Abstract
Aim:
Human cystic echinococcosis (CE), caused by the larval stage of Echinococcus granulosus cestodes, is a globally distributed chronic disease that is an important socioeconomic and public health problem in humans and livestock in developing countries, including Iran. The aim of this study was to determine the overall seroprevalence of hydatid infection in the general population of Iran.
Materials and Methods:
This systematic review began by searching electronic databases in English (PubMed, Science Direct, Scopus, and Google Scholar) and Persian (Magiran, Scientific Information Database, Iran Medex, and Iran Doc).
Results:
Our search resulted in a total of 40 reports published from 1995 to 2015. Of 49,460 individuals surveyed, 3090 cases of hydatidosis were reported. Community-based studies showed that the seroprevalence of CE in the Iranian general population was 6.0% (95% confidence interval: 5.0-7.0%). The age group with the highest CE seroprevalence was 20-40 years, and the lowest one was in the under 20 year’s group. The seroprevalence of hydatidosis in males was significantly higher than that in females. In addition, the intended rate was significantly higher in rural regions than in urban areas.
Conclusion:
Management program for developing more efficient diagnostic tests should be established. Further, cost-effective preventive approaches, including relevant research, should be considered. Finally, hydatid cyst control programs that are important for interrupting the transmission of human CE should be improved.
Keywords: cystic echinococcosis, diagnosis, general population, hydatidosis, Iran, seroprevalence
Introduction
Cystic echinococcosis (CE) or hydatidosis is a chronic disease caused by the larval stage of the Echinococcus granulosus parasite, a globally important helminth [1-3]. In addition to being a major public health problem in the world, many studies have shown that CE is an important socioeconomic concern. CE is recognized as an emerging or re-emerging disease, with a geographic distribution that is greater than previously recognized [3-6].
Humans acquire this infection by accidental ingestion of E. granulosus eggs with food, water, or contaminated soil. CE was included in the World Health Organization (WHO) initiative to assess the global burden of foodborne diseases [7]. The natural history of CE in humans usually includes several years of asymptomatic infection. The cysts are usually found in the liver (50-70%), lungs (20-30%), and, less commonly, in other organs (10%), for example, spleen, brain, kidneys, peritoneal cavity, muscle, bone, and heart [1,8,9]. Space in the body is occupied by hydatid cysts, and pressure on surrounding tissues typically causes clinical signs to develop. Anaphylactic shock and secondary CE are major complications caused by the rupture of cysts and spillage of their contents [2,10].
CE is a cosmopolitan zoonosis, with highly endemic areas in some regions of South America, North Africa, China, and the Middle East [2,10]. Iran is the only country in the Middle East where Echinococcus spp. has been found in natural host populations continuously to the present [11]. The previous studies revealed that Iran is one of the areas that has been known as hyperendemic area for CE by the WHO in terms of close relationship of a high proportion of society with animals, traditional animal husbandry, and then contact with the sources of infection, and 1% of all surgeries in this country can be attributed to CE [12-15].
Human cases of CE are regularly reported from medical centers in different parts of Iran, and the incidence of CE has been estimated at 1.18-3 per 100,000 populations [10,15,16]. Overall, the annual cost of CE is estimated at US $93.39 million for individuals living in Iran [10].
Some factors, such as exposure to contaminated soil, are closely linked to dogs, which play an essential role in the development and progression of CE [1,10]. However, many studies have examined the seroprevalence and effects of CE in Iran, and there is little information about the seroprevalence of E. granulosus infection in the general population. Therefore, the objective of the present meta-analysis was to estimate the seroprevalence of CE in the general population of Iran to evaluate the risk factors associated with this infection.
Materials and Methods
Ethical approval
This study is based on data and not on the animals so, ethical approval is not necessary to pursue such type of the study.
Study design and data sources
Publications for the present systematic review and meta-analysis were collected from four English (PubMed, ScienceDirect, Scopus, and Google Scholar) and four Persian (Magiran, Scientific Information Database, Iran Medex, and Iran Doc) databases using the following search terms: “Hydatid cyst,” “E. granulosus,” “cystic echinococcosis,” “Iran,” “general population,” “serology,” “epidemiology,” “seroepidemiology,” and “seroprevalence.” Data were collected from a wide range of literature comprising full text articles, abstracts, and proceedings from national parasitological congresses in Iran which were published from 1995 to 2015.
Study selection
To estimate the seroprevalence of CE in Iranian general population, cross-sectional studies were included in the analysis. CE was diagnosed in these studies by serological methods. Two researchers independently assessed studies for eligibility for inclusion in this analysis. Discrepancies between the researchers were resolved through discussion and consensus by a third reviewer for the accuracy and to remove conflict before starting of the study. Serological surveys carried out in other countries and studies that diagnosed infections with non-serological methods were excluded from the present study.
Data extraction
In this review to provide comprehensive awareness, all studies that were based on serological methods and carried out to estimate the seroprevalence of CE in general populations in Iran were included. A data form was used to extract data consisting of the first author, year of publication, research locations, sample size, gender, and number of samples that were found positive for infection, age distribution, and methods. Information on risk factors including fruit and vegetable washing methods, contact with dogs, area of residence, education level, and occupation was also gathered.
Statistical analysis
Since the wide variation was observed in included studies (Q=172.90, df=37, I2=98%, p<0.001), significant heterogeneity between studies was evident that is why we used to random effects instead of fixed effect. We calculated a pooled estimate of the prevalence (proportion) using a random effects model (reported as effect estimates with a 95% confidence interval [CI]). An overall seroprevalence and group-specific seroprevalences based on age (0-19, 20-40, 40-60, and ≥60 years), gender, and residential region were calculated. The heterogeneity among studies was evaluated (Der Simonian and Laird method) using the Cochran Q-test and I2 statistic. For the Q statistic, p<0.10 indicates statistically significant heterogeneity, and for the I2 statistic, I2 > 50% indicates a large degree of heterogeneity. A fixed effects model using the Mantel-Haenszel method was applied if the Q statistic was p<0.10 or I2 was >50%. The presence of heterogeneity was more through subgroups analysis and meta-regression. To evaluate the possibility of publication bias, an Egger weighted regression was performed. All statistical analyses were performed using Stata software version 11.0 (Stata Corp LP, College Station, TX, USA). p<0.05 was considered statistically significant.
Results
In the current systematic review and meta-analysis, of 1670 studies found in the literature search, 40 publications were included based on our criteria. Figure-1 shows a flowchart of the study design. Overall, 49,460 individuals, with 3090 seropositive cases, were included in the calculation of CE seroprevalence. The general characteristics and results of the studies included in this analysis are presented in Table-1[12,17-50]. To identify the sources of study heterogeneity, we performed a subgroup meta-analysis of five factors including mean age, sex, residential area, education level, and diagnostic test. There was wide variation in seroprevalence estimates across studies (Q=172.90, df=37, I2=98%, p<0.001). Using a random effects model, the seroprevalence of CE in the general population of Iran was found to be 6.0% (95% CI: 5.0-7.0%) (Figure-2). A subgroup analysis showed that the lowest and highest seroprevalences were in the age groups under 20 years (3.4%) and 20-40 years (10.6%) and that this difference was statistically significant. The seroprevalence of CE in males (9%; 95% CI: 7.0-12%) was significantly higher than that in females (8%; 95% CI: 6.0-10%) (z=51.02, df=1, p<0.001) (Figure-3 and Table-2). The seroprevalence of CE in rural regions (7.0%; 95% CI: 4.0-9.0%) was significantly higher than that in urban areas (3.0%; 95% CI: 2.0-4.0%) (z=3.90, df=1, p=0.048) (Figure-4 and Table-2).
Figure-1.
