Abstract
This study investigated the effects of supplementing sow diets with lysozyme during the late gestation to lactation stage on the performance of sows and their offspring. Sixty sows (Yorkshire × Landrace, 3 to 6 of parity) at day 85 of gestation were allocated to the following 3 dietary treatments: 1) sows fed a basal diet from late gestation to lactation (control, n = 20), 2) sows fed a basal diet with lysozyme 150 g/t (LZM 150, n = 20), and 3) sows fed a basal diet with lysozyme 300 g/t (LZM 300, n = 20). During the lactation period, sows fed diets containing lysozyme had increased average daily feed intake (ADFI) (P < 0.01) and decreased weaning-to-estrus interval (WEI, P < 0.05), but there were no significant effects on backfat during the trial among treatments. Sows fed lysozyme diets had increased (P < 0.05) serum concentration of total protein (TP) compared with those fed the control diets. Serum immunoglobulin M (IgM) of the sows increased (P < 0.05) on day 1 of lactation, immunoglobulin A (IgA) and interleukin-10 (IL-10) increased (P < 0.05) on day 7 of lactation, and immunoglobulin G (IgG) had a tendency to increase (P = 0.05) during the lactation. Milk concentration of IgA increased (P < 0.05) on day 1 and 7 of lactation and tended to be greater (P = 0.06) on day 21 of lactation. No significant differences among the dietary treatments were observed in placental tissue mRNA expression of interleukin-6 (IL-6), IL-10, tumor necrosis factor-α (TNF-α), polymeric immunoglobulin receptor (pIgR), or the concentrations of IL-6, IL-10, TNF-α, or secretory immunoglobulin A (sIgA). Moreover, there was a decrease (P < 0.05) in stillborn in sows fed lysozyme diets. The diarrhea rate decreased (P < 0.05) and serum concentrations of IgA, IgG, IgM, and IL-10 increased (P < 0.05) in piglets from sows fed the diets containing lysozyme compared with piglets from sows fed the control diet. The serum concentrations of TP increased (P < 0.05), and albumin (ALB) and globulin (GLB) had a tendency to increase (P = 0.08, P = 0.06) in piglets from sows fed the diets containing lysozyme compared with piglets from sows fed the control diet. In conclusion, this study indicates that feeding sows diets supplemented with lysozyme from the late gestation through lactation stage increased sow ADFI during the lactation, shortened the WEI, and improved the maternal and offspring health status as indicated by immunological characteristics and a reduced incidence of diarrhea in piglets.
Keywords: immunity, lysozyme, offspring, reproductive performance, sows
INTRODUCTION
Lysozyme (LZM) is a naturally occurring antimicrobial enzyme found in the tears, saliva, and milk of all mammals (Masschalck and Michiels, 2003), which is highly resistant to hydrolysis by acids and proteases and to digestion in the gastrointestinal tract (Humphrey et al., 2002). Lysozyme can act both as an enzyme and as an alternative to antibiotics (Humphrey et al., 2002; Liu et al., 2010; Nyachoti et al., 2012).
It has been demonstrated that the addition of lysozyme to weanling piglets diets improved feed efficiency by improving the gut health and nonspecific immunity, and supplementing 90 mg/kg lysozyme was as effective as antibiotics (20 mg/kg colistin sulphate + 50 mg/kg kitasamycin) in improving the growth performance of weanling piglets (Long et al., 2016). In a previous study in chickens, 40 mg lysozyme/kg diet could decrease Clostridium perfringens colonization, improve the intestinal barrier function, and improve the growth performance (Liu et al., 2010). Receiving a water-soluble lysozyme in drinking water of piglets at concentrations of 0.1 to 0.2% improved the piglet performance under challenge with Escherichia coli K88 to the same extent as antibiotics (Nyachoti et al., 2012). In addition, piglets consuming the lysozyme-treated liquid diet had improved their small intestinal morphology and growth performance (May et al., 2012; Oliver and Wells, 2013; Oliver et al., 2014). Lysozyme is a 1, 4-β-N-acetylmuramidase and functions by cleaving the β-1,4-glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine residues of the bacterial peptidoglycan, which results in the loss of cellular membrane integrity and cell death (Callewaert and Michiels, 2010). Hydrolysis of products produced from the loss of the bacterium cellular membrane stimulate immunoglobulin A (IgA) secretion, macrophage activation, and the rapid clearance of bacterial pathogens in the organism (Clarke et al., 2010; Silhavy et al., 2010). However, whether feeding lysozyme can improve the performance of sows and their offspring is still unknown.
Therefore, the objective of the present study is to investigate the effect of supplementing sow diets with lysozyme during late gestation and lactation stage on the performance and immunological characteristics of sows and their offspring.
MATERIALS AND METHOD
All animal procedures used in this study were approved by the Animal Care and Use Committee of Sichuan Agricultural University.
Animals and Experimental Designs
A total of 60 sows (Yorkshire × Landrace; 3 to 6 of parity) with an average backfat thickness at 14.07 ± 2.58 mm (mean ± SD), were randomly allocated to 3 treatment groups as follows: 1) sows fed a basal diet from day 85 of gestation to the end of weaning (on day 21 of lactation, control diet, n = 20), 2) sows fed a basal diet supplemented with 150 g/t lysozyme (LZM 150, 5,000 U/mg, Shanghai Longyou Biotechnology Co, Ltd, Shanghai, China, n = 20), and 3) sows fed a basal diet supplemented with 300 g/t lysozyme (LZM 300, n = 20). The powdered basal diet is presented in Table 1, which was formulated according to the nutrient requirements recommended by the National Research Council (NRC, 2012).
Table 1.
Ingredients composition and nutrient levels of the basal diets (as-fed basis)
| Content | ||
|---|---|---|
| Item | Gestation | Lactation |
| Ingredient, % | ||
| Corn | 60.00 | 62.00 |
| Wheat bran | 20.00 | 5.00 |
| Soybean meal (44% CP) | 16.00 | 24.00 |
| Oil powder1 | --- | 3.00 |
| Fish meal (66% CP) | --- | 2.00 |
| Vitamin and mineral premix2 | 0.18 | 0.17 |
| Limestone | 1.18 | 1.01 |
| Dicalcium phosphate | 1.08 | 1.44 |
| Salt | 0.40 | --- |
| Zeolite powder | 0.88 | 1.02 |
| L-Lys HCl (98.5%) | 0.01 | 0.07 |
| DL-Methionine (99%) | --- | 0.04 |
| L-Threonine (98.5%) | --- | 0.01 |
| Complex enzyme | --- | 0.03 |
| Oligosaccharide | --- | 0.02 |
| Vitamin E | --- | 0.02 |
| Phytase | 0.01 | 0.01 |
| Chromium picolinate | 0.01 | 0.01 |
| Sodium bicarbonate | 0.09 | --- |
| Magnesium sulfate (9%) | 0.08 | 0.07 |
| Choline chloride (50%) | 0.08 | 0.08 |
| Composition3 | ||
| DE, Mcal/kg | 3.04 | 3.29 |
| CP, % | 14.65 | 17.54 |
| Ca, % | 0.85 | 0.99 |
| P, % | 0.67 | 0.68 |
| Lys, % | 0.69 | 0.99 |
1Oil powder = 40% soybean oil + 60% expanded corn powder.
