Abstract
The objective was to examine uterine artery blood flow (UBF) as well as macroscopic and microscopic placentome vascular density in nutrient-restricted Angus and Brahman heifers. Angus (n = 6) and Brahman (n = 6) heifers were bred to a single sire and pregnancy confirmed at 30-d postbreeding. Heifers were randomly assigned to 1 of 2 dietary treatments consisting of 100% (control-fed; CON; n = 6) or 60% (total nutrient-restricted; RES; n = 6) based from net energy requirements for gestating heifers. Nutritional treatments were imposed from days 50 to 180 of gestation. On day 175 of gestation, UBF was collected ipsilateral and contralateral to the conceptus via Doppler ultrasonography. Heifers underwent Cesarean sections for collection of 2 adjacent placentomes on day 180 of gestation. The primary cotyledonary artery of 1 placentome was perfused with Alexa Fluor 647 Con A conjugate to examine macroscopic cotyledonary vascular density via an in vivo imaging system. The second placentome was fixed for microscopic immunofluorescence labeling of capillaries and separated into maternal (caruncle) and fetal (cotyledon) components for determination of angiogenic factor mRNA expression. Main effects of nutritional treatment and breed are reported in the absence of a significant nutritional treatment by breed interaction. Ipsilateral UBF was decreased (P < 0.05) by 48% in RES vs. CON, whereas breed did not influence ipsilateral UBF. Contralateral UBF was not different between nutritional treatments; however, contralateral UBF was decreased (P < 0.05) by 63% in Brahman vs. Angus cattle. Macroscopic cotyledonary vascular density was increased (P < 0.05) by 36% in RES vs. CON and 82% in Brahman vs. Angus heifers. Percent capillary area and capillary perimeter were increased (P < 0.05) in RES vs. CON and increased (P < 0.05) in Brahman vs. Angus heifers. Dietary treatments did not alter angiogenic factor expression; however, transcript abundance of caruncle and cotyledon ANGP1, FLT1, and KDR was increased (P < 0.05) in Brahman vs. Angus heifers. In summary, these data indicate compensatory responses in macroscopic and microscopic placentome blood vessel density during maternal nutrient restriction–induced reductions in UBF. Moreover, a greater macroscopic density of cotyledonary blood vessels was observed in Brahman vs. Angus heifers.
Keywords: angiogenic factors, Angus, Brahman, nutrient restriction, placentome, uterine artery
INTRODUCTION
Proper fetal nutrition via adequate uteroplacental blood flow is critical for maximizing offspring production potential while minimizing pregnancy wastage and calf morbidity and mortality. Thus, proper establishment and maintenance of the placenta is vital to fetal survival. Placental nutrient transport capacity is directly correlated with uteroplacental blood flow (Reynolds and Redmer, 1995). The rapid increase in fetal growth during the last half of gestation is supported by the growth of the placenta during early gestation and the dramatic development of uteroplacental vasculature during the last half of gestation (Reynolds and Redmer, 1995). The development of new blood vessels from preexisting vasculature, known as angiogenesis (Barcroft, 1946; Wallace, 1948; Alexander, 1964), is what allows placental blood flow to increase in the last one-half to one-third of gestation (Reynolds and Redmer, 1995). A number of growth factors have been described to promote or enhance angiogenesis such as vascular endothelial growth factor (VEGF; Ferrara et al., 1992) and angiopoietin 1 (ANGPT1; Suri et al., 1996). The efficiency of these growth factors is highly dependent on the presence and availability of their receptors which include kinase insert domain containing receptor (KDR; Terman et al., 1992) and fms-like tyrosine kinase (FLT1; Shibuya et al., 1990), which have been examined in the bovine placenta during nutrient restriction. Maternal nutrient restriction from days 30 to 125 of gestation increased placental FLT1 transcript abundance, with no alteration in placentome capillary density (Vonnahme, et al., 2007). In contrast, the same treatments were observed to increase cotyledonary vascularity at day 125 of gestation in nutrient-restricted cows (Zhu et al., 2007), implicating early gestational nutrient restriction in upregulating cotyledonary growth signaling pathways associated with angiogenesis.
Adaptations of the uteroplacenta following maternal nutrient restriction is incomplete and less is known about specific differences amongst breeds of cattle. Previous studies have found that Bos indicus cattle have longer gestation lengths (Reynolds et al., 1980) and differing fetal growth from mid to late gestation (Ferrell, 1991a) when compared with their B. taurus counterparts. Ferrell (1991a) found that placentome weights, as well as total RNA, DNA, and protein of the cotyledon and caruncle, were decreased in Brahman vs. Charolais cows. In an accompanying study, Ferrell (1991b) also found lesser uterine artery blood flow (UBF) in Brahman vs. Charolais cows at 227 d of gestation. Observations between breeds have typically shown that B. indicus perform better than B. taurus when consuming low-quality diets (Habib et al., 2011), while the underlying mechanisms are incomplete, a portion of these responses may be related to differences in the rumen microbiome between breeds (Latham et al., 2018). Therefore, breed may influence compensatory responses in uteroplacental hemodynamics and placental vascularity following early gestational nutrient restriction. Thus, we hypothesized decreased UBF during nutrient restriction and compensatory alterations to placentome angiogenesis. Moreover, we anticipated increased placental angiogenesis in B. indicus vs. B. taurus heifers.
MATERIALS AND METHODS
Animal Management and Treatments
Animal care and use was approved by the Mississippi State University Institutional Animal Care and Use Committee (#16-461). Angus (n = 30) and Brahman (n = 30) heifers were housed at the H. H. Leveck Animal Research Center (Mississippi State, MS) on dormant 80% bermudagrss (Cynodon dactylon) and 20% dallisgrass (Paspalum dilatatum) pasture and had ad libitum access to hay from the same pasture composition for 120 d prior to breeding. Animals were body condition scored prior to breeding. Angus heifers underwent a 5-d CO-Synch + CIDR estrous synchronization protocol, whereas Brahman heifers underwent a PG 5-d CO-Synch + CIDR estrous synchronization protocol. All heifers were artificially inseminated or bred to a single purebred Hereford bull. Transrectal ultrasonography was used to confirm pregnancy at 30-d postartificial insemination, at which time nonpregnant heifers were rebred via natural service to the same purebred Hereford bull. Pregnancy rates to artificial insemination were unusually low; therefore, the 12 heifers used in this project were all bred via natural service with pregnancy confirmed 30-d postbreeding via transrectal ultrasonography.
