at P < 0.05. Standard errors of means were calculated from the residual mean square in the analysis of variance.
RESULTS
Chemical Composition
Chemical compositions of the 6 roughages are presented in Table 2. DM content did not differ among the roughages (P = 0.668). CP content was the greatest in DG followed by SMS (P < 0.01). CP content in SRSP was the same as in RS (P = 0.686), lower than in other roughages (P < 0.01). NDF content did not differ between MC and RS (P = 0.181), greater than in other roughages (P < 0.01). NDF content was the same in CPP as in SRSP (P = 0.315), lower than in other roughages (P < 0.05). There were no differences on ADF content among MC, RS, and SMS (P = 0.892), and among CPP, DG, and SRSP (P = 0.533).
Table 2.
Chemical compositions of the 6 roughages
| Roughages | ||||||||
|---|---|---|---|---|---|---|---|---|
| Content (%) | MC | DG | SMS | SRSP | CPP | RS | SEM1 | P value |
| DM | 92.76 | 87.23 | 90.29 | 89.27 | 89.84 | 91.24 | 0.644 | 0.768 |
| CP (DM basis) | 3.04a | 14.20b | 7.48c | 2.69d | 4.49e | 2.73d | 1.557 | < 0.001 |
| NDF(DM basis) | 79.25a | 42.61b | 57.82c | 32.79d | 31.78d | 75.58a | 7.218 | < 0.001 |
| ADF(DM basis) | 45.80a | 34.65b | 45.51a | 27.19b | 27.23b | 45.71a | 3.163 | < 0.001 |
a-eWithin a row, means with uncommon superscripts differ (P < 0.05).
1SEM = standard error of the mean.
The Disappearance Rates of Nutrients
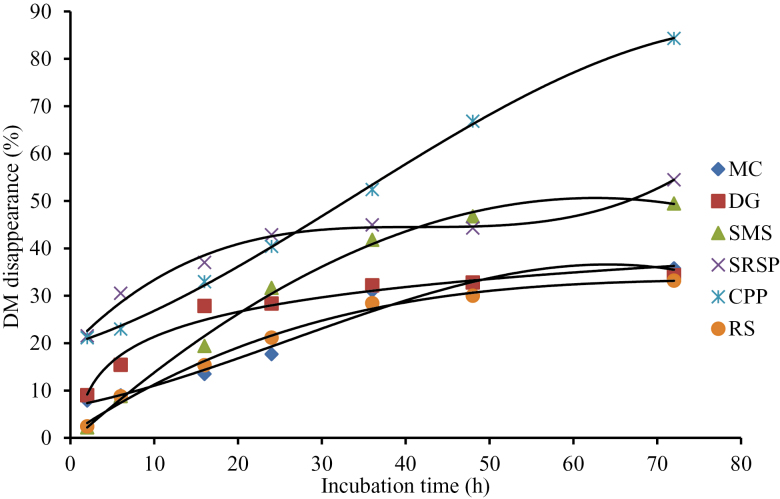
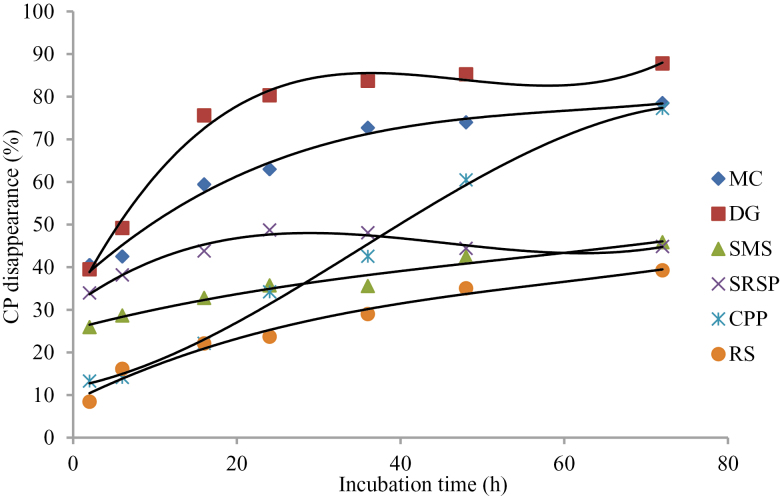
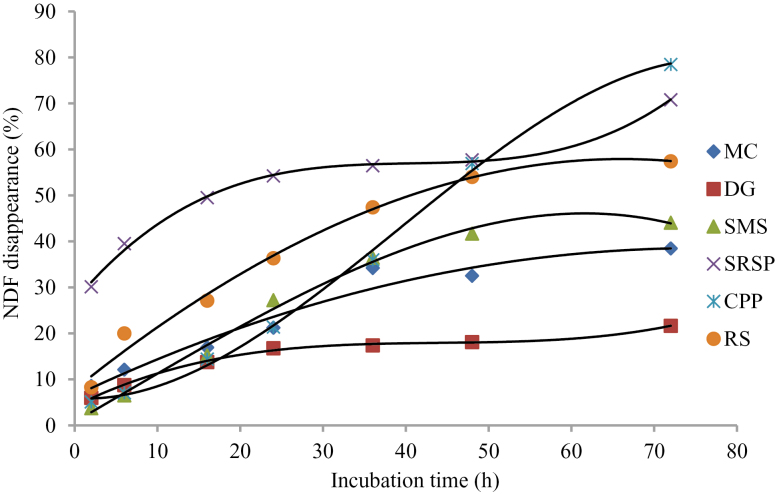
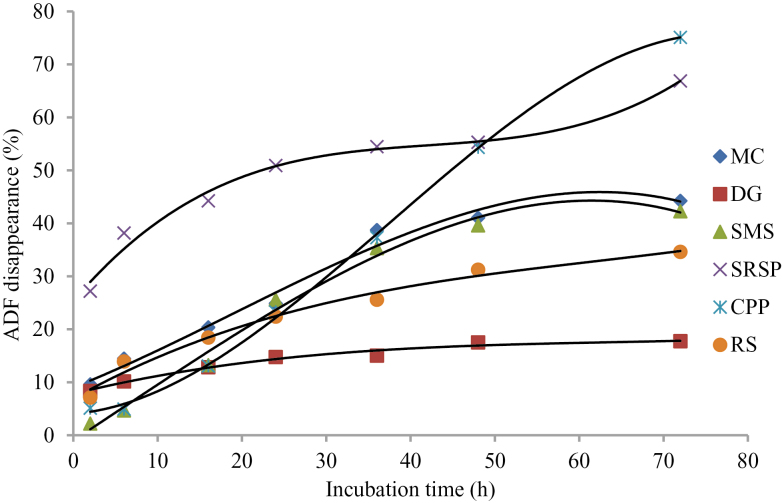
Nutrient disappearance at different incubation time is presented in Table 3. The highest DM disappearance was in SRSP (P < 0.01) at 2 and 6 h, and in CPP (P < 0.01) at 48 and 72 h. There was no difference on DM disappearance from 6- to 72-h (except for 24-h) incubation between MC and RS (P > 0.11), which was significantly lower (P < 0.001) than that in SMS, SRSP, and CPP. CP disappearance in MC was the same as (P > 0.07) that in DG at all the time point except for 16 and 24 h, higher (P < 0.01) than that in all the other roughages, and that in RS was the lowest (P < 0.01) at all the time points. NDF disappearance was the highest in SRSP (P < 0.01) at all the time points. NDF disappearance was the lowest (P < 0.01) in SMS at 2 and 6 h, and in DG after 16 h. ADF disappearance was the highest (P < 0.01) in SRSP at all the time points (P < 0.01) and the lowest in DG from 24 to 72 h.
Table 3.