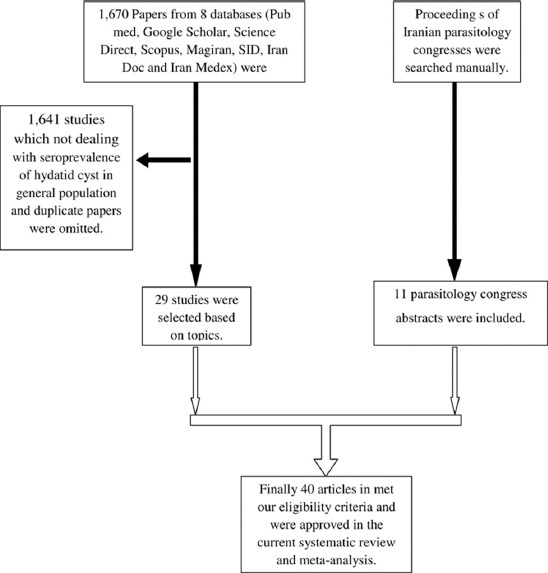
Flowchart describing the study design process.
Table-1.
General characteristics of included studies in the current systematic review and meta-analysis.
| Reference | Location (Province) | Lab method | Sample size | Positive | % (95% CI) | Gender | Residence | ||
|---|---|---|---|---|---|---|---|---|---|
| Male (%) | Female (%) | Urban (%) | Rural (%) | ||||||
| Saberi-Firouz et al. [20] | Fars | CIE | 1000 | 68 | 6.8 (5.2-8.6) | 11.80 | 15.20 | - | - |
| Saberi-Firouz et al. [20] | Fars | ELISA | 1000 | 127 | 12.7 (10.5-15.1) | 11.80 | 15.20 | - | - |
| Arbabi et al. [21] | Hamedan | IFA | 1530 | 46 | 3 (2.2-4.1) | - | 2.90 | - | - |
| Zariffard et al. [22] | Western Provinces | ELISA | 4138 | 230 | 5.5 (4.8-6.3) | 4.70 | 6.20 | 4.60 | 7 |
| Mohajeri et al. [23] | Khorasan Razavi | IFA | 1100 | 41 | 3.7 (2.6-5.1) | - | - | - | - |
| Hosseini and Masoud [24] | Kordestan | IFA | 1114 | 143 | 12.8 (10.8-15.1) | - | - | - | - |
| Sedaghat-Gohar [25] | Tehran | IFA | 1052 | 62 | 5.8 (4.5-7.5) | 4.50 | 6.36 | 4.80 | 8.10 |
| Darani et al. [26] | Chaharmahal va Bakhtiari | CIE | 2524 | 120 | 4.7 (3.9-5.6) | 4.40 | 5.10 | - | - |
| Hanilou et al. [27] | Zanjan | ELISA | 2367 | 71 | 2.9 (2.3-3.7) | 2.70 | 3.20 | - | - |
| Aflaki et al. [28] | Ilam | Dot-ELISA | 3000 | 37 | 1.2 (0.8-1.7) | 1 | 1.47 | 0.56 | 12.34 |
| Farokhzad et al. [29] | Tehran | IFA | 437 | 1 | 0.2 (0.005-1.2) | - | - | ||
| Arbabi and Hooshyar [30] | Kashan | IHA | 500 | 12 | 2.4 (1.2-4.1) | 0.90 | 3.50 | - | - |
| Asmar et al. [31] | Tehran | CIE | 233 | 39 | 16.7 (11.9-22.8) | 8.58 | 8.15 | - | - |
| Rouhnavaz et al. [32] | Tehran | IFA | 1842 | 340 | 18.4 (16.5-20.5) | - | - | - | - |
| Amiri et al. [33] | Kermanshah | IFA | 1072 | 86 | 8 (6.4-9.9) | - | - | - | - |
| Manouchehri-Naeini et al. [34] | Chaharmahal va Bakhtiari | IHA | 388 | 44 | 11.3 (8.2-15.2) | - | - | - | - |
| Baharsefat et al. [35] | Golestan | ELISA | 1024 | 22 | 2.1 (1.3-3.2) | 1.93 | 3.16 | - | - |
| Baharsefat et al. [35] | Golestan | IFA | 1024 | 24 | 2.3 (1.5-3.4) | - | - | 2.47 | 2.45 |
| Rafiei et al. [18] | South Western provinces | ELISA | 3446 | 475 | 13.7 (12.5-15.1) | 13.70 | 13.80 | - | - |
| Hadadian et al. [36] | Kordestan | ELISA | 1979 | 22 | 1.1 (0.6-1.6) | 0.45 | 1.65 | 0.90 | 1.42 |
| Moazezi et al. [37] | Kerman | ELISA | 451 | 37 | 8.2 (5.7-11.3) | 4.90 | 9.70 | - | - |
| Tavalla et al. [19] | Tehran | Dot-ELISA | 1100 | 18 | 16.3 (9.6-25.8) | - | - | - | - |
| Esmaeili and Arbabi [38] | Isfahan | IFA | 361 | 11 | 3.1 (1.5-5.4) | 2.30 | 3.70 | 2 | 4.2 |
| Mirzanejad-Asl et al. [39] | Ardebil | ELISA | 2008 | 184 | 9.1 (7.8-10.5) | 7.90 | 10 | - | - |
| Sarkari et al. [40] | Kohkiluyeh and Buyer Ahmad | ELISA | 500 | 36 | 7.2 (5.1-9.9) | 58.33 | 41.66 | - | - |
| Solhjoo et al. [41] | Fars | ELISA | 1096 | 69 | 6.2 (4.8-7.9) | 65.20 | 34.80 | 5 | 8 |
| Harandi et al. [42] | Kerman | ELISA | 1062 | 77 | 7.2 (5.7-9.1) | 2.10 | 8.30 | - | - |
| Garedaghi and Bahavarnia [43] | East Azerbaijan | ELISA | 1500 | 19 | 1.2 (0.7-1.9) | 0.83 | 1.76 | 0.93 | 1.80 |
| Heidari et al. [44] | Ardebil | ELISA | 670 | 12 | 1.7 (0.9-3.1) | 2.60 | 1.68 | 1.10 | 2.58 |
| Dadkhah et al. [45] | East Azerbaijan | IFA | 250 | 8 | 3.2 (1.3-6.3) | - | - | - | - |
| Rakhshanpour et al. [17] | Qom | ELISA | 1564 | 25 | 1.5 (1.1-2.3) | 2.20 | 0.90 | 2.10 | 1.20 |
| Asgari et al. [46] | Markazi | ELISA | 578 | 20 | 3.4 (2.1-5.3) | 2.31 | 4.15 | 1.46 | 6.98 |
| Zibaei et al. [47] | Lorestan | ELISA | 617 | 95 | 15.3 (12.4-18.8) | 5.30 | 38.90 | ||
| Shahrokhabadi et al. [48] | Kerman | ELISA | 486 | 9 | 1.8 (0.8-3.5) | 3.10 | 1.94 | 1.30 | 3.25 |
| Fallah-Omrani et al. [49] | Lorestan | ELISA | 927 | 25 | 2.6 (1.7-3.9) | 3.59 | 2.12 | 1.20 | 3.24 |
| Sadjjadi et al. [50] | Khorasan Razavi | ELISA | 1033 | 55 | 5.3 (4.1-6.9) | 4 | 5.92 | 4.10 | 6 |
| Manouchehri-Naeini et al. [34] | Chaharmahal va Bakhtiari | ELISA | 1280 | 26 | 2.1 (1.3-2.9) | - | - | - | - |
| Ilbeigi et al. [12] | Isfahan | ELISA | 635 | 7 | 1.1 (0.4-2.2) | 2.24 | 0.27 | 1.49 | - |
CI=Confidence interval, ELISA=Enzyme-linked immunosorbent assay, CIE=Counter immune electrophoresis, IFA=Indirect fluorescent antibody test, IHA=Indirect hemagglutination assay
Figure-2.
