Abstract
Pectin has been known to lower circulating cholesterol by interacting with bile acid (BA) metabolism. The current study was aimed to investigate intestinal BA transport at the molecular level in a pig model. Twelve young pigs (11.05 ± 0.11 kg) were randomly divided into 2 groups and fed corn–soybean meal diets with either 5% pectin or cornstarch for 72 d. In pigs fed with pectin, total cholesterol and low-density lipoprotein cholesterol (LDL-C) were lowered but high-density lipoprotein (HDL-C) was increased (P < 0.05). Serum triglycerides tended to be lower in the pectin-fed animals (P = 0.093), whereas no change was noted in serum total bile acid. Along the length of the intestine, the size and composition of BA pools vary. The ratio of primary, secondary, taurine-conjugated, and glycine-conjugated BAs in the ileal pool was about 46:15:9:30, whereas it was 28:61:1:11 in the cecum and 22:65:3:9 in the colon (P < 0.05). In the feces, lithocholic acid and ursodeoxycholic acid (UDCA) made up of over 97% of the total BA pool. Overall, the ileum had the greatest expression of farnesoid X receptor (FXR) and apical sodium-coupled bile acid transporter (ASBT) than the duodenum, jejunum, cecum, and colon (P < 0.05), whereas organic solute transporters α/β (OSTα/β) gene expression was peaked in the ileum and jejunum (P < 0.05). Expression multidrug resistance protein 2 (MRP2) gradually decreased towards the end of the intestine (P < 0.05). Greater expression of G protein-coupled bile acid receptor and multidrug resistance protein 3 (MRP3) was found in the cecum and colon (P < 0.05). In pigs fed with 5% pectin, only cecal UDCA (P = 0.097) and hyocholic acid (P = 0.088) showed a decreasing tendency. But FXR, ASBT, and MRP2 were upregulated in the ileum and FXR, OSTα/β, MRP2, and MRP3 in the cecum of PEC-fed pigs (P < 0.05). Liver enzymes involved in BA biosynthesis (CYP7A1, CYP27A1, bile acid-CoA synthase, and bile acid-CoA:amino acid N acyltransferase) were not affected by pectin consumption. In conclusion, the abundant distribution of BA transporters and the greater BA pool size suggests the ileum as the major site for intestinal BA reabsorption in pigs. In the ileum, pectin increased in-and-out BA transport on the apical membrane by increasing ASBT and MRP2, but it increased the overall BA transport in the cecum by increasing OSTα/β and MRP3.
Keywords: bile acid, cecum, farnesoid X receptor, ileum, transporter
INTRODUCTION
Daily supplementation of soluble dietary fibers is highly recommended to lower the risks of metabolic syndromes and cardiovascular diseases. Specifically pectin extracted from fruits can lower cholesterol by interacting with bile acid (BA) metabolism. In the liver, about 30% to 40% of cholesterol is catabolized into BAs. The biosynthesis of BAs involves the neutral or alternate pathways. Upon stimulatory effects of cholecystokinin, they are released from the gallbladder into the duodenum via the common bile duct. Through a combination of passive absorption in the proximal small intestine and active transport in the distal ileum, about 95% of BAs will be recycled to maintain the homeostasis of BA pools (Alrefai and Gill, 2007; Dawson et al., 2009; Dawson and Karpen, 2015). The fecal excretion of BAs is almost equal to the BAs synthesized in the liver. Therefore, increased excretion of BAs in the feces will promote the conversion from cholesterol to BAs and consequently reduce its release into the systemic circulation. Many studies have demonstrated that fecal BA excretion is increased by pectin consumption (Matheson and Story, 1994; Matheson et al., 1995; Ghaffarzadegan et al., 2016; Zhu et al., 2017b). Yet it is not fully understood how pectin interacts with intestinal BA transport.
BAs are amphiphilic molecules and their entering and exiting cells need transporters. Several BA transporters have been found in the intestine (Dawson et al., 2009; Dawson and Karpen, 2015). On the apical membrane, apical sodium-coupled bile acid transporter (ASBT) imports luminal BAs (mainly taurocholates) to the enterocytes. In the cell, BAs are shuttled by cytosolic binding protein fatty acid binding protein 6 (FABP6) to the basolateral membrane and then exported via the organic solute transporters α/β (OSTα/β) and multidrug resistance protein 3 (MRP3). BA transporters are heterogeneously expressed along the length of intestine in human and mice (Dawson et al., 2005; Landrier et al., 2006). Expression of ASBT and FABP6 is highly abundant in the ileum, which well supports the fact that ileum is the major site for BA recycling. Alteration in intestinal BA transport is closely related to expressional changes in BA transporters. Gao et al. (2017) showed that aged mice have less ASBT and taurocholate transport in the ileum. Zhu et al. (2017a) found consumption of modified pectin downregulate FABP6 and OSTα/β to inhibit absorption of BAs in the ileum.
Most of the studies used the murine model to investigate the interaction of dietary fiber and BA metabolism. In mice, over 95% of biliary BAs are conjugated with taurine. In contrast, biliary BAs of human are more abundant in glycine-conjugated BAs (Dawson et al., 2009; Si et al., 2015). The compositional difference may result in great differences in BA transport kinetics between mice and human because the conjugation of BA greatly affects the hydrophobicity of the molecule and the affinity to transporters (Aldini et al., 1996). The pig shares similarity anatomy in the gastrointestinal tract as the human. In addition, it was also shown to have greater glycocholates than taurocholates in the biliary BAs (Si et al., 2015; Mi et al., 2016). Therefore, the pig can serve as a better medical model to characterize the effects of dietary pectin on BA transport. However, the knowledge on intestinal BA transporters and composition is rare in pigs. The current study was aimed to 1) profile the BAs and their transporters in the pigs and 2) investigate the impact of pectin supplement on the intestinal BA transport.
MATERIALS AND METHODS
Animals and Sample Collection
Male Duroc × Large White crossbred piglets with an average body weight of 11.05 ± 0.11 kg were randomly divided into 2 groups of 6 animals each and allowed to adapt for 1 wk before the start of the 72-d experiment. Each group of piglets was fed with corn–soybean meal diets containing 5% apple pectin (PEC, purchased from Yuzhong Biotech Corporation, Henan, China) or cornstarch (purchased from Yufeng Cornstarch and Sugar Company, Hebei, China) as the control (CON). The diets (Table 1) had been formulated to meet the nutritional requirements suggested by NRC (Council, 2012) for pigs within this weight range.