After confirmation of pregnancy, heifers began a 10-d Calan feeding system (American Calan, Northwood, NH) prior to treatment initiation. From days 50 to 180 days of gestation, 6 Angus heifers and 6 Brahman heifers were randomly assigned to 1 of 2 treatments equally consisting of 100% (control-fed; CON; n = 6) or 60% (nutrient-restricted; RES; n = 6) of net energy requirements for gestating cattle (NRC, 2000). Based from NRC (2000), net energy requirements were calculated to be 0.018 Mcal/kg body weight (BW) for maintenance and to be 0.012 Mcal/kg BW for gain with a target gain of 1.0 kg/d. Animals were fed a TMR (87.5% DM, and on a DM basis 10.7% protein, 43.8% NDF, 30.1% ADF) at a rate of 2.0% BW/day and chopped hay (90.5% DM, and on a DM basis 7.1% protein, 73% NDF, 42.4% ADF) at a rate of 0.5 % BW/d for CON. For RES, TMR was fed at a rate of 1.2 % BW/d and hay at 0.3 % BW/d. Both TMR (1.0% BW and 0.6% BW for CON and RES, respectively) and hay (0.25% BW and 0.15% BW for CON and RES, respectively) were offered twice daily and heifers had ad libitum access to water. Amounts (kg/d) of TMR and hay offered were adjusted after each weigh day. The TMR consisted of corn, cotton seed hulls, soy hull pellets, molasses, limestone, and salt. These diets were consistent with other previously conducted bovine nutrient restriction studies (Long et al., 2010; Gonzalez et al., 2013). BW and BCS were recorded for each animal on days 49, 64, 77, 88, 117, 148, and 175 of gestation on average.
Color Doppler Ultrasonography
On day 175 of gestation, 5 d prior to Cesarean section surgeries, heifers underwent Doppler ultrasonography for measurements of uteroplacental hemodynamics. Ultrasonography measurements took approximately 10 min per animal and were conducted using the techniques described by Brockus et al. (2016). Uterine artery hemodynamics, ipsilateral and contralateral to the conceptus, were determined via color Doppler ultrasonography (M-Turbo, Sonosite, Bothell, WA) using a transrectal probe (Linear Endorectal L52× probe, Sonosite, Bothell, WA). The uterine artery was identified transrectally by following the abdominal aorta toward the origin of the external iliac artery. The internal iliac artery was identified by moving the probe caudally. The left and right uterine arteries were identified as major branches of the internal iliac arteries. Moreover, the uterine arteries were palpated to assure pliability and pulsatility, which is easily observed during the last half of pregnancy. Cardiac cycle waveforms from at least 2 independent ultrasound scans were used to calculate systolic velocity (s; cm/s), diastolic velocity (d; cm/s), s:d ratio, pulsatility index, and resistance index using preset functions on the Doppler ultrasound. Mean velocity was calculated using the equation: (s – d)/pulsatility index. UBF was calculated using the following equation: mean velocity × vessel area × 60 s.
Cesarean Sections
On day 180 of gestation, 130-d posttreatment initiation, heifers underwent a Cesarean section for collection of placentomes. Surgeries were performed at the H. H. Leveck Animal Research Center; therefore, animals were not transported prior to surgery. For this project, the registered Brahman heifers were 4 mo older than the Angus heifers on average. Cesarean sections were performed on the standing dam following a paravertebral or inverted-L block with 2% lidocaine. After the skin surrounding the incision site was prepared for aseptic surgery, an incision was made 10 to 15 cm ventral to the transverse processes of the lumbar vertebrae midway between the last rib and the tuber coxae and extended sufficiently to allow extraction of the fetus. A left oblique celiotomy approach was utilized and the abdominal wall incision was extended cranioventrally at a 45-degree angle. This surgical approach permitted easier access to the gravid uterus compared with more traditional vertical incisions in the paralumbar fossa. One of the fetal limbs was identified and used as a handle to deliver the uterus to the abdominal incision. After the uterus was incised, the umbilical cord was located and clamped off in 2 locations approximately 10 cm from the fetus and 10 cm from the major branch points feeding the cotyledons and cut between the 2 clamps. After successful removal of the fetal calf, two placentomes were excised nearest to the entry of the umbilical cord. The 2 placentomes were submerged in PBS and immediately transported back to the Wise Center (Mississippi State, MS). One of the placentomes was left intact and used for fluorescence cotyledonary artery perfusion for imaging (described below). The other placentome was processed for fixation and freezing. Briefly, a 1- by 1-cm placentome section was placed in Optimal Cutting Temperature (OCT) tissue embedding media (Fisher Scientific, Pittsburgh, PA) and frozen by submersion in supercooled isobutene and stored at −80 °C. The remainder of this placentome was separated into caruncle (maternal) and cotyledon (fetal) portions and snap frozen in liquid nitrogen and stored at −80 °C for later processing of angiogenic factor mRNA expression.
Placentome Perfusion and Macroscopic Imaging
The cotyledonary artery of intact placentomes was dissected and catheterized using a 20-g by 2-inch Exel Safelet Cath (Exelint International, Los Angeles, CA). Following catheterization, approximately 20 to 30 mL of PBS was perfused through the cotyledonary artery and determined to be exiting via the cotyledonary vein. Once vessels were cleared of blood, the catheters were perfused with a sufficient volume of 200-µg/mL Concanavalin A, Alexa Fluor 647 Conjugate (ThermoFisher Scientific, Waltham, MA) to ensure drainage of the fluorophore through the cotyledonary vein. Following perfusion, the cotyledonary artery and cotyledonary vein were ligated with silk suture. Placentomes were immediately imaged using an in vivo imaging system (IVIS) Lumina XRMS Series III (PerkinElmer, Waltham, MA). A negative control placentome was imaged following PBS perfusion alone. The radiance signal (photon/s/cm2/sr) from each sample was obtained from IVIS system and assessment of the radiance signal per gram was performed to correct for any differences in total placentome weight between CON and RES or between Angus and Brahman heifers.
