Abstract
Introduction:
This study aimed to determine if endogenous gonadal hormones affect the intracellular mechanisms in the spinal cord that control inflammatory pain responses.
Methods:
We analyzed behavioral responses to, and changes in, serum levels of prostaglandin E2, estradiol, progesterone, and corticosterone after administration of 5% formalin in intact and ovariectomized (OVX) female rats.
Results:
OVX females displayed significantly more flinching than did intact females during Phase I, and after formalin administration their corticosterone levels were significantly lower. No differences were seen across COX-1 and COX-2 protein expression in the spinal cord of either naïve or formalin-treated rats. However, subsequent to formalin a main effect of gonadectomy was seen in prostaglandin E2 levels; OVX animals had significantly lower prostaglandin E2 levels than intact animals.
Conclusions:
These results indicate that in female rats nociceptive responses to formalin are regulated through the levels of prostaglandin E2, an important mediator in inflammation, whereas protein levels of COX-1 and COX-2 play a more limited role.
Keywords: Chronic, Pain, Sex, Differences, Hormones, Inflammation
Introduction
Sex differences in responses to inflammatory pain have been attributed to gonadal hormone effects that are most evident during fluctuations in pain sensitivity across the female estrous cycle and after gonadectomy.1,6 Although the role of endogenous hormones in acute pain responses has been extensively studied and reviewed in the literature, the few studies that have examined the role of endogenous hormones during inflammatory and chronic pain have not always been consistent. Ovariectomy had no effect on behavioral responses to 2% formalin during Phase I and II,4 but it significantly increased subject’s time spent licking its injected paw after 10% formalin.3 On the other hand, ovariectomy decreased thermal hyperalgesia after exposure to capsaicin.2
Prostaglandins, especially prostaglandin E2 (PGE2), are important mediators of inflammatory responses in that after a nociceptive stimulus they are released at the site of injury.17 Moreover, PGE2 levels closely follow formalin-induced behavioral responses.8 Cyclooxygenase (COX) is the rate-limiting enzyme involved in prostaglandin metabolism during inflammatory responses.17 Both isoforms of COX (COX-1 and COX-2) mediate responses to inflammatory stimuli including formalin administration.17 For example, indomethacin (a nonselective COX-1 and COX-2 inhibitor), diclofenac and FR122047 (selective COX-1 inhibitors), and celecoxib (a selective COX-2 inhibitor) attenuate flinching responses after formalin administration.16,18 Furthermore, it has been postulated that anti-inflammatory responses after inflammatory stimuli involve the activation of COX enzymes by corticosterone, an inhibitor that in turn decreases PGE2 release. The aim of this study was to determine if gonadal hormones contribute to the reported sex differences in response to inflammatory pain through endogenous differences in COX activation, COX protein levels, and/or release of prostaglandins.
Methods
Animals
Eight-week-old intact and ovariectomized (OVX) female Sprague-Dawley rats were purchased from Taconic (Germantown, NY). Rats were double-housed with a 12-hour light/12-hour dark cycle (lights on 8 AM) with food and water ad libitum. Animals were randomly assigned to experimental groups (n=8–10 per group) and run in two separate cohorts. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication 85–23, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee at Hunter College of The City University of New York. All chemicals, unless otherwise stated, were purchased from Sigma Aldrich (St. Louis, MO).
Nociceptive Testing
An automated nociceptive analyzer was used to determine overall behavioral activity after formalin administration as defined by—all movement of the hind paw including flinches, paw licks and any other motion.19 Prior to formalin injection, a soft metal band was placed on the right hind paw with the opening positioned at the plantar surface of the paw as previously described.5,10,11 The formalin assay was always carried out between 9:00 AM and 3:00 PM. Rats were placed inside the testing chamber for a total of 30 minutes before the formalin injection to minimize novelty of the testing environment and the band. Five percent formalin was injected intra-plantar on the banded right hind paw in a volume of 50 μL. Rats were then returned to the testing chamber, and data were collected at 1-minute intervals for a total of 60 minutes.
Radioimmunoassay and Enzyme Immunoassay
Sixty minutes after formalin injection rats were sacrificed by decapitation, after a brief exposure to CO2 (20 seconds), and trunk blood was collected, centrifuged (3,000 RPM for 30 minutes at 4°C), and stored at −80°C. Serum was analyzed using Coat-A-Count radioimmunoassay kits for corticosterone, progesterone, and estradiol (Diagnostic Products Corporation, Los Angeles, CA). Serum was also analyzed for PGE2 concentration with use of an enzyme immunoassay kit from Cayman Chemical (Ann Arbor, MI). Results for these assays were determined by means of a log-logit analysis within GraphPad Prism Software (San Diego, CA). Intra-assay coefficients of variation averaged 10.0% ± 1.0%.
