Abstract
Pain and stress assessment in animals is considered an imperative issue and also a difficult challenge. Unfortunately, no gold standard technique for pain and stress assessment in animals has been validated nowadays. A new tool to assess stress in animals consists of measuring the leukocyte coping capacity (LCC). The aim of this study was to evaluate the whole-blood LCC chemiluminescence as an innovative tool for stress and pain assessment in the bovine species undergoing ring castration. Twenty 2-mo-old male mix-breed Piemontese-Angus-Belgian Blue calves (Bos taurus) weighing 90 ± 4 kg were used. The animals were randomly allocated in 2 groups composed of 10 subjects each as follows: ring castration group (CAS) and sham castration group (SHAM). Blood drawing, scrotal and perineal temperature recording, scrotal lesion score, pain assessment, and LCC Chemiluminescence were performed at different time points, which were as follows: 1 h before castration/sham (−1 h), 30 min postcastration/sham (30 min), 3 d postcastration/sham (3 d), 7 d postcastration/sham (7 d), 14 d postcastration/sham (14 d). Results showed that in CAS LCC values significantly increased (P < 0.05) at 3 d and decreased at 7 d, whereas in SHAM, LCC values did not significantly vary between the study times. Significant differences in LCC values between CAS and SHAM were seen at 7 d (P < 0.0001). In the CAS group, scrotal lesion was scored as 0, 0, 3.8, 2.7, and 0.2 at −1 h, 30 min, 3 d, 7 d, and 14 d, respectively, whereas in SHAM, its score was 0 at every time point. Perineal temperatures did not vary throughout all the study times in both CAS and SHAM. Differences among the 2 groups were noted in scrotal temperatures only at 3, 7, and 14 d (P < 0.05). In CAS, the percentage of animals which obtained a pain score ≥ 1 was: 10% at −1 h, 30% at 30 min, 20% at 3 and 7 d, and 10% at 14 d, whereas in SHAM, no pain signs were noted at any time point. No significant difference between CAS and SHAM was recorded in cortisol blood level at any time point. No stress leukogram nor variation in neutrophil/lymphocyte ratio was noted at any of the time points in both CAS or SHAM. Our results suggest that ring castration might cause long-lasting pain in calves, but its magnitude is not easily detected by conventional methods. We argue that whole-blood LCC chemiluminescence might be a useful tool for detecting pain and stress in calves undergoing ring castration.
Keywords: assessment, calves, LCC, pain, ring castration, stress
INTRODUCTION
Pain and stress assessment in animals is considered an imperative issue and also a difficult challenge. Objective, quantitative, and practicable measures of sufferance are crucial to studies in many branches, including animal husbandry (Flecknell and Roughan, 2004). The pain response in animals is currently assessed using a variety of techniques, including physiological, hematological, and behavioral parameters (Weary et al., 2006). Unfortunately, no gold standard technique for pain and stress assessment in animals has been validated nowadays. For this reason, researching effective and innovative methods is essential.
A new tool to assess stress in animals consists of measuring the leukocyte coping capacity (LCC). The LCC is defined as the ability of an animal’s leukocytes to produce a respiratory burst in response to a bacterial-type challenge and has been used as a measure of stress (McLaren et al., 2003; Honess et al., 2005; Moorhouse et al., 2007; Shelton-Rayner et al., 2011; Esteruelas et al., 2016; Huber et al., 2017). This immune challenge is triggered, in vitro, by the use of a chemical stimulator and compared with the individuals’ own baseline level of immune system activity (McLaren et al., 2003). It is reported how LCC results vary according to different stress levels. In fact, lower LCC values have been reported in stressful situations, thus proving how these conditions impair reactive oxygen species (ROS) production by the subject’s immune system (mainly by granulocytes) (Shelton-Rayner et al., 2011). Studies in humans revealed that pain and stress are 2 distinguished yet overlapping processes sharing multiple conceptual and physiological patterns (Abdallah and Geha, 2017). Moreover, both can be considered double edged swords, as they can result in adaptive or maladaptive changes required to regain homeostasis and/or stability (Sinha and Jastreboff, 2013). If maladaptive changes occur, changes in physiology and behavior can be observed, resulting in suffering and compromised well-being of the subject (Knaster et al., 2012).
No reports on whole-blood chemiluminescence in the bovine species are available in the literature available to date; thus, how the magnitude of ROS production varies after stress and/or pain is still unknown.
The primary objective of this research was to investigate the validity and feasibility of this new methodology for pain assessment. In this experimental study, ring castration of calves, which has been considered by Marti et al. (2010) a source of pain, was used as nociceptive stimulus.
Furthermore, comparisons between LCC results and some established methods to measure pain and pain-related stress were executed.
We hypothesized that 1) calves after castration would show lower LCC values than before castration, 2) noncastrated animals would present higher LCC values compared to treated ones, and 3) there would be a meaningful correlation between LCC results and other parameters routinely used for stress (blood cortisol level, leukogram) and pain (pain scale) assessment.
MATERIALS AND METHODS
Study Design
The study was performed after the approval of the Animal-welfare Body of the University of Padua in a farm located in the Veneto region (Italy) between March and May 2017. Twenty (n = 20) 2-mo-old male mix-breed Piemontese-Angus-Belgian Blue calves (Bos taurus) weighing 90 ± 4 kg (mean ± SD) were used. The animals were housed in 2 large group pens, fed mashed weaning feed and milk replacer; they also had free access to water and contact with herd mates and mothers. The animals had been living with the herd for at least 30 d when they entered the study. Calves were considered healthy on the basis of clinical examination and blood exams results. The animals were randomly allocated in 2 groups composed of 10 subjects each as follows: ring castration group (CAS) and sham castration group (SHAM).
One hour before castration or sham procedure, all calves were assessed for pain using the UNESP-Botucatu unidimensional composite pain scale (de Oliveira et al., 2014). Before any manipulation, every animal was evaluated for pain assessment, using the above-mentioned scale that was performed for 25 min by 3 trained observers who did not interfere with the animals and used a scoring system ranging from 0 to 10. Each animal was then gently captured by means of a rope and restrained by 2 people for weighing, blood sampling, temperature measuring, and scrotal clinical evaluation. Scrotal and perineal (used as control measure) temperatures were recorded by means of an infrared thermometer (DT8380, CAMMUO, SKU009011, China) at a distance of 20 cm from the skin. The scrotum was evaluated for clinical conditions using a scoring system ranging from 0 to 6 (Table 1).
Table 1.