2Supplied the following per kg of diet: Zn 67 mg; Cu 13 mg; Fe 73 mg; Mn 33 mg; Co 0.13 mg; I 0.33 mg; Se 0.27 mg; vitamin A 12500 IU; vitamin D3 2000 IU; vitamin E 60 mg; vitamin K3 2.5 mg; vitamin B1 2.5 mg; vitamin B2 6.3 mg; vitamin B3 20 mg; vitamin B6 2.5 mg; vitamin B12 0.03 mg; Niacin 35 mg; Folic acid 3.0 mg; Biotin 0.3 mg.
3Crude protein (CP), Ca, P, and Lys are analyzed values. Digestible energy (DE), is a calculated value.
Sows were housed in individual gestation stalls (2.20 by 0.65 m) from day 85 until 106 of gestation. On day 107 of gestation, sows were moved to farrowing crates (2.50 by 1.80 m) which had a piglet creep area provided with a heat lamp and nipple waterer. Sows were fed an average of 3.5 kg/d before farrowing. During lactation, sows were fed 3 times daily at 0730, 1200, and 1600 h and had access to water ad libitum. On the parturition day, sows were fed 0.5 kg and the ration was gradually increased by 1.0 kg/d until the maximum ration was reached. Then, the sows had free access to feed during the following days of lactation. On the morning of weaning (day 21 of lactation), the sows were deprived of feed and moved into gestation stalls. Cross-fostering took place in a few cases (within 24 h of farrowing) within the same treatment group. The number of piglets per sow ranged from 9 to 12 piglets.
Sample Collection and Measurements
The backfat thickness of each sow was measured at the P2 position (left side of the 10th rib and 6 cm lateral to the spine) with a Digital Backfat Indicator (Renco Lean-Meater, Minneapolis, MN, USA) on d 85 and 100 of gestation and d 1 and 21 of lactation. After the sows farrowed, the number of total born, live born, stillborn, and neonatal weight (before colostrum consumption) were recorded. After weaning, the number of piglets that survived and the weaning weight were recorded. The diarrhea rate in piglets during lactation was recorded. The feed intake of the sows from parturition to weaning was recorded. Feed disappearance was determined every day during lactation; the average daily feed intake (ADFI) was calculated. The weaning-to-estrus interval (WEI) was monitored daily at 0830 and 1530 h by boar stimulation. In order to calculate the WEI and breeding rate (BR), the beginning of the estrus period was characterized as the midpoint between the time of the first observed positive response to back pressure (immobilization reflex). Thirty days after mating, a Digital Handheld Scanner (KX5100, Xuzhou Kaixin Electronic Instrument Co., Ltd, Jiangsu, China) was used to check whether the sows conceived to calculate the conception rate (CR).
On day 1, 7, and 21 of lactation, 10 mL blood samples were collected from the ear vein of 8 sows per treatment after an overnight fast (12 h). On the day of weaning, 5 mL blood samples were collected from the jugular vein of 8 piglets (the 8 piglets were from the same 8 sows for blood collection, from each sow randomly selected 1 piglet) per treatment. Blood samples were centrifuged at 4 °C, 3,000 × g for 15 min to obtain the serum which was stored at −20 °C for further analysis. Approximately 30 mL of milk was collected from 8 sows per treatment on day 1, 7, and 21 of lactation by infusing the sow with 1 mL of oxytocin (Ningbo Sansheng Pharmaceutical Co. Ltd., Ningbo, China) via ear vein injection and then manually milking from the first and fourth mammary glands on one side of the sow. Approximately 5 mL of the milk was collected in tubes, followed by centrifugation at 8,000 × g at 4 °C for 15 min to obtain milk serum. Milk and milk serum samples were immediately frozen at −20 °C until further analysis. After parturition, placental tissue samples (the end of the part) were immediately collected from 8 sows per treatment and frozen in liquid nitrogen and stored at −80 °C until analysis. Placental tissue was homogenized in 10 volumes (w/v) of ice-cold physiological saline, centrifuged at 3,000 × g for 15 min at 4 °C; then, the supernatant was conserved for enzyme activity analysis.
Milk and Serum Analyses
The milk samples were thawed at 4 °C, then the lactose, fat, protein, and total solids were determined using an infrared milk analyzer (MilkoScan, Foss Electric, Hillerød, Denmark). The serum samples were thawed at 4 °C, then total protein (TP), albumin (ALB), globulin (GLB), glutamate pyruvic transaminase (GPT), and glutamate oxaloacetic transaminase (GOT) were determined using an Automatic Analyzer (Hitachi 7020, Hitachi High-Technologies Corporation, Tokyo, Japan). The lysozyme content in blood samples of sows and weaned piglets and milk serum of sows were determined using commercial kits (Jiancheng Institute of Biological Technology, Nanjing, China), according to the manufacturer’s instructions, and the absorbance was determined using a spectrophotometer (Beckman Coulter DU-800; Beckman Coulter, Inc., CA, USA).
Immunoglobulins and Cytokines Analysis
Blood samples from the sows and piglets were analyzed for the concentrations of IgA, immunoglobulin G (IgG), immunoglobulin M (IgM), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α), placental tissue supernatant was analyzed for the concentrations of secretory immunoglobulin A (sIgA), IL-6, IL-10, TNF-α, and the milk serum concentration of IgA were measured using enzyme-linked immunosorbent assay (ELISA) Kits (Jiancheng Institute of Biological Technology, Nanjing, China). All of the immunoglobulin and cytokine analysis was done referring to the manufacturer’s instructions, and each sample was analyzed in duplicate. Briefly, 50 µL of the standard solution or the serum samples were added to plate wells, then 50 µL of biotinylated antibody working solution was added, the plate was sealed and mixed before incubation at 37 °C for 60 min, after the incubation completely washed 5 times by washing buffer. Fifty microliters of horseradish peroxidase were then added to each well, the plate was sealed, and mixed before incubation at 37 °C for 60 min, after the incubation completely washed 5 times by washing buffer. Then, 50 µL of Chromogen Solution A and 50 µL of Chromogen Solution B were added to each well and incubated at 37 °C in the dark for 15 min, then 50 µL stop solution was added. After completely mixing, the optical density (OD) values of standards and serum samples were determined at 450 nm within 15 min (MuLtisKan MK3, Thermo Labsystems, CA, USA). The concentrations of serum IgA, IgG, IgM, IL-6, IL-10, and TNF-α were quantified according to the produced standard curve. The minimal detection limit for IgA, IgG, IgM, IL-6, IL-10, and TNF-α was as follows: 3.12 μg/mL, 15.6μg/mL, 2.5μg/mL, 12.5 ng/L, 5 ng/L, and 7 ng/L.
