Abstract
Background:
Many experts believe that hospitals with more frequent hospital readmissions provide lower quality of care, but little is known about how the preventability of readmissions might change over the post-discharge timeframe.
Objective:
To determine whether readmissions within 7 days of discharge are different from readmissions between 8 and 30 days after discharge with respect to preventability.
Design:
Prospective cohort study.
Setting:
10 US academic medical centers.
Patients:
822 adults readmitted to a general medicine service.
Measurements:
At each site, 2 physician assessors used a structured survey instrument to determine whether each readmission was preventable and to measure other characteristics of the readmission.
Results:
36.2% of early readmissions versus 23.0% of late readmissions were preventable (median risk difference 13.0%, 25th, 75th percentile 5.5, 26.4). The hospital was identified as a better location to prevent an early readmission than a late readmission (47.2% vs. 25.5%, [median risk difference 22.8%, 25th, 75th percentile 17.9, 31.8]). In contrast, the outpatient clinic (15.2% vs. 6.6%, [median risk difference 10%, 25th, 75th percentile 4.6, 12.2]) and home (19.4% vs. 14%, [median risk difference 5.6%, 25th, 75th percentile −6.1, 17.1]) were identified as better locations to prevent late readmissions than early readmissions.
Limitations:
Physician assessors were not blinded to readmission timing. In addition, community hospitals were not included in the study, and readmissions to non-study hospitals were not included in the results.
Conclusions:
Early readmissions were more likely to be preventable and amenable to hospital-based interventions. Late readmissions were less likely to be preventable and were more amenable to ambulatory and home-based interventions.
INTRODUCTION
Each year, hospital readmissions affect 18.2% of Medicare beneficiaries (1) and cost Medicare between $15 and $17 billion (2). Effective October 1, 2012, Section 3025 of the Patient Protection and Affordable Care Act established the Hospital Readmissions Reduction Program, authorizing the Centers for Medicare and Medicaid Services to impose financial penalties on hospitals for excessive readmissions within 30 days of hospital discharge (3).
The Act specified 30 days because lawmakers sought to identify a window of time during which a hospital readmission was likely attributable to the quality of care during the index hospitalization, thus representing a preventable outcome. However, there is little scientific basis for this choice (2, 4–7). The 30-day period does not correlate with quality indicators (8,9) or inpatient mortality rates (10–12), and readmissions during this period are influenced by the ambulatory care environment, chronic illness burden, and social determinants of health (13–23). Furthermore, one recent single-center study found that readmissions within 7 days of discharge were more highly associated with factors influenced by the index hospitalization than readmissions that occurred from 8–30 days after discharge (13). Moreover, it is uncertain whether preventability varies during the 30 days (24). One way to choose the ideal period would be to use a measure that identifies preventable readmissions that are directly influenced by hospital factors such as physician decision-making, processes of inpatient care, and transitional care planning while striking a balance between validity and simplicity (2). The lack of a clear relationship between preventability and the 30-day period has led experts to propose a 3-, 7-, or 14-day windows rather than a 30-day window (4–7, 13) but without direct evidence on why these shorter periods may be better.
The aim of this study was to compare patients readmitted within 7 days of hospital discharge to patients readmitted 8–30 days after discharge using measures of preventability. We hypothesized that early readmissions would be more preventable than late readmissions and that more of them would be caused by factors directly related to the index hospitalization.
METHODS
Setting and Study Cohort
The Hospital Medicine Reengineering Network (HOMERuN) is composed of 12 US academic medical centers (25). Our study is limited to the 10 centers that include readmission timing in their databases (Appendix Table 1). Patients were eligible for inclusion in the study if they were at least 18 years of age; spoke English as their primary language; and were discharged from a general medicine service and had an unplanned readmission within 30 days between April 1, 2012 and March 31, 2013. We used a random-digit generator to select up to 5 patients per week at each site. If a patient declined an interview, was too sick to participate, or was unavailable, we tried to enroll the next randomly selected patient.
Institutional review boards at UCSF (the data coordinating center) and all participating sites approved this study.
Data collection
Trained research assistants performed structured medical record review to collect demographic data and information regarding medical comorbidities, medications, and measures of transitions in care. We developed survey instruments to identify factors that might contribute to readmission, and research assistants administered these surveys to each patient’s primary care physician, the attending physicians for the index hospitalization, and the attending physician for the readmission. Research assistants used similar survey instruments to interview readmitted patients (22, 25).
The primary outcome of this study was preventability, which we defined as a rating of ≥4 out of 6 on an ordinal scale (26–30) (See footnote of table 2 for a description of the scale). At each site, 2 physicians from among the 3–10 physician adjudicators at the site used a standard approach (26–28) to review all available data and make a joint determination of the preventability rating for each readmission with adjudication by the lead physician when needed (22, 25). These physicians also decided the location where an intervention to prevent the readmission would have been most effective and identified factors that contributed to the readmission. These factors were based on the Ideal Transition in Care framework (29) and included the following categories: monitoring/managing symptoms after discharge, social and community supports, self-management instruction, continuity of care, end of life and advance care planning, diagnostic and therapeutic problems, decision-making concerning the readmission, and medication problems and adverse events. We defined an early readmission as occurring within days 0–7 days after discharge and a late readmission as occurring 8–30 days after discharge.