Placentome Immunohistochemistry
Placentomes embedded in OCT molds were sectioned into four 10-μm cryosections using a CRYOSTAR NX50 (Thermo Scientific, Waltham, MA) and positioned on positively charged microscope slides. For immunofluorescence imaging of blood vessels, slides were blocked for 30 min with 10% goat serum in PBS with the addition of 0.2% Tween-20. Next, slides were treated with the primary antibody, Anti-Von Willebrand Factor (ab6994; Abcam, Cambridge, MA) followed by secondary antibody, Goat Anti-Rabbit IgG H&L (Alexa Fluor 594; ab150080; Abcam). To counterstain caruncular epithelium and fetal villi, slides were incubated with 10-μg/mL Fluorescein-labeled Griffonia Simplicifolia Lectin I (FL-1101; Vector Laboratories, Burlingame, CA). Lastly, tissue sections were treated with Fluoroshield mounting medium with DAPI (ab104139; Abcam) for nuclear staining. Images were captured using an EVOS microscope (AMAFD1000; Life Technologies, Carlsbad, CA) with 10× magnification. At least 10 representative photomicrographs were captured per animal. Images captured with the EVOS Texas Red light cube (Alexa Fluor 594) were then analyzed using ImageJ (https://imagej.nih.gov/ij/download.html). Total capillary number per tissue area (vessel number/mm2), percent capillary area (%/mm2), average capillary size (μm2), and average capillary perimeter per tissue area (mm/mm2 or mm−1) were recorded.
Placentome RT-PCR
Transcript abundances of angiogenic factors were determined in frozen caruncular and cotyledonary tissues. Approximately 1 g of placental tissue was homogenized with 1 mL of PBS. Nucleic acids were isolated by the placental homogenate using QIAzol Lysis Reagent (QIAGEN, Hilden, Germany) followed by purification using a miRNeasy Mini Kit (QIAGEN, Hilden, Germany). Extracted total RNA was quantified using a NanoDrop One spectrophotometer (Thermo scientific, Waltham, MA). Samples exhibiting 260/280-nm ratios between 1.9 and 2.1 were deemed acceptable and stored at −80 °C. Synthesis of cDNA was conducted using High-Capacity cDNA Reverse Transcription Kit (Thermo Fischer Scientific, Auburn, AL). RT-PCR was performed using Custom TaqMan Gene Expression Assays (Thermo Fischer Scientific, Auburn, AL) according to the manufacturer protocol using a QuantiStudio 3 (Applied Biosystems, Foster City, CA) for real-time qPCR. Thermal cycling parameters used consisted of a hold stage at 50 °C for 2 min, polymerase activation hold at 95 °C for 20 s, and PCR stage with step 1 at 95 °C for 1 s followed by step 2 at 60 °C for 20 s for 40 cycles. The genes of interest for caruncle and cotyledon tissues were angiopoeitin 1 (ANGPT1), vascular endothelial growth factor A (VEGFA), kinase insert domain containing receptor (KDR), and fms-like tyrosine kinase (FLT1). Beta actin (ACTB) and glyceraldehyde 3 phosphate dehydrogenase (GAPDH) served as housekeeping genes. Duplexing of genes were performed according to manufacturers’ instructions. Assays for the genes of interest (Table 1) were validated for efficiency and specificity prior to qPCR. Primer efficiencies were determined by plotting the threshold cycle vs. the log of the input concentrations for 6 serial dilutions of pooled caruncle or cotyledon tissue cDNA ran in duplicate. Efficiency was calculated by raising 10 to the power of −1 divided by the slope of the line (E = 10[−1/slope]). Primer sets and assays with efficiencies between 1.8 and 2.2 (90%–110%) were considered acceptable for real-time PCR analysis. Replicate CT values were averaged and used for relative quantification using the 2−ΔΔCt method where the geometric mean of GAPDH and ACTB was used as a reference to normalize all of the genes of interest.
Table 1.
Assays, accession, amplicon length, and efficiency (10[−1/slope]) of primers used for TaqMan real-time PCR quantification of placental gene expression
| Gene | Assay ID | Accession | Amplicon | Efficiency | |
|---|---|---|---|---|---|
| Caruncle | Cotyledon | ||||
| ACTB | Bt03279174 | NP_776404.2 | 141 | 2.05 | 2.04 |
| GAPDH | Bt03210913 | NP_001029106.1 | 66 | 2.04 | 2.09 |
| ANGPT1 | Bt03249559 | NP_001070265.1 | 147 | 2.10 | 2.05 |
| VEGFA | Bt03213282 | NP_776641.1 | 59 | 2.20 | 2.14 |
| KDR | Bt03258885 | NP_001103470.1 | 68 | 2.09 | 2.06 |
| FLT1 | Bt04302190 | NP_001178061.2 | 101 | 2.04 | 2.08 |
Statistical Analysis
Maternal BW was analyzed using repeated measures of ANOVA (SAS software version 9.4, SAS Institute, Cary, NC). The model statement included day of gestation, treatment, breed, and their respective interactions. Fetal weight, uterine hemodynamics, macroscopic cotyledonary blood vessel density, microscopic placentome capillary density, and angiogenic factor transcript abundance were analyzed using ANOVA (SAS software version 9.4). The class statement included heifer, breed, treatment, and fetal sex. The model statement included treatment, breed, treatment by breed interaction, and fetal sex. Fetal sex was removed from the model statement if determined to have no influence (P > 0.25) on the dependent variable. Briefly, the only variables influenced (P < 0.25) by fetal sex were measurements of microscopic placentome capillary density. Means were not separated across fetal sex and therefore not reported. The fetal sex distribution for each breed and treatment was Angus-CON (n = 2 male; n = 1 female), Brahman-CON (n = 1 male; n = 2 female), Angus-RES (n = 2 male; n = 1 female), and Brahman-RES (n = 1 male; n = 2 female). Least-square means and standard error of the means are reported. Main effects of dietary treatment or breed are reported in the absence of a significant dietary treatment by breed interaction. Statistical significance was declared at P ≤ 0.05, whereas tendencies were declared at P > 0.05 and P < 0.10. To further test the validity of the novel placentome perfusion technique, Pearson correlation coefficients were determined for macroscopic cotyledonary blood vessel density vs. cotyledonary angiogenic factor transcript abundance using the CORR procedure of SAS (SAS software version 9.4).
RESULTS
A dietary treatment by gestational day interaction (P = 0.027) was observed for maternal BW, which was decreased in RES dams (523 ± 23 kg) vs. CON dams (593 ± 23 kg) at 148 d of gestation and remained decreased in RES dams (540 ± 23 kg) vs. CON dams (611 ± 23 kg) at 175 d of gestation. Breed did not interact with any of the variables tested for maternal BW. Maternal BW was not different (P = 0.787) between Angus (509 ± 22 kg) and Brahman (518 ± 22 kg) heifers. Fetal weight at day 180 was decreased (P = 0.047) in RES (7.35 ± 0.68 kg) compared with CON (9.56 ± 0.61 kg), whereas fetal weight was not different (P = 0.388) between Angus (8.04 ± 0.68 kg) and Brahman (8.88 ± 0.61 kg) heifers.