In situ nutrient disappearance of 6 roughages at different incubation times
| Incubation time (h) | Roughages | |||||||
|---|---|---|---|---|---|---|---|---|
| MC | DG | SMS | SRSP | CPP | RS | SEM1 | P-value | |
| DM | ||||||||
| 2 | 7.89a | 9.03a | 2.24b | 21.55c | 21.16c | 2.45b | 2.998 | < 0.001 |
| 6 | 9.00a | 15.43b | 8.83a | 30.53c | 22.99d | 8.79a | 3.135 | < 0.001 |
| 16 | 13.5a | 27.86b | 19.43c3 | 7.04d | 32.97d | 15.36a | 3.347 | < 0.001 |
| 24 | 17.68a | 28.37b | 31.67b | 42.87c | 40.44c | 21.17d | 3.481 | < 0.001 |
| 36 | 31.06a | 32.21a | 41. 83b | 44.96bc | 52.41d | 28.44a | 3.246 | < 0.001 |
| 48 | 30.31a | 32.81a | 46.77b | 44.32b | 66.84c | 30.03a | 4.902 | < 0.001 |
| 72 | 35.8a | 34.35a | 49.49b | 54.49b | 84.31c | 33.21a | 6.751 | < 0.001 |
| CP | ||||||||
| 2 | 40.52a | 39.50a | 25.96b | 33.96c | 13.30d | 8.46e | 4.680 | < 0.001 |
| 6 | 42.52ab | 49.19a | 28.69c | 38.19b | 14.14d | 16.16d | 4.937 | < 0.001 |
| 16 | 59.43a | 75.62b | 32.82c | 43.82d | 22.14e | 22.12e | 7.414 | < 0.001 |
| 24 | 63.00a | 80.35b | 35.75c | 48.75d | 34.24c | 23.71e | 7.239 | < 0.001 |
| 36 | 72.71a | 83.75a | 35.59b | 48.09c | 42.57c | 29.02d | 7.462 | < 0.001 |
| 48 | 73.99a | 85.25a | 42.44b | 44.44b | 60.50c | 35.05d | 6.815 | < 0.001 |
| 72 | 78.51a | 87.82a | 45.87b | 44.87b | 77.28a | 39.27b | 7.307 | < 0.001 |
| NDF | ||||||||
| 2 | 8.56a | 5.98b | 3.74c | 30.15d | 5.10e | 8.27a | 7.620 | < 0.001 |
| 6 | 12.12a | 8.75b | 6.53c | 39.53d | 7.08c | 20.01e | 4.381 | < 0.001 |
| 16 | 16.94a | 13.77b | 15.21ab | 49.51c | 14.41b | 27.13d | 4.821 | < 0.001 |
| 24 | 21.24a | 16.78b | 27.24c | 54.24d | 21.39a | 36.35e | 4.781 | < 0.001 |
| 36 | 34.23a | 17.40b | 36.47a | 56.47c | 36.00a | 47.42d | 4.564 | < 0.001 |
| 48 | 32.54a | 18.11b | 41.72c | 57.72d | 56.95d | 54.04d | 5.482 | < 0.001 |
| 72 | 38.46a | 21.65b | 44.09a | 70.79c | 78.47c | 57.41d | 7.319 | < 0.001 |
| ADF | ||||||||
| 2 | 9.60a | 8.35a | 2.20b | 27.20c | 5.13d | 7.11e | 3.054 | < 0.001 |
| 6 | 14.47a | 10.16b | 4.68c | 38.18d | 4.93c | 13.93a | 4.276 | < 0.001 |
| 16 | 20.37a | 12.85b | 13. 25b | 44.25c | 13.08b | 18.45a | 4.181 | < 0.001 |
| 24 | 24.85a | 14.76b | 25.61a | 50.91c | 23.27a | 22.39a | 4.263 | < 0.001 |
| 36 | 38.74a | 15.04b | 35.32a | 54.48c | 37.30a | 25.58d | 4.588 | < 0.001 |
| 48 | 41.06a | 17.52b | 39.62a | 55. 31c | 54.35c | 31.24d | 4.937 | < 0.001 |
| 72 | 44.26a | 17.76b | 42.28a | 66.88c | 75.11c | 34.62d | 7.276 | < 0.001 |