Analyses
We describe the preventability of early and late readmissions using the median of the risk differences and the 25th and 75th percentiles across study sites. We used logistic regression to model preventability of the readmission based on early versus late timing with hospital site as a fixed variable to adjust for site-specific differences in patient characteristics, hospital care processes, the adjudication process, and other unknown variables. We also included patient age and variables describing each patient’s transitions in care as covariates in our model. To identify the optimal cut-point for separating early vs. late readmissions, we visually inspected a graph of the adjusted probability of preventability by post-discharge day. We report the frequencies of each of the potential causative factors during the early and late periods, along with the median risk difference across sites with the 25th and 75th percentiles. We managed the data and conducted the analyses using SAS v.9.2 (Cary, NC).
Role of the Funding Source
None of the funding sources had a role in the design, conduct, collection, management, analysis, or interpretation of the data or in the preparation, review or approval of the manuscript.
RESULTS
We identified 890 patients who were eligible for the study, but we subsequently excluded 54 patients with missing age values and 14 patients due to data entry errors for date of discharge, date of readmission, or both. Of the 822 patients who remained, 301 (36.6%) were readmitted 0–7 days post-discharge, and 521 (63.4%) were readmitted 8–30 days post-discharge. Patients who were readmitted early and late had similar baseline characteristics, comorbidities, social factors, and process of care variables (Table 1). However, more results of diagnostic studies were pending at hospital discharge for early readmissions (27.6%) versus late readmissions (20.0%) (Table 1). In addition, there were differences in patient characteristics and processes of care by study site (Appendix Table 2).
Table 1:
Initial Hospitalization and Patient Characteristics by Readmission Timing
| Early Readmissions (0–7d post- discharge) n=301, 36.6% |
Late Readmissions (8–30d post- discharge) n=521, 63.4% |
|
|---|---|---|
| Age mean, (SD) | 54.7 (17.3) | 55.5 (18.3) |
| Index length of stay, mean, (SD) | 5.8 (7.7) | 5.5 (4.9) |
| Pre-admission Disposition* (%) | ||
| Homeless | 6.3 | 5.8 |
| Home without services | 68.8 | 69.9 |
| Home with services | 11.6 | 12.7 |
| Home Hospice | 0.7 | 0.2 |
| Home, services unspecified | 5.0 | 3.8 |
| Rehabilitation Facility | 5.0 | 3.1 |
| Chronic Care Facility | 1.0 | 2.9 |
| Other | 1.0 | 1.5 |
| Married or living as married (%) | 36.5 | 33.2 |
| Status of inpatient work-up (%) | ||
| Studies pending at discharge† | 27.6 | 20.0 |
| Diagnostic work-up as outpatient‡ | 34.9 | 31.9 |
| Did the patient have any of the following terminal illnesses? |
||
| Stage III or IV congestive heart failure | 7.0 | 5.4 |
| Hemorrhagic or ischemic stroke, degenerative central nervous system disorder |
7.3 | 6.1 |
| Cancer | 2.0 | 3.3 |
| Severe COPD§ | 16.9 | 17.5 |
| Stable IV chronic renal failure|| | 5.7 | 6.1 |
| Stage III or IV congestive heart failure | 15.0 | 12.9 |
| Treatment indicating chronic illness | ||
| Dialysis | 7.6 | 6.0 |
| Chemotherapy | 1.0 | 1.0 |
| Anticoagulation | 14.3 | 14.8 |
| Opioids | 49.2 | 51.6 |
| Insulin | 17.3 | 17.9 |
| Lasix | 17.3 | 18.2 |
| English as a primary language (%) | 90.4 | 93.5 |
| Patient understood how to execute care plan (%) | 92.0 | 91.5 |
| Difficulty with transportation access (%) | 20.3 | 21.6 |
| Income vulnerability | ||
| Homeless (%) | 4.7 | 4.2 |
| Difficulty meeting basic needs (%) | 11.4 | 10.8 |
| Social supports lacking (%) | 16.7 | 15.6 |
| Substance use disorder (%) | 9.0 | 6.6 |
| Process of care variables | ||
| Primary MD contacted at admission | 63.8 | 59.5 |
| Follow-up call to patient | 13.6 | 14.8 |
| Discharge summary within 24h | 78.1 | 75.8 |
| Post-discharge appointment made | 66.8 | 61.0 |
| Medication reconciliation | 86.7 | 84.3 |
| Primary MD contacted at discharge | 48.5 | 41.3 |
At the time of the readmission
Did discharge documentation note that test results were pending at time of discharge?
Were there directions in discharge documentation that additional diagnostic work-up was to be completed as an outpatient?