Table 1.
The composition of experimental diet
| Ingredient, % | D1-30 | Ingredient, % | D31-72 | ||
|---|---|---|---|---|---|
| CON | PEC | CON | PEC | ||
| Corn | 40.41 | 40.41 | Corn | 58.32 | 58.32 |
| Extruded soybean | 6.00 | 6.00 | Soybean meal | 28.47 | 28.47 |
| Soybean meal | 19.80 | 19.80 | Soybean oil | 4.81 | 4.81 |
| Fish meal | 5.00 | 5.00 | Calcium phosphate | 1.37 | 1.37 |
| Whey powder | 14.80 | 14.80 | Limestone | 0.68 | 0.68 |
| Soybean oil | 1.15 | 1.15 | Sodium chloride | 0.30 | 0.30 |
| Calcium phosphate | 0.50 | 0.50 | L-lysine | 0.05 | 0.05 |
| Limestone | 1.03 | 1.03 | Premix1 | 1.00 | 1.00 |
| Sodium chloride | 0.5 | 0.5 | Cornstarch | 5.00 | – |
| DL-Methionine | 0.10 | 0.10 | Pectin | – | 5.00 |
| L-lysine | 0.40 | 0.40 | |||
| L-Threonine | 0.11 | 0.11 | |||
| Sucrose | 2.50 | 2.50 | |||
| Glucose | 2.50 | 2.50 | |||
| Premix1 | 0.20 | 0.20 | |||
| Cornstarch | 5.00 | – | |||
| Pectin | – | 5.00 | |||
1Vitamin and mineral premixes were obtained from Yinong Forage Ltd. and supplied per kg of diet as-fed: Mn (MnSO4.H2O), 25.0 mg; Zn (ZnSO4), 100.0 mg; Cu (CuSO4.5H2O), 50.0 mg; Se (Na2SeO3), 0.15 mg; Fe (FeSO4), 80.0 mg; I (KI), 0.5 mg; choline, 600.0 mg; vitamin A (all-trans retinyl acetate), 2.84 mg; vitamin D3, 0.22 mg; α-tocopherol, 40 mg; phylloquinone, 2.0 mg; thiamin, 1.0 mg; riboflavin, 5.0 mg; nicotinic acid, 15.0 mg; pantothenic acid, 15.0 mg; pyridoxine, 2.0 mg; vitamin B12, 0.025 mg; folic acid, 2 mg; and biotin, 0.2 mg.
The piglets were fed twice each day, at 8:30 and 15:30, with an amount of feed that provided 1.13-fold digestible energy required for the normal growth. The amount of food provided was adjusted every week. Water was provided ad libitum. On day 72, blood samples were collected from the jugular vein before the morning meal, and animals were sacrificed 2 h after the meal. Digesta were collected from the ileum, cecum, and proximal colon and stored at −80 °C for later BA quantification. Mucosa of the duodenum, jejunum, ileum, cecum, and proximal colon were scraped, snap-frozen, and stored at −80 °C for mRNA measurements. Liver samples were also harvested to quantify gene expression involved in BA biosynthesis. Fresh faces were sampled on the previous day before scarification. All procedures had been approved by the Committee of Animal Welfare in the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, following the Guide for the Care and Use of Laboratory Animals.
Lipid Profiles
Serum triglyceride (TG), total cholesterol (TC), HDL-C, and LDL-C were measured at the fasting status by biochemical methods as previously described by Wu et al. (Wu et al., 2016). Total BA was determined, using the interaction of NAD to produce NADH, which further oxidizes iodonitrotetrazolium chloride to produce red formazan. Commercial kits for TG, TC, HDL-C, LDL-C, and total bile acid (TBA) were purchased from Nanjing Jiancheng Bioegineering Institute (Nanjing, China).
Quantification of BAs by LC/MS
Sample preparation.
Minor adjustments were made to BA quantification procedures described by Kakiyama et al. (2014). Lyophilized luminal contents or feces were suspended in 50-mM cold sodium acetate buffer (pH 5.6) and then refluxed with ethanol for 1 h. After centrifugation at 20,000 × g for 15 min, the supernatant was diluted 4 times with water and applied to a Bond Elute C18 cartridge (500 mg/6 mL; Varian, Harbor City, CA). The cartridge was then washed with 20% ethanol, and BAs were eluted with methanol. After the solvent was removed under nitrogen gas, the residue was dissolved in 1 mL of methanol. Precipitated solids were removed by filtration through a 0.45-μm Millipore filter (Millex-LG; Billerica, MA). All chemicals used were of HPLC grade purchased from Thermo Fisher.
Ultraperformance liquid chromatography/mass spectrometry.
BAs were quantified by a Waters Xevo TQ LC/MS mass spectrometer with an ESI source. A 10-μL aliquot of the filtrate was injected into the LC-ESI-MS/MS system. All chromatographic separations were performed with a ZORBAX Eclipse plus C18 column (95 Å, 1.8 µm, 2.1 × 100 mm) from Agilent (Santa Clara). The mobile phase was composed of 2 extremes, 5% acetonitrile and 0.1% formic acid (mobile phase A) and 95% acetonitrile and 0.1% formic acid (mobile phase B), in a gradient system at a total flow rate of 0.4 mL/min. The proportions of mobile phases A and B were gradually changed as follows: mobile phase A:B (9:1, vol/vol) from 0 to1 min, mobile phase A:B (7:3, vol/vol) from 1 to 1.5 min, mobile phase A:B (2:3, vol/vol) from 1.5 to 5.5 min, and mobile phase A:B (9:1, vol/vol) from 5.5 to 7.0 min. The total run time was 7 min. To operate the LC-ESI-MS/MS, the spray voltage and vaporizer temperature were set at 2.91 kV and 500 °C, respectively. The gas flow was set at 550 L/h. All chemicals used were of HPLC grade purchased from Thermo Fisher.
Quantification by standard curves.