Scrotal lesion score used to assess the scrotal condition in calves undergoing rubber ring castration and sham castration
| Score | Description |
|---|---|
| 0 | Clinically normal/wound healed |
| 1 | Small wound |
| 2 | Mummified scrotum |
| 3 | Mummified and shrunk scrotum with partial detachment from the abdominal wall |
| 4 | Dry scrotum |
| 5 | Swollen and dry scrotum, ulceration |
| 6 | Swollen, moist and hot scrotum |
Castration was performed as described by Stafford et al. (2002). Briefly, while the animal was being restrained, the skin of the scrotum was disinfected with povidone iodine solution (Betadine 10%, Meda Manufacturing, Merignac, France) for 3 min and 2 rubber castration rings (Allflex New Zealand, Palmerston North, New Zealand) were placed simultaneously on the neck of the scrotum just proximal to the testes using an elastrator (Elastrator, Blenheim, New Zealand). After 10 d, the devitalized tissues of the scrotum and testes were surgically removed, before they naturally fell off, to spare the animal any kind of unnecessary discomfort accountable to tissues degeneration. Fourteen days after castration/sham, because of the absence of the scrotum, the wound temperature was evaluated instead.
Pain assessment, blood sampling, scrotal, perineal, and environmental temperature recording, and scrotal clinical evaluation were repeated as follows: 1 h before castration (−1 h), 30 min after castration (30 min) and at day 3 (3 d), 7 (7 d), and 14 (14 d) after rings application.
An identical study design was applied to SHAM group (iodine solution application included), except for castration which was replaced with an equally long testicular manipulation.
Blood Samples Processing
Three and 9-mL blood samples were aseptically collected from the external jugular vein in evacuated tubes containing 5.4 mg of K3EDTA (Vacumed, FL MEDICAL s.r.l., Italy) and in evacuated tubes containing serum clot activator (Vacuette Z Serum Separator Clot Activator, Preanalitica s.r.l., Italy), respectively. A small amount of blood stored in K3EDTA tubes was used in the field for chemiluminescence assay, while the residual was immediately refrigerated at 4 °C until blood count test was performed. A full hematological profile was provided by means of an automated cell counter (ADVIA 120 Hematology System, Siemens Healthcare GmbH, Germany). Clotted blood samples were centrifuged at 3,500 rpm for 15 min to obtain serum, which was stored at −20 °C and then thawed for cortisol concentrations to be measured by immunoassay (COBAS 6000-c601, Roche Diagnostics S.p.A., Italy).
Whole Blood Chemiluminescence Assay
In order to understand an individual’s LCC, the whole blood of the same individual was divided into 2 samples: the nonstimulated sample (ns) and the stimulated one (s).
The nonstimulated sample provided a baseline measure of the individual’s LCC response. The sample was prepared as described by Shelton-Rayner et al. (2012): 10 µL of whole blood-K3EDTA were transferred into a silicon antireflective tube (Lumivial, EG & G Berthold, Germany); 90 µL of 10−4 mol L−1 luminol (5-amino-2.3-dihydrophthalzine; Sigma A8511, Sigma-Aldrich, Oslo, Norway) diluted in phosphate-buffered saline (PBS) (Sigma, Sigma-Aldrich) were added and 10 µL of fresh PBS were used to bring the solution to a total volume of 110 µL. The tube was gently shaken to mix the reagents. Because it is known that luminol chemiluminesces if combined with an oxidizing agent to produce a low-intensity light reaction (Whitehead et al., 1992), phorbol 12-myristate 13-acetate (PMA, Sigma P8139, Sigma-Aldrich) was used as an activator to challenge granulocytes in the stimulated sample. The preparation of the latter was identical to that of the unstimulated sample, except for the replacement of fresh PBS with 10 µL of PMA at a concentration of 10−5 mol L−1.
The PMA solution had been prepared in advance by diluting 1 mg of PMA in 8.106 mL of dimethyl sulfoxide (Sigma D 5879, Sigma-Aldrich) creating 0.1 mL aliquots (stock solutions) which were stored at −20 °C as long as necessary. A working solution of 10−5M was produced daily, by adding 9.9 mL of fresh PBS to 0.1 mL of 10−3M PMA. PBS was prepared by adding 1 tablet to 200 mL distilled water and stored at −20 °C until needed. Stock solution of 10−2M luminol was produced by dissolving 0.0177 g of luminol in 1 mL of dimethyl sulfoxide and 9 mL of fresh PBS, using a magnetic hotplate stirrer. Attention was paid to prevent luminol to be exposed to light and 0.2 mL aliquots were stored, wrapped in foil, at −20 °C. A luminol working solution of 10−4M was produced from stock solution by dilution with 19.8 mL PBS and was refrigerated in the dark until required.
Chemiluminescence of both samples (ns and s), measured in relative light units (RLU), was recorded at intervals of 5 min using a high sensitivity portable chemiluminometer (Junior LB 9509, E G & G Berthold, Germany) for a total of 45 min in order to produce a luminescence profile. The measurements were done in the field immediately after the blood samples were collected. When not in the chemiluminometer, tubes were incubated at 37 °C in a lightproof water bath.
Statistics
Data were analyzed using the SAS statistical software (version 9.3, SAS Inst. Inc., Cary. NC). Normality of data distribution was assessed by adopting the Shapiro-Wilk test. For LCC data, some values were calculated: Delta LCC (the difference in response at each time interval between ns and s measures), Max Delta LCC (maximum Delta LCC value per time point), and AUC (area under the Delta LCC curve).
Analysis of repeated and normally distributed data (Delta LCC and white blood cells count) was performed through a repeated type mixed model analysis of variance. Time points (−1 h, 30 min, 3 d, 7 d, 14d), CAS/SHAM groups and their interaction were included in the model as fixed effects, intervals (0, 5, 10, up to 45 min) as time effect and animal as random repeated effect. Nonrepeated and normally distributed measures (Max Delta LCC, AUC, scrotal, perineal and environmental temperature) were analyzed using a mixed model with time points (−1 h, 30 min, 3 d, 7 d, 14 d), CAS/SHAM groups and their interaction as fixed effects and animal as random effect. Non-normally distributed data were first log-transformed before being analyzed through the same model.
Data were reported as least squares means ± standard error for normally distributed variables (transformed data were back log transformed).
Correlation among variables was calculated. Pearson coefficient was used for normally distributed data, whereas Spearman rank correlation index was applied to non-normally distributed variables.
Pain scores were analyzed by computing the percentage of scores ≥ 1. These percentages were compared using chi-square test and Marascuilo procedure.
RESULTS
LCC
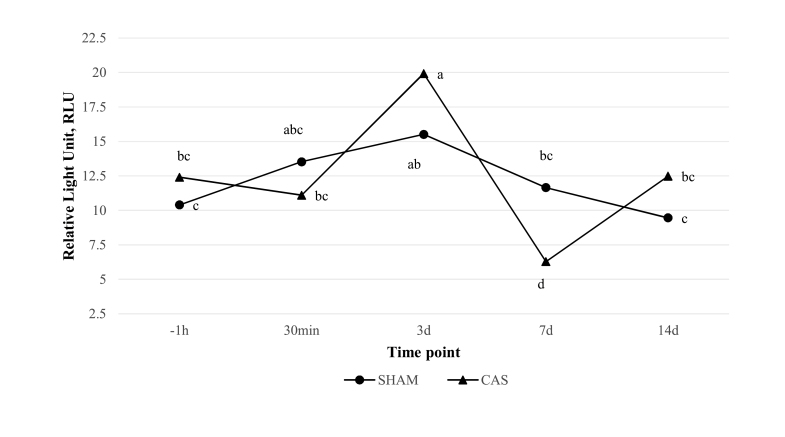
Analysis of data for CAS group revealed that Delta LCC values were associated with a significant (P < 0.05) increase at 3 d from −1 h and 30 min, and reduction at 7 d from −1 h, 30 min, and 3 d. Delta LCC values at −1 h, 30 min, and 14 d showed no statistically significant difference. Analysis of data for SHAM group revealed that Delta LCC values did not significantly vary among the study times. Furthermore, significant differences in Delta LCC values between CAS and SHAM were noted only at 7 d (P < 0.0001); no differences between the experimental groups were documented at the other time points (Fig. 1).