Chronic obstructive pulmonary disorder, oxygen-dependent or forced expiratory volume in 1second< 1L
Glomerular filtration rate< 30 or current hemodialysis
Missing data for all variables was <3% (Length of stay- 0.5%, patient understood how to execute care plan- 0.7%, difficulty with transportation access- 2.8%, difficulty meeting basic needs-0.7%, social supports lacking-0.5%, substance use disorder-0.9%)
Overall, 229 (27.9%) readmissions were preventable. There was a difference in preventability for early versus late readmission: 36.2% of early readmissions versus 23.0% of late readmissions were preventable. There was variability in preventability between early and late readmissions across study sites (Figure 1, Appendix Table 2), although preventability was rated as higher for early readmissions for 9 of 10 study sites. The median risk difference across sites was 13.0% [25th,75th percentile 5.5, 26.4]) (Table 2). In adjusted analyses, early readmissions were significantly more likely to be preventable (OR 2.0 [95%CI 1.5–2.8]) (Table 2). A sensitivity analysis using a more stringent cut-off for preventability produced similar results (Table 2). We observed a clear reduction in preventability after day 7 (Figure 2).
Figure 1:
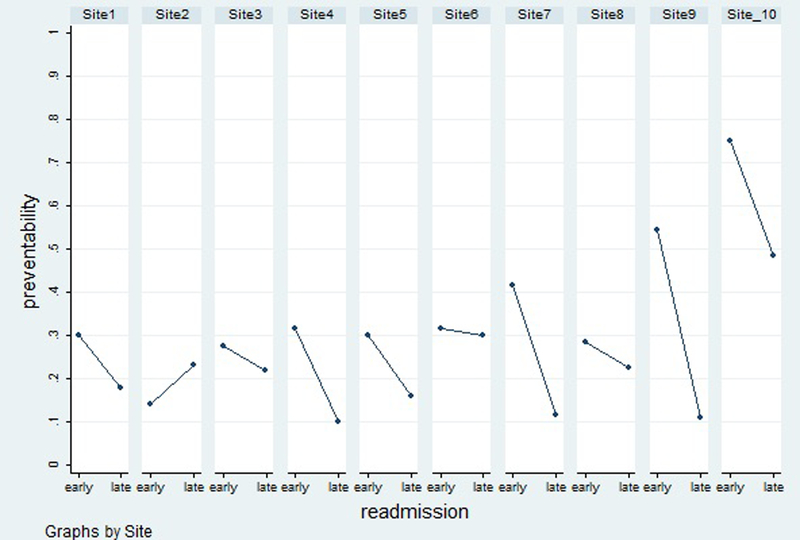
Proportion of early and late readmissions that were preventable at each of the ten hospital sites.
Table 2:
Physician-adjudicated Readmission Preventability by Readmission Timing
| Early Readmissions (0–7d post- discharge) n=301, 36.6% |
Late Readmissions (8–30d post- discharge) n=521, 63.4% |
Median of Risk Differences Across Sites (25th,75th percentile) |
|
|---|---|---|---|
| Preventability (%)* | 36.2 | 23.0 | 13.0 (5.5, 26.4) |
| Preventability, alternate definition (%)† | 22.6 | 12.5 | 12.4 (1.6, 21.6) |
| Preventability (OR, 95% CI)‡ | 2.0 (1.5–2.8) | Reference | |
| Ideal location for an intervention to prevent a readmission (%)?|| |
|||
| Hospital | 47.2 | 25.5 | 22.8 (17.9, 31.8) |
| Home | 14.0 | 19.4 | −5.6 (−17.1, 6.1) |
| Outpatient Clinic | 6.6 | 15.2 | −10.0 (−12.2, −4.6) |
| Emergency Department | 3.7 | 4.0 | −2.0 (−3.1, 1.5) |
| Other | 14.6 | 18.4 | −6.6 (−11.8, 1.5) |
Defined as a preventability score of ≥4 on a 6 point ordinal scale, (1= No evidence for preventability, 2= slight evidence of preventability, 3= preventability less than 50–50 but close call, 4= preventability more than 50–50 but close call, 5= strong evidence for preventability, 6= virtually certain evidence for preventability), which is the standard approach
Defined as a preventability score of ≥5 on this scale, presented as a sensitivity analysis
Using logistic regression to model the odds of a preventable readmission for early vs. late readmissions, adjusted for hospital site, patient age, and all process of care variables listed in Table 1
After reviewing each admission and readmission pair, adjudicators determined where an intervention to prevent the readmission would have been most effective.