Total RNA Extraction and Real-Time RT-PCR
Total RNA was extracted from the frozen samples of placental tissue of sow using Trizol reagent (TaKaRa Biotechnology, Dalian, China), according to the manufacturer’s instructions. Agarose gel electrophoresis was conducted to detect the integrity of the RNA. RNA purity was determined using a nucleic acid/protein analyzer (Beckman DU-800, Beckman Coulter, Inc., CA, USA) by evaluating the OD260/OD280 ratio. Both genomic DNA removal and reverse transcription (RT) were performed using a PrimeScript RT reagent kit with gDNA eraser (TaKaRa Biotechnology, Dalian, China) according to the manufacturer’s instructions. Real-time PCR was performed to analyze mRNA expression using SYBR Premix Ex TaqTM Kits (TaKaRa Biotechnology, Dalian, China). A total volume of 10 µL reaction system contained 5.0 μL SYBR Premix Ex Taq (2×), 0.4 μL forward primer (10 μM, Table 2), 0.4 μL reverse primer (10 μM), 0.2 μL ROX Reference Dye (50×), 1.0 μL cDNA, and 3.0 μL double-distilled water. Cycling conditions were 95 °C for 30 s, then 40 cycles at 95 °C for 5 s, and 60 °C for 34 s. At the end of amplification, melting curve analysis was performed to verify specific amplifications by an ABI-7900HT Fast Real-Time PCR System (Applied Biosystems, CA, USA). The products were electrophoresed on agarose gel to confirm the product size also. The identity of each product was further confirmed by DNA sequence analysis. Negative controls were performed in which water was substituted for cDNA. The experiment was repeated 3 times. The β-actin was used as a housekeeping gene to normalize the expression of target genes according to the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Primer sequences of the target and reference genes
| Gene1 | Primer sequence (5′-3′) | Product (bp) | GenBank accession |
|---|---|---|---|
| IL-6 | Forward: CAAAGCCACCACCCCTAACC Reverse: GACGGCATCAATCTCAGGTG |
180 | NM_214399.1 |
| IL-10 | Forward: GGGTGTGCCCTATGGTGTTC Reverse: GGGTGGGTAGGCTTGGAATG |
112 | NM_214041.1 |
| TNF-α | Forward: CGACTCAGTGCCGAGATCAA Reverse: CTCACAGGGCAATGATCCCA |
85 | NM_214022.1 |
| pIgR 2 | Forward: ACGAGAGAACGAAGGTGTGG Reverse: AAATCGTCTCCCCATCCACC |
95 | XM_021102216.1 |
| β-actin | Forward: GGCGCCCAGCACGAT Reverse: CCGATCCACACGGAGTACTTG |
102 | DQ452569.1 |
1 IL-6 = interleukin-6, IL-10 = interleukin-10, TNF-α = tumor necrosis factor-α, pIgR = polymeric immunoglobulin receptor.
2It was observed that the exercise-induced secretory immunoglobulin A (sIgA) suppression was probably caused by a decline in pIgR mRNA expression (Kimura et al., 2008). Wu et al. (2016) used the pIgR mRNA expression to reflect the expression of sIgA. Herein, we use pIgR mRNA to reflect the expression of sIgA in placental tissue.
Statistical Analysis
Sows and their litters were regarded as the experimental units. Original data were checked using Grubbs’ test method. If |Xp-¯X|>λ(α, n) S, Xp was considered as the outlier. Descriptive statistics was performed to check for normality and homogeneity of variances. All of the data were tested for normal distribution; then, data were analyzed by General Linear Model procedures of SAS (V9.3, SAS Institute Inc., Cary, NC, USA). The least significant difference test was used to compare the group means when the F test in the analysis of variance table was significant. Then, multiple comparison by DUNCAN analysis was used to determine statistical differences between groups. The results were presented as the mean values with pooled SEM. Differences at P < 0.05 were considered to be statistically significant, whereas a tendency was considered when 0.05 < P < 0.10.
RESULTS
Sow and Litter Growth Performance
As shown in Table 3, sows fed 150 and 300 g/t lysozyme diets both increased ADFI during the lactation period (P < 0.01) and decreased WEI (P < 0.05) compared with the control diet, but there was no difference between the 2 lysozyme treatments. However, supplemented 150 or 300 g/t lysozyme had no effect on sow’s BR and CR. In addition, over the late gestation and lactation period, sows fed lysozyme diets had no effect on the backfat thickness.
Table 3.
Effects of supplementation with lysozyme during the late gestation and lactation on the performance of sows and piglets1
| Treatment | |||||
|---|---|---|---|---|---|
| Item | Control | LZM 150 | LZM 300 | SEM | P-value |
| Sows2 | |||||
| Backfat thickness, mm | |||||
| Day 85 of gestation | 14.56 | 14.35 | 14.45 | 0.29 | 0.96 |
| Day 100 of gestation | 15.72 | 15.40 | 15.70 | 0.31 | 0.89 |
| Day 1 of lactation | 16.06 | 16.25 | 16.50 | 0.32 | 0.86 |
| Weaning | 13.22 | 13.25 | 13.80 | 0.31 | 0.69 |
| Gestation increase kg | 1.50 | 1.90 | 2.05 | 0.14 | 0.29 |
| Lactation loss kg | 2.83 | 3.00 | 2.70 | 0.26 | 0.89 |
| ADFI3, kg/d | 5.26b | 5.70a | 5.80a | 0.06 | <0.01 |
| WEI, d | 5.14a | 4.58b | 4.56b | 0.10 | 0.04 |
| BR, % | 90.00 | 95.00 | 95.00 | 0.03 | 0.76 |
| CR, % | 76.80 | 90.00 | 85.00 | 0.04 | 0.34 |
| Litter size | |||||
| Total born | 11.71 | 11.88 | 11.94 | 0.31 | 0.95 |
| Alive at birth | 10.31 | 10.21 | 11.00 | 0.33 | 0.56 |
| Weakling | 0.39 | 0.15 | 0.40 | 0.09 | 0.46 |
| Stillborn | 0.89a | 1.10a | 0.15b | 0.16 | 0.03 |
| Piglets4 | |||||
| Neonatal weight, kg | 1.54 | 1.57 | 1.64 | 0.03 | 0.39 |
| Weaning weight, kg | 6.21 | 6.39 | 6.40 | 0.07 | 0.34 |
| Diarrhea rate 5 (%) | 2.24a | 1.67b | 1.41b | 0.01 | 0.03 |
1LZM 150 = control diet + lysozyme 150 g/t, LZM 300 = control diet + lysozyme 300 g/t. ADFI = average daily feed intake, WEI = weaning-to-estrus interval, BR = rebreeding rate, CR = conception rate.