Standards for hyocholic acid (HCA) and taurohyocholic acid (THCA) were obtained from Steroloids, Inc. (Newport) and other 14 BA standards were purchased from Sigma-Aldrich (Steinheim, Germany). The quantification of each BA was based upon the series dilutions of available standards and good linearities have been confirmed.
qPCR to Measure Abundance of BA-Related Genes
Total RNA was extracted from the mucosa of the 5 intestinal segments (duodenum, jejunum, ileum, cecum, and proximal colon) and liver using Qiagen kits (RNeasy Mini Kit, Cat # 74104). The integrity of RNA was checked by electrophoresis before reverse transcription. cDNA was transcribed using a kit (PrimeScript RT reagent kit with gDNA Eraser, Cat# RRO47A, China) containing genomic DNA wipeout buffer to eliminate potential contamination. ASBT, multidrug resistance protein 2 (MRP2), MRP3, OSTα, OSTβ, farnesoid X receptor (FXR), G protein-coupled bile acid receptor (TGR5), FABP6, and fibroblast growth factor 19 (FGF19) were quantified in the intestine, and FXR, TGR5, small heterodimer partner (SHP), fibroblast growth factor receptor 4 (FGFR4), Klotho β (KLβ), CYP7A1, CYP27A1, bile acid-CoA synthase (BACS), and bile acid-CoA:amino acid N acyltransferase (BAAT) were quantified in the liver. The efficacy of each pair of primers (Table 2) was validated within 90% to 110%. Real-time PCR was run with kits from Takara (SYBRPremix Ex Taq, Tli RNaseH Plus, Cat #RR420A, China) using the SYBR green method. The single product of each PCR was verified by a dissociation curve. β-Actin and GAPDH were used as reference genes and the geometric mean was employed in the normalization. The relative abundance of each gene was determined using the 2−ΔΔCt method. The control group and the duodenum were used to normalize the different treatment groups and various intestinal sections, respectively.
Table 2.
Primes for investigated genes related to BA transport, receptors, signaling, and biosynthesis
| Gene | Accession No. | Primer sequences (5′-3′) | |
|---|---|---|---|
| BA transport | ASBT | NM_001244463.1 | F: TGGATCTGAGCATCAGCATGAC |
| R: GGCACAGCGGCATCATTC | |||
| OSTα | NM_001244266.1 | F: TGTACAAGAACACTCGCTGC | |
| R: GAACACACACACTATCGTGGG | |||
| MRP2 | DQ530510.1 | F: GAACAGGTTTGCTGGCGATATT | |
| R: GCCAGGAGCGCAAAGACA | |||
| MRP3 | XM_003131575.5 | F: TGGACAAAGGGACAATAGCTGAGT | |
| R: TGGCCATCCCGTAGAAGATG | |||
| FABP6 | NM_214215.2 | F: GTGAACAGCCCCAACTACCA | |
| R: CCACTAGCTCATGCCAGCTT | |||
| BA receptors | FXR | NM_001287412.1 | F: TATGAACTCAGGCGAATGCCTGCT |
| R: ATCCAGATGCTCTGTCTCCGCAAA | |||
| TGR5 | XM_013984487.1 | F: TGCTGTCCCTCATCTCATTGG | |
| R: TGTGTAGCGATGATCACCCAG | |||
| BA signaling | FGF19 | XM_003122420.3 | F: AAGATGCAAGGGCAGACTCA |
| R: AGATGGTGTTTCTTGGACCAGT | |||
| SHP | AH014861.3 | F: GCCTACCTGAAAGGGACCAT | |
| R: CAACGGGTGTCAAGCCTTTA | |||
| FGFR4 | XM_003123682.5 | F: GCTCAGAGGTGGAGGTCCTA | |
| R: GCCTGCCAGACAGGTGTATT | |||
| KLβ | XM_003482367.4 | F: GCACCGAGTGGAAGGAGT | |
| R: TTGCCAGTAGGAAGGATTG | |||
| BA biosynthesis | CYP7A1 | NM_001005352.3 | F: GAAAGAGAGACCACATCTCGG |
| R: GAATGGTGTTGGCTTGCGAT | |||
| CYP27A1 | NM_001243304.1 | F: ACTGAAGACCGCGATGAAAC | |
| R: CAAAGGCGAATCAGGAAGGG | |||
| BAAT | XM_021066831.1 | F: GGCTGATGATCCGAGAAGGG | |
| R: ATGCCCCCAAACAAGTCGAT | |||
| BACS | XM_005658625.1 | F: CTGGCTCCCTGCCTATGCT | |
| R: GAACGTGCTTGTGGTCTCCAA |
Statistical Analyses
All statistics were derived using JMP10.0 (SAS Institute, Inc., Cary, NC). Each animal was considered as an experimental unit. The appropriate sample size of 6 animals per group was estimated using total plasma cholesterol as the primary outcome and assuming an average difference of 22% (effect size) and an equal SD of 12% to yield a statistical power of 0.8 for an α error of 0.05, according to our previous experiment that evaluated the effect of dietary fiber supplementation on plasma lipid profile and metabolomics (Wu et al., 2016). Student’s t-test was used to determine the treatment effects on growth performance, plasma lipid profiles, BA compositions, and gene expression in a specific section of intestine. One-way ANOVA was employed to determine section effects, and post hoc Fisher LSD was used to compare the differences among the 5 investigated intestinal sections. Equality of group was tested by the Levene test, and data were subjected to logistic transformation if the test was significant. All data were expressed as mean ± SEM. Statistical significance was considered when P < 0.05. A tendency to differ was claimed when 0.05 < P < 0.1 and power analyses were conducted to verify the least significant number with JMP.