Uterine Artery Hemodynamics
Data for UBF are included in Figure 1. Absolute ipsilateral UBF was decreased (P < 0.05) in RES compared with CON and not different (P = 0.12) between breeds (Figure 1A). Ipsilateral UBF relative to fetal weight tended to be decreased (P = 0.058) in RES compared with CON and tended to be decreased (P = 0.054) in Brahman compared with Angus (Figure 1B). The area of the ipsilateral uterine artery was decreased (P < 0.05) in RES (0.22 ± 0.02 cm2) vs. CON (0.30 ± 0.02 cm2) and tended to be decreased (P = 0.08) in Brahman (0.23 ± 0.02 cm2) vs. Angus (0.30 ± 0.02 cm2) heifers. Pulsatility index, resistance index, and s:d ratio of the ipsilateral uterine artery were not different (P > 0.10) between dietary treatments or breeds (data not shown).
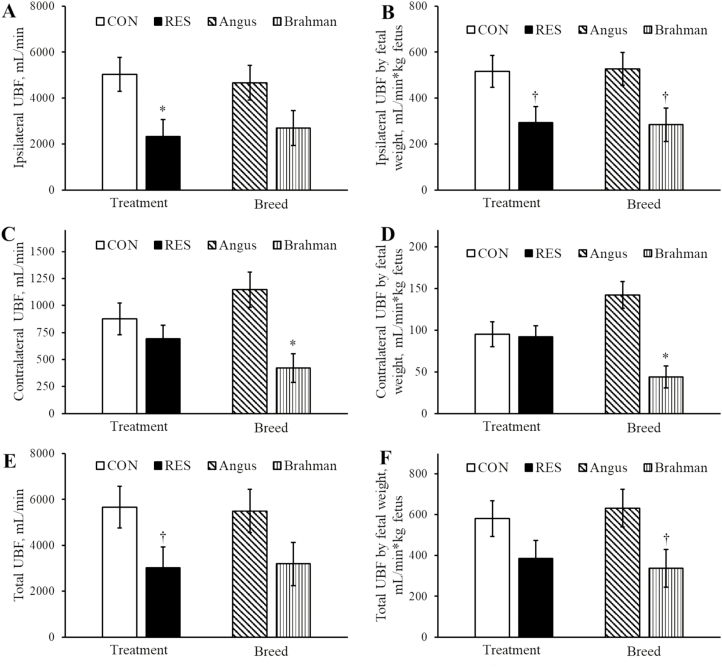
Figure 1.
UBF ipsilateral and contralateral to the conceptus as well as the summation of ipsilateral and contralateral UBF (total UBF) in RES and CON-fed Angus and Brahman heifers at day 175 of pregnancy. Fetal body weight was determined at Cesarean section on day 180 of pregnancy. All treatment by breed interactions for uterine hemodynamics were P > 0.34. Main effects of dietary treatment and breed of absolute ipsilateral UBF (A), ipsilateral UBF relative to fetal body weight (B), absolute contralateral UBF (C), contralateral UBF relative to fetal body weight (D), total UBF (E), and total UBF relative to fetal body weight (F). Asterisk (*) represents a difference (P < 0.05) between dietary treatment groups or between breeds. Dagger (†) represents a tendency (P < 0.10) between dietary treatment groups or between breeds.
Absolute contralateral UBF was not different (P = 0.38) between dietary treatments and decreased (P < 0.05) in Brahman compared with Angus heifers (Figure 1C). Similarly, contralateral UBF relative to fetal weight was not different (P = 0.88) between dietary treatments and decreased (P < 0.005) in Brahman compared with Angus heifers (Figure 1D). The area of the contralateral uterine artery was not different (P = 0.87) between RES (0.13 ± 0.01 cm2) vs. CON (0.13 ± 0.01 cm2) and decreased (P < 0.05) in Brahman (0.12 ± 0.01 cm2) vs. Angus (0.16 ± 0.01 cm2) heifers. Pulsatility index, resistance index, and s:d ratio of the contralateral uterine artery were not different (P > 0.10) between dietary treatments or breeds (data not shown).
Absolute total UBF tended to be decreased (P = 0.079) in RES compared with CON and was not different (P = 0.136) between breeds (Figure 1E). In contrast, total UBF relative to fetal weight was not different (P = 0.162) between dietary treatments and tended to be decreased (P = 0.062) in Brahman compared with Angus heifers (Figure 1F). Heifer heart rate at the time of ultrasonography was not different (P = 0.98) between RES (82 ± 5 bpm) vs. CON (82 ± 6 bpm) and not different (P = 0.50) between Brahman (79 ± 6 bpm) vs. Angus (85 ± 5 bpm).
Placentome Vascularity
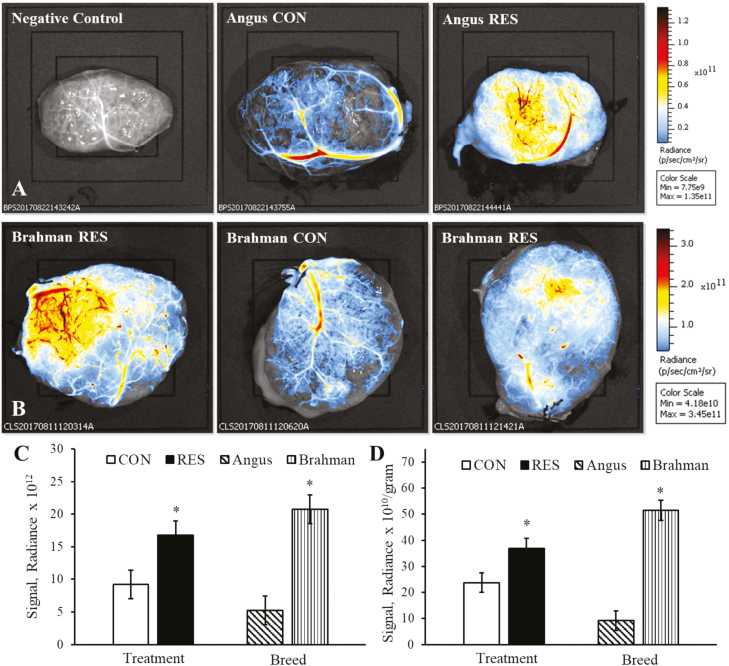
Representative images of macroscopic cotyledonary vascular density of Angus and Brahman heifers are illustrated in Figure 2A and B, respectively. Fluorescent signal radiance was increased (P < 0.05) in RES compared with CON and increased (P < 0.005) in Brahman compared with Angus heifers (Figure 2C). Similarly, fluorescent signal radiance relative to placentome weight was increased (P < 0.05) in RES compared with CON and increased (P < 0.0001) in Brahman compared with Angus heifers (Figure 2D).