Figure 1.
Difference in leukocyte coping capacity (LCC) luminescence values at each time point between non-stimulated and stimulated measures in both castrated (CAS) and control (SHAM) groups (Delta LCC). Results are reported in Relative Light Unit (RLU). Lowercase letters indicate differences between and within groups at different time points (P < 0.05). Time points are reported as follows: −1 h (1 h before castration/manipulation), 30 min, 3 d, 7 d, 14 d (30 min, 3, 7, and 14 d postcastration/manipulation).
As far as Max Delta LCC is concerned, there were no differences neither inside each group nor between CAS and SHAM. Recorded peak values were 37 and 29 RLU in CAS and SHAM, respectively, at 3 d.
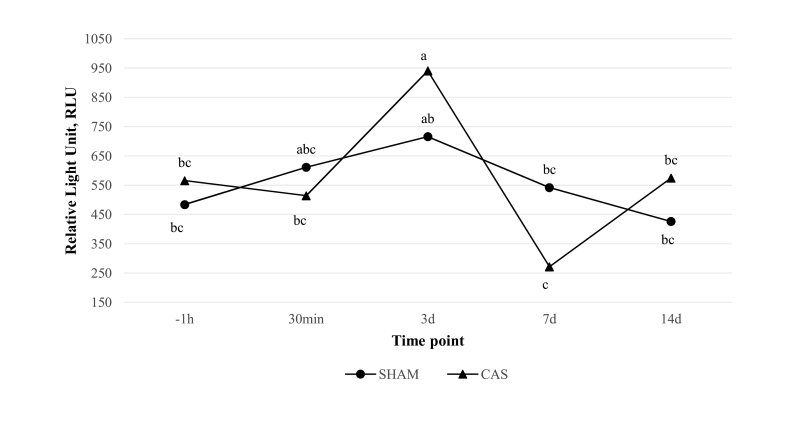
AUC data analysis resulted similar to the one provided by Delta LCC for CAS and SHAM among time points: AUC values in CAS increased with statistical significance at 3 d and significantly diminished at 7 d (P < 0.05). Whereas, for SHAM, no statistically significant variation was noted among time points (Fig. 2).
Figure 2.
Area under the Delta LCC curve (AUC) values at each time point in both castrated (CAS) and control (SHAM) groups. Results are reported in Relative Light Unit (RLU). Lowercase letters indicate differences between and within groups at different time points (P < 0.05). Time points are reported as follows: −1 h (1 h before castration/manipulation), 30 min, 3 d, 7 d, 14 d (30 min, 3, 7, and 14 d postcastration/manipulation).
Scrotal Lesion Score
No scrotal abnormalities were recorded at the first time point in CAS; all animals’ scrotal sacs were considered to be in good condition after clinical examination (all scores were 0). At 30 min, no gross lesions were noted and the scrota presented a clinical condition similar to that recoded at −1 h (all scores were 0). Scrotal swelling was documented at 3 d; the scrotal surface was dry and without any other apparent sign of inflammation (average score 3.8; range 3 to 4). Mummification and partial detachment of the scrotum were the most evident clinical signs at 7 d. Also, the scrotal sacs were cold and stiff at palpation (average score 2.7; range 2 to 3). After the surgical removal of strangled portion at day 10, the abdominal wall began a healing process, resulting in a neat circular scar at 14 d (average score 0.2; range 0 to 1).
SHAM exhibited normal scrotal condition throughout all the time points (all scores were 0) (Table 2).
Table 2.
Total white blood cells (WBC) count, different WBC populations (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), blood cortisol, pain scale score, and scrotal lesion score at each time point in CAS and SHAM group
| Time point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −1 h | 30 min | 3 d | 7 d | 14 d | ||||||
| CAS | SHAM | CAS | SHAM | CAS | SHAM | CAS | SHAM | CAS | SHAM | |
| WBC (103 cells/µL) | 8.90 ± 2.67 | 9.39 ± 2.49 | 8.89 ± 2.67 | 9.39 ± 2.49 | 9.01 ± 2.26 | 9.36 ± 1.48 | 9.37 ± 1.99 | 9.36 ± 1.48 | 10.65 ± 3.72 | 8.45 ± 1.69 |
| Neutrophils (103 cells/µL) | 2.14 ± 1.43 | 2.66 ± 1.39 | 2.14 ± 1.43 | 2.66 ± 1.39 | 2.37 ± 1.37 | 2.65 ± 0.92 | 2.44 ± 1.13 | 2.65 ± 0.92 | 2.99 ± 2.78 | 2.10 ± 0.71 |
| Lymphocytes (103 cells/µL) | 6.04 ± 1.23 | 5.84 ± 1.55 | 6.04 ± 1.23 | 5.84 ± 1.55 | 5.80 ± 1.66 | 5.90 ± 0.98 | 6.12 ± 1.52 | 5.89 ± 0.98 | 7.05 ± 1.55 | 5.57 ± 1.21 |
| Monocytes (103 cells/µL) | 0.47 ± 0.19 | 0.52 ± 0.17 | 0.47 ± 0.19 | 0.52 ± 0.17 | 0.61 ± 0.20 | 0.39 ± 0.11 | 0.58 ± 0.20 | 0.39 ± 0.11 | 0.28 ± 0.15 | 0.42 ± 0.17 |
| Eosinophils (103 cells/µL) | 0.15 ± 0.12 | 0.23 ± 0.28 | 0.15 ± 0.12 | 0.23 ± 0.28 | 0.10 ± 0.06 | 0.31 ± 0.24 | 0.12 ± 0.06 | 0.31 ± 0.24 | 0.20 ± 0.10 | 0.24 ± 0.26 |
| Basophils (103 cells/µL) | 0.07 ± 0.02 | 0.11 ± 0.04 | 0.07 ± 0.02 | 0.11 ± 0.04 | 0.08 ± 0.04 | 0.10 ± 0.04 | 0.07 ± 0.03 | 0.10 ± 0.04 | 0.12 ± 0.04 | 0.08 ± 0.02 |
| Blood cortisol level (nmol/L) | 7.54 ± 5.82 | 8.48 ± 8.24 | 11.08 ± 8.00 | 2.91 ± 3.82 | 8.60 ± 7.75 | 10.98 ± 9.61 | 13.02 ± 12.74 | 5.28 ± 5.36 | 8.73 ± 7.41 | 13.06 ± 9.46 |
| Pain Scores | 0.3 (0–2) | 0 (0–0) | 0.6 (0–4) | 0 (0–0) | 0.3 (0–2) | 0 (0–0) | 0.2 (0–1) | 0 (0–0) | 0.1 (0–1) | 0 (0–0) |
| Scrotal lesion score | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 3.8 (3–4) | 0 (0–0) | 2.7 (2–3) | 0 (0–0) | 0.2 (0–1) | 0 (0-0) |
Temperatures
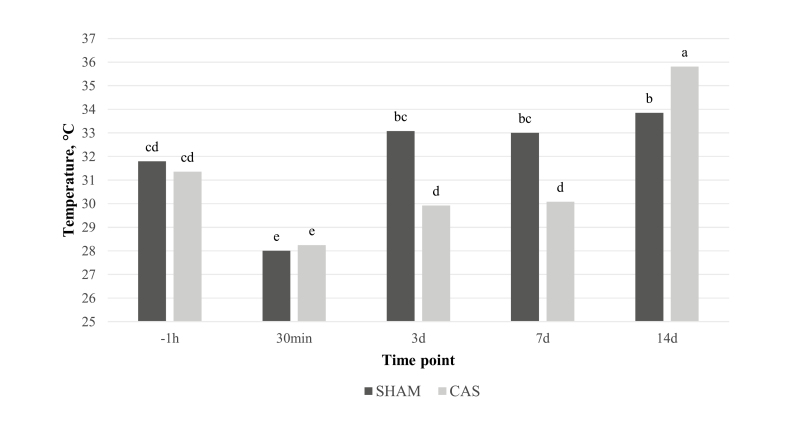
No significant variation was noted in perineal temperature (35.5 ± 0.8 °C) at any time point within SHAM group. In the same group, scrotal temperature significantly decreased at 30 min (28 ± 2.3 °C; P < 0.05) from −1 h (31.8 ± 1.8 °C), increased at 3 d (33.1 ± 1.9 °C; P < 0.05) from 30 min and at 14 d (average 33.8 ± 1.6 °C; P < 0.05) from −1 h and 30 min. No significant variation was noted in perineal temperature within CAS group (36.1 ± 1.3 °C), whereas the scrotal temperature significantly decreased at 30 min (28.2 ± 2.8 °C; P < 0.05) from −1 h (31.4 ± 3.2 °C), increased at 3 d (29.9 ± 1.9 °C; P < 0.05) from 30 min, but still remained significantly lower than at −1 h. Also, the temperature of the wound recorded at 14 d (35.8 ± 0.9 °C; P < 0.05) increased from the scrotal temperature registered at 7 d (30.1 ± 1.7 °C).
Differences among the 2 groups were noted in scrotal temperatures only at 3, 7, and 14 d (P < 0.05) (Fig. 3).
Figure 3.