2Data are means of 20 sows.
3Average daily feed intake during the lactation.
4Data are means of 20 litters from 20 sows.
5Diarrhea rate = total diarrhea piglets × diarrhea days / [litter size at birth (live) × trial days].
a,bWithin a row, means with different superscripts are different (P < 0.05).
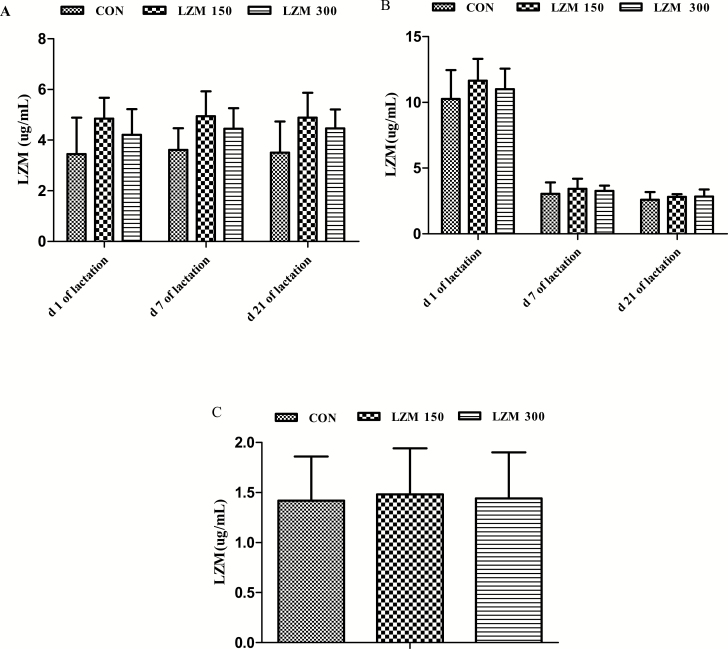
Sows fed 300 g/t lysozyme diets had decreased stillborn piglets (P < 0.05) when compared to sows fed a control diet and the 150 g/t lysozyme diets. But no difference in total born, alive at birth, weakling piglets, and neonatal weight, weaning weight was observed among the treatments. Additionally, the diarrhea rate of piglets from sows fed 150 or 300 g/t lysozyme diets was markedly reduced (P < 0.05) when compared with that in the control treatment, although lysozyme concentration was not different in serum and milk of sows and serum of weaned piglets (Fig. 1) among the treatments.
Figure 1.
The effect of feeding sows diets supplemented with lysozyme on concentrations of lysozyme in serum (A) and milk (B) of sows and serum of weaned piglets (C). CON = control, LZM 150 = control diet + lysozyme 150 g/t, LZM 300 = control diet + lysozyme 300 g/t. Data is presented as mean ± standard error. Data are means of 8 sows (A and B) or 8 piglets from 8 sows (C).
Composition of Colostrum and Milk
There were no differences among the treatments in the composition of colostrum with regards to fat, lactose, protein, and total solids (Table 4). In addition, for milk on day 7 and 21 of lactation, no significant difference in the milk composition of fat, lactose, protein, and total solids were observed among the treatments.
Table 4.
Effects of feeding sows diets supplemented with lysozyme on the composition of milk and milk concentrations of immunoglobulin1
| Treatment | |||||
|---|---|---|---|---|---|
| Item | Control | LZM 150 | LZM 300 | SEM | P-value |
| Colostrum, % d 1 of lactation | |||||
| Fat | 3.11 | 3.28 | 3.30 | 0.15 | 0.88 |
| Lactose | 12.62 | 12.17 | 12.22 | 0.47 | 0.93 |
| Protein | 8.15 | 7.75 | 7.82 | 0.21 | 0.73 |
| Total solids | 22.01 | 20.95 | 20.15 | 0.48 | 0.30 |
| IgA (mg/mL) | 3.21b | 3.54a | 3.51a | 0.06 | 0.04 |
| Milk, % | |||||
| Day 7 of lactation | |||||
| Fat | 6.35 | 6.49 | 7.03 | 0.16 | 0.23 |
| Lactose | 6.06 | 6.10 | 6.10 | 0.19 | 0.99 |
| Protein | 3.82 | 3.9 | 3.92 | 0.07 | 0.86 |
| Total solids | 10.81 | 10.70 | 10.55 | 0.13 | 0.71 |
| IgA (mg/mL) | 1.84b | 2.13a | 2.11a | 0.05 | 0.03 |
| Day 21 of lactation | |||||
| Fat | 5.04 | 5.84 | 5.40 | 0.23 | 0.38 |
| Lactose | 5.49 | 5.16 | 5.24 | 0.19 | 0.79 |
| Protein | 3.73 | 3.60 | 3.63 | 0.08 | 0.83 |
| Total solids | 10.00 | 9.40 | 9.50 | 0.20 | 0.47 |
| IgA (mg/mL) | 1.91 | 2.14 | 2.18 | 0.05 | 0.06 |
1LZM 150 = control diet + lysozyme 150 g/t, LZM 300 = control diet + lysozyme 300 g/t, IgA = immunoglobulin A. Data are means of 8 sows.
a,bWithin a row, means with different superscripts are different (P < 0.05).”
Compared with control diets, sows fed 150 and 300 g/t lysozyme diets had increased (P < 0.05) milk concentrations of IgA on day 1 and 7 of lactation and tended to have increased IgA (P = 0.06) on day 21 of lactation. However, there was no significant difference in milk IgG and IgM content among the treatments (data not shown).
Serum Concentrations of Biochemistry
Compared with control diets, sows fed 150 and 300 g/t lysozyme diets both had increased the serum concentration of TP on day 1 of lactation (Table 5). However, there were no significant differences in TP, ALB, GLB, GPT, and GOT among the treatments on day 7 and 21 of lactation. On the day of weaning, piglets from sows fed the 150 and 300 g/t lysozyme diets both increased the serum concentration of TP (P < 0.05), ALB (P = 0.08), and GLB (P = 0.06) compared with piglets from sows fed the control diet.
Table 5.