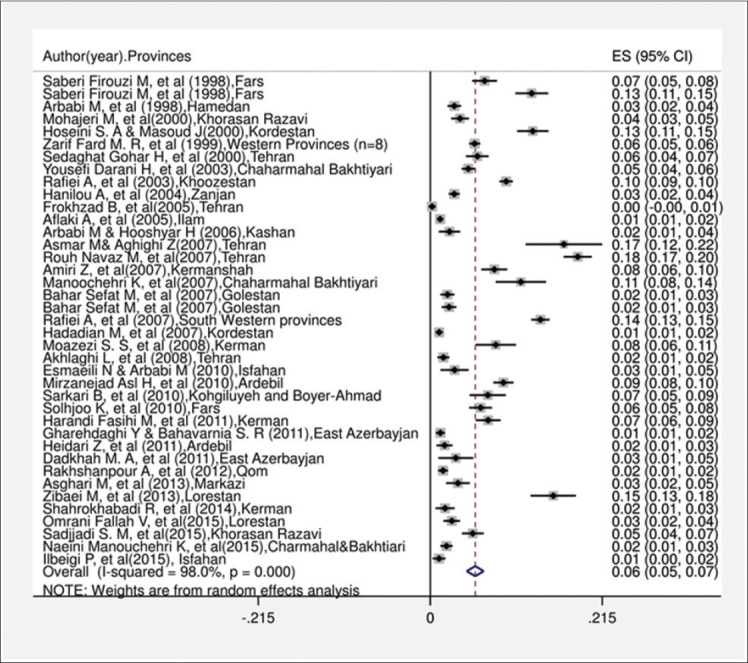
Forest plot for the prevalence of serology hydatidosis in general population in Iran.
Figure-3.
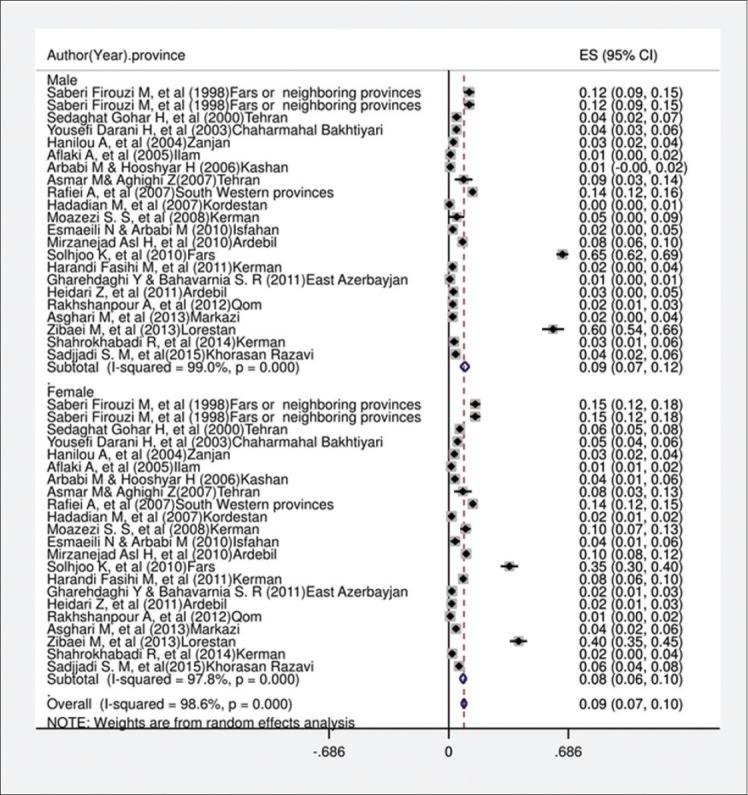
Forest plot for distribution seroprevalence of hydatidosis in male and female groups in Iran.
Table-2.
Subgroup meta-analysis of the prevalence of hydatid cyst serology for characteristics of the included studies.
| Variable | n | Prevalence (%) | 95% CI | I2 (%) | p-value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age (year) | ||||||
| <20 | 19 | 3.4 | 0.7 | 12.4 | 89.4 | p<0.001 |
| 20-40 | 20 | 10.6 | 2.4 | 14.1 | 90.1 | |
| 41-60 | 21 | 7.5 | 4.1 | 13.3 | 88.8 | |
| >60 | 16 | 5.4 | 2.7 | 8.4 | 87.8 | |
| Sex | ||||||
| Male | 22 | 9.2 | 6.7 | 11.8 | 99.8 | p<0.001 |
| Female | 22 | 8.3 | 6.4 | 10.2 | 99.8 | |
| Residence | ||||||
| Urban | 8 | 3.0 | 1.8 | 4.2 | 88.6 | p=0.048 |
| Rural | 8 | 6.5 | 4.0 | 9.1 | 96.0 | |
| Lab method | ||||||
| ELISA | 22 | 5.4 | 4.0 | 6.9 | 98.0 | p<0.001 |
| CIE | 3 | 8.4 | 4.7 | 12.1 | 92.8 | |
| IFA | 10 | 6.0 | 3.2 | 8.9 | 98.5 | |
| Others* | 4 | 3.2 | 1.4 | 4.9 | 92.7 | |
| Education | ||||||
| Illiterate | 10 | 6.9 | 2.3 | 11.5 | 93.6 | p<0.001 |
| School | 6 | 8.3 | 4.4 | 12.6 | 90.1 | |
| Diploma | 7 | 5.6 | 1.7 | 8.6 | 96 | |
| University | 7 | 4.3 | 1.5 | 7.1 | 98.4 | |
IHA=Dot ELISA, CI=Confidence interval, ELISA=Enzyme-linked immunosorbent assay, CIE=Counter immune electrophoresis, IFA=Indirect fluorescent antibody test, IHA=Indirect hemagglutination assay
Figure-4.
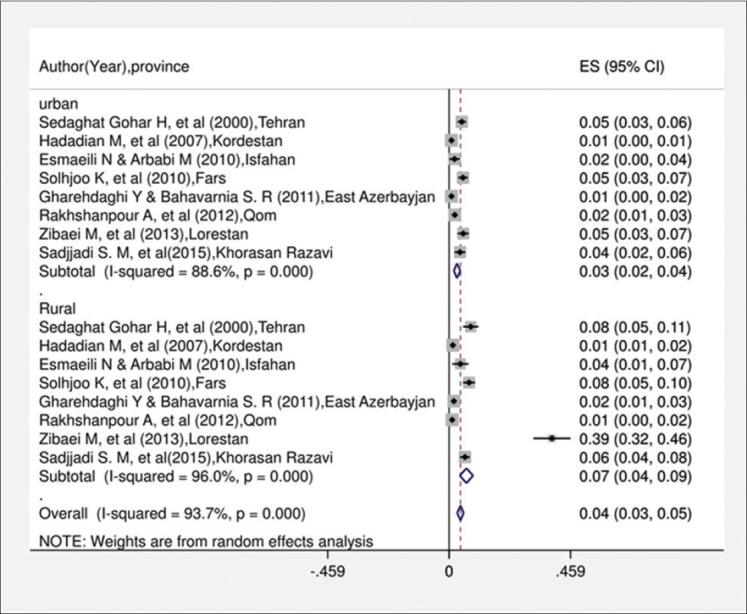
Forest plot for distribution seroprevalence of hydatidosis in urban and rural groups in Iran.
Five types of serological diagnostic assays including enzyme-linked immunosorbent assay (ELISA), indirect fluorescent antibody test (IFA), indirect hemagglutination assay (IHA), dot-ELISA, and counter immune electrophoresis (CIE) were conducted in the different studies included in this analysis. The CIE method was used in three studies, the IFA test in 10, ELISA in 22, and other serological methods in 22. A subgroup analysis of methods is shown in Figure-5 and Table-2.
Figure-5.