a-eWithin a row, means with uncommon superscripts differ (P < 0.05).
1SEM = standard error of the mean.
The Degradation Kinetic Parameters and ED
The degradation kinetic parameters and ED are summarized in Table 4. Parameter a was the greatest (P < 0.01) for DM, CP, NDF, and ADF in SRSP, the lowest (P < 0.01) for DM and ADF in SMS, and the lowest (P < 0.01) for CP and NDF in CPP. Parameter b was the greatest (P < 0.01) for DM, CP, NDF, and ADF in CPP, the lowest (P < 0.01) for DM, NDF, and ADF in DG, and the lowest (P < 0.01) for CP in SRSP. The greatest c for DM was in DG and SMS (P < 0.05), for CP was in DG (P < 0.01), for NDF was in SMS and RS (P < 0.05), and for ADF was in DG (P < 0.01). The lowest c for DM was in SRSP and CPP (P < 0.05), for CP was in SMS and CPP (P < 0.05), for NDF was in SRSP (P < 0.01), and for ADF was in SRSP and CPP (P < 0.05).
Table 4.
Ruminal degradation kinetics and effective degradability of nutrients of 6 roughages
| Roughage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Item | MC | DG | SMS | SRSP | CPP | RS | SEM1 | P value |
| a, % | ||||||||
| DM | 1.03a | 9.47b | −11.92c | 25.07d | 5.97e | 0.02f | 4.228 | <0.001 |
| CP | 34.89a | 39.82b | 25.02b | 33.60a | −0.56c | 8.31d | 5.584 | <0.001 |
| NDF | 3.10a | 6.76b | −10.01c | 33.70d | −14.98e | 0.03f | 5.892 | <0.001 |
| ADF | 0.00a | 5.45b | −5.90c | 30.68d | 12.43e | 5.54b | 4.375 | <0.001 |
| b, % | ||||||||
| DM | 37.88a | 25.19b | 62.58b | 37.78a | 93.56d | 35.05a | 8.702 | <0.001 |
| CP | 45.44a | 48.40b | 25.90b | 15. 56c | 00.04d | 36.51e | 10.171 | <0.001 |
| NDF | 37.97a | 17.10b | 55.47cd | 51.28c | 113.61e | 59.63df | 11.120 | <0.001 |
| ADF | 45.88a | 12.48b | 50.92a | 48.48a | 111.34c | 31.51d | 11.477 | <0.001 |
| c,h-1 | ||||||||
| DM | 0.034a | 0.60b | 0.055b | 0.020c | 0.023c | 0.041d | 0.006 | <0.001 |
| CP | 0.044a | 0.65b | 0.023cd | 0.050e | 0.020df | 0.026c | 0.006 | <0.001 |
| NDF | 0.036a | 0.027b | 0.052c | 0.017d | 0.022be | 0.049cf | 0.005 | <0.001 |
| ADF | 0.046a | 0.60b | 0.043ac | 0.018d | 0.020de | 0.35cf | 0.006 | <0.001 |
| ED, % | ||||||||
| DM | 20.90a | 26.10b | 28.15b | 39.84c | 46.02d | 20.20ae | 3.633 | <0.001 |
| CP | 61.57a | 72.65b | 36.00b | 43.22c | 38.19bc | 24.97d | 6.103 | <0.001 |
| NDF | 23.58a | 14.79b | 24.72b | 51.79c | 31.80d | 35.56d | 4.388 | <0.001 |
| ADF | 27.48a | 13.67b | 23.54ac | 48.68d | 55.83d | 22.34c | 5.706 | <0.001 |
a-eWithin a row, means with uncommon superscripts differ (P < 0.05).