Effects of feeding sows diets supplemented with lysozyme on serum biochemistry of sows and piglets1
| Treatment | |||||
|---|---|---|---|---|---|
| Item | Control | LZM 150 | LZM 300 | SEM | P-value |
| Sows2 | |||||
| Day 1 of lactation | |||||
| TP (g/L) | 70.42b | 75.83a | 75.46a | 0.81 | <0.01 |
| ALB (g/L) | 35.40 | 37.84 | 37.27 | 0.52 | 0.13 |
| GLB (g/L) | 35.01 | 38.15 | 38.19 | 0.76 | 0.14 |
| GPT (U/L) | 46.02 | 43.24 | 42.88 | 1.47 | 0.66 |
| GOT (U/L) | 38.86 | 35.33 | 34.76 | 1.75 | 0.60 |
| Day 7 of lactation | |||||
| TP (g/L) | 81.77 | 82.73 | 85.51 | 0.98 | 0.28 |
| ALB (g/L) | 36.83 | 36.31 | 36.79 | 0.36 | 0.82 |
| GLB (g/L) | 44.95 | 46.42 | 48.73 | 1.02 | 0.33 |
| GPT (U/L) | 47.74 | 41.09 | 40.72 | 2.01 | 0.30 |
| GOT (U/L) | 32.23 | 28.80 | 28.74 | 1.07 | 0.33 |
| Day 21 of lactation | |||||
| TP (g/L) | 80.07 | 82.61 | 84.06 | 0.79 | 0.10 |
| ALB (g/L) | 35.69 | 37.30 | 38.40 | 0.57 | 0.14 |
| GLB (g/L) | 44.39 | 45.30 | 45.65 | 0.84 | 0.82 |
| GPT (U/L) | 45.47 | 44.82 | 41.83 | 2.01 | 0.76 |
| GOT (U/L) | 30.76 | 27.83 | 25.59 | 1.56 | 0.39 |
| Piglets3 | |||||
| Day 21 of lactation | |||||
| TP (g/L) | 57.02b | 63.70a | 63.97a | 0.98 | <0.01 |
| ALB (g/L) | 33.04 | 36.08 | 35.10 | 0.58 | 0.08 |
| GLB (g/L) | 23.98 | 27.62 | 28.87 | 0.92 | 0.06 |
| GPT (U/L) | 58.23 | 53.21 | 53.57 | 2.61 | 0.73 |
| GOT (U/L) | 58.67 | 53.09 | 55.17 | 3.16 | 0.79 |
1LZM 150 = control diet + lysozyme 150 g/t, LZM 300 = control diet + lysozyme 300 g/t.
ALB = albumin, GLB = globulin, GPT = glutamate pyruvic transaminase, GOT = glutamate oxaloacetic transaminase, TP = total protein.
2Data are means of 8 sows.
3Data are means of 8 piglets from 8 sows.
a,bWithin a row, means with different superscripts are different (P < 0.05).
Serum Concentrations of Immunoglobulins and Cytokines
Compared with control diets, sows fed 150 and 300 g/t lysozyme diets both increased (P < 0.05) the serum concentration of IgM on day 1 of lactation and increased (P < 0.05) the serum concentration of IgA and IL-10 on day 7 of lactation (Table 6), but there was no difference between the 2 lysozyme treatments. Compared with sows fed control diets, sows fed the lysozyme diets had a tendency to improve serum concentrations of IgA, IgG, and IL-10 (P = 0.06, P = 0.05, P = 0.08) on day 1 of lactation, and had a tendency to increase serum concentrations of IgG, IgM (P = 0.08, P = 0.09) on day 7 and IgM (P = 0.05) on day 21 of lactation.
Table 6.
Effects of feeding sows diets supplemented with lysozyme on serum immunoglobulin and cytokines of sows and piglets1
| Treatment | |||||
|---|---|---|---|---|---|
| Item | Control | LZM 150 | LZM 300 | SEM | P-value |
| Sows2 | |||||
| Day 1 of lactation | |||||
| IgA (mg/mL) | 3.85 | 4.21 | 4.23 | 0.08 | 0.06 |
| IgG (mg/mL) | 0.64 | 0.86 | 0.87 | 0.04 | 0.05 |
| IgM (mg/mL) | 0.81b | 1.01a | 0.98a | 0.03 | <0.01 |
| IL-6 (ng/L) | 58.41 | 56.48 | 56.08 | 2.06 | 0.90 |
| IL-10 (ng/L) | 250.78 | 280.4 | 274.06 | 5.82 | 0.08 |
| TNF-α (ng/L) | 122.68 | 119.54 | 121.1 | 2.26 | 0.86 |
| Day 7 of lactation | |||||
| IgA (mg/mL) | 4.31b | 4.72a | 4.70a | 0.07 | 0.01 |
| IgG (mg/mL) | 0.60 | 0.69 | 0.72 | 0.02 | 0.08 |
| IgM (mg/mL) | 0.89 | 0.89 | 0.93 | 0.02 | 0.09 |
| IL-6 (ng/L) | 67.09 | 63.88 | 63.58 | 0.91 | 0.23 |
| IL-10 (ng/L) | 266.67b | 287.36a | 284.09a | 3.50 | 0.02 |
| TNF-α (ng/L) | 123.38 | 119.54 | 121.65 | 3.6 | 0.98 |
| Day 21 of lactation | |||||
| IgA (mg/mL) | 4.54 | 4.81 | 4.89 | 0.15 | 0.62 |
| IgG (mg/mL) | 0.60 | 0.65 | 0.66 | 0.02 | 0.57 |
| IgM (mg/mL) | 0.74b | 0.85a | 0.91a | 0.02 | 0.05 |
| IL-6 (ng/L) | 72.91 | 70.70 | 71.17 | 1.24 | 0.76 |
| IL-10 (ng/L) | 235.84 | 251.11 | 250.36 | 3.89 | 0.20 |
| TNF-α (ng/L) | 107.76 | 108.13 | 106.69 | 1.98 | 0.95 |
| Piglets3 | |||||
| Day 21 of lactation | |||||
| IgA (mg/mL) | 2.16b | 2.62a | 2.56a | 0.07 | 0.01 |
| IgG (mg/mL) | 2.25b | 2.77a | 2.65a | 0.08 | <0.01 |
| IgM (mg/mL) | 23.98b | 27.62a | 28.87a | 0.06 | 0.04 |
| IL-6 (ng/L) | 52.82 | 50.70 | 50.77 | 0.64 | 0.34 |
| IL-10 (ng/L) | 209.60b | 256.59a | 239.21a | 6.96 | 0.01 |
| TNF-α (ng/L) | 54.42 | 51.79 | 51.59 | 1.32 | 0.66 |
1LZM 150 = control diet + lysozyme 150 g/t, LZM 300 = control diet + lysozyme 300 g/t, IgA = immunoglobulin A, IgG = immunoglobulin G, IgM = immunoglobulin M, IL-6 = interleukin-6, IL-10 = interleukin-10, TNF-α = tumor necrosis factor-α.