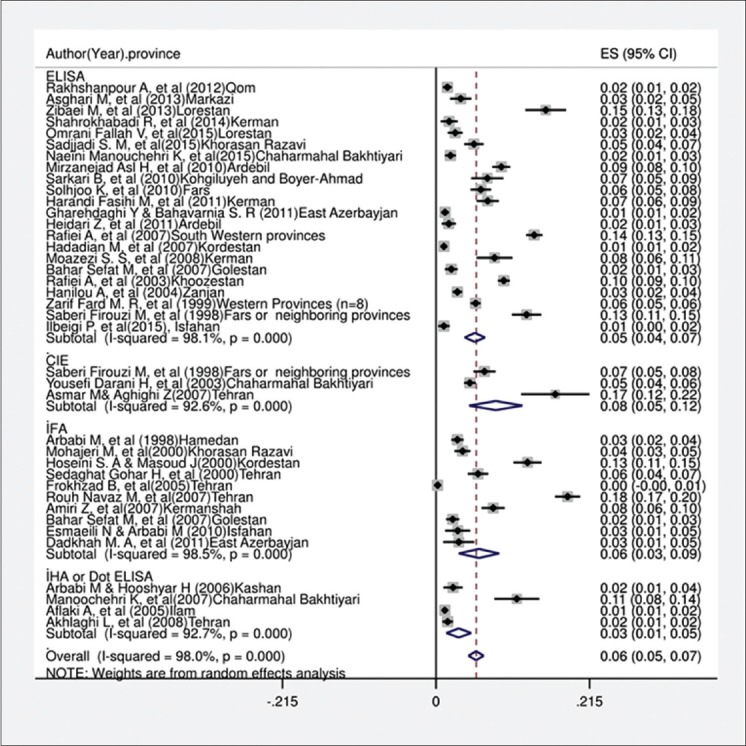
Forest plot for distribution hydatid cyst serology in terms of lab methods in Iran.
The seropositivity rate of human CE infection in some provinces was determined (Figure-6). Based on available information, CE infection was more common in warm and humid climates than in colder and drier regions.
Figure-6.
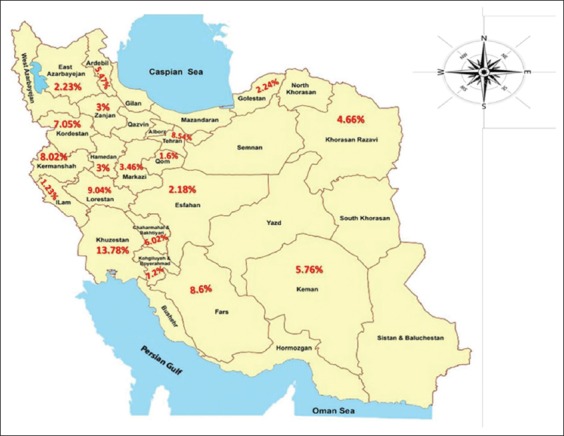
Distribution of Iranian cystic echinococcosis seroprevalences in different provinces.
The seroprevalence of CE among people who had close contact with dogs, consumed raw or uncooked vegetables, farmers and housewives and at last who had low level of education were significantly higher than that of others groups. Begg’s funnel plot (Figure-7) and the Egger weighted regression test showed that there was a significant publication bias (p<0.001).
Figure-7.
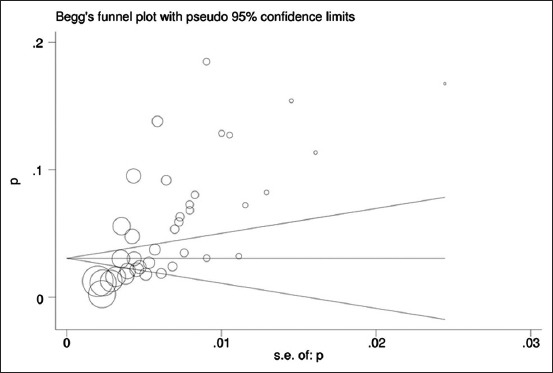
Begg’s funnel plot for assessing publication bias in the seroprevalence analysis of hydatidosis.
Discussion
Since the geographical distribution of CE is worldwide, it is crucial to determine the status of CE in humans. This systematic review and meta-analysis were performed by reviewing published literature. From 40 selected studies and 49,460 participants, 3090 CE seropositive cases were identified, resulting in a CE seroprevalence in the general population of 6% (95% CI: 5.0-7.0%).
Iran has three water borders, namely the Caspian Sea, Oman Sea, and Persian Gulf. There are different geographical regions with distinct climates in Iran [51,52]. In Iran, three distinct cycles of E. granulosus have been identified: A domestic cycle between dogs and livestock, a desert cycle between dogs and camels, and a sylvatic cycle between wild carnivores and wild ruminants [53].
The seroprevalence of CE varies by region in Iran, as a result of differences in climate and other conditions. CE infection was found to occur more often in warm and humid climates than in colder and drier regions [14]. The highest prevalences of CE in human and animal are found in countries in temperate zones, including the Mediterranean region, southern and central Russia, Central Asia, China, Australia, some regions of South America, and northern and eastern regions of Africa [9,54,55].
The annual incidence of human CE in Europe varies between 1 and 8 per 100,000 populations, with the exceptions of Ireland, Iceland, and Denmark [2,17,56,57]. Studies on different endemic areas have determined CE seroprevalences in Peru (2.6%), Spain (3.4%), Brazil (3.5-6%), India (5-9.23%), Jordan (2.4-11.4%), China (9.5-25.5%), and Greece (up to 29%) [58-67].
Human CE has been associated with several risk factors including gender, age, residential area, climate, contact with dogs, soil exposure, livestock ownership, herding occupation, hunting, eating habits (e.g., raw or unwashed vegetables), and level of education and knowledge. Of course, when risk factors are combined, they can shape the epidemiologic pattern of the disease in that region [66,68-72].
Our meta-analysis of community-based surveys showed that males had a significantly higher seroprevalence of CE infection than females (p<0.001). This may be a result of gender roles and cultural differences in endemic regions, with men more involved in farming, hunting, and herding livestock, and in closer contact with dogs. Similar differences were seen in India [66,73].
Age is one of the major factors associated with CE, with the seroprevalence of human CE increasing with age. The development of clinical symptoms takes a long time in humans, making a determination of the true age of infection difficult. Since hydatid cysts grow slowly and immune responses to CE infection in childhood persist, long-term CE may be diagnosed in adulthood [5,56,66,73,74]. The present study showed that the age groups 0-20 and 20-40 years had the lowest and highest CE seroprevalences, respectively. Similar results were seen in Pakistan [75].
Another important risk factor is living in a rural region. The higher seroprevalence of CE in rural regions than in urban areas found in this study may be attributed to rural populations being closely associated with the Echinococcus lifecycle. Other factors that may be responsible for the high prevalence in rural residents include low education levels, poor economic conditions and medical services, and farming and herding livestock as main occupations. Moreover, soil contaminated by dog feces and even dust containing eggs aspirated during rural activities can be major reasons for transmission of E. granulosus [66,72]. In this analysis, the seroprevalence of CE infection in rural regions was found to be significantly higher (p=0.048) than that of urban areas.
Climate has an effect on the geographical distribution of CE. The dominant climate in Iran is cold and arid. Our study revealed that Khuzestan Province has the highest seroprevalence (13.78%) of CE in Iran. Khuzestan Province has high humidity and suitable temperature for the maintenance of E. granulosus eggs and continuation of the parasite’s lifecycle. In contrast, Qom Province has low humidity and is a semi-desert climate; thus, agriculture and husbandry are not possible; correspondingly, Qom Province had the lowest CE seroprevalence (1.6%) in Iran [17,18].
Dogs that guard livestock are an important source of E. granulosus infections. Interactions between humans and livestock, particularly in rural areas, as well as close contact with dogs can increase the rate of CE seroprevalence [72,76].