Figure 2:
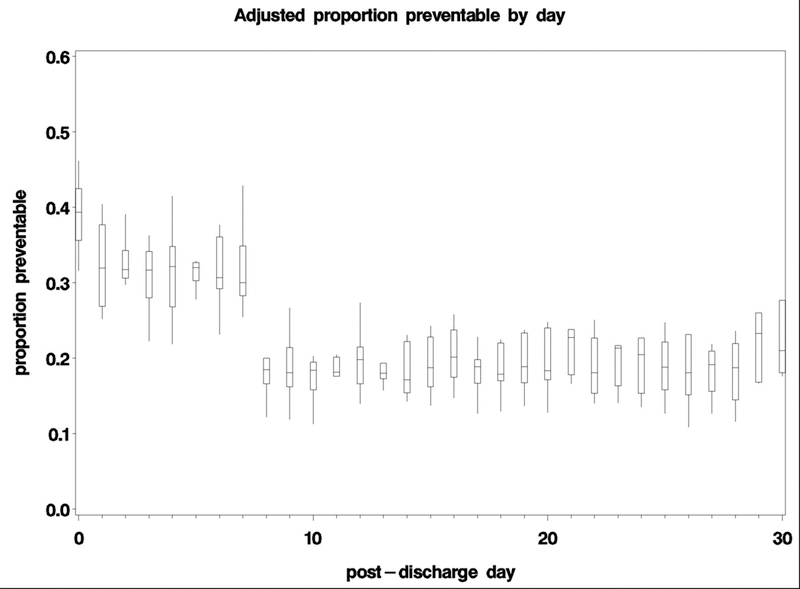
Pooled, adjusted proportion of readmissions ascertained as preventable using a standard algorithm and physician adjudication at all ten hospital sites. The bottom and top edges of the boxes represent the pooled 25th and 75th percentiles, the center horizontal line is drawn at the 50th percentile (median), and the vertical lines represent the most extreme observations.
The hospital was more likely to be identified as the ideal location for an intervention to prevent early compared to late readmissions (47.2% vs. 25.5%, median risk difference 22.8%). In contrast, the outpatient clinic (6.6% vs. 15.2%, median risk difference −10%) and home (14.0% vs. 19.4%, median risk difference −5.6%) were more likely to be identified as the ideal location for an intervention to prevent late compared to early readmissions (Table 2). There was variability by study site for the ideal location for an intervention (Appendix Table 3), but hospital was more frequently identified as the ideal location for early versus late readmissions in 9 out of 10 study sites, and home and outpatient clinic were more frequently identified as the ideal location in late versus early readmissions in 7 and 9 (respectively) out of 10 study sites.
Problems with physician decision making related to diagnosis and management were more frequently identified as causal factors for early versus late readmissions (28.9% vs. 11.5%, median risk difference 14.1%) (Table 3). The differences by specific problem were as follows: missed diagnoses (10.6 vs. 4.0%, median risk difference 6.7%), inadequate treatment of active medical conditions during the index admission (14.3 vs. 7.1%, median risk difference 4.6%) and patients being discharged too soon from the hospital (16.3 vs. 3.7%, median risk difference 13.6%).
Table 3.
Adjudicators’ Assessment of Factors Contributing to Readmission by Readmission Timing
| Early Readmissions (0–7d post- discharge) n=301, 36.6% |
Late Readmissions (8–30d post- discharge) n=521, 63.4% |
Median of Risk Differences Across Sites (25th,75th percentile) |
|
|---|---|---|---|
| Monitoring and managing symptoms after discharge* | 25.3 | 33.2 | −11.8 (−17.1, 2.3) |
| Inappropriate choice of discharge location | 9.3 | 5.6 | −0.1 (−2.0, 8.9) |
| Inappropriately long time between discharge and first follow-up with outpatient provider(s) | 5.0 | 10.0 | −2.9 (−4.9, −0.7) |
| Patient was not able to keep post-discharge appointments | 5.7 | 10.9 | −5.3 (−9.3, −1.4) |
| Discharge without needed procedure | 3.0 | 2.5 | 0.5 (−1.8, 3.2) |
| Lack of disease monitoring | 11.6 | 12.7 | −1.8 (−4.9, 5.5) |
| Social and Community Supports | 24.6 | 22.1 | 0.9 (−4.5, 7.3) |
| Patient required additional or different home services than those included in discharge plans | 12.3 | 9.8 | 3.0 (−1.1, 6.4) |
| Patient was not able to access services at home | 4.0 | 4.0 | 1.6 (−3.4, 2.5) |
| Patient required additional help from others that was not available or sufficient | 12.3 | 13.6 | −2.3 (−6.3, 2.0) |
| Patient required community programs not included in discharge plans | 5.3 | 4.2 | 2.1 (−1.5, 2.5) |
| Inpatient assessment of physical needs were incomplete | 4.0 | 3.5 | 1.3 (−2.1, 3.9) |
| Self Management Instruction | 49.8 | 45.9 | 6.4 (−8.1, 12.1) |
| Patient lacked awareness of who to contact, when to go to the emergency department | 7.3 | 9.6 | −0.6 (−6.1, 1.8) |
| Patient lacked awareness of post-discharge plans | 2.7 | 5.6 | −4.7 (−7.9, −0.9) |
| Patient or family had difficulty managing symptoms at home | 39.2 | 33.4 | 6.6 (−6.4, 14.3) |
| Patient or family had difficulty managing other self-care activities at home | 18.9 | 17.3 | 3.5 (0.7, 5.1) |
| Continuity of Care | 16.9 | 18.8 | −0.2 (−9.6, 11.3) |
| Team did not ensure that the patient had a primary care physician | 1.7 | 2.5 | −1.8 (−3.6, 2.5) |
| Follow-up appointments were not scheduled prior to discharge | 10.0 | 10.4 | 1.5 (−3.9, 6.5) |
| Follow-up appointments were not sufficiently soon after discharge | 5.6 | 8.8 | −2.2 (−5.5, 2.1) |
| Team did not relay important information to outpatient providers | 5.7 | 4.6 | 1.6 (−2.1, 8.7) |
| Patient unable to be reached for post-discharge care coordination | 1.7 | 1.2 | 2.0 (−4.2, 3.2) |
| Test results ordered by initial team were not followed up appropriately | 0.3 | 0.6 | −1.5 (−1.8, 0) |
| End of Life/Advance Care Planning | 8.0 | 13.8 | −3.9 (−10.7, −1.0) |
| Patient nearing end of life but still wants hospitalization and full treatment measures | 5.3 | 8.6 | −3.6 (−6.1, −0.4) |