1SEM = standard error of the mean.
The highest ED of DM was found in CPP (P < 0.05), followed by SRSP (P < 0.05), and the lowest was in MC and RS (P < 0.01). The ED of CP was the greatest in DG and MC (P < 0.01), and the lowest in RS (P < 0.01). There was no difference (P = 0.14) on ED of CP between SRSP and CPP. There was no difference on ED of NDF between MC and SMS (P = 0.35), and between CPP and RS (P = 0.16). The highest ED of NDF was in SRSP (P < 0.01), and the lowest in DG (P < 0.01). The highest ED of ADF was in SRSP and CPP (P < 0.05), and the lowest in DG (P < 0.01). There was no difference on ED of ADF between MC and SMS (P = 0.08), and between SMS and RS (P = 0.32).
Intestinal Digestibility of RUP and the IDCP Content of Feeds
The intestinal digestibility of RUP of the 6 roughages and the IDCP content are presented in Table 5. The intestinal digestibility of 16-h RUP of MC was the highest (P < 0.05). There was no difference on intestinal digestibility of 16-h RUP between SRSP and CPP (P = 0.12), and among DG, SMS, and RS (P > 0.55). The content of IDCP was the highest in DG (P < 0.01), followed by SMS (P < 0.01), and the lowest in RS (P < 0.05).
Table 5.
Intestinal digestibility of rumen undegradable protein (RUP) and intestinal digestible CP (IDCP) content of the 6 roughages
| Roughages | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | MC | DG | SMS | SRSP | CPP | RS | SEM1 | P value |
| CP content % | 3.04a | 14.20b | 7.48c | 2.69d | 4.49e | 2.73d | 1.557 | < 0.001 |
| ED % | 61.57a | 72.65a | 36.00b | 43.22b | 38.19b | 24.97c | 6.103 | < 0.001 |
| 16-h RDP, % | 59.43a | 75.62b | 32.82c | 43.82d | 22.14e | 22.12e | 7.414 | < 0.001 |
| Idg of RUP, % | 95.28a | 37.23b | 38.72b | 48.06c | 54.49c | 37.88b | 7.70 | < 0.001 |
| IDCP16-hRUP, g/kg | 23.65a | 83. 63b | 35.63c | 15.03d | 25.60a | 12.03e | 9.09 | < 0.001 |
| from RDP, g/kg | 12.33a | 67.96b | 17.74c | 7.66d | 11.30a | 4.49e | 8.21 | < 0.001 |
| from RUP, g/kg | 11.13a | 14.46b | 18.54c | 7.34d | 15.12b | 7.76d | 1.53 | < 0.001 |
| IDCPED, g/kg | 23.46a | 82.42b | 36.27c | 15.00d | 26.42a | 12.25e | 8.92 | < 0.001 |
| Relative error2 | −0.0082 | −0.0147 | 0.0178 | −0.0019 | 0.0311 | 0.0178 | – | – |
a-eWithin a row, means with uncommon superscripts differ (P < 0.05).
1SEM = standard error of the mean.
2Relative error = (IDCPED – IDCP16-hRUP)/IDCPED.
Distillers grains provided the vast amount of IDCP, followed by SMS, and the lowest in RS. Among the IDCP contained in DG (83.63%), 81.3% was from rumen degradable protein (RDP). IDCP content in CPP was 25.60%, 59.1% of what was from RUP.