RESULTS
Growth Performance and Plasma Lipid Profiles
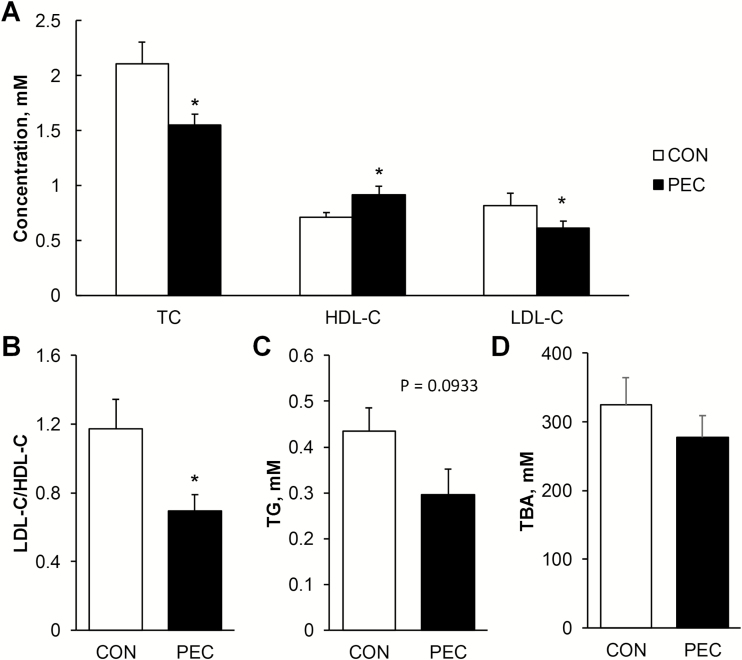
Growth performance was not affected by the inclusion of 5% pectin in the conventional corn–soybean meal diet over the 72-d trial period (Table 3). In PEC-fed pigs, plasma TC (P = 0.029), LDL-C (P = 0.035), and TG (P = 0.093) were found to be less, whereas plasma HDL-C was greater by 30% than for the controls (Figure 1A and C). Therefore, the ratio of LDL-C/HDL-C was significantly lowered in the PEC group (Figure 1B, P = 0.038). Serum TBA was not statistically different between pectin-fed animals and controls (Figure 1D).
Table 3.
Growth performance of pigs fed with 5% pectin for 72 d
| CON | PEC | P value | |
|---|---|---|---|
| Body weight (D0), g | 11.38 ± 0.22 | 10.99 ± 0.14 | 0.242 |
| Body weight (D72), g | 64.60 ± 3.04 | 57.9 ± 1.47 | 0.259 |
| Average daily feed intake, g | 1331 ± 70.7 | 1419 ± 7.19 | 0.352 |
Figure 1.
Effects of 5% pectin inclusion on fasting cholesterol (A and B), triglycerides (C), and total bile acids (D) in the plasma of young pigs fed with corn–soybean meal (n = 6). *Indicates the significant difference between pigs on the pectin and control diets.
Alterations in BA Profiles After Pectin Consumption
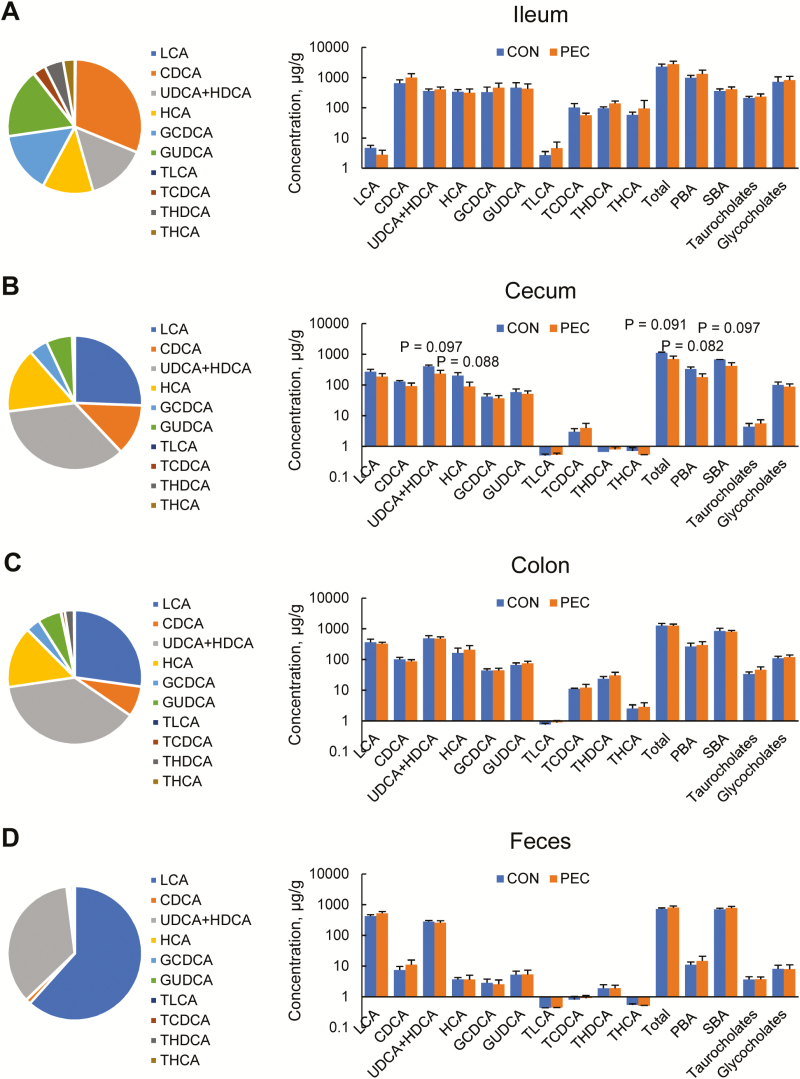
BA composition exhibited great variations among different intestinal sections and feces. In the ileum, chenodeoxycholic acid (CDCA), ursodeoxycholic acid (UDCA) + hyodeoxycholic acid (HDCA), HCA, glycochenodeoxycholic acid (GCDCA), and glycoursodeoxycholic acid (GUDCA) were the most abundant BAs, making up 72.7% of the BA pool, when lithocholic acid (LCA), CDCA, UDCA + HDCA, and HCA made up 88.5% and 87.5% of the cecal and colonic BAs (Figure 2). The ratio of primary, secondary, taurine-conjugated, and glycine-conjugated BAs in the ileal pool was about 46:15:9:30, whereas it was 28:61:1:11 in the cecum and 22:65:3:9 in the colon. All 4 BA categories significantly differed between the ileal pool and the cecal and colonic pools (P < 0.001). In the feces, the major BAs were LCA and UDCA + HDCA, making up 96.79% of total BAs.
Figure 2.
Compositions of bile acid pools in the ileum (A), cecum (B), colon (C), and feces (D) of young pigs fed with 5% pectin (n = 6).