| Patient receiving palliative or hospice care, but unable to manage symptoms | 0 | 0.6 | −1.8 (−2.0, −1.7) |
| Patient with end-stage illness but palliative care not consulted | 3.0 | 5.4 | −3.6 (−4.1, 2.5) |
| Patient with end-stage illness and goals of care discussion not documented | 3.0 | 5.2 | −4.0 (−5.1, 0.1) |
| Diagnostic or Therapeutic Problems | 28.9 | 11.5 | 14.1 (9.8, 26.5) |
| Missed diagnosis during the index admission | 10.6 | 4.0 | 6.7 (1.1, 8.9) |
| Inadequate treatment of medical conditions during the index admission | 14.3 | 7.1 | 4.6 (1.0, 15.7) |
| Inadequate treatment of pain during index admission | 4.0 | 2.3 | 1.8 (−2.0, 5.0) |
| Patient discharged too soon from index hospitalization | 16.3 | 3.7 | 13.6 (2.6, 23.0) |
| Decision-Making Concerning Readmission | 7.6 | 5.6 | 2.9 (−2.6, 7.9) |
| Patient inappropriately sent from sub-acute facility to emergency department | 0.7 | 0.6 | −1.8 (−1.8, 2.6) |
| Patient inappropriately told to come to emergency department from home | 1.0 | 1.3 | −1.4 (−1.8, 1.0) |
| ED inappropriately decided to admit patient | 7.0 | 4.4 | 2.9 (−2.0, 7.9) |
| Medication Problem or Adverse Drug Event | 18.6 | 21.7 | 0 (−10.4, 2.2) |
| Errors in taking the preadmission medication history during index admission | 1.3 | 0.6 | 3.3 (0.8, 4.8) |
| Errors in discharge orders | 2.3 | 2.1 | −1.1 (−1.8, 5.0) |
| Drug-Drug or drug-disease interaction | 2.7 | 5.0 | −2.7 (−5.5, 0.2) |
| Patient/caregiver misunderstanding of discharge medication regimen | 5.3 | 4.8 | 2.4 (−0.7, 3.0) |
| Patient/caregiver inability to manage medications at home/inadequate drug level monitoring | 9.3 | 11.9 | −2.9 (−5.2, 2.0) |
| Inadequate monitoring for side effects or non-adherence | 6.6 | 6.7 | −0.4 (−2.4, 1.1) |
| Inadequate steps to ensure patient could afford medications | 1.7 | 2.7 | −1.8 (−2.0, −0.9) |
The top row of each category represents the frequency with which at least 1 factor from this category was selected by physician adjudicators
Issues with monitoring and managing symptoms after discharge (33.2 vs. 25.3%, median risk difference 11.8%) and issues with end of life care and advance care planning (13.8 vs. 8.0%, median risk difference 3.9%) were more frequently identified as causal factors for late versus early readmissions. The differences by specific problem were inappropriately long wait times for post-discharge appointments (10.0 vs. 5.0%, median risk difference 2.9%) and patients not being able to keep their post-discharge follow-up visits (10.9 vs. 5.7%, median risk difference 5.3%). Patients nearing the end of life but still desiring hospitalization and full treatment measures were more frequently identified as causes of late versus early readmissions (8.6 vs. 5.3%, median risk difference 3.6%) (Table 3). An analysis of causation including only preventable readmissions produced similar results except issues with end of life care/advance care planning was no longer significantly different, and medication problems/adverse drug events were more likely to be identified as causal factors for late than early readmissions (36.7 vs. 27.5%, median risk difference 2.9%; Appendix Table 4)
DISCUSSION
In this cohort derived from ten academic medical centers consisting of readmitted general medicine patients, we found a significant difference in rates of preventability between an early and late period within the 30 days following hospital discharge, with early readmissions associated with double the odds of preventability compared to late readmissions, and a clear decline in adjusted preventability rates after post-discharge day 7. Physician adjudicators were more likely to consider the hospital to be the optimal site to implement interventions to prevent early readmissions (between days 0–7), and the outpatient clinic and home environments to implement interventions to prevent late readmissions (between days 8–30). Lastly, we found that problems with physician decision making related to diagnosis and management during the index hospitalization and premature discharge were significantly more likely to be identified as the cause of the readmission in the early period, while problems with post-discharge follow-up and monitoring and end of life issues were more likely to be identified as the cause of readmissions in the late period. Taken together, these findings suggest that readmissions occurring within the week following hospital discharge are more preventable and more likely to be caused by factors over which the hospital has direct control than those occurring later in the 30-day window following hospital discharge.