The prevalence of E. granulosus infection in definitive hosts was 19.1% in dogs, 2.3% in golden jackals, and 5% in red foxes, whereas the prevalences in intermediate hosts, namely sheep (11.1%), goats (6.3%), cattle (16.4%), and buffaloes (12.4%), in Lorestan Province have been reported [53,77]. In addition, a survey in western provinces of Iran showed that the prevalence of E. granulosus infection in stray dogs and red foxes was 13.25% and 4.54%, respectively [78].
A majority of dog owners, especially in rural areas, neglected to take precautions against infection such as care in feeding their dogs, maintaining the place where they kept them, proper handling of their feces, and regular medical checkups [55]. With intimate contact between children and dogs, including playing, there is the possibility of parasite transmission through accidentally swallowed eggs[79]. Eggs adhere to hairs around an infected dog’s anus and are found on the muzzle and paws. Indirect transfer of eggs either through contaminated water and uncooked infected vegetables or arthropods intermediates such as flies can also result in infection of humans [3,69]. It is crucial that slaughterhouse scraps, which may include cyst-infected livers and lung tissues, be kept away from dogs and be disposed of properly [15,53,80,81].
Antibody assays are useful serological tests to detect prior E. granulosus infection, based on their low cost and ease of use. However, some patients with CE do not demonstrate a detectable immune response [81,82]. According to epidemiological investigations, ELISA test was principal test used by researchers. Therefore, this could be the most important test to evaluate the relative importance of different sources of hydatidosis infection use in CE. CE serological tests have been useful in diagnosis of CE in humans, but, in terms of both specificity and sensitivity, there are remarkable differences among the various tests. An optimal test should have both high specificity and high sensitivity. Earlier CE diagnostic tests with low sensitivity and low specificity, including the Casoni intradermal test, the complement fixation test, IHA, and the latex agglutination test, have been replaced by ELISA, IFA, immunoelectrophoresis, and immunoblotting basic methods as routine tests [72,83-86].
Based on the results of this analysis, the rate of CE was determined from several studies in several regions of Iran. Seroprevalences ranged from 1.2% to 21.4% based on serological methods, mainly ELISA [14,17]. According to studies conducted between 1998 and 2007, the most commonly used serodiagnostic test was IFA. This test was used to detect CE in some areas of Iran [87].
IFA is a useful and cost-effective test, but it is difficult to perform in a routine laboratory. The sensitivity of IFA is between 82.5% and 91.6%, and the specificity is between 83% and 100% [87,88]. However, from 2007 to 2015, ELISAs have been used for CE screening. Several studies have indicated that the ELISA technique shows greater sensitivity (87.5-96.7%), specificity (89.7-100%), and 92.3% diagnostic efficacy for CE than other serological methods [81,89-94]. Moreover, ELISA allows large numbers of sample to be tested at the same time, representing a major advantage over other serological studies.
In the case of IHA, sensitivities have been found to range between 78.1 and 90%, with specificities of 93.9-97.5% [87,94,95]. However, it seems that Dot-ELISA, with a sensitivity range of 86-100% and a specificity range of 90-99.5%, has greater diagnostic value than other tests [19,96,97].
Conclusion
This analysis synthesizes valuable information from prior studies. Our results indicated that there is a high seroprevalence of CE in the general population of Iran and that this country should be considered an endemic area of E. granulosus infection. This point is worthwhile to mention that ELISA is more sensitive and specificity than other immune assays in CE diagnosis, and also, the present study provides a comprehensive view of the seroepidemiology of CE in the Iranian general population. Considering the high prevalence of prior E. granulosus infection in the definitive and intermediate hosts and the distribution of this parasite in Iran, defining this country as endemic for CE can be justified. Due to the significance of this disease, proper preventive strategies should be considered.
Authors’ Contributions
SG and MS conceived the idea for this review. AT and MR searched the databases for potentially eligible articles based on their titles and abstracts. SS and AD participated in the study design and wrote the manuscript. SS and SM critically reviewed the manuscript. All authors read and approved the final manuscript for publication.
Acknowledgments
The authors are thankful to Vice Chancellors for Research of Mazandaran University of Medical Sciences, Sari, Iran for financial support (Grant number: 2606).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Harandi MF, Moazezi SS, Saba M, Grimm F, Kamyabi H, Sheikhzadeh F, Sharifi I, Deplazes P. Sonographical and serological survey of human cystic echinococcosis and analysis of risk factors associated with seroconversion in rural communities of Kerman, Iran. Zoonoses Public Health. 2011;58(8):582–588. doi: 10.1111/j.1863-2378.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 2.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17(1):107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moro P, Schantz PM. Echinococcosis: A review. Int. J. Infect. Dis. 2009;13(2):125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RC, McManus DP. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 2002;18(10):452–457. doi: 10.1016/s1471-4922(02)02358-9. [DOI] [PubMed] [Google Scholar]
- 5.Torgerson PR, Budke CM. Echinococcosis-an international public health challenge. Res. Vet. Sci. 2003;74(3):191–202. doi: 10.1016/s0034-5288(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 6.Dakkak A. Echinococcosis/hydatidosis: A severe threat in Mediterranean countries. Vet. Parasitol. 2010;174(1-2):2–11. doi: 10.1016/j.vetpar.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Budke CM, Carabin H, Ndimubanzi PC, Nguyen H, Rainwater E, Dickey M, Bhattarai R, Zeziulin O, Qian MB. A systematic review of the literature on cystic echinococcosis frequency worldwide and its associated clinical manifestations. Am. J. Trop. Med. Hyg. 2013;88(6):1011–1027. doi: 10.4269/ajtmh.12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King S, Hutchinson G. Agfact series. 2nd ed. 2005. Hydatids-you, too, can be affected. A0. 9.43. [Google Scholar]
- 9.Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS. WHO/OIE manual on echinococcosis in humans and animals: A public health problem of global concern. World Organ. Anim. Health. 2001:20–66. [Google Scholar]