Scrotal and wound temperature variation between time points in castrated (CAS) and control (SHAM) groups. Results are reported in Celsius degrees (°C). Lowercase letters indicate differences between and within groups at different time points (P < 0.05). Time points are reported as follows: −1 h (1 h before castration/manipulation), 30 min, 3 d, 7 d, 14 d (30 min, 3, 7, and 14 d postcastration/manipulation).
Environmental temperature recorded throughout the study did not change significantly at any time point (22.1 ± 0.7 °C).
Pain Scores
All 3 observers gave the animals the same score at each time point and, as expected, no pain signs were noted in the SHAM at any time point (all animals were scored 0 at each time point). The animals belonging to CAS showed very little pain signs at each experimental time. For this group, the mean value was 0.3 out of 10 at −1 h (range 0 to 2), 0.6 at 30 min (range 0 to 4), 0.3 at 3 d (range 0 to 2), 0.2 at 7 d (range 0 to 1), and 0.1 at 14 d (range 0 to 1) (Table 2). The percentage of animals which obtained ≥ 1 was: 10% at −1 h, 30% at 30 min, 20% at 3 and 7 d, and 10% at 14 d.
Blood Cortisol Level
Cortisol data did not show any significant variation within CAS between time points. The average values were 7.54, 11.09, 8.60, 13.02, and 8.73 nmol/L at −1 h, 30 min, 3 d, 7 d, and 14 d, respectively. Whereas, in SHAM, differences were noted between 30 min and 3 d (P < 0.05) and also 30 min and 14 d (P < 0.05). The average values per time point were 8.48, 2.91, 10.98, 5.28, and 13.06 nmol/L at −1 h, 30 min, 3 d, 7 d, and 14 d, respectively (Table 2). No significant differences were recorded comparing CAS and SHAM at any time point. Spearman test did not reveal any correlation between cortisol and LCC AUC data, nor between cortisol and Max Delta LCC data.
White Blood Cells
Complete blood counts and biochemistry parameters were within the range of reference for the species (Kessell, 2015). All animals were considered to be in good health status. No stress leukograms were noted at any of the time points in either CAS or SHAM. The prevailing white blood cell type was represented by lymphocytes (range 63.0 to 69.4%), followed by neutrophils (range 22.3 to 27.9%), monocytes (range 2.8 to 7.1%), eosinophils (range 1.2 to 3.1%), and basophils (0.8 to 1.1%).
In addition, no significant variation in white blood cells count was registered neither inside each group, nor between CAS and SHAM (range 8.90 to 10.65 × 103 cells µL−1 in CAS; range 8.45 to 9.39 × 103 cells µL−1 in SHAM) (Table 2).
No statistically significant differences were documented in the Neutrophil/Lymphocyte ratio (N:L) between time points in any of the groups (range 0.34 to 0.42 for CAS; range 0.39 to 0.49 for SHAM).
After correlating max Delta and AUC values with the number of both total white blood cells and every single white blood cell population, we observed that only a moderate correlation exists (Spearman correlation: WBC-maxDelta 45.99%; WBC-AUC 45.30%).
DISCUSSION
To our knowledge, this paper is the first to present the whole-blood LCC assay in the bovine species in field conditions, although ROS production from isolated bovine leukocytes has been evaluated in the past years (Hoeben et al., 2000; Mehrzad et al., 2005; Rinaldi et al., 2007; Pang et al., 2009). The validity of this technique seems to reside in maintaining leukocytes in their natural environments, preserving cellular integrity, thus minimizing potential disruption to cell signaling pathways (Shelton-Rayner et al., 2011; Shelton-Rayner et al., 2012; Huber et al., 2017). Studies on human leukocytes demonstrated that their surface is covered with over 150 receptors (Mian et al., 2005), capable of interacting with a large number of stress-sensitive factors, like: endocrine factors in the plasma, cytokines, and substances released from circulating and noncirculating cells (e.g., endothelial cells), and also changes in erythrocyte hemodynamics, blood biochemistry, hypothalamic-pituitary-adrenal axis, and the sympathetic nervous system. The constant exposure to each of these stimuli pertains to their effectiveness as stress indicators (Shelton-Rayner et al., 2012). In our study, LCC values at 3 d of the animals undergoing ring castration showed an increase when compared to their own LCC values at other time points and also to values of SHAM group animals. This result might seem in contrast with our first and second hypothesis. In fact, we would have expected a reduction in LCC values in CAS group after castration. Rubber ring gradual constriction of the scrotum might be responsible for the nonsignificant change in LCC values seen at 30 min. Whereas, a possible explanation to the augment in LCC values at 3 d might be attributable to the systemic diffusion of inflammatory factors coming from the scrotal sac (edematous scrotal sac). We believe that the local inflammation/ischemia started a chain reaction that eventually led to a systemic activation of the immune system, inducing leukocytes to temporarily augment their ROS production (which is what we observed at 3 d). This fact might have led to a subsequent impairment in ROS production at 7 d, causing an “exhaustion state” in granulocytes, thus potentially resulting in augmented susceptibility to infections (McLaren et al., 2003). Also, at 7 d, in addition to the persistent inflammation, mummification and partial detachment of the scrotum might have caused a stressful condition leading to a decrease in ROS production.