DISCUSSION
Chemical Composition of the Roughages
Chemical composition (CP, NDF, and ADF) of most of the roughages investigated in this study was within the range of other reports (Table 6). It should be noted that CP content in DG was much lower, whereas ADF content was much greater, in this study, than those in Preston et al. (2011) and Spanghero et al. (2010), respectively. Similar circumstances were found in CPP, in which CP content was much less while ADF content was much greater than those reported by Preston et al. (2011) and Pereira et al. (2004), respectively. Chemical composition of roughages was influenced by many factors such as plant varieties, different parts of plant, harvest time, climatic conditions, soil conditions, fertilizer, and maturity level. It is possible that the different raw materials of DG lead to the great discrepancy of nutrient contents between this study and other reports. Spiehs et al. (2002) and Fastinger et al. (2006) reported that the nutritional composition and nutritional value of dried distillers grains with solubles (DDGS) differed greatly due to the different raw materials used in United States. CP and NDF content of SMS in this study was within the ranges reported by Bae et al. (2006). ADF content of SMS was lower than the results of Bae et al. (2006) and Kwak et al. (2008). The chemical composition of SMS was affected mainly by the primary culture ingredient rather than mushroom species. Different components of SMS may lead to such differences in nutrient contents. There was no other report on nutrient content of SRSP.
Table 6.
Nutritional composition of feedstuffs compared with other researches (DM basis)
| Feedstuff | CP | NDF | ADF | Researcher |
|---|---|---|---|---|
| MC | ||||
| 2.50% | – | 45.80% | Kaliyan et al. (2010) | |
| 2.60% | 78.80% | 43.70% | Spanghero et al. (2010) | |
| 3.04% | 79.25% | 45.80% | This study | |
| DG | ||||
| 28.00% | 40.00% | 18.00% | Preston et al. (2011) | |
| 28.90% | 35. 80% | 18.60% | Spanghero et al. (2010) | |
| 14.20% | 42.61% | 34.65% | This study | |
| SMS | ||||
| 7.2–11.1% | 64–78.2% | 42. 8–66.3% | Bae et al. (2006) | |
| 5.90% | 76.10% | 59.10% | Kwak et al. (2008) | |
| 7.48% | 57.82% | 45.51% | This study | |
| CPP | ||||
| 7.00% | 21.00% | 20.00% | Preston et al. (2011) | |
| 6.54% | 25.90% | 16.10% | Pereira et al. (2004) | |
| 4.49% | 31.78% | 27.23% | This study | |
| RS | ||||
| 4.00% | 72.00% | 47.00% | Preston et al. (2011) | |
| 5.60% | 76.90% | 44.60% | Eun et al. (2006) | |
| 2.73% | 75.58% | 45.71% | This study | |
Nutrient Disappearance of the Feeds
Kamalak et al. (2005) reported that the rate and extent of DM fermentation in rumen were important determinants for the nutrients absorbed by ruminants. DM disappearance of SRSP and CPP was higher than that of other feeds at all incubation times (Figure 1). DM disappearance of DG, MC, and RS tended to be overlap after 36-h incubation and lower than other roughages. These results indicated that SRSP and CPP were more digestible, and DG, MC, and RS were less digestible than other roughages. It should be noted that CP disappearance of DG and MC, especially DG, was considerably higher than other roughages (Figure 2) despite the very low DM disappearance, which illustrated that CP in DG and MC was utilized well in rumen. The low DM disappearance in DG and MC may be due to the low NDF and ADF disappearance (Figures 3 and 4) in these 2 roughages. In this study, NDF and ADF disappearance of DG was the lowest after 16-h incubation. NDF and ADF disappearances were important parameters that reflect the degree of difficulty the feed is digested.
Figure 1.
DM disappearance of 6 roughages in the rumen of Xuanhan steer.
Figure 2.
CP disappearance of 6 roughages in the rumen of Xuanhan steer.
Figure 3.
NDF disappearance of 6 roughages in the rumen of Xuanhan steer.
Figure 4.
ADF disappearance of 6 roughages in the rumen of Xuanhan steer.
The ED of the Roughages
The ED of DM of DG was 48.08% in this study. Batajoo et al. (1998) reported that the ED of DM of distillers dried grain (DDG) was 58.3% at an outflow rate of 0.07/h. The great discrepancy of ED of DM between the present study and other reports was mainly due to the various outflow rates used in different reports (Table 7). Martins et al. (1999) studied the ED of DM of CPP and found that the value was 79.8%, 67.5%, and 61.7%, when the outflow rate was 0.02, 0.05, and 0.08 per h, correspondingly. The ED of DM of DG and CPP in this study were lower than that in the reports of Batajoo et al. (1998) and Martins et al. (1999), respectively.
Table 7.