2Data are means of 8 sows.
3Data are means of 8 piglets from 8 sows.
a,bWithin a row, means with different superscripts are different (P < 0.05).
On the day of weaning, piglets farrowed by sows which were fed lysozyme diets had increased (P < 0.05) serum concentrations of IgA, IgG, IgM, and IL-10 when compared with the piglets farrowed by sows fed the control diets. But there was no difference between the 2 levels of lysozyme treatments.
Cytokine Gene Expression in the Placental Tissue
There was no difference in the mRNA expression of IL-6, IL-10, TNF-α, and pIgR (Table 7) in the placental tissue of sows among the dietary treatments. Similarly, no difference was found in the concentrations of IL-6, IL-10, TNF-α, and sIgA in the placental tissue of sows among the treatments.
Table 7.
Effects of feeding sows diets supplemented with lysozyme on placental tissue of sows mRNA expression, and concentration of cytokines and immunoglobulin1
| Treatment | |||||
|---|---|---|---|---|---|
| Item | Control | LZM 150 | LZM 300 | SEM | P-value |
| mRNA expression | |||||
| IL-6 | 1.00 | 1.01 | 0.96 | 0.06 | 0.95 |
| IL-10 | 1.00 | 1.09 | 1.13 | 0.06 | 0.60 |
| TNF-α | 1.00 | 0.96 | 0.95 | 0.07 | 0.95 |
| pIgR | 1.00 | 1.05 | 1.02 | 0.06 | 0.92 |
| Concentration of cytokines and sIgA | |||||
| IL-6, ng/g | 673.86 | 681.47 | 688.19 | 10.83 | 0.87 |
| IL-10, mg/g | 4.17 | 4.54 | 4.49 | 0.10 | 0.28 |
| TNF-α, ng/g | 624.1 | 612.39 | 610.12 | 10.54 | 0.87 |
| sIgA, ng/g | 128.98 | 132.03 | 133.58 | 3.31 | 0.86 |
1LZM 150 = control diet + lysozyme 150 g/t, LZM 300 = control diet + lysozyme 300 g/t, IL-6 = interleukin-6, IL-10 = interleukin-10, TNF-α = tumor necrosis factor-α, pIgR = polymeric immunoglobulin receptor, sIgA = secretory immunoglobulin A. Data are means of 8 sows.
DISCUSSION
Previous studies have shown the beneficial effects of lysozyme supplementation on the growth performance (May et al., 2012; Oliver et al., 2014; Long et al., 2016), intestinal morphology (May et al., 2012; Oliver and Wells, 2013), intestinal microbiota (Cooper et al., 2013), and immunity (Nyachoti et al., 2012; Cooper et al., 2013) of piglets. However, there are few reports investigating lysozyme use as an additive in the diet of sows. In the present study, we showed that sows consuming diets with lysozyme supplementation had increased ADFI during the lactation period and decreased WEI when compared with sows fed the diets without lysozyme supplementation. Similar to a previous report showing that piglets fed the diets supplemented with lysozyme had increased ADFI (May et al., 2012). However, there are also inconsistent reports showing that piglets consuming diets with lysozyme did not increase ADFI (Oliver and Wells, 2013; Oliver et al., 2014). The discrepancies between studies might be due to the variations of lysozyme levels in the treatment diets, furthermore, the physiological stage (age) of the pig and the feeding length were different. For the WEI, this could be attributed to high ADFI reducing the weight loss of the sows during the lactation period, so that the sows have better physical condition resulting in entering estrus earlier.
In addition, sows fed lysozyme supplemented diets had decreased stillborn piglets. However, there were no differences in litter size, weight at birth, and weight at weaning among the dietary treatments. In agreement with the findings of the present study, Maga et al. (2006) and Brundige et al. (2008) did not notice any differences in the growth rate of pigs consuming human lysozyme from transgenic goat milk. The relatively short timeframe, of lysozyme supplementation, may explain there being no change in the litter size and weight at birth. It is also important to note that it is not clear how much lysozyme in the sow milk was actually consumed by the piglets. In this study, there were no effects of sows fed lysozyme supplemented diets on the concentrations of lysozyme in the serum and milk of sows and serum of weaned piglets among the treatments. The amount of lysozyme was sufficient to alter intestinal morphology and immunity but may not have been enough to improve their growth performance (Oliver and Wells, 2013). Lysozyme supplementation in this study also had decreased diarrhea rate in piglets when compared with sows fed a control diet. Similarly, Wells et al. (2015) added lysozyme to the diet of piglets and found that the amount of Campylobacter in their feces was significantly reduced. Cooper et al. (2013) showed that piglets fed the milk from transgenic goats, which produce in their milk human lysozyme (hLZ-milk), had a significantly lower diarrhea rate than the control group.
Sows consuming nutritive supplemented diets have shown altered milk composition (Lauridsen and Danielsen, 2004). However, in this study, sows fed lysozyme diets had no effect on milk composition. Currently, in the literature, there are few reports investigating the milk composition of sows consuming lysozyme rich diets. It appears the amount or the nature of the lysozyme supplementations has not been adequate to change the milk composition. It has been found that lysozyme supplementation does not change the composition of milk unlike some nutrients, such as fat (Chapman et al., 2000; Jones et al., 2002; Wang et al., 2017).
The concentrations of TP, ALB, GLB, GPT, and GOT in serum are indicators of the level of liver metabolism and health. The metabolic response of pigs to a low protein diet was abnormal, as indicated by lower ALB concentrations in their serum and GOT, GPT in their liver (Gómez et al., 2002; Figueroa et al., 2003; Che et al., 2017). However, there are not many reports about serum biochemical indexes of sows. In this study, compared with control diets, sows fed lysozyme diets had increased serum concentration of TP on day 1 of lactation. The piglets from sows fed lysozyme diets had increased serum concentration of TP, ALB, and GLB compared with piglets from sows fed the control diets. These results indicate that lysozyme may be beneficial to metabolism in the liver and the health of the sows.