These findings suggest that hospitals are more likely to be successful at preventing readmissions occurring within the first week after discharge, after which interventions to prevent readmission may be more effective when targeted to the ambulatory care environment. This is consistent with our prior work, which showed that factors related to the index hospitalization such as acute illness burden and discharge timing were more closely associated with early readmissions than late readmissions (13), and our follow-up work, which showed that mean preventability scores were significantly higher in this early period compared to the late period by blinded physician review in a single center study (31). In the current study, we were able to address our hypothesis directly with a geographically diverse multicenter sample, improving upon external validity. Our findings are also supportive of prior work by others’ who demonstrated the inter-hospital variability in readmissions is highest during post-discharge days 0–7, suggesting this is a more ideal timeframe to capture hospital-attributable readmissions (7).
Our assessment of causality provides more insight into potential targets to prevent readmissions in these two timeframes. Compared to late readmissions, early readmissions were more likely to be caused by problems with physician decision making related to diagnosis and treatment during the index admission. Specifically, the adjudicators cited missed diagnoses and inadequate treatment of the admitting condition as reasons for early readmissions significantly more frequently than for late readmissions. The adjudicators also cited premature discharge as more likely to be the cause for early readmissions. This may be explained by the fact that more patients in the early cohort had incomplete diagnostic work-ups on the day of discharge than those in the late cohort. Although physician cognitive error may play a role in premature discharge, hospitalists face a number of significant pressures in our current healthcare system that could be influencing discharge timing decisions, including external pressure to drive down length of stay and shift non-urgent evaluation and treatment from the inpatient to outpatient setting. This points to a potential source of bias regarding optimal discharge timing that is potentially harmful to patients and should be explored further.
The analysis of causality also found inadequate monitoring and management of symptoms post-discharge as significantly more prevalent for late readmissions. Specifically, we found the long wait times and inability to keep post-discharge follow-up appointments with primary care providers were more often cited as causing late readmissions than early readmissions. These findings also support our hypothesis that late readmissions are driven by factors outside of the hospital and in the ambulatory care environment, where post-discharge monitoring and follow-up care could be better tracked and managed. This presents a potential area for intervention in ambulatory care clinics.
Finally, we found issues related to end of life care and advance care planning more likely to be cited as a cause for late readmissions. Specifically, we found that presence of a terminal illness with a preference to pursue aggressive medical care rather than palliative care was significantly more likely to be considered a contributing factor for late than early readmissions. These findings also support our conceptual model and prior work (13), in that a patient with a terminal illness who desires aggressive care has a high likelihood of being readmitted as a function of progression of their disease rather than hospital or ambulatory-sensitive care processes, leading to inevitable readmissions rather than preventable ones.
The most important limitation of our study is that the physician adjudicators were not blinded to the timing of the readmission, as this was not a pre-specified analysis. While the adjudicators were not explicitly instructed to make note of readmission timing as an aim of the original study, knowledge of readmission timing could have biased their assessment of preventability or informed their choice of location where an intervention to prevent the readmission would have been most effective. It should be noted that while the possibility of bias is present, the current study is consistent with our findings in our prior single center study where the methodology for physician adjudication did involve blinding the adjudicators to the timing of the readmission (31). Additionally, all of the sites included in our population were academic medical centers, where patients are often not local, and carrying out usual transitions in care processes can be a challenge. These out-of-network patients were not included in the cohort unless they were readmitted to one of the sites in our dataset, thus potentially limiting generalizability. These findings should be validated in community hospitals and hospitals with full access to readmission data.
It is also important to note that there can be a great deal of disagreement among adjudicators in preventability determinations, which may have contributed to the heterogeneity in our outcomes across sites (32). Accordingly, differences in preventability by site can reflect either true differences in preventability, or differences in calibration of the adjudicators in their probability of assigning preventability. We attempted to mitigate this by employing an in-depth training process for adjudicators, a dual-physician review process to allow for some degree of internal calibration, and providing descriptive analyses stratified by hospital site. Although we observed substantial site-level variability in the magnitude of differences in preventability and ideal location for an intervention between early and late readmissions, the direction of the relationship was consistent across the majority of sites. Nonetheless, we cannot rule out that differences in care processes or differences in preventability determinations between sites could have confounded our results.