Figure 2.
Fluorescent detection of macroscopic cotyledonary blood vessel density in RES and CON-fed Angus and Brahman heifers at day 180 of pregnancy. Representative placentome images from Angus (A; top three images and scale bar) and Brahman heifers (B; middle three images and scale bar) with cotyledonary surface up. All treatment by breed interactions for macroscopic blood vessel density were P > 0.15. Main effects of dietary treatment and breed of absolute fluorescent signal intensity (C) and fluorescent signal intensity relative to individual placentome weights (D). Asterisk (*) represents a difference (P < 0.05) between dietary treatment groups or between breeds.
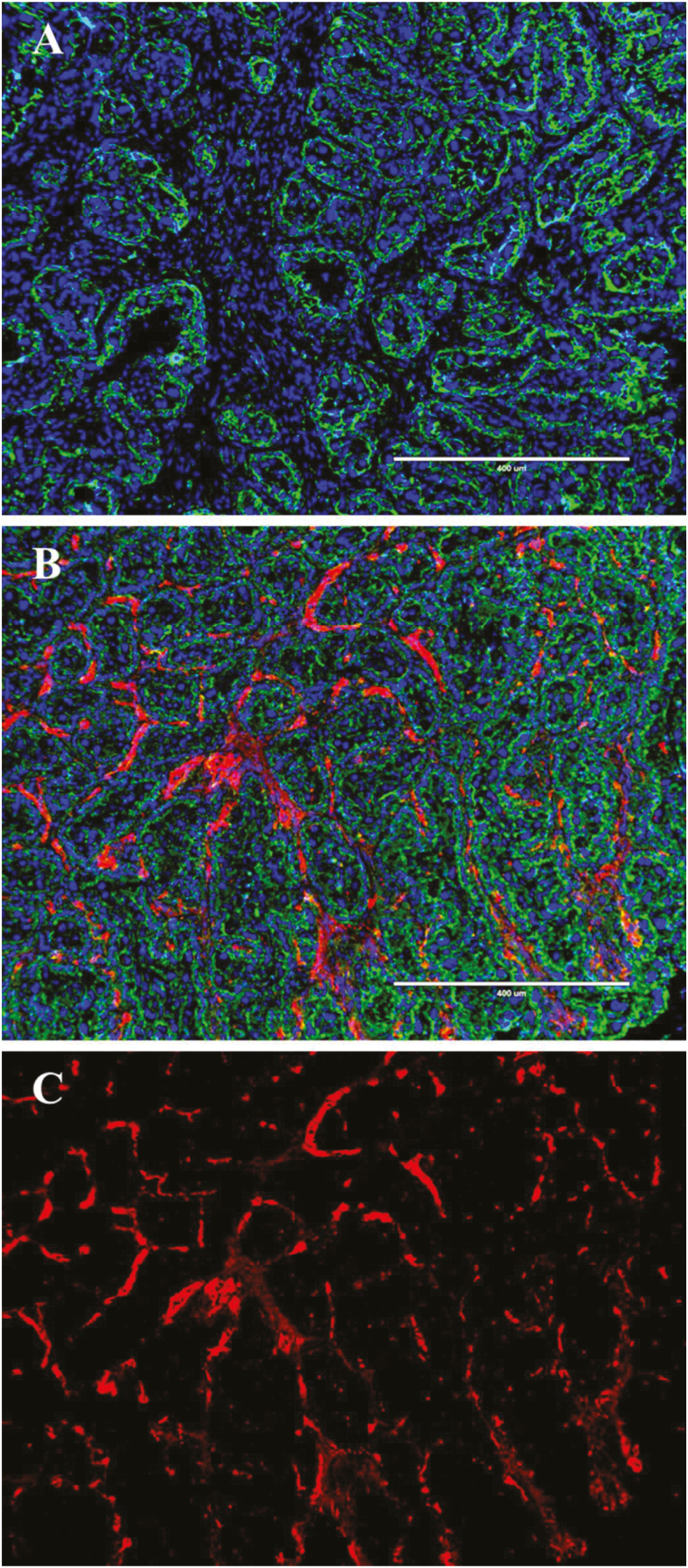
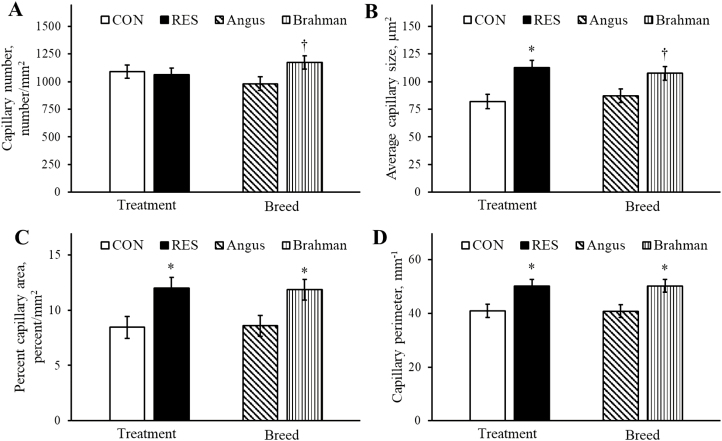
Representative images of microscopic placentome blood vessel density are illustrated in Figure 3A–C. Capillary number per mm2 was not different (P = 0.76) between dietary treatments and tended to be increased (P = 0.068) in Brahman compared with Angus heifers (Figure 4A). Average capillary size (mm2) was increased (P < 0.05) in RES compared with CON and tended to be increased (P = 0.055) in Brahman compared with Angus heifers (Figure 4B). Percent capillary area was increased (P < 0.05) in RES compared with CON and increased (P < 0.05) in Brahman compared with Angus heifers (Figure 4C). Similarly, capillary perimeter was increased (P < 0.05) in RES compared with CON and increased (P < 0.05) in Brahman compared with Angus heifers (Figure 4D).
Figure 3.