In the ileum (Figure 2A), colon (Figure 2C), and feces (Figure 2D), there were no significant differences in each BA between PEC-fed pigs and controls. In the cecum (Figure 2B), pigs of the PEC group tended to have less UDCA + HDCA (P = 0.097) and HCA (P = 0.088). Since these 2 BAs were abundant in the cecal pool, the total (P = 0.091), primary (P = 0.082) and secondary (P = 0.097) all tended to be less in the PEC-fed pigs than controls. Power analyses showed that the significance of PEC supplement could be revealed when the sample size reaches 7 animals per group.
Distribution of BA-Related Genes in the Intestine
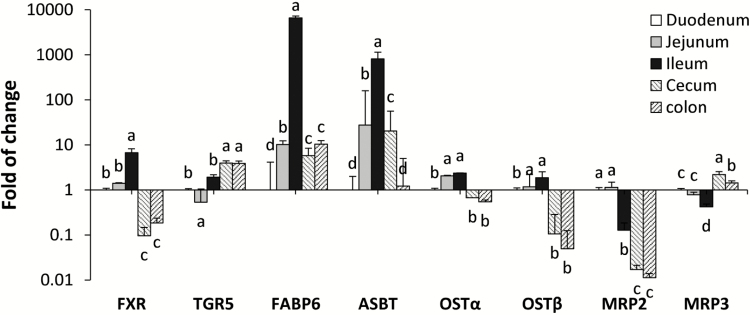
FXR and TGR5 are different BA receptors located in the nuclei and on the membrane, respectively. Along the length of intestine, FXR was highly expressed in the ileum, followed by the jejunum, duodenum, and then the cecum and colon (P < 0.001, Figure 3). In contrast, greater expression of TGR5 is noted in the cecum and colon than the small intestinal sections (P < 0.01, Figure 3). In addition to FXR, the ileal section also possesses exclusive distribution of FGF19 and greatest expression of FABP6 (P < 0.001) and ASBT (P < 0.001). The exporter complex on the basolateral membrane, OSTα and OSTβ, was greatly expressed in the ileum and jejunum than other intestinal sections (P < 0.001). MRP2, the exporter on the apical membrane, gradually reduced from the proximal small intestine to the large intestine (P < 0.001), whereas MRP3, the other basolateral exporter, was more abundant in the cecum and the colon than duodenum, jejunum, and ileum (P < 0.001).
Figure 3.
Spatial distributions of bile acid receptors and transporters along the intestinal tract (n = 12). Different letters indicate significant differences in each gene abundance among intestinal sections.
Changes in BA-Related Genes in Different Intestinal Sections After Pectin Consumption
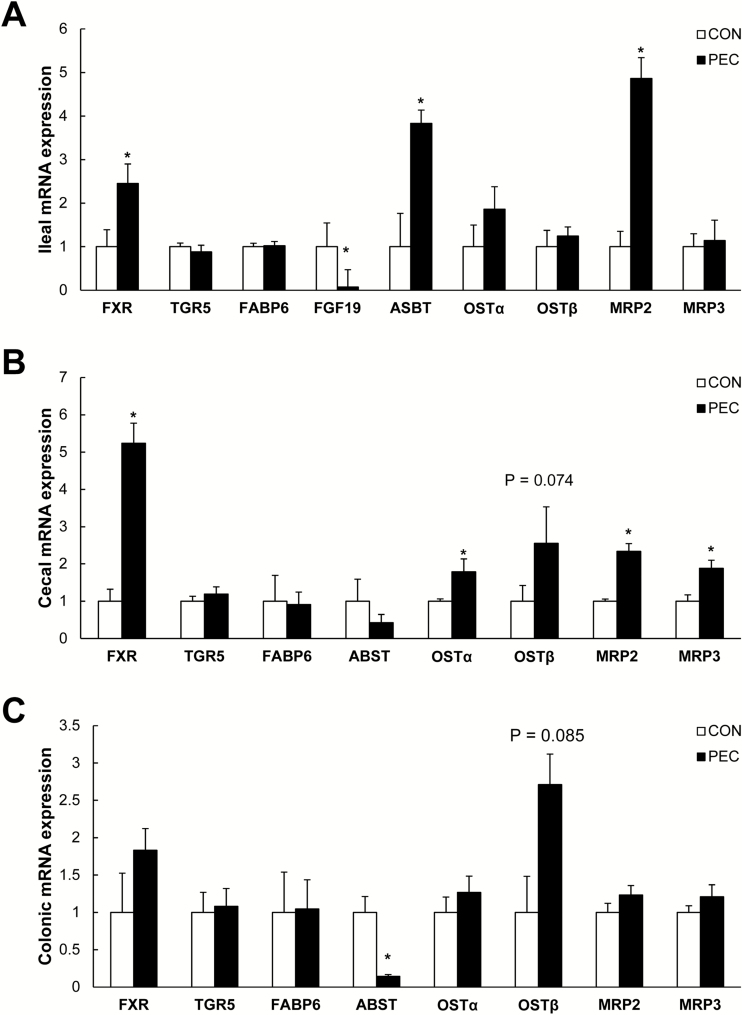
In the ileum (Figure 4A), the magnitude of expression of the nuclear receptor, FXR, was about 250% greater in the PEC-fed pigs than the controls (P = 0.019), whereas site-specific expression of FGF19 was lowered by 90% (P = 0.006). Expression of the apical BA transporters, ASBT (P = 0.027) and MRP2 (P = 0.019), increased by 400% and 500%, respectively, but transporters (OSTα, OSTβ, and MRP3) on the basolateral membrane were not affected. In the cecum (Figure 4B), FXR was about 5 times greater in PEC-fed pigs than controls (P < 0.001). Efflux receptors for BAs, OSTα (P = 0.025), OSTβ (P = 0.074), MRP3 (P = 0.015) on the basolateral membrane, and MRP2 (P = 0.005) on the apical membrane were also upregulated in this section. In the colon (Figure 4C), expression of ASBT (P = 0.036) was less, and OSTβ (P = 0.085) tended to be greater in the PEC-fed pigs than those in the controls.
Figure 4.
Bile acid receptors and transporters in the ileum (A), cecum (B), and colon (C) of young pigs fed with 5% pectin (n = 6). *Indicates the significant group difference.
Hepatic biosynthesis of BAs after pectin consumption.