Our findings have several additional implications. An ideal accountability metric should reflect a care process or system over which the organization or individual that is penalized has direct control. As such, the timeframe used for this accountability metric is critically important, specifically given the concerns that the 30-day readmission rate has introduced disparities in penalties, with the highest burden affecting hospitals caring for the most socially disadvantaged patients (33). The timeframe used must strike a balance between simplicity and validity (2). It is not feasible to recommend direct clinician review of the medical record to assess preventability. However, until hospital discharge is viewed as a population management task that will only succeed with successful integration between hospital and primary care teams, and where penalties are equally shared by the inpatient and ambulatory environments, a more evidence-based timeframe to capture this difficult construct could strike this balance.
We believe that using a 7-day cut-off would continue to incentivize hospitals to develop processes of care to reduce readmissions, while simultaneously avoiding inappropriate penalization. This idea is supported by our finding that the hospital was identified as the ideal location for an intervention to reduce early readmissions nearly half of the time compared to about one quarter for late readmissions. Taken together, we believe that our findings provide strong evidence for a 7-day readmission rate as a superior accountability measure for the hospital setting.
However, while changing the timeframe may address the problems faced by hospitals as a result of potentially undeserved financial penalties, a simple cut-off is unlikely to be the answer to providing our patients with the most high-quality and safe transitions in care at discharge. This effort will require a multi-faceted integration between hospitals and primary care offices, and better quality measurement. In addition to best practices for discharge planning, our results suggest that hospitalists should focus on interventions to reduce cognitive errors that affect diagnosis and treatment planning. The extent to which incentives imposed by hospital systems to increase throughput result in premature discharge and readmission should be further examined. Outpatient systems should prioritize the development of multi-disciplinary care management systems for post-discharge monitoring, and expanded access to the primary care team for timely post-discharge follow-up appointments. And finally, we believe that the quality metric used to measure and promote success in this realm must change. Shared accountability over the 30-days between hospitals and outpatient practices, possibly with weighted penalties by readmission timing, would engage outpatient practices in readmission reduction efforts, and reduce unfair financial penalties on hospitals, which have negative downstream effects on the patients they serve.
In summary, in a cohort derived from 10 academic medical centers, we found that readmissions within the first seven days following hospital discharge were more likely to be preventable than those occurring within a late period of 8–30 days. Early readmissions were more likely to be amenable to interventions within the hospital, and more likely to be caused by factors for which the hospital is directly accountable, such as problems with physician decision-making and premature discharge. Late readmissions were more likely to be amenable to interventions outside of the hospital, and were more likely to be caused by factors for which the hospital has less direct control, such as appropriate monitoring and managing of patients’ symptoms after discharge by the primary care team, and end of life preferences. We believe it is time to change the model for patient outcomes after hospital discharge to one that recognizes shared accountability for readmissions among the entire spectrum of care. If this cannot be achieved in the short term, our findings suggest that a 7-day readmission window will more accurately capture preventable hospital readmissions.
Supplementary Material
ACKNOWLEDGEMENTS
Funding Information: Support for this study was through an unrestricted research grant from the American Association of Medical Colleges, and in part by 2 UL1 TR000445–06. This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center through National Institutes of Health Award UL1 TR001102 and financial contributions from Harvard University and its affiliated academic health care centers. Dr. Graham is funded by the Eleanor and Miles Shore 50th Anniversary Fellowship Program for Scholars in Medicine at Beth Israel Deaconess Medical Center and Harvard Medical School. Dr. Herzig is funded by the National Institute on aging NIA K23AG042459. Dr. Marcantonio was supported in part by grants No. R01AG030618 (ERM) and K24AG035075 (ERM) from the National Institute on Aging. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health award #K23AG040157, the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC).
Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies.
Dr. Graham had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest: No authors report any conflicts of interest
This work has not been previously presented.