Lysozyme is an important modulator of nonspecific immunity in the host animal (Saurabh and Sahoo, 2008; Moynihan and Clarke, 2011) and mammalian milk (Salmon et al., 2009). Cytokines such as IL-1β and TNF-α are known to mediate and have function in the inflammatory response (Dinarello, 1988; Akira et al., 1990). In addition to regulating the immune response, cytokines have a profound effect on nutrient metabolism. Proinflammatory cytokines redirect nutrients toward the immune response and away from growth processes (Johnson, 1997; Spurlock, 1997). In the previous studies, piglets offered lysozyme had no effects on the concentration of serum IgA over the course of the trials (May et al., 2012; Oliver and Wells, 2013). However, the effect of oral lysozyme on systemic nonspecific immunity in sows is still not conclusive. In the present research, sows fed lysozyme diets had increased the serum concentration of IgM on day 1 of lactation, IgA and IL-10 on day 7 of lactation, and had a tendency to increase serum concentration of IgA, IgG, IL-10, and IgM during the lactation period. Sows fed lysozyme diets had an increased concentration of IgA in their milk on day 1 and 7 of lactation and tended to have greater IgA in their milk on day 21 of lactation. The possible reason for a lack of response in piglets may be due to differing amounts of lysozyme consumed, and differences in age and body condition between sows and piglets. In this study, the piglets from sows fed lysozyme diets had increased serum concentrations of IgA, IgG, IgM, and IL-10. May et al. (2012) found that pigs fed diets supplemented with lysozyme had no effect on the serum concentration of IgA, whereas Ma et al. (2017) noted that piglets fed diets supplemented with lysozyme showed a significant decrease in the serum concentration of IgM but no effect on IgG content, which is likely due to lower immune activation by the lysozyme supplementation. In our study, the amount of lysozyme seems sufficient to alter sow immunity and provides high-quality breast milk (higher IgA) for piglets, consequently improving the immunity of piglets. This is similar to a study conducted by Cooper et al. (2013), which reported that sustainably providing a direct source of lysozyme rich milk from transgenic goats to piglets can improve the piglets’ health.
Immunoglobulins and cytokines are important indicators of immune status and response to inflammation (Ye et al., 2006; Praveena et al., 2010). For example, in a colitis porcine model, lysozyme was observed to upregulate the mRNA abundance of the anti-inflammatory cytokines IL-4 and TGF-β (Lee et al., 2009). Moreover, another study reported that pigs fed lysozyme diets had lower TNF-α (Oliver et al., 2014). In their study, most piglets received lysozyme at a younger age and for a shorter time, during which time the piglets are more susceptible to stress from the rearing environment. However, in our study, we did not see a difference in the mRNA expression of IL-6, IL-10, TNF-α, and pIgR in the placental tissue of sows among the dietary treatments. And there was no significant difference in the concentrations of IL-6, IL-10, TNF-α, and sIgA in the placental tissue of sows. These findings are similar to previous studies reporting that piglets fed lysozyme diets had no change in the mRNA abundance of TNF-α and IL-8 in gut tissue (Cooper et al., 2013; Long et al., 2016). This may be caused by the difference in the age and physiological state of the sows and piglets; compared with piglets, sows have a more mature immune system able to respond to inflammation.
CONCLUSION
The present study demonstrates that supplementing lysozyme in the diet of sows from the late gestation to lactation stage increased the performance and immunity of both the sows and piglets. These beneficial effects of lysozyme on sows and their offspring may be attributable to the following factors: promoted sow reproductive performance, which is indicated by the increased ADFI and shortened WEI of the sows; improved immunity of the sows, which is indicated by the increased concentrations of serum TP, IgM, IgA, and IL-10 and milk IgA; and improved offspring performance, which is indicated by the reduction in stillborns and piglets’ diarrhea rate; improved immunity of the offspring, which is indicated by the increased serum concentrations of TP, IgA, IgG, IgM, and IL-10 in piglets. Therefore, lysozyme may be included in sow diets as a dietary strategy to improve the performance of sows and their offspring.
Footnotes
The authors would like to thank Shanghai Longyou Biotechnology Co. Ltd for financial support of this study. This work was also supported by the Application and Demonstration of High-efficiency and Safe Breeding Technology for High-yield Breeding Pigs Project (2018YFD0501005) and Sichuan Agricultural University Double Support Project. The authors would like to thank Dr. Paul Dyce for his careful editing of this manuscript. Author disclosures: S.Y.X., J.K.S., X.L.S., Y.P.D., X.L.W., Z.M.L., Z.F.F., Y.L., L.Q.C., J.L., B.F., J.P.W., D.W., and Y.P.S. declare no conflicts of interest.
LITERATURE CITED
- Akira S., Hirano T., Taga T., and Kishimoto T.. 1990. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). Faseb J. 4:2860–2867. doi:10.1096/fasebj.4.11.2199284 [PubMed] [Google Scholar]
- Brundige D. R., Maga E. A., Klasing K. C., and Murray J. D.. 2008. Lysozyme transgenic goats’ milk influences gastrointestinal morphology in young pigs. J. Nutr. 138:921–926. doi: 10.1093/jn/138.5.921 [DOI] [PubMed] [Google Scholar]
- Callewaert L. and Michiels C. W.. 2010. Lysozymes in the animal kingdom. J. Biosci. 35:127–160. doi:10.1007/s12038-010-0015-5 [DOI] [PubMed] [Google Scholar]
- Chapman C., Morgan L. M., and Murphy M. C.. 2000. Maternal and early dietary fatty acid intake: changes in lipid metabolism and liver enzymes in adult rats. J. Nutr. 130:146–151. doi: 10.1093/jn/130.2.146 [DOI] [PubMed] [Google Scholar]
- Che L. Q., Peng X., Hu L., Wu C., Xu Q., Fang Z. F., Lin Y., Xu S. Y., Li J., Feng B., et al. 2017. The addition of protein-bound amino acids in low-protein diets improves the metabolic and immunological characteristics in fifteen- to thirty-five-kg pigs. J. Anim. Sci. 95:1277–1287. doi: 10.2527/jas.2016.0990 [DOI] [PubMed] [Google Scholar]
- Clarke T. B., Davis K. M., Lysenko E. S., Zhou A. Y., Yu Y., and Weiser J. N.. 2010. Recognition of peptidoglycan from the microbiota by nod1 enhances systemic innate immunity. Nat. Med. 16:228–231. doi: 10.1038/nm.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. A., Garas Klobas L. C., Maga E. A., and Murray J. D.. 2013. Consuming transgenic goats’ milk containing the antimicrobial protein lysozyme helps resolve diarrhea in young pigs. Plos One 8:e58409. doi: 10.1371/journal.pone.0058409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. 1988. Biology of interleukin 1. Faseb J. 2:108–115. doi: 10.1096/fasebj.2.2.3277884 [PubMed] [Google Scholar]