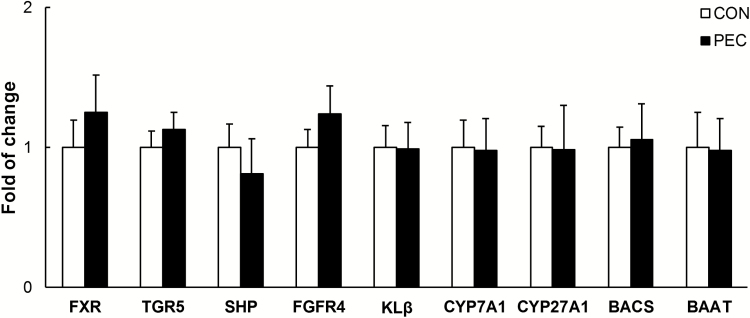
In the liver, expression of critical enzymes involved in biosynthesis (CYP7A1 and CYP27A1) and conjugation (BACS and BAAT) also remained unaffected in the PEC-fed pigs (Figure 5). No difference has been noted in liver FXR, TGR5, SHP, FGFR4, and KLβ either.
Figure 5.
Hepatic expression of bile acid-related genes in young pigs fed with pectin or control diets (n = 6).
DISCUSSION
In the current study, serum cholesterol and TG were lowered by 25% when pigs were put on a conventional corn–soybean meal diet supplemented with 5% pectin. This finding suggested that pectin supplementation can also benefit cholesterol metabolism with normal diets. Pectin consumption resulted in minor alterations in the BA composition and upregulation of intestinal BA transporters and receptors. Unexpectedly, genes encoding the critical enzymes for liver BA biosynthesis were not affected by pectin inclusion at the transcriptional level.
To investigate BA transport, BA profiling is an important step. Each BA molecule has different hydrophobicity, which is one of the determinant factors for transporter affinity and transport kinetics (Aldini et al., 1996). Characterization of BAs can be very challenging due to the structural similarity of different BA. Taking advantage of chromatography and mass spectrometry technique, we were able to quantify 10 BAs in the digesta and feces with the solid phase extraction and LC-MS/MS method. Consistent with the composition of biliary BAs (Si et al., 2015; Mi et al., 2016), glycocholates were also found to be the major conjugation form of intestinal BAs. It provides additional evidence to show the similarity in the BA composition between the pig and the human. It was shown that BA composition varies greatly in each intestinal segment. Secondary and unconjugated BAs occupied a greater portion in the cecum and colon than that in the ileum, whereas primary BA had a reverse pattern. Commensal microbiota in the large intestine, harboring bile salt hydrolase (BSH) and BA-inducible genes, actively participate in the processes of deconjugation and dehydroxylation of BAs (Wahlstrom et al., 2016; Zhang et al., 2018). Pectin, as a probiotic, can promote the growth of Prevotella and Dialister in the cecum of pigs (Tian et al., 2017), and Prevotellaceae in the large intestine was associated with increased secondary BA and decreased primary and conjugated BAs (Zhang et al., 2018). In line with its probiotic effect, HCA and UDCA + HDCA tended to be decreased by pectin consumption in the cecum. Therefore, microbial activities could be an important route for pectin to interfere with the BA metabolism in the large intestine. Furthermore, in the same intestinal section, basolateral BA exporters, OSTα/β, and MRP3 were also upregulated, reflecting an increase in overall BA transport. It is not clear about the biological consequences followed by the increased BA transport in the cecum. It may also contribute to fecal excretion of BAs. Or it could interfere with secretion of glucagon-like protein 1 (GLP-1) in the cecum. It has been known that BAs can stimulate GLP-1 via TGR5 in the lower intestine (Katsuma et al., 2005; Brighton et al., 2015). This peptide is one of the powerful regulators for obesity development (Holst, 2007; Greiner and Backhed, 2016), and thus, pectin would interfere with lipid metabolism via GLP-1.
The ileum has been confirmed as the major site of BA recycling in human and mice. The ileum has been shown to have abundant expression of ASBT and FABP6 as well as FGF19/fibroblast growth factor 15 (FGF15) (Alrefai and Gill, 2007; Dawson et al., 2009; Dawson and Karpen, 2015). Besides recycling BAs, the ileum can also regulate liver BA synthesis via FGF19/FGF15 (Dawson et al., 2005; Landrier et al., 2006). In pigs, we found the greatest expression of ASBT, FXR, FABP6, and FGF19 in the ileum. Along with the greatest size of the BA pool, the ileum of pigs is also the major site for BA recycling. Gunness and his coworkers also showed the largest BA pool at 3/4 of the small intestine, which is approximately the junction of jejunum and ileum (Gunness et al., 2016a; Gunness et al., 2016b).We found that OSTα/β was comparable in the jejunum to the ileum. Therefore, the jejunum maybe a secondary region for BA recycling in pigs. Pectin consumption did not affect ileal BA consumption, but upregulated apical transporters ASBT and MRP2 by 3.8- and 5-fold, respectively. ASBT is the major transporter to import BAs into the cell and hence its upregulation strongly suggests an increased import of BAs in the ileum. However, the concurrent up-regulation of MRP2, an exporter on the apical membrane, can significantly reduce the net transport of BAs. Thus, the cytosolic and basolateral transporters (FABP6, OSTα/β, and MRP3) remained unaltered when ASBT was increased by pectin consumption.
BA transporters need to be highly coordinated. Knowledge is growing and FXR is considered as the core of the regulatory network. First of all, FXR is a sensor of changes in BAs as it has a ligand binding domain for BA binding (Tu et al., 2000; Modica et al., 2010). Secondly, by interacting with FXR response element FXR can transcriptionally regulate a wide range of genes related to BA transport and biosynthesis, including OSTα/β (Landrier et al., 2006), FGF19 (Miyata et al., 2014), CYP7A1 (Goodwin et al., 2000; Lu et al., 2000), BACS, and BAAT (Pircher et al., 2003). We found that FXR was also increased in the ileum and cecum when BA transporters were upregulated by pectin consumption. Pectin is likely to increase BA influx to induce FXR and consequently trigger the transcription of BA transporters. Unlike other BA transporters, the activation of ASBT gene transcription is independent of FXR (Duane et al., 2007). It has been reported that FXR can indirectly inhibit ASBT expression via the SHP-RAR pathway (Neimark et al., 2004; Zhu et al., 2017b). FGF19 is a stimulator for SHP (Sinha et al., 2008). The downregulated FGF19 in the ileum may decrease the production of SHP locally and thereby increase the ASBT expression in the ileum after pectin consumption.