- 10.Harandi MF, Budke CM, Rostami S. The monetary burden of cystic echinococcosis in Iran. PLoS Negl. Trop. Dis. 2012;6(11):e1915. doi: 10.1371/journal.pntd.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmena D, Cardona GA. Echinococcosis in wild carnivorous species: Epidemiology, genotypic diversity, and implications for veterinary public health. Vet. Parasitol. 2014;202(3-4):69–94. doi: 10.1016/j.vetpar.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Ilbeigi P, Mohebali M, Kia EB, Saber-Inasab M, Aryaeipour M, Bizhani N, Rokni MB. Seroepidemiology of human hydatidosis using AgB-ELISA test in Isfahan City and Suburb Areas, Isfahan Province, Central Iran. Iran. J. Public Health. 2015;44(9):1219–1224. [PMC free article] [PubMed] [Google Scholar]
- 13.Hadighi R, Mirhadi F, Rokni MB. Evaluation of a dot-ELISA for the serodiagnosis of human hydatid diseases. Pak. J. Med. Sci. 2003;19(4):268–271. [Google Scholar]
- 14.Rokni MB. Echinococcosis/hydatidosis in Iran. Iran. J. Parasitol. 2009;4(2):1–16. [Google Scholar]
- 15.Sadjjadi SM. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol. Int. 2006;55(1):S197–S202. doi: 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Rokni MB. The present status of human helminthic diseases in Iran. Ann. Trop. Med. Parasitol. 2008;102(4):283–295. doi: 10.1179/136485908X300805. [DOI] [PubMed] [Google Scholar]
- 17.Rakhshanpour A, Harandi MF, Moazezi S, Rahimi M, Mohebali M, Mowlavi G, Babaei Z, Ariaeipour M, Heidari Z, Rokni MB. Seroprevalence of human hydatidosis using ELISA method in Qom province, central Iran. Iran. J. Parasitol. 2012;7(3):10–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Rafiei A, Hemadi A, Maraghi S, Kaikhaei B, Craig PS. Seroepidemiological survey to assess human hydatidosis in nomads of Southwest Iran. East. Mediterr. Health J. 2007;13(1):41–48. [PubMed] [Google Scholar]
- 19.Tavalla M, Akhlaghi L, Ourmazdi H, Sarvi SH, Razmjoo E, Shokrabi M, Siavoshi M, Beiromvand M. Using dot-ELISA method to study the prevalence of human hydatidosis in people referred to blood transfusion center in Tehran, 2005-2006. Razi J. Med. Sci. 2010;16(67):52–58. [Google Scholar]
- 20.Saberi-Firouzi M, Kaffashian F, Hayati E, Ghaderi AA, Keshavarz H, Arshadi S, Arshadi C, Sotudehmaram MS, Massarrat MS, Ghalambor MN. Prevalence of hydatidosis in nomadic tribes of Southern Iran. Med. J. Islam. Repub. Iran. 1998;12(2):113–118. [Google Scholar]
- 21.Arbabi M, Masoud J, Dalimi A, Sajadi M. Seroepidemiologic prevalence of hydatid cyst in Hamadan 1991. Feyz J. Kashan Univ. Med. Sci. 1998;2(2):43–50. [Google Scholar]
- 22.Zariffard MR, Abshar N, Akhavizadegan MA, Motamedi GR. Seroepidemiological survey of human hydatidosis in Western part of Iran. Arch. Razi Ins. 1999;50:113. [Google Scholar]
- 23.Mohajeri M, Masoud J, Saber B. Seroepidemiology of Hydatid Cyst in Mashhad by IFA Method. In: Proceeding of the 3rd National Congress of Parasitology and Parasitic Diseases in Iran. Sari, Mazandaran, Iran: 2001. [Google Scholar]
- 24.Hosseini SA, Masoud J. Seroepidemiological Study of Hydatidosis in Divandarreh, Kurdistan and Sannandaj. In: Proceeding of the 3rd National Congress of Parasitology and Parasitic Disease in Iran Sari, Mazandaran, Iran. 2001 [Google Scholar]
- 25.Sedaghat-Gohar M, Masoud J, Rokni M, Kia EB. Seroepidemiologic survey of human hydatidosis in Shahriar region. J. Kerman Univ. Med. Sci. 2001;1(1):44–49. [Google Scholar]
- 26.Darani HY, Avijgan M, Karimi K, Manouchehri K, Masoud J. Seroepidemiology of hydatid cyst in Chaharmahal va Bakhtiari Province, Iran. Iran. J Public Health. 2003;32(2):31–33. [Google Scholar]
- 27.Hanilou A, Badali H, Esmaeilzadeh AR. Seroepidemiological study of hydatidosis in Zanjan (Islam-Abad 2002) J. Zanjan Univ. Med. Sci. Health Serv. 2004;46(12):41–46. [Google Scholar]
- 28.Aflaki A, Ghaffarifar F, Dalimi-Asl A. Seroepidemiological survey of hydatidosis by Dot- ELISA in Ilam province. J. Med. Sci. Modares. 2005;8(1):1–6. [Google Scholar]
- 29.Farokhzad B, Gachkar L, Mosafa N, Nazaripouya M. Seroepidemiologic survey of hydatid cyst in rural area of Shemiranat and determining the efficacy of IFA test. Res. Med. 2006;30(3):241–243. [Google Scholar]
- 30.Arbabi M, Hooshyar H. Survey of echinococcosis and hydatidosis in Kashan region, central Iran. Iran. J Publ. Health. 2006;35(1):75–81. [Google Scholar]
- 31.Asmar M, Aghighi Z. Serological review hydatid cyst by CCIE methods in patients referred to the Pasteur Institute of Iran. In: Proceeding of national congress on hydatid cyst. Yasuj, Iran (Abstract) Armaghane Danesh J. Yasuj Univ. Med. Sci. 2007;12(1):34. [Google Scholar]
- 32.Rouhnavaz M, Masoud J, Momeni Z. Seroepidemiology of hydatid cyst by IFA method in patients referred to the health faculty of Tehran University of Medical Sciences (2000-2005). In: Proceeding of national congress on hydatid cyst. Yasuj, Iran. (Abstract) Armaghane Danesh J. Yasuj Univ. Med. Sci. 2007;12(1):33. [Google Scholar]
- 33.Amiri Z, Massoud J, Sohrabi N, Kia EB. Seroepidemiological survey of human hydatid cyst in the city population of Kermanshah Province. In: Proceedings of national congress on hydatid cyst, Yasuj, Iran (Abstract) Armaghane Danesh J. Yasuj Univ. Med. Sci. 2007;12(1):P72. [Google Scholar]
- 34.Manouchehri-Naeini K, Jaffari M, Kheiri S. Sero-Prevalence and Risk Factors of Human Hydatidosis in Chaharmahal VA Bakhtiyari Province, South West of Iran using ELISA Method, 2014-2015. In: Proceeding of 2th International and 9th National Iranian Congress of Parasitology and Parasitic Diseases. Guilan, Iran (Abstract) 2015:235. [Google Scholar]
- 35.Baharsefat M, Massoud J, Mobedi I, Farahnak A, Rokni M. Seroepidemiology of cystic echinococcosis in referred patients to health centers in Golestan province using ELISA and IFA. Iran. J. Parasitol. 2007;2(2):20–24. [Google Scholar]
- 36.Hadadian M, Ghaffarifar F, Dalimi A, Roudbar-Mohammadi S. Seroepidemiological survey of hydatid cyst by ELISA in Kordestan Province. Modares J. Med. Sci.: Pathobiol. 2008;10(3-4):13–18. [Google Scholar]
- 37.Moazezi SS, Harandi MF, Saba M, Kamyabi H, Sheikhzadeh F. Sonographic and serological survey of hydatid disease in rural regions of Shahdad and Chatroud, Kerman Province, 2006-2007. J. Kerman Univ. Med. Sci. 2009;16(1):25–34. [Google Scholar]
- 38.Esmaeili N, Arbabi M. Seroepidemiology of hydatidosis among adult human at Kashan region, Iran in 2008. Feyz J. Kashan Univ. Med. Sci. 2010;13(4):321–326. [Google Scholar]
- 39.Mirzanejad-Asl H, Harandi MF. Seroepidemiological survey of human cystic echinococcosis with ELISA method in Moghan Plain, Ardabil Province. J. Ardabil. Univ. Med. Sci. 2010;9(4):334–346. [Google Scholar]
- 40.Sarkari B, Sadjjadi SM, Beheshtian MM, Aghaee M, Sedaghat F. Human cystic echinococcosis in Yasuj district in Southwest of Iran: An epidemiological study of seroprevalence and surgical cases over a ten-year period. Zoonoses Public Health. 2010;57(2):146–150. doi: 10.1111/j.1863-2378.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- 41.Solhjoo K, Kazemi A, Jelodari S. Seroepidemiology of human hydatid cyst in Jahrom. J. Jahrom Univ. Med. Sci. 2010;8(3):18–24. [Google Scholar]