Representative immunofluorescence images of heifer placentomes at 180 d of gestation. Cryosectioned placentomes were stained for capillaries with Anti-Von Willebrand Factor (red, Alexa Fluor 594), caruncular and chorionic epithelium (green, FITC), and nuclei (blue, DAPI). (A) represents a negative control–treated without Anti-Von Willebrand Factor. (B) represents a fully stained image with all 3 fluorescent channels overlaid and (C) represents the Texas Red channel only from (B). The white scale bar represents 400 μm.
Figure 4.
Main effects of dietary treatment and breed for placentome capillary number (A), average capillary size (B), percent capillary area (C), and capillary perimeter (D). All treatment by breed interactions for microscopic capillary measurements were P > 0.65. Asterisk (*) represents a difference (P < 0.05) between dietary treatment groups or between breeds. Dagger (†) represents a tendency (P < 0.10) between breeds.
Data for mRNA transcript abundance are included in Table 2. Dietary treatment did not alter (P > 0.15) transcript abundance of ANGPT1, VEGF, FLT1, and KDR in caruncular tissue. Caruncular transcript abundance of ANGPT1, FLT1, and KDR was increased (P < 0.05) in Brahman compared with Angus heifers. Caruncular VEGF transcript abundance was not different (P = 0.234) between breeds. Dietary treatment did not alter (P > 0.15) transcript abundance of ANGPT1, VEGF, FLT1, and KDR in cotyledonary tissue. Cotyledonary transcript abundance of ANGPT1, FLT1, and KDR was increased (P < 0.05) in Brahman compared with Angus heifers. Cotyledonary VEGF transcript abundance was not different (P = 0.18) between breeds.
Table 2.
Transcript abundance of angiogenic factors in caruncle and cotyledon of control-fed (CON) and nutrient-restricted (RES) Angus and Brahman heifers on day 180 of pregnancy
| Item | Treatment | Breed | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | RES | SEM | P value | Angus | Brahman | SEM | P value | |
| No. of heifers | 6 | 6 | 6 | 6 | ||||
| Caruncle | ||||||||
| ANGPT1 | 1.93 | 1.01 | 0.40 | 0.181 | 0.38 | 2.56 | 0.38 | 0.009 |
| VEGF | 1.13 | 0.93 | 0.17 | 0.464 | 0.87 | 1.19 | 0.16 | 0.234 |
| FLT1 | 1.06 | 0.97 | 0.14 | 0.710 | 0.68 | 1.36 | 0.14 | 0.019 |
| KDR | 1.12 | 0.96 | 0.21 | 0.634 | 0.58 | 1.51 | 0.21 | 0.022 |
| Cotyledon | ||||||||
| ANGPT1 | 1.13 | 1.05 | 0.18 | 0.762 | 0.59 | 1.59 | 0.17 | 0.007 |
| VEGF | 0.98 | 1.00 | 0.13 | 0.895 | 0.86 | 1.13 | 0.13 | 0.180 |
| FLT1 | 1.16 | 1.01 | 0.19 | 0.594 | 0.73 | 1.44 | 0.19 | 0.029 |
| KDR | 1.19 | 0.90 | 0.28 | 0.476 | 0.58 | 1.52 | 0.28 | 0.042 |
All treatment by breed interactions for transcript abundance were P > 0.15.
Using the correlation procedure of SAS, significant positive correlations were observed between macroscopic fluorescent signal intensity of the cotyledonary blood vessels with cotyledonary transcript abundance of ANGPT1 (r = 0.757; P = 0.004), VEGF (r = 0.562; P = 0.057), FLT1 (r = 0.656; P = 0.021), and KDR (r = 0.618; P = 0.032).
DISCUSSION
The present study demonstrated decreased ipsilateral UBF in RES heifers compared with CON-fed heifers, whereas contralateral blood flow was not different between dietary treatments. This is different from previous work in lactating Simmental cows, where ipsilateral UBF was not different following nutrient restriction from days 30 to 140 of gestation compared with control-fed cows (Camacho et al., 2014). However, following realimentation of nutrient restricted cows, the authors observed an increase in ipsilateral UBF in realimented cows vs. control-fed. Although breed and parity may contribute to these different responses during early gestational nutrient restriction, it is important to note that the current study also extended nutrient restriction to day 175 of pregnancy. In addition, the previous work in Simmental cows demonstrated a trend for decreased contralateral UBF in nutrient-restricted cows compared with control-fed cows, with similar responses carried into late gestation following nutrient realimentation (Camacho et al., 2014). In the current work, nutrient restriction did not alter contralateral UBF, although significant differences in contralateral UBF were observed between Angus and Brahman heifers. Interestingly, the observed increase in uteroplacental blood flow and hemodynamics following realimentation of early gestational nutrient-restricted cows has not been fully explained, even though these important compensatory responses may enhance late gestational conceptus development.
Early research described the bovine placenta as fully developed around 170 d of gestation, where growth slows towards the periphery of the placentome, forming a more mushroom-like appearance (Bjorkman 1954, 1969; Leiser et al., 1997). Granted total bovine placental weight increases exponentially throughout gestation; however, this rate of exponential growth is far exceeded by the exponential increase in fetal weight throughout gestation (Reynolds and Redmer, 1995). In addition, caruncular weight was greater than cotyledonary weight during the last two-thirds of pregnancy, while caruncular DNA concentration remains relatively constant from days 100 to 250, whereas cotyledonary DNA concentration increases nearly 2-fold over the same span of time, suggesting altered cellular density and potential rearrangement of the cotyledon to keep pace with fetal growth (Reynolds and Redmer, 1995). The microvasculature changes of the bovine placenta in relation to the development of the fetal villi were outlined by Leiser et al. (1997). Similar to our own sampling, the most developmentally advanced placentomes were selected by Leiser et al. (1997), which correspond to the midsection of the ipsilateral uterine horn, where conceptus attachment first occurs (Leiser, 1975). Therefore, placentomes located in the ipsilateral and contralateral uterine horns, which are generally smaller and less developmentally advanced, were excluded from the Leiser et al. (1997) study as well as the current study. The major findings from Leiser et al. (1997) indicated transformation of the placentome villous trees, which included altered branching patterns of the microvasculature as well as an improved spatial relationship between endothelial cells and the trophoblast.