Our results at 7 d seem to confirm that LCC is affected by pain-related discomfort/stress, leading us to confirm our first and second hypothesis. In fact, we think that LCC technique might be able to detect physiological changes accountable to stress/pain caused by ring castration, especially during the degenerative phase of the scrotal tissues starting from the 7th day after castration. This is very similar to what was reported by Molony et al. (1995). He demonstrated that methods of castration involving the use of rubber rings in calves produced a long-lasting inflammation. Furthermore, he observed that if the scrotum is left untouched, wound healing could be incomplete even after 51 d, with a peak in lesion severity between 27 and 30 d. Similar results were obtained by Fisher et al. (2001) and Thüer et al. (2007). Considering this, we wanted to avoid unnecessary pain and stress to the animals opting for surgical removal of the degenerating tissues after 10 d from castration, leading to a complete wound sealing at 14 d.
Whole-blood LCC chemiluminescence seems to be an interesting tool for stress/pain assessment in calves after castration, even though it must be pointed out that results coming from this technique in the bovine species are not always easy to be interpreted. In fact, bovines are known to have an “inverted” white blood cell formula, in which lymphocytes are predominant whereas neutrophils are the second population in order of numerosity (Sjaastad et al., 2010; Roland et al., 2014). This fact might explain the lower whole blood chemiluminescence values, because of the lower number of neutrophils (which are known to be the main ROS producers) per blood volume, if compared to values coming from other mammalian species like bears, badgers, bank voles, or humans (Montes et al., 2004; Gelling et al., 2009; Shelton-Rayner et al., 2010; Shelton-Rayner et al., 2011; Esteruelas et al., 2016). Because this is the first study that evaluates whole-blood LCC after castration, the exact time at which LCC values would have changed from baseline was not known to the authors. In fact, our study times were chosen on the basis of what reported in the literature about blood cortisol changes after castration (Molony et al., 1995; Fisher et al., 2001; Stafford et al., 2002; Marti et al., 2010; Becker et al., 2012). Hence, to better understand the effect of ring castration on ROS production observed in this study, it would be interesting to evaluate changes in LCC values at more frequent intervals.
As for temperature, its decrease recorded in both groups at 30 min may be accountable to the presence of the povidone-iodine detergent previously applied on the scrotal surface at castration and that led to heat loss. Furthermore, compression caused by rubber ring is known to be gradual and not sudden (Stafford et al., 2002), thus the temperature decrease seen at 30 min should not be due to blood flow impairment. Temperature returned to baseline values at 3 and 7 d in SHAM, but not in CAS. This result can be explained by the blood flow impairment at the level of the testicular artery caused by mechanical compression of the rubber ring, leading to hypoxia of the tissues (Marti et al., 2010; Fubini and Ducharme, 2017). The temperature increase registered at 14 d in CAS from all other time points might be accountable to the healing process of the tissues resected at day 10 postcastration and the fact that the measurement was performed at the level of the wound, because of the absence of the scrotal sac.
Diagnosing pain in nonverbal patients has always been a challenge for veterinary practitioners. In fact, pain in animals can only be measured indirectly using pain scoring systems or pain scales based on behavioral assessment. These tools represent a valuable diagnostic aid, as they provide pain assessors with objective, ready-to-use tools (della Rocca et al., 2017). Because methods of castration are typically associated with physical, chemical, or hormonal damage to the testicles (Stafford and Mellor, 2005), it is legitimate to discuss whether these procedures can be a source of pain for the animal and, if necessary, it is important to efficiently assess its presence. Animal behavior has been shown to be a sensitive indicator of pain in response to castration in cattle (Robertson et al., 1994). In 2014, the UNESP-Botucatu Unidimensional Composite Pain Scale (UCPS) for assessing postoperative pain in cattle was developed and validated by de Oliveira et al. This pain scale is nowadays considered as the only tool specifically designed for pain assessment in bovine species (della Rocca et al., 2017) although it is validated for postoperative pain only. It is not clear whether ring castration causes pain and what type of pain (e.g., chronic, acute or both) in calves (Mellor et al., 1991; Robertson et al., 1994; Molony et al., 1995; Stafford et al., 2002; Boesch et al., 2006; Thüer et al., 2007; Becker et al., 2012; Marti et al., 2017). A major hypothesis points out that occlusion of the blood vessels to the testes might not immediately disable the afferent nerves or nociceptors and an increased afferent activity may be accountable to the sensitization of nociceptors after hypoxia of tissues (Gebhart and Ness, 1991; Handwerker and Reeh, 1991). Also, failure to seal the distal scrotal portion from the rest of the body might lead to exposure of living tissues to algogens, pathogens, and toxins carried by fluids coming from the degenerating scrotum (O’connor et al., 1993). Interestingly, in our study, the UNESP-Botucatu pain scale did not measure highly painful conditions at any time point. The highest score was 4 out of 10 measured at 30 min in 1 animal (CAS group) showing discomfort signs, probably due to the new condition caused by the rubber ring and povidone solution application, such as: tail flicking, licking its scrotum and slight apathy. These results might be explained by the intrinsic nature of calves. Cattle are known to be grazing animals which are generally predated upon, and man might be easily considered a predator. For this reason, pain experiences can be expected to have different priorities in these species and to influence behavior in different ways (Molony et al., 1995). In accordance to this, we believe that the incidence of pain manifestation in these animals might be low and subjected to a high variability. Thus, it is recommended to extend the observation period, provide different schedules of observation and always compare findings with physiological changes. Also, it must be taken into account that the UNESP-Botucatu pain scale is specifically designed to measure postoperative pain. In fact, the low pain scale values can be attributable to a bias due to the ring-castrated calves experiencing a different kind of pain to which the scale is not sensitive enough.