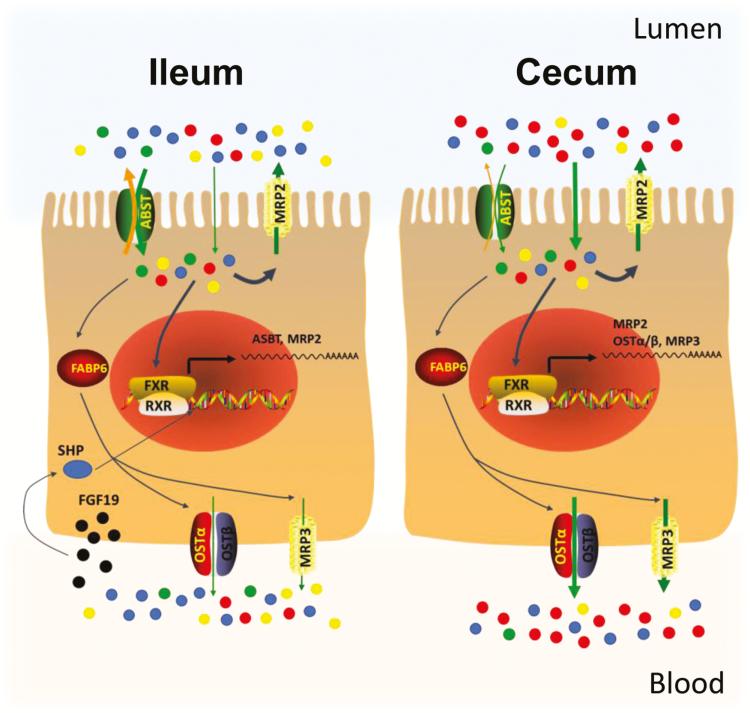
In the current study, we fully characterized the composition of intestinal BAs and the distribution of BA receptors and transporters along the length of swine intestine. The pig shared many similarities in the BA composition and BA transporters with the human, suggesting that it is a good biomedical model in BA-related research. Pectin can interact with intestinal BA metabolism and transport in a site-specific manner. In the ileum, the major site to recycle BA, pectin increased in-and-out transport of BA just across the apical membrane by upregulating ASBT and MRP2, whereas in the cecum, it increased the total BA transport across the epithelium by increasing OSTα/β and MRP3 (Figure 6).
Figure 6.
Illustration of intestinal bile acid transport after pectin consumption. In the ileum, pectin upregulates importer ASBT and exporter MRP2 on the apical membrane, which enhances in-and-out bile acid transport just across the apical membrane without affecting the cytosolic and basolateral transport of bile acids. In the cecum, pectin upregulates OSTα/β, MRP3, and MRP2 to enhance the efflux of bile acids across the epithelial cells. Dots in blue, red, green, and yellow represent the primary, secondary, taurine-conjugated, and glycine-conjugated bile acids, respectively.
ACKNOWLEDGMENTS
We thank Dr. Kolapo Ajuwon for reviewing the manuscript. Drs. Curtin Miller and Xiaohui Feng were acknowledged for the language editing. Tian Qiao helped with operating LC-MS.
Conflict of interest statement. None declared.
LITERATURE CITED
- Aldini R., Roda A., Montagnani M., Cerrè C., Pellicciari R., and Roda E.. 1996. Relationship between structure and intestinal absorption of bile acids with a steroid or side-chain modification. Steroids 61:590–597. doi:10.1016/S0039-128X(96)00119-5 [DOI] [PubMed] [Google Scholar]
- Alrefai W. A., and Gill R. K.. 2007. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm. Res. 24:1803–1823. doi: 10.1007/s11095-007-9289-1 [DOI] [PubMed] [Google Scholar]
- Brighton C. A., Rievaj J., Kuhre R. E., Glass L. L., Schoonjans K., Holst J. J., Gribble F. M., and Reimann F.. 2015. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology 156:3961–3970. doi: 10.1210/en.2015-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council N. R. 2012. Nutrient requirements of swine. Natl. Acad. Press, Washington, D.C. [Google Scholar]
- Dawson P. A., Hubbert M., Haywood J., Craddock A. L., Zerangue N., Christian W. V., and Ballatori N.. 2005. The heteromeric organic solute transporter alpha-beta, ostalpha-ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 280:6960–6968. doi: 10.1074/jbc.M412752200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. A., and Karpen S. J.. 2015. Intestinal transport and metabolism of bile acids. J. Lipid Res. 56:1085–1099. doi: 10.1194/jlr.R054114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. A., Lan T., and Rao A.. 2009. Bile acid transporters. J. Lipid Res. 50:2340–2357. doi: 10.1194/jlr.R900012-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane W. C., Xiong W., and Wolvers J.. 2007. Effects of bile acids on expression of the human apical sodium dependent bile acid transporter gene. Biochim. Biophys. Acta 1771:1380–1388. doi: 10.1016/j.bbalip.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Gao T., Feridooni H. A., Howlett S. E., and Pelis R. M.. 2017. Influence of age on intestinal bile acid transport in C57BL/6 mice. Pharmacol. Res. Perspect. 5:e00287. doi: 10.1002/prp2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffarzadegan T., Marungruang N., Fåk F., and Nyman M.. 2016. Molecular properties of guar gum and pectin modify cecal bile acids, microbiota, and plasma lipopolysaccharide-binding protein in rats. PLoS ONE 11:e0157427. doi: 10.1371/journal.pone.0157427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B., Jones S. A., Price R. R., Watson M. A., McKee D. D., Moore L. B., Galardi C., Wilson J. G., Lewis M. C., Roth M. E., et al. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6:517–526. doi:10.1016/S1097-2765(00)00051-4 [DOI] [PubMed] [Google Scholar]
- Greiner T. U., and Bäckhed F.. 2016. Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 5:753–758. doi: 10.1016/j.molmet.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunness P., Michiels J., Vanhaecke L., De Smet S., Kravchuk O., Van de Meene A., and Gidley M. J.. 2016a. Reduction in circulating bile acid and restricted diffusion across the intestinal epithelium are associated with a decrease in blood cholesterol in the presence of oat β-glucan. Faseb J. 30:4227–4238. doi: 10.1096/fj.201600465R [DOI] [PubMed] [Google Scholar]
- Gunness P., Williams B. A., Gerrits W. J., Bird A. R., Kravchuk O., and Gidley M. J.. 2016b. Circulating triglycerides and bile acids are reduced by a soluble wheat arabinoxylan via modulation of bile concentration and lipid digestion rates in a pig model. Mol. Nutr. Food Res. 60:642–651. doi: 10.1002/mnfr.201500686 [DOI] [PubMed] [Google Scholar]