The ED of DM of feedstuffs compared with other research results
| Feedstuff | Breeds | Outflow rate | ED | Researcher |
|---|---|---|---|---|
| DDG | Holstein cow | 0.07 | 58.30% | Batajoo et al. (1998) |
| DG | Xuanhan steers | 0.031 | 48.08% | This study |
| Citrus pulp | Holstein cow | 0.02 | 79.80% | Martins et al. (1999) |
| Citrus pulp | Holstein cow | 0.05 | 67.50% | Martins et al. (1999) |
| Citrus pulp | Holstein cow | 0.08 | 61.70% | Martins et al. (1999) |
| Citrus pulp | Xuanhan steers | 0.031 | 46.02% | This study |
The RDP of DDGS was reported to be from 28% (Archibeque et al., 2008) to 44% of CP (Kelzer et al., 2010a), quite lower than the ED of CP of DG in this study. Rumen degradation characteristics of protein contained in DDGS may be influenced by fermentation material (Spiehs et al., 2002). Protein in DDGS originating from yeast may resist rumen degradation (Castillo-Lopez et al., 2010). Bateman et al. (2005) reported that dairy cattle would have a greater passage rate than steers, and therefore, different animal breeds may also contribute to the variability of ED of CP. Satter (1986) concluded that ED of CP negatively correlated to passage rate. The ED of CP of DG in this study was 83.5% higher (Table 8) than that reported by Batajoo et al. (1998). The ED of CP of DG in this study was 61.2% when the outflow rate was 0.07/h, which was still 54.5% higher than the result of Batajoo et al. (1998).
Table 8.
The ED of CP of feedstuffs compared with other research results
| Feedstuff | Variety | Out of rate | ED | Researcher |
|---|---|---|---|---|
| DDG | Holstein cow | 0.07 | 39.6% | Batajoo |
| DG | Xuanhan steer | 0.031 | 72.6% | This study |
| DG | Xuanhan steer | 0.07 | 61.2% | This study |
| DG | Holstein cow | 0.07 | 39.6% | Batajoo et al. (1998) |
| Citrus pulp | Holstein cow | 0.02 | 70.4% | Martins et al. (1999) |
| Citrus pulp | Holstein cow | 0.05 | 62.1% | Martins et al. (1999) |
| Citrus pulp | Holstein cow | 0.08 | 59.1% | Martins et al. (1999) |
| Citrus pulp | Xuanhan steer | 0.031 | 40.0% | This study |
NDF and ADF represent the fraction of feedstuffs uneasily digested by livestock. The facts that both disappearance and ED of NDF and ADF were the highest in SRSP indicated that SRSP was easily degraded in rumen and may have greater digestibility. Similarly, these observations were the lowest in DG reflecting the high level of lignification which should retard the rumen degradation and the further digestibility. But the lignification may not trap CP from microbial degradation because both disappearance and ED of CP were the highest in DG.
The Intestinal Digestibility of RUP
Hvelplund et al. (2000) reported that the intestinal digestibility determined at 16 h could apply to all feeds and reflect the metabolic situation in rumen before the feeds reached the small intestine, and researchers Calsamiglia and Stern (1995) and Promkot and Wanapat (2003) applied rumen undegraded residues from bags of 16-h ruminal incubation for estimating intestinal digestion of protein in ruminants by in vitro pepsin-pancreatin digestion. This study investigated for the first time the intestinal digestibility of RUP in MC, SMS, SRSP, CPP and RS. Negi et al. (1988) reported that the protein escaping rumen degradation was combined with lignin and therefore not easy to be digested in small intestine. The intestinal digestibility of most of the roughages investigated in this study was indeed low (less than 55%), with the exclusion of MC. The RUP intestinal digestibility of MC (95.3%) was higher than all the other roughages, illustrating the RUP in MC could be digested well in small intestine. Because of the high CP content (14.2%) and the high ED of CP (72.65%), DG was considered to be of good quality, though the intestinal digestibility of RUP was low (37.23%). IDCP includes RUP and MCP synthesized from RDP reflecting the total digestible CP reaching the small intestine. The difference between IDCP16-hRUP and IDCPED lies in the calculation of RDP (for calculating MCP production), which was based on 16-h RUP in IDCP16-hRUP and ED of CP in IDCPED. This study revealed the trivial differences between IDCP16-hRUP and IDCPED in all the roughages investigated, with the biggest relative error of less than 3.11% (Table 5). Both IDCP16-hRUP and IDCPED can be used to reflect the total digestible CP through the gastrointestinal tract of ruminants. In this study, DG provided the vast amount of IDCP, followed by SMS, and the lowest in RS (Table 5).
CONCLUSION
SRSP and CPP were easily digested in rumen, whereas DG, MC, and RS were resistant to digestion in rumen. CP in DG could be easily degraded in rumen, but RUP in DG was uneasy to be digested in intestine. CP in MC was utilized well both in rumen and in intestine. Distillers grain was considered to be of good quality for its high IDCP content.
IMPLICATION
Our results revealed that DM degradability may not synchronize with CP (or other nutrients) degradability. The digestibility of CP bypassing the rumen varied greatly with feedstuffs. This pattern indicates highly complicated nutrient utilization in rumen and in intestinal tract, which encourages researchers to make out the different portions of nutrient utilized in different parts of gastrointestinal tract for a better understanding of nutrient supply of feedstuffs. The higher rumen degradability and higher intestinal digestibility of CP in DG suggest that DG feeds the rumen microbes with more nitrogen and subsequently supplies sufficient digestible CP (both from microbial CP and from bypass CP) for host animal.