Serum cortisol measurement has been widely used as an indicator of stress in animals (Möstl and Palme, 2002). Specifically, some reports using blood cortisol level as a tool for comparing different methods of castration in cattle are documented in the literature (Cohen et al., 1990; King et al., 1991; Faulkner et al., 1992; Robertson et al., 1994; Fisher et al., 1996; Fisher et al., 1997; Fisher et al., 2001; Stafford et al., 2002; Thüer et al., 2007; Becker et al., 2012). Also, it is reported that rubber banding castration may be, if performed appropriately, less stressful than surgical castration (Bretschneider, 2005). Conflicting results can be found in the literature regarding blood cortisol variations after rubber ring castration. In fact, some authors report an increase in blood cortisol level which is greater in castrated animals than in control calves (Stafford et al., 2002; Thüer et al., 2007; Pang et al., 2009; González et al., 2010), whereas others did not find any significant change between the 2 groups (Mellor et al., 1991; Fisher et al., 2001; Becker et al., 2012). In our study, no significant difference in blood cortisol changes was found between the castrated and the control group. This result is in accordance with what already documented by Mellor et al. (1991), Fisher et al. (2001), and Becker et al. (2012). It is important to remember that cortisol is rapidly cleared from the bloodstream (Plumb, 1994); therefore, the timing of blood sampling after ring castration might influence the interpretation of the results (Bretschneider, 2005). For this reason, to better evaluate the cortisol response, the time intervals between blood samplings could be narrowed. In addition, no significant blood cortisol peaks were documented within animals from CAS group and their average values at 30 min (11.09 ± 8.00 nmol/L) postcastration were lower than those reported by other authors in rubber ring castrated cattle at the same study time (range 40 to 76 nmol/L), which seems to be the time at which blood cortisol peaks (Stafford et al., 2002; Thüer et al., 2007). Evaluating blood cortisol level is indeed important when assessing stress in animals. Unfortunately, it is not a perfect tool. In fact, blood cortisol level might be influenced by many factors, including the mental status of the animal at sampling and a high individual variability. Furthermore, low blood cortisol values may be due to individuals having high pain thresholds (Stafford and Mellor, 2005) and being less likely to get stressed by noxious stimuli like the one caused by rubber ring castration. Serum cortisol levels must be interpreted with caution, as they may not always accurately reflect the extent of the pain response in animals, because of this variability (Coetzee, 2011). As a confirmation of this, blood cortisol levels of SHAM group significantly fluctuated between the study times even if no painful stimulus was inflicted to these animals.
Stress is known to cause neutrophilia, lymphopenia, and eosinopenia in the bovine species (Kessell, 2015), conditions characterizing the so called “stress leukogram.” As a consequence of this, an increased N:L can be observed (Tornquist and Rigas, 2010). In the present study, neither stress leukogram nor altered N:L was documented at any time point in castrated animals or in SHAM group. This finding is in accordance with what reported by Wistuba et al., (2004) and Pang et al., (2009) who did not find any white blood cell count variation in calves after band castration.
Even though HPA-mediated stress is known to be involved in several aspects of pain appraisal in animals and humans (Blackburn-Munro, 2004) and down-regulate immune system function (Sapolsky et al., 2000; Tsigos and Chrousos, 2002), LCC values (AUC and max Delta LCC) did not correlate with cortisol values and weakly correlated with both the total number of white blood cells and each white blood cell population. Therefore, we rejected our third hypothesis that LCC values would correlate with the other physiological parameters. This is in accordance with what was found by Shelton-Rayner et al. (2012) and Esteruelas et al. (2016). They attributed the absence of correlation between LCC values and physiological variables to the fact that they are influenced by a wide range of factors in addition to stress. Moreover, because of the weak correlation between LCC values and granulocytes, we hypothesize that, in our study, ROS production, and thus LCC values documented, might not have been predominantly related to the number of granulocytes present in the chemiluminescence tube, but rather to their current status and capability of producing ROS.
Gaining more insight into stress and pain caused by routine procedures in animal husbandry is fundamental, especially nowadays that societal concern regarding the moral and ethical treatment of animals has become more relevant (Rollin, 2004). The model of pain we chose in this study is just one of the many procedures that veterinarians and zootechnicians must deal with during everyday practice. As suggested by our results, ring castration seems to cause long-lasting pain in calves, but its magnitude may not be easily detected by conventional pain assessment methods. For this reason, we argue that whole-blood LCC chemiluminescence might be a new useful tool for assessing pain and stress in farm animals undergoing ring castration and further studies should be carried out to test its efficacy on other routine husbandry procedures that are considered to be painful. Furthermore, as reported in the literature and suggested by our results, stress measures based on immune system alterations seem to be valid alternatives to measures based on the HPA axis, and may even be more suitable in certain circumstances (McLaren et al., 2003; Gelling et al., 2009). Nevertheless, given the complexity of the subject and to provide a clearer picture of the multifaceted effects of stress and pain, we suggest a combined approach using more than one parameter.
Footnotes
Acknowledgments: The authors declare that no conflicts of interest are related to the research presented in the manuscript.
LITERATURE CITED
- Abdallah C. G., and Geha P.. 2017. Chronic pain and chronic stress: two sides of the same coin?Why Stress and Pain? Chronic Stress. 1:1–10. doi: 10.1177/2470547017704763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J., Doherr M. G., Bruckmaier R. M., Bodmer M., Zanolari P., and Steiner A.. 2012. Acute and chronic pain in calves after different methods of rubber-ring castration. Vet. J. 194:380–385. doi: 10.1016/j.tvjl.2012.04.022 [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G. 2004. Hypothalamo-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr. Pain Headache Rep. 8:116–124. doi: 10.1007/s11916-004-0025-9 [DOI] [PubMed] [Google Scholar]