- 42.Harandi MF, Moazezi SS, Saba M, Grimm F, Kamyabi H, Sheikhzadeh F, Sharifi I, Deplazes P. Sonographical and serological survey of human cystic echinococcosis and analysis of risk factors associated with seroconversion in rural communities of Kerman, Iran. Zoonoses Public Health. 2011;58(8):582–588. doi: 10.1111/j.1863-2378.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 43.Garedaghi Y, Bahavarnia SR. Seroepidemiological study of hydatid cyst by ELISA method in East-Azarbaijan Province (2009) J. Kerman Univ. Med. Sci. 2011;18(2):172–181. [Google Scholar]
- 44.Heidari Z, Mohebali M, Zarei Z, Aryayipour M, Eshraghian M, Kia E, Shodajei S, Abdi J, Rakhshanpour A, Rokni M. Seroepidemiological study of human hydatidosis in Meshkinshahr district, Ardabil province, Iran. Iran. J. Parasitol. 2011;6(3):19–25. [PMC free article] [PubMed] [Google Scholar]
- 45.Dadkhah MA, Yeganehzad M, Nadery B. Survey on hydatid cyst infestation in Sarab city (Northwest of Iran) using epidemiological and seroepidemiological criteria. JAVA. 2011;10(16):2099–2101. [Google Scholar]
- 46.Asgari M, Mohebali M, Kia EB, Farahnak A, Aryaeipour M, Asadianm S, Rokni MB. Seroepidemiology of human hydatidosis using AgB-ELISA test in Arak, central Iran. Iran. J. Public Health. 2013;42(4):391–396. [PMC free article] [PubMed] [Google Scholar]
- 47.Zibaei M, Azargoon A, Ataie-Khorasgani M, Ghanadi K, Sadjjadi SM. The serological study of cystic echinococcosis and assessment of surgical cases during 5 years (2007-2011) in Khorram Abad, Iran. Niger. J. Clin. Pract. 2013;16(2):221–225. doi: 10.4103/1119-3077.110156. [DOI] [PubMed] [Google Scholar]
- 48.Shahrokhabadi R, Rahimi E, Poursahebi R. Seroepidemiological study of human hydatidosis in Rafsanjan, Kerman. Zahedan J. Res. Med. Sci. 2014;16(4):46–46. [Google Scholar]
- 49.Fallah-Omrani V, Kheirandish F, Rouhani S, Kazemi B, Seyed-Tabaei SJ, Bandehpour M. Seroepidemiological Study of Human Hydatid Cyst by ELISA Method using Recombinant AgB in Doroud City, Lorestan Province, Western Iran. In: Proceeding of 2th International and 9th National Iranian Congress of Parasitology and Parasitic Diseases. Guilan, Iran. 2015:218. [Google Scholar]
- 50.Sadjjadi SM, Noormohammadi A, Mohammadzadeh T, Fata A. Seroepidemiological Study of Hydatid Cyst using AgB ELISA in Torbat-Jaam, Khorasan Razavi Province, Iran. In: Proceeding of 2th International and 9th National Iranian Congress of Parasitology and Parasitic Diseases. Guilan, Iran (Abstract) 2015:228. [Google Scholar]
- 51.Rahimi MT, Sharifdini M, Ahmadi A, Laktarashi B, Mahdavi SA, Kia EB. Hydatidosis in human and slaughtered herbivores in Mazandaran, Northern Iran. Asian Pac. J. Trop. Dis. 2011;1(3):212–215. [Google Scholar]
- 52.Sarvi S, Daryani A, Sharif M, Rahimi MT, Azami D, Marhaba Z, Ahmadpour E, Mizani A. Domestic dog as a human health hazard in North of Iran. J. Parasit. Dis. 2014;38(4):1–5. doi: 10.1007/s12639-014-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalimi A, Motamedi G, Hosseini M, Mohammadian B, Malaki H, Ghamari Z, Ghaffarifar F. Echinococcosis/hydatidosis in Western Iran. Vet. Parasitol. 2002;105(2):161–171. doi: 10.1016/s0304-4017(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 54.Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J. Gastroenterol. 2012;18(13):1425–1437. doi: 10.3748/wjg.v18.i13.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang YR, Sun T, Li Z, Zhang J, Teng J, Liu X, Zhao R, Jones MK, Wang Y, Hao W, Xiaohui F, Qin Z, Yumin Z, Dazhong S, Brigitte B, Dominique AV, David P, Patrick G, Akira I, Mark FD, Belchis B, Philip SC, Gail MW, Donald P. Community surveys and risk factor analysis of human alveolar and cystic echinococcosis in Ningxia Hui Autonomous Region, China. Bull. World Health Organ. 2006;84(9):714–721. doi: 10.2471/blt.05.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 2006;12(2):296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kayal A, Hussain A. A comprehensive prospective clinical study of hydatid disease. ISRN Gastroenterol. 2014;2014(514757):5. doi: 10.1155/2014/514757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moosa RA, Abdel-Hafez SK. Serodiagnosis and seroepidemiology of human unilocular hydatidosis in Jordan. Parasitol. Res. 1994;80(8):664–671. doi: 10.1007/BF00932950. [DOI] [PubMed] [Google Scholar]
- 59.Bai Y, Cheng N, Wang Q, Cao D. An epidemiological survey of cystic echinococcosis among Tibetan school pupils in West China. Ann. Trop. Paediatr. 2001;21(3):235–238. doi: 10.1080/027249301200777817. [DOI] [PubMed] [Google Scholar]
- 60.Gutiérrez MP, Ramírez I, Zarzosa-Mdel P, Fernández JM, Dueñas AI, Mantecón MA, Almaraz A, Rodríguez-Recio MJ, Marcos A, Alonso P, Bratos MA, Orduña A, Rodríguez-Torres A, Grupo de Epidemiologos del Servicio de Epidemiologia de la Junta de Castilla y Leon Seroprevalence of infection due to Echinococcus granulosus in the population of Castilla and León (Spain) Enferm. Infect. Microbiol. Clin. 2003;21(10):563–567. doi: 10.1016/s0213-005x(03)73010-9. [DOI] [PubMed] [Google Scholar]
- 61.Pastore R, Vitali LH, Macedo VO, Prata A. A serological survey of the infection by Echinococcus sp. In the municipal ity of Sena Madureira, AC. Rev. Soc. Bras. Med. Trop. 2003;36(4):473–477. doi: 10.1590/s0037-86822003000400007. [DOI] [PubMed] [Google Scholar]
- 62.Qaqish AM, Nasrieh MA, Al-Qaoud KM, Craig PS, Abdel-Hafez SK. The seroprevalences of cystic echinococcosis, and the associated risk factors, in rural-agricultural, Bedouin and semi-bedouin communities in Jordan. Ann. Trop. Med. Parasitol. 2003;97(5):511–520. doi: 10.1179/000349803225001436. [DOI] [PubMed] [Google Scholar]
- 63.Sotiraki S, Himonas C, Korkoliakou P. Hydatidosis–echinococcosis in Greece. Acta Trop. 2003;85(2):197–201. doi: 10.1016/s0001-706x(02)00273-5. [DOI] [PubMed] [Google Scholar]
- 64.Moro PL, Garcia HH, Gonzales AE, Bonilla JJ, Verastegui M, Gilman RH. Screening for cystic echinococcosis in an endemic region of Peru using portable ultrasonography and the enzyme-linked immunoelectrotransfer blot (EITB) assay. Parasitol. Res. 2005;96(4):242–246. doi: 10.1007/s00436-005-1350-6. [DOI] [PubMed] [Google Scholar]
- 65.Yu SH, Wang H, Wu XH, Ma X, Liu PY, Liu YF, Zhao YM, Yasuyuki M, Mansanori K. Cystic and alveolar echinococcosis: An epidemiological survey in a Tibetan population in Southeast Qinghai, China. Jpn. J. Infect. Dis. 2008;61(3):242–246. [PubMed] [Google Scholar]
- 66.Fomda BA, Khan A, Thokar MA, Malik AA, Fazili A, Dar RA, Sharma M, Malla N. Sero-epidemiological survey of human cystic echinococcosis in Kashmir, North India. PLoS One. 2015;10(4):e0124813. doi: 10.1371/journal.pone.0124813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fotiou V, Malissiova E, Minas A, Petinaki E, Hadjjchristodoulou C. Seroprevalence of IgG antibodies against Echinococcus granulosus in the population of the region of Thessaly, Central Greece. PLoS One. 2012;7(5):e37112. doi: 10.1371/journal.pone.0037112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. The Lancet. 2003;362(9392):1295–1304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 69.Schantz PM, Wang H, Qiu J, Liu FJ, Saito E, Emshoff A, Ito A, Roberts JM, Delker C. Echinococcosis on the Tibetan Plateau: Prevalence and risk factors for cystic and alveolar echinococcosis in Tibetan populations in Qinghai Province, China. Parasitology. 2003;127(Suppl):S109–S120. [PubMed] [Google Scholar]