In the current study, we utilized a novel macroscopic approach to generate data related to cotyledonary blood vessel densities. This approach focused on arterial perfusion of a fluorescent substrate through the cotyledonary artery followed by image capture of the total placentome. In general, placentome comparisons between dietary treatments and breed were vastly different in their perfusion profiles, whereby RES placentomes and placentomes from Brahman cattle showed greater macroscopic fluorescent perfusion, indicating significant blood perfusion activity in specific areas of the placentome compared with their counterparts. The inclusion of microscopic measurements of placentome blood vessels, specifically capillary densities, is important to note because macroscopic perfusion measurements may be better indicative of in vivo blood perfusion profiles. For example, the remodeling of the villous tree could result in transformations of cellular structures related to arteriole constriction, coiling of capillary loops, or precapillary sphincters, which may redistribute blood to specific regions of the placentome. Although precapillary sphincters have not been reported in the placentome, the current data set indicates dramatic shunting of blood at the macroscopic level of the placentome. In addition, capillary sinusoids or dilations in the terminal villi of the bovine placentome (Leiser et al., 1997) may slow blood flow locally, which should not influence gas exchange, but may facilitate slow-transported solutes. This reduction in vascular resistance due to widening of the vascular lumen can be further attributed to anastomosis of the capillary bed and venous system of the cow placenta (Leiser et al., 1997). Moreover, these alterations may be less apparent when examining microscopic capillary densities via immunofluorescence compared with total placentome imaging following ex vivo fluorescent perfusion of the cotyledonary artery.
Microscopic capillary area density, surface density, and number of the cotyledon and caruncle portions of the placentome were not different at day 125 in Angus × Gelbvieh cows nutrient restricted from days 30 to 125 of pregnancy (Vonnahme et al., 2007). Using immunofluorescent techniques, capillary area density, surface density, and number of the cotyledon and caruncle were not different between nutrient-restricted and control-fed sheep at day 130 of pregnancy (Eifert et al., 2015). Using similar techniques, microscopic measurements of cotyledon and caruncle capillary densities were not different between control-fed vs. placentomes from fetal growth–restricted pregnancies brought about by over-nourishment in adolescent ewe lambs (Carr et al., 2016). However, in this same study, the transcript abundance of several angiogenic factors was decreased in the caruncle of over-nourished vs. control-fed lambs (Carr et al., 2016). In the current study, we utilized similar staining techniques with FITC-labeled lectin to differentiate the trophoblast layer of the ovine placentome; however, micrographs showed abundant FITC staining around the fetal villous tree and caruncular epithelial cell layer of the bovine placentome. Therefore, this did not permit differential examination of microscopic capillary densities in the cotyledon vs. the caruncle of the bovine placentome. Irrespective of these study differences, total placentome capillary density from the current study was increased in RES vs. CON heifers as well as Brahman vs. Angus heifers. Although the magnitude difference was lower compared with our novel macroscopic imaging techniques, the directional changes were similar across the variables of interest. Moreover, the increase in placentome capillary density may be attributed to compensatory responses from the cotyledon, as evidenced by the increase in macroscopic blood vessel density of the cotyledon.
Placental expression of FLT1 was increased at day 125 in Angus × Gelbvieh cows nutrient restricted from days 30 to 125 of pregnancy; however, transcript abundance of VEGF and KDR were unaffected by dietary treatments (Vonnahme et al., 2007). In the current study, angiogenic factor expression was similar between RES and CON heifers; however, both caruncle and cotyledonary expressions of ANGPT1, KDR, and FLT1 were increased in Brahman vs. Angus heifers. Utilizing multiple regression, the relative contribution of several angiogenic factors to placental capillary measurements has been explained in the sheep placenta (Reynolds et al., 2010). In the caruncle, VEGF, NOS3, and ANGPT2 transcript abundance showed a positive relationship with capillary area density, whereas FGF, ANGPT1, and TIE2 showed a negative relationship. Interestingly, none of the angiogenic or vasoactive transcripts showed a positive relationship with capillary density of the ovine cotyledon (Reynolds et al., 2010). In addition, UBF was not associated with measurements of capillary density of the caruncle; however, melatonin supplementation increased both umbilical artery blood flow and capillary number density of the cotyledon in sheep (Eifert et al., 2015). In the current study, significant positive correlations were observed between macroscopic fluorescent signal intensity of the cotyledonary blood vessels with cotyledonary transcript abundance of tested angiogenic factors.
In conclusion, ipsilateral UBF is decreased following nutrient restriction from days 50 to 175 of gestation, irrespective of breed. This decrease in blood flow may signal compensatory adaptations of the cotyledon to increase blood perfusion to specific zones compared with CON-fed heifers. Moreover, Brahman heifers exhibited increased cotyledonary blood vessel density irrespective of dietary treatments. These responses could be attributed to an increase in cotyledonary transcript abundance of angiogenic factors in Brahman vs. Angus. It is also important to note that B. indicus cattle typically have longer gestation lengths; therefore, the greater placental angiogenesis at 180 d in Brahman vs. Angus heifers may be even more apparent between breeds when considering the adjusted gestational age as a percent competed. Compensatory responses in ipsilateral UBF have been observed in cattle subjected to nutrient restriction during early gestation followed by realimentation for the last half of pregnancy. These compensatory mechanisms may develop in the cotyledon, although further investigations into late pregnancy realimentation are warranted. Lastly, the novel techniques used in detecting blood vessel density of the cotyledon appear promising in detecting specific macroscopic zones of increased angiogenesis.
Footnotes
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station. This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession number 1011100. Additional funding was provided by the U.S. Department of Agriculture, Agricultural Research Service, Biophotonic Initiative number 58-6402-3-018.