- Boesch D., Steiner A., and Stauffacher M.. 2006. [Castration of calves: a survey among swiss suckler beef farmers]. Schweiz. Arch. Tierheilkd. 148:231–6, 238. doi: 10.1024/0036-7281.148.5.231 [DOI] [PubMed] [Google Scholar]
- Bretschneider G. 2005. Effects of age and method of castration on performance and stress response of beef male cattle: a review. Livest. Prod. Sci. 97:89–100. doi: 10.1016/j.livprodsci.2005.04.006. [DOI] [Google Scholar]
- Coetzee J. F. 2011. A review of pain assessment techniques and pharmacological approaches to pain relief after bovine castration: practical implications for cattle production within the United States. Appl. Anim. Behav. Sci. 135:192–213. doi: 10.1016/j.applanim.2011.10.016 [DOI] [Google Scholar]
- Cohen R. D. H., King B. D., Thomas L. R., and Janzen E. D.. 1990. Efficacy and stress of chemical versus surgical castration of cattle. Can. J. Anim. Sci. 70:1063–1072. doi: 10.4141/cjas90-129 [DOI] [Google Scholar]
- Della Rocca G., Brondani J. T., de Oliveira F. A., Crociati M., Sylla L., Elad Ngonput A., Di Salvo A., and Luna S. P. L.. 2017. Validation of the Italian version of the UNESP-Botucatu unidimensional composite pain scale for the assessment of postoperative pain in cattle. Vet. Anaesth. Analg. 44:1253–1261. doi: 10.1016/j.vaa.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Esteruelas N. F., Huber N., Evans A. L., Zedrosser A., Cattet M., Palomares F., Angel M., Swenson J. E., and Arnemo J. M.. 2016. Leukocyte coping capacity as a tool to assess capture- and handling-induced stress in Scandinavian brown bears (Ursus arctos). J. Wildl. Dis. 52(2 Suppl):S40–S53. doi: 10.7589/52.2S.S40 [DOI] [PubMed] [Google Scholar]
- Faulkner D. B., Eurell T., Tranquilli W. J., Ott R. S., Ohl M. W., Cmarik G. F., and Zinn G.. 1992. Performance and health of weanling bulls after butorphanol and xylazine administration at castration. J. Anim. Sci. 70:2970–2974. doi: 10.2527/1992.70102970x [DOI] [PubMed] [Google Scholar]
- Fisher A. D., Crowe M. A., Alonso de la Varga M. E., and Enright W. J.. 1996. Effect of castration method and the provision of local anesthesia on plasma cortisol, scrotal circumference, growth, and feed intake of bull calves. J. Anim. Sci. 74:2336–2343. doi: 10.2527/1996.74102336x [DOI] [PubMed] [Google Scholar]
- Fisher A. D., Crowe M. A., O’Nualláin E. M., Monaghan M. L., Prendiville D. J., O’Kiely P., and Enright W. J.. 1997. Effects of suppressing cortisol following castration of bull calves on adrenocorticotropic hormone, in vitro interferon-gamma production, leukocytes, acute-phase proteins, growth, and feed intake. J. Anim. Sci. 75:1899–1908. doi: 10.2527/1997.7571899x [DOI] [PubMed] [Google Scholar]
- Fisher A. D., Knight T. W., Cosgrove G. P., Death A. F., Anderson C. B., Duganzich D. M., and Matthews L. R.. 2001. Effects of surgical or banding castration on stress responses and behaviour of bulls. Aust. Vet. J. doi: 10.1111/j.1751-0813.2001.tb11981.x [DOI] [PubMed] [Google Scholar]
- Flecknell P. A., and Roughan J. V.. 2004. Assessing pain in animals - putting research into practice. Anim. Welf. 13:71–75. ISSN 0962-7286. [Google Scholar]
- Fubini S. L., and Ducharme N.. 2017. Farm animal surgery. In: Fubini, S. L. and Ducharme N., editors. Surgery of the bovine reproductive system and urinary tract. 2nd ed. (Fubini S. L. and Ducharme N., editors.). Elsevier, St. Louis, Missouri. [Google Scholar]
- Gebhart G. F., and Ness T. J.. 1991. Central mechanisms of visceral pain. Can. J. Physiol. Pharmacol. 69:627–634. doi: 10.1139/y91-093 [DOI] [PubMed] [Google Scholar]
- Gelling M., Mclaren G., Mathews F., Mian R., and Macdonald D.. 2009. Impact of trapping and handling on leukocyte coping capacity in bank voles (Clethrionomys glareolus) and wood mice (Apodemus sylvaticus). Anim. Welf. 18:1–7. ISSN 0962-7286. [Google Scholar]
- González L. A., Schwartzkopf-Genswein K. S., Caulkett N. A., Janzen E., McAllister T. A., Fierheller E., Schaefer A. L., Haley D. B., Stookey J. M., and Hendrick S.. 2010. Pain mitigation after band castration of beef calves and its effects on performance, behavior, Escherichia coli, and salivary cortisol. J. Anim. Sci. 88:802–810. doi: 10.2527/jas.2008-1752 [DOI] [PubMed] [Google Scholar]
- Handwerker H. O., and Reeh P. W.. 1991. Pain and inflammation. In: Bond M. R., Charlton J. E., and Woolf C. J., editors, Proceedings of the VIth World Congress on Pain. Elsevier, Amsterdam: p. 59–70. [Google Scholar]
- Hoeben D., Monfardini E., Opsomer G., Burvenich C., Dosogne H., De Kruif A., and Beckers J. F.. 2000. Chemiluminescence of bovine polymorphonuclear leucocytes during the periparturient period and relation with metabolic markers and bovine pregnancy-associated glycoprotein. J. Dairy Res. 67:249–259. doi: 10.1017/s0022029900004052 [DOI] [PubMed] [Google Scholar]
- Honess P., Marin C., Brown A., and Wolfensohn S.. 2005. Assessment of stress in non-human primates: application of the neutrophil activation test. Anim. Welf. 14:291–295. [Google Scholar]
- Huber N., Vetter S. G., Evans A. L., Kjellander P., Küker S., Bergvall U. A., and Arnemo J. M.. 2017. Quantifying capture stress in free ranging European roe deer (Capreolus capreolus). BMC Vet. Res. 13:127. doi: 10.1186/s12917-017-1045-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessell A. 2015. Bovine haematology and biochemistry. In: Cockcroft P. D., editor. Bovine medicine. 3rd ed. John Wiley & Sons, Ltd, Oxford, UK, p. 146–160. [Google Scholar]
- King B., Cohen R., Guentherr C., and Ianzen E.. 1991. The effect of age and method of castration on plasma cortisol in beef calves. Can. J. Anim. Sci. 71:257–263. doi: 10.4141/cjas91-033 [DOI] [Google Scholar]
- Knaster P., Karlsson H., Estlander A. M., and Kalso E.. 2012. Psychiatric disorders as assessed with SCID in chronic pain patients: the anxiety disorders precede the onset of pain. Gen. Hosp. Psychiatry 34:46–52. doi: 10.1016/j.genhosppsych.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Marti S., Velarde A., de la Torre J. L., Bach A., Aris A., Serrano A., Manteca X., and Devant M.. 2010. Effects of ring castration with local anesthesia and analgesia in Holstein calves at 3 months of age on welfare indicators. J. Anim. Sci. 88:2789–2796. doi: 10.2527/jas.2009-2408 [DOI] [PubMed] [Google Scholar]
- Marti S., Meléndez D. M., Pajor E. A., Moya D., Heuston C. E. M., Gellatly D., Janzen E. D., and Schwartzkopf-Genswein K. S.. 2017. Effect of band and knife castration of beef calves on welfare indicators of pain at three relevant industry ages: II. Chronic pain. J. Anim. Sci. 95:4367–4380. doi: 10.2527/jas2017.1763 [DOI] [PubMed] [Google Scholar]