Conflict of interest statement. None declared.
Footnotes
This work was supported by the Local Innovation Team of Sichuan Agricultural Bureau, Sichuan Province, China.
LITERATURE CITED
- AOAC 1990. Official methods of analysis. 15th ed. Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- Archibeque S. L., Freetly H. C., and Ferrell C. L.. 2008. Feeding distillers grains supplements to improve amino acid nutriture of lambs consuming moderate-quality forages. J. Anim. Sci. 86:691–701. doi:10.2527/jas.2007-0139 [DOI] [PubMed] [Google Scholar]
- Bae J. S., Kim Y. I., Jung S. H., Oh Y. G., and Kwak W. S.. 2006. Evaluation on feed-nutritional value of spent mushroom (Pleurotus osteratus, Pleurotus eryngii, Flammulina velutupes substrates as a roughage source for ruminants). J. Anim. Sci. and Technol. 48:237–246. doi:10.5187/JAST. 2006. 48. 2. 237 [Google Scholar]
- Batajoo K. K., and Shaver R. D.. 1998. In situ dry matter, crude protein, and starch degradabilities of selected grains and by-product feeds. Anim. Feed Sci. and Technol. 71:165–176. doi:10.1016/S0377-8401(97)00132-6 [Google Scholar]
- Bateman H. G. 2nd, Clark J. H., and Murphy M. R.. 2005. Development of a system to predict feed protein flow to the small intestine of cattle. J. Dairy Sci. 88:282–295. doi: 10.3168/jds.S0022-0302(05)72686-2 [DOI] [PubMed] [Google Scholar]
- Calsamiglia S., and Stern M. D.. 1995. A three-step in vitro procedure for estimating intestinal digestion of protein in ruminants. J. Anim. Sci. 73:1459–1465. doi:10.2527/1995. 7351459X [DOI] [PubMed] [Google Scholar]
- Castillo-Lopez E., Kononoff P. J., and Miner J. L.. 2010. Short communication: detection of yeast DNA in omasal digesta of dairy cows consuming dried distillers grains and solubles. J. Dairy Sci. 93:5926–5929. doi: 10.3168/jds.2010-3302 [DOI] [PubMed] [Google Scholar]
- Cozzi G., Andrighetto I., Berzaghi P., and Polan C. E.. 1995. In situ ruminal disappearance of essential amino acids in protein feedstuffs. J. Dairy Sci. 78:161–171. doi: 10.3168/jds.S0022-0302(95)76626-7 [DOI] [PubMed] [Google Scholar]
- Erasmus L. J., Botha P. M., Cruywagen C. W., and Meissner H. H.. 1994. Amino acid profile and intestinal digestibility in dairy cows of rumen-undegradable protein from various feedstuffs. J. Dairy Sci. 77:541–551. doi: 10.3168/jds.S0022-0302(94)76982-4 [DOI] [PubMed] [Google Scholar]
- Eun J.-S., Beauchemin K. A., Hong S.-H., and Bauer M. W.. 2006. Exogenous enzymes added to untreated or ammoniated rice straw: Effects on in vitro fermentation characteristics and degradability. Anim. Feed Sci. Technol. 131(1-2):87–102. doi:10.1016/j.anifeedsci.2006.01.026 [Google Scholar]
- Fastinger N. D., and Mahan D. C.. 2006. Determination of the ileal amino acid and energy digestibilities of corn distillers dried grains with solubles using grower-finisher pigs. J. Anim. Sci. 84:1722–1728. doi: 10.2527/jas.2005-308 [DOI] [PubMed] [Google Scholar]
- Gargallo S., Calsamiglia S., and Ferret A.. 2006. Technical note: a modified three-step in vitro procedure to determine intestinal digestion of proteins. J. Anim. Sci. 84:2163–2167. doi: 10.2527/jas.2004-704 [DOI] [PubMed] [Google Scholar]
- Hvelplund T., and Nørgaard P.. 2003. Ruminant nutrition and physiology. Nutrient turnover and feed evaluation. DJF Rapport Husdyrbrug; 53 DJF, Tjele, Denmark, p. 601. [Google Scholar]
- Kamalak A. D. E. M., Canbolat O., Gurbuz Y., and Ozay O.. 2005. Comparison of in vitro gas production tenhnique with in situ nylon bag technique to estimate dry matter degradation. Czech J. Anim. Sci. 50:60–67. doi: 10.17221/3996-cjas [Google Scholar]
- Kaliyan N., and Morey R. V.. 2010. Densification characteristics of corn cobs. Fuel Process. Technol. 91:5, 559–565. doi:10.1016/j.fuproc.2010.01.001 [Google Scholar]