- Figueroa J. L., Lewis A. J., Miller P. S., Fischer R. L., and Diedrichsen R. M.. 2003. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J. Anim. Sci. 81:1529–1537. doi: 10.2527/2003.8161529x [DOI] [PubMed] [Google Scholar]
- Gómez R. S., Lewis A. J., Miller P. S., and Chen H. Y.. 2002. Growth performance, diet apparent digestibility, and plasma metabolite concentrations of barrows fed corn-soybean meal diets or low-protein, amino acid-supplemented diets at different feeding level. J. Anim. Sci. 80:644–653. doi:10.2527/2002.803644x [DOI] [PubMed] [Google Scholar]
- Humphrey B. D., Huang N., and Klasing K. C.. 2002. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J. Nutr. 132:1214–1218. doi: 10.1093/jn/132.6.1214 [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. doi:10.2527/1997.7551244x [DOI] [PubMed] [Google Scholar]
- Jones G. M., Edwards S. A., Sinclair A. G., Gebbie F. E., Rooke J. A., Jagger S., and Hoste S.. 2002. The effect of maize starch or soya-bean oil as energy sources in lactation on sow and piglet performance in association with sow metabolic state around peak lactation. Anim. Sci. 75:76–82. doi:10.1017/S1357729800052838 [Google Scholar]
- Kimura F., Aizawa K., Tanabe K., Shimizu K., Kon M., Lee H., Akimoto T., Akama T., and Kono I.. 2008. A rat model of saliva secretory immunoglobulin: a suppression caused by intense exercise. Scand. J. Med. Sci. Sports 18:367–372. doi: 10.1111/j.1600-0838.2007.00642.x [DOI] [PubMed] [Google Scholar]
- Lauridsen C., and Danielsen V.. 2004. Lactational dietary fat levels and sources influence milk composition and performance of sows and their progeny. Livest. Prod. Sci. 91:95–105. doi:10.1016/j.livprodsci.2004.07.014 [Google Scholar]
- Lee M., Kovacs-Nolan J., Yang C., Archbold T., Fan M. Z., and Mine Y.. 2009. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Agric. Food Chem. 57:2233–2240. doi: 10.1021/jf803133b [DOI] [PubMed] [Google Scholar]
- Liu D., Guo Y., Wang Z., and Yuan J.. 2010. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 39:17–24. doi: 10.1080/03079450903447404 [DOI] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Long Y., Lin S., Zhu J., Pang X., Fang Z., Lin Y., Che L., Xu S., Li J., Huang Y., et al. 2016. Effects of dietary lysozyme levels on growth performance, intestinal morphology, non-specific immunity and mRNA expression in weanling piglets. Anim. Sci. J. 87:411–418. doi: 10.1111/asj.12444 [DOI] [PubMed] [Google Scholar]
- Ma X. K., Zhang S., Pan L., and Piao X. S.. 2017. Effects of lysozyme on the growth performance, nutrient digestibility, intestinal barrier, and microbiota of weaned pigs fed diets containing spray-dried whole egg or albumen powder. Can. J. Anim. Sci. 97:466–475. doi: 10.1139/CJAS-2016-0171 [Google Scholar]
- Maga E. A., Walker R. L., Anderson G. B., and Murray J. D.. 2006. Consumption of milk from transgenic goats expressing human lysozyme in the mammary gland results in the modulation of intestinal microflora. Transgenic Res. 15:515–519. doi: 10.1007/s11248-006-0014-3 [DOI] [PubMed] [Google Scholar]
- Masschalck B. and Michiels C. W.. 2003. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 29:191–214. doi: 10.1080/713610448 [DOI] [PubMed] [Google Scholar]
- May K. D., Wells J. E., Maxwell C. V., and Oliver W. T.. 2012. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J. Anim. Sci. 90:1118–1125. doi: 10.2527/jas.2011-4297 [DOI] [PubMed] [Google Scholar]
- Moynihan, P. J. and Clarke A. J.. 2011. O-acetylated peptidoglycan: controlling the activity of bacterial autolysins and lytic enzymes of innate immune systems. Int. J. Biochem. Cell Biol. 43:1655–1659. doi: 10.1016/j.biocel.2011.08.007 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Nyachoti C. M., Kiarie E., Bhandari S. K., Zhang G., and Krause D. O.. 2012. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J. Anim. Sci. 90:252–260. doi: 10.2527/jas.2010-3596 [DOI] [PubMed] [Google Scholar]
- Oliver W. T. and Wells J. E.. 2013. Lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology in nursery pigs. J. Anim. Sci. 91:3129–3136. doi: 10.2527/jas.2012-5782 [DOI] [PubMed] [Google Scholar]
- Oliver W. T., Wells J. E., and Maxwell C. V.. 2014. Lysozyme as an alternative to antibiotics improves performance in nursery pigs during an indirect immune challenge. J. Anim. Sci. 92:4927–4934. doi: 10.2527/jas.2014-8033 [DOI] [PubMed] [Google Scholar]
- Praveena P. E., Periasamy S., Kumar A. A., and Singh N.. 2010. Cytokine profiles, apoptosis and pathology of experimental Pasteurella multocida serotype A1 infection in mice. Res. Vet. Sci. 89:332–339. doi: 10.1016/j.rvsc.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Salmon H., Berri M., Gerdts V., and Meurens F.. 2009. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 33:384–393. doi: 10.1016/j.dci.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Saurabh S., and Sahoo P. K.. 2008. Lysozyme: an important defence molecule of fish innate immune system. Aquac. Res. 39:223–239. doi:10.1111/j.1365-2109.2007.01883.x [Google Scholar]
- Silhavy T. J., Kahne D., and Walker S.. 2010. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2:a000414. doi: 10.1101/cshperspect.a000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlock M. E. 1997. Regulation of metabolism and growth during immune challenge: an overview of cytokine function. J. Anim. Sci. 75:1773–1783. doi: 10.2527/1997.7571773x [DOI] [PubMed] [Google Scholar]
- Wang C. Q., Bai Y. S., Zhao X., Shi B. M., Meng X. Y., and Shan A. S.. 2017. Effects of feeding sodium stearoyl-2-lactylate diets to lactating sows on performance, digestibility of nutrients, composition, and fat globule size in milk. J. Anim. Sci. 95:5091–5099. doi: 10.2527/jas2017.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. E., Berry E. D., Kalchayanand N., Rempel L. A., Kim M., and Oliver W. T.. 2015. Effect of lysozyme or antibiotics on faecal zoonotic pathogens in nursery pigs. J. Appl. Microbiol. 118:1489–1497. doi: 10.1111/jam.12803 [DOI] [PubMed] [Google Scholar]
- Wu M., Xiao H., Liu G., Chen S., Tan B., Ren W., Bazer F. W., Wu G., and Yin Y.. 2016. Glutamine promotes intestinal siga secretion through intestinal microbiota and IL-13. Mol. Nutr. Food Res. 60:1637–1648. doi: 10.1002/mnfr.201600026 [DOI] [PubMed] [Google Scholar]
- Ye D., Ma I., and Ma T. Y.. 2006. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G496–G504. doi: 10.1152/ajpgi.00318.2005 [DOI] [PubMed] [Google Scholar]