- McLaren G. W., Macdonald D. W., Georgiou C., Mathews F., Newman C., and Mian R.. 2003. Leukocyte coping capacity: a novel technique for measuring the stress response in vertebrates. Exp. Physiol. 88:541–546. doi: 10.1113/eph8802571 [DOI] [PubMed] [Google Scholar]
- Mehrzad J., Duchateau L., and Burvenich C.. 2005. High milk neutrophil chemiluminescence limits the severity of bovine coliform mastitis. Vet. Res. 36:101–116. doi: 10.1051/vetres:2004055 [DOI] [PubMed] [Google Scholar]
- Mellor D. J., Molony V., and Robertson I. S.. 1991. Effects of castration on behaviour and plasma cortisol concentrations in young lambs, kids and calves. Res. Vet. Sci. 51:149–154. doi: 10.1016/0034-5288(91)90005-9 [DOI] [PubMed] [Google Scholar]
- Mian R, McLaren G, Macdonald DW. 2005. Of stress, mice and men: a radical approach to old problems. In: Oxington K. V., editor. Stress and Health: New Research. Nova Biomerical Books. Nova Sience Publishers, New York, p. 61–79. [Google Scholar]
- Molony V., Kent J. E., and Robertson I. S.. 1995. Assessment of acute and chronic pain after different methods of castration of calves. Appl. Anim. Behav. Sci. Anim. Behav. Sci. 46:33–48. doi: 10.1016/0168-1591(95)00635-4 [DOI] [Google Scholar]
- Montes I., McLaren G., Macdonald D., and Mian R.. 2004. The effect of transport stress on neutrophil activation in wild badgers (Meles meles). Anim. Welf. 3:355–359. [Google Scholar]
- Moorhouse T. P., Gelling M., McLaren G. W., Mian R., and MacDonald D. W.. 2007. Physiological consequences of captive conditions in water voles (Arvicola terrestris). J. Zool. doi: 10.1111/j.1469-7998.2006.00175.x [DOI] [Google Scholar]
- Möstl E., and Palme R.. 2002. Hormones as indicators of stress. Domest. Anim. Endocrinol. 23:67–74. doi: 10.1016/s0739-7240(02)00146-7 [DOI] [PubMed] [Google Scholar]
- O’connor B., Leavitt S., and Parker K.. 1993. Alberta. Tetanus in feeder calves associated with elastic castration. Can. Vet. J. 34:311–312. PMID 17424228. [PMC free article] [PubMed] [Google Scholar]
- de Oliveira F. A., Luna S. P., do Amaral J. B., Rodrigues K. A., Sant’Anna A. C., Daolio M., and Brondani J. T.. 2014. Validation of the UNESP-botucatu unidimensional composite pain scale for assessing postoperative pain in cattle. BMC Vet. Res. 10:200. doi: 10.1186/s12917-014-0200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang W. Y., Earley B., Sweeney T., Pirani S., Gath V., and Crowe M. A.. 2009. Effects of banding or burdizzo castration of bulls on neutrophil phagocytosis and respiratory burst, CD62-L expression, and serum interleukin-8 concentration. J. Anim. Sci. 87:3187–3195. doi: 10.2527/jas.2009-1905 [DOI] [PubMed] [Google Scholar]
- Plumb D. C. 1994. Veterinary drug handbook. 2nd ed. Iowa State University Press, Ames. [Google Scholar]
- Rinaldi M., Moroni P., Paape M. J., and Bannerman D. D.. 2007. Evaluation of assays for the measurement of bovine neutrophil reactive oxygen species. Vet. Immunol. Immunopathol. 115:107–125. doi: 10.1016/j.vetimm.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Robertson I. S., Kent J. E., and Molony V.. 1994. Effect of different methods of castration on behaviour and plasma cortisol in calves of three ages. Res. Vet. Sci. 56:8–17. doi: 10.1016/0034-5288(94)90189-9 [DOI] [PubMed] [Google Scholar]
- Roland L., Drillich M., and Iwersen M.. 2014. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Invest. 26:592–598. doi: 10.1177/1040638714546490 [DOI] [PubMed] [Google Scholar]
- Rollin B. E. 2004. Annual meeting keynote address: animal agriculture and emerging social ethics for animals. J. Anim. Sci. 82:955–964. doi: 10.2527/2004.823955x [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., and Munck A. U.. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21:55–89. doi: 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Shelton-Rayner G. K., Macdonald D. W., Chandler S., Robertson D., and Mian R.. 2010. Leukocyte reactivity as an objective means of quantifying mental loading during ergonomic evaluation. Cell. Immunol. 263:22–30. doi: 10.1016/j.cellimm.2010.02.011 [DOI] [PubMed] [Google Scholar]
- Shelton-Rayner G. K., Mian R., Chandler S., Robertson D., and Macdonald D. W.. 2011. Quantifying transient psychological stress using a novel technique: changes to PMA-induced leukocyte production of ROS in vitro. Int. J. Occup. Saf. Ergon. 17:3–13. doi: 10.1080/10803548.2011.11076866 [DOI] [PubMed] [Google Scholar]
- Shelton-Rayner G. K., Mian R., Chandler S., Robertson D., and Macdonald D. W.. 2012. Leukocyte responsiveness, a quantitative assay for subjective mental workload. Int. J. Ind. Ergon. doi: 10.1016/j.ergon.2011.11.004 [DOI] [Google Scholar]
- Sinha R., and Jastreboff A. M.. 2013. Stress as a common risk factor for obesity and addiction. Biol. Psychiatry 73:827–835. doi: 10.1016/j.biopsych.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaastad O., Hove K., and Sand O.. 2010. Physiology of domestic animals. 2nd ed., Scandinavian Veterinary Press Oslo, NO. [Google Scholar]
- Stafford K. J., and Mellor D. J.. 2005. The welfare significance of the castration of cattle: a review. N. Z. Vet. J. 53:271–278. doi: 10.1080/00480169.2005.36560 [DOI] [PubMed] [Google Scholar]
- Stafford K. J., Mellor D. J., Todd S. E., Bruce R. A., and Ward R. N.. 2002. Effects of local anaesthesia or local anaesthesia plus a non-steroidal anti-inflammatory drug on the acute cortisol response of calves to five different methods of castration. Res. Vet. Sci. 73:61–70. doi: 10.1016/s0034-5288(02)00045-0 [DOI] [PubMed] [Google Scholar]
- Thüer S., Mellema S., Doherr M. G., Wechsler B., Nuss K., and Steiner A.. 2007. Effect of local anaesthesia on short- and long-term pain induced by two bloodless castration methods in calves. Vet. J. 173:333–342. doi: 10.1016/j.tvjl.2005.08.031 [DOI] [PubMed] [Google Scholar]
- Tornquist S., and Rigas R.. 2010. Interpretation of ruminant leukocyte responses. In: Weiss D. J. and Wardrop K. J., editors. Schalm’s veterinary hematology. 6th ed. Blackwell Publishing Ltd, Ames, Iowa: p. 307–316. [Google Scholar]
- Tsigos C., and Chrousos G. P.. 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 53:865–871. doi: 10.1016/s0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- Weary D. M., Niel L., Flower F. C., and Fraser D.. 2006. Identifying and preventing pain in animals. Appl. Anim. Behav. Sci. doi: 10.1016/j.applanim.2006.04.013 [DOI] [Google Scholar]
- Whitehead T. P., Thorpe G., and Maxwell S.. 1992. Enhanced chemiluminescent assay for antioxidant capacity in biological fluids. Anal. Chim. Acta. 266:265–277. doi: 10.1016/0003-2670(92)85052-8 [DOI] [Google Scholar]
- Wistuba T. J., Kegley E. B., Davis M. E., and Krumpelman S.. 2004. Influence of castration method on receiving calf performance and immune characteristics. J. Anim. Sci. 82:102.14753353 [Google Scholar]