- 70.Torgerson PR, Deplazes P. Echinococcosis: Diagnosis and diagnostic interpretation in population studies. Trends Parasitol. 2009;25(4):164–170. doi: 10.1016/j.pt.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Yang YR, Craig PS, Vuitton DA, Williams GM, Sun T, Liu TX, Boufana B, Giraudoux P, Jing T, Yanbin L, Ling H, Wei Z. Serological prevalence of echinococcosis and risk factors for infection among children in rural communities of Southern Ningxia, China. Trop. Med. Int. Health. 2008;13(8):1086–1094. doi: 10.1111/j.1365-3156.2008.02101.x. [DOI] [PubMed] [Google Scholar]
- 72.Acosta-Jamett G, Weitzel T, Boufana B, Adones C, Bahamonde A, Abarca K, Craig PS, Reiter-Owona I. Prevalence and risk factors for echinococcal infection in a rural area of Northern Chile: A household-based cross-sectional study. PLoS Negl. Trop. Dis. 2014;8(8):e3090. doi: 10.1371/journal.pntd.0003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cappello E, Cacopardo B, Caltabiano E, Li-Volsi S, Chiara R, Sapienza M, Nigro L. Epidemiology and clinical features of cystic hydatidosis in Western Sicily: A ten-year review. World J. Gastroenterol. 2013;19(48):9351–9358. doi: 10.3748/wjg.v19.i48.9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fahim F, Al Salamah SM. Cystic echinococcosis in Central Saudi Arabia: A 5-year experience. Turk. J. Gastroenterol. 2007;18(1):22–27. [PubMed] [Google Scholar]
- 75.Mumtaz K, Kamani L, Chawla T, Hamid S, Jafri W. Hepatic cystic echinococcosis: Clinical characteristics and outcomes in Pakistan. Trop. Doct. 2009;39(4):215–217. doi: 10.1258/td.2009.080463. [DOI] [PubMed] [Google Scholar]
- 76.Azlaf R, Dakkak A. Epidemiological study of the cystic echinococcosis in Morocco. Vet. Parasitol. 2006;137(1-2):83–93. doi: 10.1016/j.vetpar.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Dalimi A, Hosseini M, Motamedi G. The potential role of stray dog and red fox in echinococcosis/hydatidosis life cycle in Lorestan Province, Western Iran. J. Vet. Res. 2002;57(3):81–86. [Google Scholar]
- 78.Dalimi A, Sattari A, Motamedi G. A study on intestinal helminthes of dogs, foxes and jackals in the Western part of Iran. Vet. Parasitol. 2006;142(1-2):129–133. doi: 10.1016/j.vetpar.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 79.Abdybekova AM, Torgerson PR. Frequency distributions of helminths of wolves in Kazakhstan. Vet. Parasitol. 2012;184(2-4):348–351. doi: 10.1016/j.vetpar.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Wang LJ, Wu WP, Zhu XH. The endemic status of hydatidosis in China from 2004 to 2008. Chin. J. Zoonoses. 2010;26(7):699–702. [Google Scholar]
- 81.Sbihi Y, Rmiqui A, Rodriguez-Cabezas MN, Orduña A, Rodriguez-Torres A, Osuna A. Comparative sensitivity of six serological tests and diagnostic value of ELISA using purified antigen in hydatidosis. J. Clin. Lab. Anal. 2001;15(1):14–18. doi: 10.1002/1098-2825(2001)15:1<14::AID-JCLA3>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang W, McManus DP. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol. Med. Microbiol. 2006;47(1):24–41. doi: 10.1111/j.1574-695X.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 83.Lightowlers MW, Gottstein B. Echinococcosis/Hydatidosis: Antigens, Immunological and Molecular Diagnosis. Wallingford, UK: CAB International; 1995. pp. 355–410. [Google Scholar]
- 84.Harandi MF, Hobbs RP, Adams PJ, Mobedi I, Morgan-Ryan UM, Thompson RC. Molecular and morphological characterization of Echinococcus granulosus of human and animal origin in Iran. Parasitology. 2002;125(Pt 4):367–373. doi: 10.1017/s0031182002002172. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin. Microbiol. Rev. 2003;16(1):18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiter-Owona I, Grüner B, Frosch M, Hoerauf A, Kern P, Tappe D. Serological confirmatory testing of alveolar and cystic echinococcosis in clinical practice: Results of a comparative study with commercialized and in-house assays. Clin. Lab. 2009;55(1-2):41–48. [PubMed] [Google Scholar]
- 87.Dalimi A, Madani R, Ghorbankhani D, Salami S. Comparative evaluation of serodiagnostic techniques in cystic hydatid disease. Arch. Inst. Razi. 2000;51:85–93. [Google Scholar]
- 88.Akpolat N, Gedik E. The importance of indirect fluorescent antibody test in the diagnosis of cystic echinococcosis. Turk. Klin. Tip Etigi. Hukuku. Tarihi. 2009;29(6):1594–1597. [Google Scholar]
- 89.Kaddah MH, Maher KM, Hassanein HI, Farrag AI, Shaker ZA, Khalafallah AM. Evaluation of different immunodiagnostic techniques for diagnosis of hydatidosis in Egypt. J. Egypt Soc. Parasitol. 1992;22(3):653–665. [PubMed] [Google Scholar]
- 90.Poretti D, Felleisen E, Grimm F, Pfister M, Teuscher F, Zuercher C, Ju¨rg R, Bruno G. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am. J. Trop. Med. Hyg. 1999;60(2):193–198. doi: 10.4269/ajtmh.1999.60.193. [DOI] [PubMed] [Google Scholar]
- 91.Zarzosa MP, Orduña-Domingo A, Gutiérrez P, Alonso P, Cuervo M, Prado A, Bratos MA, García-Yuste M, Ramos G, Rodríguez TA. Evaluation of six serological tests in diagnosis and postoperative control of pulmonary hydatid disease patients. Diagn. Microbiol. Infect. Dis. 1999;35(4):255–262. doi: 10.1016/s0732-8893(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 92.Ortona E, Riganò R, Buttari B, Delunardo F, Ioppolo S, Margutti P, Profumo E, Teggi A, Vaccari S, Siracusano A. An update on immunodiagnosis of cystic echinococcosis. Acta Trop. 2003;85(2):165–171. doi: 10.1016/s0001-706x(02)00225-5. [DOI] [PubMed] [Google Scholar]
- 93.Siavashi MR, Taherkhani H, Rezaei K, Razavi-Deligani MR, Assmar M. Comparison of dot-ELISA and sandwich ELISA diagnostic tests in detection of human hydatidosis. Iran. Biom. J. 2005;9(2):91–94. [Google Scholar]
- 94.El-Shazly AM, Saad RM, Belal US, Sakr T, Zakae HA. Evaluation of ELISA and IHAT in serological diagnosis of proven cases of human hydatidosis. J. Egypt. Soc. Parasitol. 2010;40(2):531–538. [PubMed] [Google Scholar]
- 95.Bilge UE, Ozdemir M, Baykan M. Comparison of commercial IFA, IHA and in-house IFA tests in the diagnosis of cystic echinococcosis. Turkiye Parazitol. Derg. 2009;33(3):195–198. [PubMed] [Google Scholar]
- 96.Rogan MT, Craig PS, Zeyhle E, Romig T, Lubano GM, Deshan L. Evaluation of a rapid dot-ELISA as a field test for the diagnosis of cystic hydatid disease. Trans. R. Soc. Trop. Med. Hyg. 1991;85(6):773–777. doi: 10.1016/0035-9203(91)90451-4. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Bradshaw H, Rogan MT, Craig PS. Rapid dot-ELISA for the detection of specific antigens in the cyst fluid from human cases of cystic echinococcosis. Ann. Trop. Med. Parasitol. 2002;96(7):691–694. doi: 10.1179/000349802125001717. [DOI] [PubMed] [Google Scholar]