- Kelzer J. M., Kononoff P. J., Tedeschi L. O., Jenkins T. C., Karges K., and Gibson M. L.. 2010. Evaluation of protein fractionation and ruminal and intestinal digestibility of corn milling co-products. J. Dairy Sci. 93:2803–2815. doi: 10.3168/jds.2009-2460 [DOI] [PubMed] [Google Scholar]
- Kwak W. S., Jung S. H., and Kim Y. I.. 2008. Broiler litter supplementation improves storage and feed-nutritional value of sawdust-based spent mushroom substrate. Bioresour. Technol. 99:2947–2955. doi: 10.1016/j.biortech.2007.06.021 [DOI] [PubMed] [Google Scholar]
- Martins A. de S., Zeoula L. M., Prado I. N. do, Martins E. N., and Loyola V. R.. 1999. Degradabilidade ruminal In Situ da matéria seca e proteína bruta das silagens de milho e sorgo e de alguns alimentos concentrados. Revista Brasileira de Zootecnia, 28(5), 1109–1117. doi:10.1590/s1516-35981999000500029 [Google Scholar]
- Mehrez A. Z., Ørskov E. R., and McDonald I.. 1977. Rates of rumen fermentation in relation to ammonia concentration. Br. J. Nutr. 38:437–443. doi:10.1079/bjn19770108. [DOI] [PubMed] [Google Scholar]
- Negi S. S., Singh B., and Makkar H. P. S.. 1988. An approach to the determination of rumen degradability of nitrogen in low-grade roughages and partition of nitrogen therein. J. Agri. Sci. 111:487–494. doi:10.1017/S0021859600083684 [Google Scholar]
- NRC 1989. Nutrient requirement of dairy cattle. (6th rev. ed). Natl. Acad. Press, Washington, DC. [Google Scholar]
- NRC 2001. Nutrient requirements of dairy cattle. (7th rev. ed). Natl. Acad. Press, Washington, DC. [Google Scholar]
- Ørskov E. R., and Mcdonald I.. 1979. The estimation of protein degradability in the rumen from incubation measure-ments weighted according to rate of passage. J. Agric. Sci. 92:499–503. doi:10.1017/S0021859600063048 [Google Scholar]
- Ørskov E. R., Ojwang I., and Reid G. W.. 1988. A study on consistency of differences between cows in rumen outflow rate of fibrous particles and other substrates and consequences for digestibility and intake of roughages. Anim. Prod. 47:45–51. doi:10.1017/S000335610003703X [Google Scholar]
- Pearson E. S., Pearson K. and Hartley H. O.. 1966. Biometrika tables for statisticians. 21:5, 171–172. [Google Scholar]
- Pereira J. C., and González J.. 2004. Rumen degradability of dehydrated beet pulp and dehydrated citrus pulp. Anim. Research. 53:99–110. doi:10.1051/animres:2004005 [Google Scholar]
- Preston R. L. 2011. 2011 Feed composition tables. Beef Magazine. 47:43–47. [Google Scholar]
- Promkot C., and Wanapat M.. 2003. Ruminal degradation and intestinal digestion of crude protein of tropical protein resources using nylon bag technique and three-step in Vitro procedure in dairy cattle. Livest. Res. Rural Dev. 15, article 81. http://www.lrrd.org/lrrd15/11/prom1511.htm [Google Scholar]
- Satter L. D. 1986. Protein supply from undegraded dietary protein. J. Dairy Sci. 69:2734–2749. doi: 10.3168/jds.S0022-0302(86)80722-6 [DOI] [PubMed] [Google Scholar]
- Schingoethe D. J. 1996. Balancing the amino acid needs of the dairy cow. Anim. Feed Sci. Technol. 60:153–160. doi:10.1016/0377-8401(96)00976-5 [Google Scholar]
- Spanghero M., Berzaghi P., Fortina R., Masoero F., Rapetti L., Zanfi C., Tassone S., Gallo A., Colombini S., and Ferlito J. C.. 2010. Technical note: precision and accuracy of in vitro digestion of neutral detergent fiber and predicted net energy of lactation content of fibrous feeds. J. Dairy Sci. 93:4855–4859. doi: 10.3168/jds.2010-3098 [DOI] [PubMed] [Google Scholar]
- Spiehs M. J., Whitney M. H., and Shurson G. C.. 2002. Nutrient database for distiller’s dried grains with solubles produced from new ethanol plants in Minnesota and South Dakota. J. Anim. Sci. 80:2639–2645. doi:10.2527/2002.80102639x [DOI] [PubMed] [Google Scholar]
- Storm E., and Orskov E. R.. 1983. The nutritive value of rumen micro-organisms in ruminants. 1. Large-scale isolation and chemical composition of rumen micro-organisms. Br. J. Nutr. 50:463–470. doi:10.1079/bjn19840128 [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]