- Holst J. J. 2007. The physiology of glucagon-like peptide 1. Physiol. Rev. 87:1409–1439. doi: 10.1152/physrev.00034.2006 [DOI] [PubMed] [Google Scholar]
- Kakiyama G., Muto A., Takei H., Nittono H., Murai T., Kurosawa T., Hofmann A. F., Pandak W. M., and Bajaj J. S.. 2014. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: validation by GC-MS and LC-MS. J. Lipid Res. 55:978–990. doi: 10.1194/jlr.D047506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma S., Hirasawa A., and Tsujimoto G.. 2005. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 329:386–390. doi: 10.1016/j.bbrc.2005.01.139 [DOI] [PubMed] [Google Scholar]
- Landrier J. F., Eloranta J. J., Vavricka S. R., and Kullak-Ublick G. A.. 2006. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G476–G485. doi: 10.1152/ajpgi.00430.2005 [DOI] [PubMed] [Google Scholar]
- Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., and Mangelsdorf D. J.. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 6:507–515. doi:10.1016/S1097-2765(00)00050-2 [DOI] [PubMed] [Google Scholar]
- Matheson H. B., Colón I. S., and Story J. A.. 1995. Cholesterol 7 alpha-hydroxylase activity is increased by dietary modification with psyllium hydrocolloid, pectin, cholesterol and cholestyramine in rats. J. Nutr. 125:454–458. doi: 10.1093/jn/125.3.454 [DOI] [PubMed] [Google Scholar]
- Matheson H. B., and Story J. A.. 1994. Dietary psyllium hydrocolloid and pectin increase bile acid pool size and change bile acid composition in rats. J. Nutr. 124:1161–1165. doi: 10.1093/jn/124.8.1161 [DOI] [PubMed] [Google Scholar]
- Mi S., Lim D. W., Turner J. M., Wales P. W., and Curtis J. M.. 2016. Determination of bile acids in piglet bile by solid phase extraction and liquid chromatography-electrospray tandem mass spectrometry. Lipids 51:359–372. doi: 10.1007/s11745-016-4125-1 [DOI] [PubMed] [Google Scholar]
- Miyata M., Hata T., Yamazoe Y., and Yoshinari K.. 2014. SREBP-2 negatively regulates FXR-dependent transcription of FGF19 in human intestinal cells. Biochem. Biophys. Res. Commun. 443:477–482. doi: 10.1016/j.bbrc.2013.11.126 [DOI] [PubMed] [Google Scholar]
- Modica S., Gadaleta R. M., and Moschetta A.. 2010. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl. Recept. Signal. 8:e005. doi: 10.1621/nrs.08005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neimark E., Chen F., Li X., and Shneider B. L.. 2004. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology 40:149–156. doi: 10.1002/hep.20295 [DOI] [PubMed] [Google Scholar]
- Pircher P. C., Kitto J. L., Petrowski M. L., Tangirala R. K., Bischoff E. D., Schulman I. G., and Westin S. K.. 2003. Farnesoid X receptor regulates bile acid-amino acid conjugation. J. Biol. Chem. 278:27703–27711. doi: 10.1074/jbc.M302128200 [DOI] [PubMed] [Google Scholar]
- Si G. L., Yao P., and Shi L.. 2015. Rapid determination of bile acids in bile from various mammals by reversed-phase ultra-fast liquid chromatography. J. Chromatogr. Sci. 53:1060–1065. doi: 10.1093/chromsci/bmu167 [DOI] [PubMed] [Google Scholar]
- Sinha J., Chen F., Miloh T., Burns R. C., Yu Z., and Shneider B. L.. 2008. Beta-klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 295:G996–G1003. doi: 10.1152/ajpgi.90343.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Bruggeman G., van den Berg M., Borewicz K., Scheurink A. J., Bruininx E., de Vos P., Smidt H., Schols H. A., and Gruppen H.. 2017. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol. Nutr. Food Res. 61:1–10. doi: 10.1002/mnfr.201600186 [DOI] [PubMed] [Google Scholar]
- Tu H., Okamoto A. Y., and Shan B.. 2000. FXR, a bile acid receptor and biological sensor. Trends Cardiovasc. Med. 10:30–35. [DOI] [PubMed] [Google Scholar]
- Wahlström A., Sayin S. I., Marschall H. U., and Bäckhed F.. 2016. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24:41–50. doi: 10.1016/j.cmet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Wu W., Xie J., and Zhang H.. 2016. Dietary fibers influence the intestinal scfas and plasma metabolites profiling in growing pigs. Food Funct. 7:4644–4654. doi: 10.1039/c6fo01406b [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu W., Lee Y.-K., Xie J., and Zhang H.. 2018. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front Microbiol. 9. (Original Research) doi: 10.3389/fmicb.2018.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R., Hou Y., Sun Y., Li T., Fan J., Chen G., and Wei J.. 2017a. Pectin penta-oligogalacturonide suppresses intestinal bile acids absorption and downregulates the FXR-FGF15 axis in high-cholesterol fed mice. Lipids 52:489–498. doi: 10.1007/s11745-017-4258-x [DOI] [PubMed] [Google Scholar]
- Zhu R. G., Sun Y. D., Hou Y. T., Fan J. G., Chen G., and Li T. P.. 2017b. Pectin penta-oligogalacturonide reduces cholesterol accumulation by promoting bile acid biosynthesis and excretion in high-cholesterol-fed mice. Chem. Biol. Interact. 272:153–159. doi: 10.1016/j.cbi.2017.05.018 [DOI] [PubMed] [Google Scholar]