REFERENCES
- 1.Gerhart G, Yemane A, Hickman P, Oelschlaeger A, Rollins E, Brennan N for the Centers for Medicare and Medicaid Services. Medicare readmission rates showed meaningful decline in 2012. Medicare & Medicaid Research Review 2013. 3 (2). E1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavenberg JG, Leas B, Umscheid CA, Williams K, Goldmann DR, Kripalani S. Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med 2014. September;9(9):598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services (CMS), HHS. Medicare program: hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates. Federal Register 2011. August 18;76(160):51476–846. [PubMed] [Google Scholar]
- 4.Joynt KE, Jha AK. Thirty-day readmissions--truth and consequences. N Engl J Med 2012. April 12; 366(15): 1366–9 [DOI] [PubMed] [Google Scholar]
- 5.Fontanarosa PB, McNutt RA. Revisiting hospital readmissions. JAMA 2013. January 23; 309(4): 398–400. [DOI] [PubMed] [Google Scholar]
- 6.Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med 2013. March 28; 368(13): 1175–7 [DOI] [PubMed] [Google Scholar]
- 7.Chin DL, Bang H, Manickam RN, Romano PS. Rethinking Thirty-Day Hospital Readmissions: Shorter Intervals Might Be Better Indicators Of Quality Of Care Health Aff 2016. October 35:10 1867–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefan MS, Pekow PS, Nsa W, Priya A, Miller LE, Bratzler DW, Rothberg MB, Goldberg RJ, Baus K, Lindenauer PK. Hospital performance measures and 30-day readmission rates. J Gen Intern Med 2013. March; 28(3): 377–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical-readmission rates and quality of hospital care. Tsai TC N Engl J Med 2013. September 19;369(12):1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med 2010. July 15; 363(3): 297–8 [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM, Lin Z, Keenan PS, Chen J, Ross JS, Drye EE, Bernheim SM, Wang Y, Bradley EH, Han LF, Normand SL. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA 2013. February 13; 309(6): 587–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parina RP, Chang DC, Rose JA, Talamini MAJ, Is a low readmission rate indicative of a good hospital? Am Coll Surg 2015. February;220(2):169–76. [DOI] [PubMed] [Google Scholar]
- 13.Graham KL, Wilker EH, Howell MD, Davis RB, Marcantonio ER. Differences between early and late readmissions among patients: a cohort study. Ann Intern Med 2015. June 2; 162 (11): 741–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahlon S, Pederson J, Majumdar SR, Belga S, Lau D, Fradette M, Boyko D, Bakal JA, Johnston C, Padwal RS, McAlister FA. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ 2015. August 11;187(11):799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijlaars LP, Hardelid P, Woodman J, Allister J, Cheung R, Gilbert R.Contribution of recurrent admissions in children and young people to emergency hospital admissions: retrospective cohort analysis of hospital episode statistics. Arch Dis Child 2015. September;100(9):845–9. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med 2015. April;43(4):738–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med 2014. December 2;161(11):765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odonkor CA, Hurst PV, Kondo N, Makary MA, Pronovost PJ.Beyond the Hospital Gates: Elucidating the Interactive Association of Social Support, Depressive Symptoms, and Physical Function with 30-Day Readmissions. Am J Phys Med Rehabil 2015. July;94(7):555–67. [DOI] [PubMed] [Google Scholar]
- 19.Lindenauer PK, Lagu T, Rothberg MB, Avrunin J, Pekow PS, Wang Y, Krumholz HM. Income inequality and 30 day outcomes after acute myocardial infarction, heart failure, and pneumonia: retrospective cohort study. BMJ 2013. February 14;346:f521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvillo-King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H, Halm EA. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med 2013. February; 28(2): 269–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA 2011. February 16; 305(7): 675–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzig SJ, Schnipper JL, Doctoroff L, Kim CS, Flanders SA, Robinson EJ, Ruhnke GW, Thomas L, Kripalani S, Lindenauer PK, Williams MV, Metlay JP, Auerbach AD. Physician Perspectives on Factors Contributing to Readmissions and Potential Prevention Strategies: A Multicenter Survey. J Gen Intern Med 2016. November;31(11):1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrin J, St Andre J, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res 2015. Feb;50(1):20–39. doi: 10.1111/1475-6773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011. April 19;183(7): E1067–71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auerbach AD, Kripalani S, Vasilevskis EE, Sehgal N, Lindenauer PK, Metlay JP, Fletcher G, Ruhnke GW, Flanders SA, Kim C, Williams MV, Thomas L, Giang V, Herzig SJ, Patel K, Boscardin WJ, Robinson EJ, Schnipper JL. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med 2016. April;176(4):484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kripalani S, Roumie CL, Dalal AK, et al. ; PILL-CVD (Pharmacist Intervention for Low Literacyin Cardiovascular Disease) Study Group. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med 2012;157(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006;166(5):565–571. [DOI] [PubMed] [Google Scholar]
- 28.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003;138(3):161–167. [DOI] [PubMed] [Google Scholar]
- 29.Burke RE, Kripalani S, Vasilevskis EE, Schnipper JL. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med 2013;8(2):102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan TA, Leape LL, Laird NM, Herbert L, Localio R, Lawthers AG, Newhouse JP, Weiler PC, Hiatt HH Incidence of adverse events and negligence in hospitalized patients. NEJM 1991. February; 324 (6): 370–6 [DOI] [PubMed] [Google Scholar]
- 31.Graham KL, Dike O, Doctoroff L, Vanka A, Jupiter M, Davis RB, Marcantonio ER Preventability of early vs. late readmissions at tan academic medical center. 2016. PLoS One June 2017; 12(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Localio AR, Weaver SL, Landis JR, Lawthers AG, Brennan TA, Hebert L, Sharp MS. Identifying Adverse Events Caused by Medical Care: Degree of Physician Agreement in a Retrospective Chart Review. Ann Intern Med 1996:125:457–464 [DOI] [PubMed] [Google Scholar]
- 33.Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA 2013. January 23; 309(4): 342–3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