LITERATURE CITED
- Alexander G. 1964. Studies on the placenta of the sheep (Ovis aries L.). Placental size. J. Reprod. Fertil. 7:289–305. doi:10.1530/jrf.0.0070289 [DOI] [PubMed] [Google Scholar]
- Barcroft J. 1946. Researches on pre-natal life. Charles C Thomas, Springfield, IL. [Google Scholar]
- Bjorkman N. 1954. Morphological and histochemical studies on the bovine placenta. Acta Anat. (Basel). 22:1–91. [PubMed] [Google Scholar]
- Bjorkman N. H. 1969. Light and electron microscopic studies on cellular alterations in the normal bovine placentome. Anat. Rec. 163:17–29. doi: 10.1002/ar.1091630103 [DOI] [PubMed] [Google Scholar]
- Brockus K. E., Hart C. G., Gilfeather C. L., Fleming B. O., and Lemley C. O.. 2016. Dietary melatonin alters uterine artery hemodynamics in pregnant Holstein heifers. Domest. Anim. Endocrinol. 55:1–10. doi: 10.1016/j.domaniend.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Camacho L. E., Lemley C. O., Prezotto L. D., Bauer M. L., Freetly H. C., Swanson K. C., and Vonnahme K. A.. 2014. Effects of maternal nutrient restriction followed by realimentation during midgestation on uterine blood flow in beef cows. Theriogenology 81:1248–56.e1. doi: 10.1016/j.theriogenology.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Carr D. J., David A. L., Aitken R. P., Milne J. S., Borowicz P. P., Wallace J. M., and Redmer D. A.. 2016. Placental vascularity and markers of angiogenesis in relation to prenatal growth status in overnourished adolescent ewes. Placenta 46:79–86. doi: 10.1016/j.placenta.2016.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifert A. W., Wilson M. E., Vonnahme K. A., Camacho L. E., Borowicz P. P., Redmer D. A., Romero S., Dorsam S., Haring J., and Lemley C. O.. 2015. Effect of melatonin or maternal nutrient restriction on vascularity and cell proliferation in the ovine placenta. Anim. Reprod. Sci. 153:13–21. doi: 10.1016/j.anireprosci.2014.11.022 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Houck K., Jakeman L., and Leung D. W.. 1992. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 13:18–32. doi: 10.1210/edrv-13-1-18 [DOI] [PubMed] [Google Scholar]
- Ferrell C. L. 1991a. Maternal and fetal influences on uterine and conceptus development in the cow: I. Growth of tissues of the gravid uterus. J. Anim. Sci. 69:1945–1953. doi:10.2527/1991.6951945x [DOI] [PubMed] [Google Scholar]
- Ferrell C. L. 1991b. Maternal and fetal influences on uterine and conceptus development in the cow: II. Blood flow and nutrient flux. J. Anim. Sci. 69:1954–1965. doi:10.2527/1991.6951954x [DOI] [PubMed] [Google Scholar]
- Gonzalez J. M., Camacho L. E., Ebarb S. M., Swanson K. C., Vonnahme K. A., Stelzleni A. M., and Johnson S. E.. 2013. Realimentation of nutrient restricted pregnant beef cows supports compensatory fetal muscle growth. J. Anim. Sci. 91:4797–4806. doi: 10.2527/jas.2013-6704 [DOI] [PubMed] [Google Scholar]
- Habib M., Pollott G., and Leaver D.. 2011. Digestibility and nitrogen balance of high- and low quality forages supplemented with high- and low-protein concentrates fet to two breeds of cattle. J. Appl. Anim. Res. 39:303–310. doi:10.1080/09712119.2011.607891 [Google Scholar]
- Latham E. A., Weldon K. K., Wickersham T. A., Coverdale J. A., and Pinchak W. E.. 2018. Responses in the rumen microbiome of Bos taurus and indicus steers fed a low-quality rice straw diet and supplemented protein. J. Anim. Sci. 96:1032–1044. doi: 10.1093/jas/sky023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser R. 1975. [Development of contact between trophoblast and uterine epithelium during the early stages on implantation in the cow]. Zentralbl. Veterinarmed. C. 4:63–86. [PubMed] [Google Scholar]
- Leiser R., Krebs C., Klisch K., Ebert B., Dantzer V., Schuler G., and Hoffmann B.. 1997. Fetal villosity and microvasculature of the bovine placentome in the second half of gestation. J. Anat. 191:517–527. doi:10.1046/j.1469-7580.1997.19140517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long N. M., Prado-Cooper M. J., Krehbiel C. R., DeSilva U., and Wettemann R. P.. 2010. Effects of nutrient restriction of bovine dams during early gestation on postnatal growth, carcass and organ characteristics, and gene expression in adipose tissue and muscle. J. Anim. Sci. 88:3251–3261. doi: 10.2527/jas.2009-2512 [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutritional requirements of beef cattle. 7th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Reynolds L. P., Borowicz P. P., Caton J. S., Vonnahme K. A., Luther J. S., Buchanan D. S., Hafez S. A., Grazul-Bilska A. T., and Redmer D. A.. 2010. Uteroplacental vascular development and placental function: an update. Int. J. Dev. Biol. 54:355–366. doi: 10.1387/ijdb.082799lr [DOI] [PubMed] [Google Scholar]
- Reynolds W. L., DeRouen T. M., Moin S., and Koonce K. L.. 1980. Factors influencing gestation length, birth weight and calf survival of Angus, Zebu and Zebu cross beef cattle. J. Anim. Sci. 51:860–867. doi: 10.2527/jas1980.514860x [DOI] [PubMed] [Google Scholar]
- Reynolds L. P., and Redmer D. A.. 1995. Utero-placental vascular development and placental function. J. Anim. Sci. 73:1839–1851. doi:10.2527/1995.7361839x [DOI] [PubMed] [Google Scholar]
- Shibuya M., Yamaguchi S., Yamane A., Ikeda T., Tojo A., Matsushime H., and Sato M.. 1990. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5:519–524. [PubMed] [Google Scholar]
- Suri C., Jones P. F., Patan S., Bartunkova S., Maisonpierre P. C., Davis S., Sato T. N., and Yancopoulos G. D.. 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87:1171–1180. doi:10.1016/S0092-8674(00)81813-9 [DOI] [PubMed] [Google Scholar]
- Terman B. I., Dougher-Vermazen M., Carrion M. E., Dimitrov D., Armellino D. C., Gospodarowicz D., and Böhlen P.. 1992. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 187:1579–1586. doi:10.1016/0006-291X(92)90483-2 [DOI] [PubMed] [Google Scholar]
- Vonnahme K. A., Zhu M. J., Borowicz P. P., Geary T. W., Hess B. W., Reynolds L. P., Caton J. S., Means W. J., and Ford S. P.. 2007. Effect of early gestational undernutrition on angiogenic factor expression and vascularity in the bovine placentome. J. Anim. Sci. 85:2464–2472. doi: 10.2527/jas.2006-805 [DOI] [PubMed] [Google Scholar]
- Wallace L. R. 1948. The growth of lambs before and after birth in relation to the level of nutrition. Part III. Agric. Sci. 38:367–381. doi:10.1017/S0021859600006079 [Google Scholar]
- Zhu M. J., Du M., Hess B. W., Means W. J., Nathanielsz P. W., and Ford S. P.. 2007. Maternal nutrient restriction upregulates growth signaling pathways in the cotyledonary artery of cow placentomes. Placenta 28:361–368. doi: 10.1016/j.placenta.2006.04.005 [DOI] [PubMed] [Google Scholar]