Western Blots
After decapitation of the rats, the lumbosacral region of their spinal cords was rapidly dissected and stored at −80°C until use. Protein levels of COX-1 and COX-2 were analyzed using Western blot techniques as previously described.5 Briefly, 30 μg of protein samples were resolved in gradient SDS-PAGE gels (4%–15%) and transferred to nitrocellulose membranes. Membranes were then blocked with 5% nonfat dry milk for 30 minutes and incubated with COX-1 or COX-2 antibodies (1:1000; Cayman Chemical, Ann Arbor, MI) for 1 hour at room temperature or overnight at 4°C, respectively. After washing in TBST, membranes were incubated with appropriate secondary antibodies (1:1000) for 1 hour at room temperature. Band intensities were detected with an enhanced chemiluminescence kit from Amersham (Piscataway, NJ) and quantified with a Molecular Dynamic Computer Densitometer and Image Quant Program. α-tubulin antibody (1:1000; Sigma Aldrich, St. Louis, MO) was used to normalize protein concentrations.
Statistics
Consistent with Yaksh et al,19 data were analyzed as the mean number of flinches (±SEM) during Phase I (0–9 min) and Phase II (10–60 min) of the nociceptive response to formalin. Twoway ANOVAs were used to test for significant differences in the sum of the flinching responses across groups for Phase I and Phase II. Fisher’s least significant difference post-hoc testing was done when appropriate. To determine significant differences in serum levels of corticosterone, estradiol, progesterone, or PGE2 for each treatment (formalin vs. no formalin), ANOVAs were done. Linear regressions were run comparing PGE2 levels with each of the hormones and with flinching behaviors. To determine if significant differences were observed in COX-1 or COX-2 protein levels among groups, independent sample t-tests were used (for each protein in each experimental manipulation comparisons were made between intact vs OVX rats). For all comparisons, significance was at the .05 level.
Results
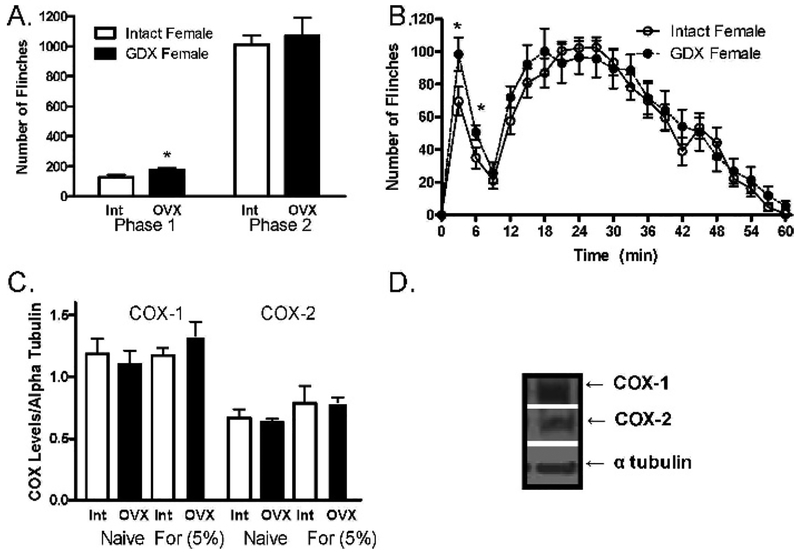
Nociceptive behavior during Phase I was greater in OVX rats than in the respective intact groups (F (1, 40) =5.18; P=.02; Figure 1A, B). An interaction between gonadectomy and corticosterone serum levels was observed (F (1, 40)=15.47; P<.01; Table 1); after formalin, corticosterone serum levels were lower in OVX than intact females (P<.05). In both naïve and formalin-treated animals, intact females had significantly higher progesterone and steroidal serum levels than did all other groups (P<.05; Table 1). Furthermore, in both naive and formalin-treated animals, PGE2 levels were significantly lower in OVX female rats than in intact groups (F (1, 40)=8.23; P<.01; Table 1). As shown in Table 2, significant correlations between behavioral responses during Phase I and progesterone (P=.0171) as well as between these behavioral responses and corticosterone (P=.039) were observed. However, no significant correlations between serum levels of PGE2 and any of the measured hormones were observed in any of the experimental groups (Table 3). COX-1 and COX-2 protein levels in the spinal cord did not significantly differ basally or after formalin administration in any of the experimental groups (Figure 1C, D).
Fig 1.
(A) Total sum number of flinches (±SEM) across phase I (0–9 min) and phase II (10–60 min) in intact and OVX female rats after 5% formalin administration (n=8/group). (B) Formalin-induced responses across time (n=8/group). (C) COX1-COX2 protein levels in the spinal cord in intact and OVX female rats, basal and after 5% formalin administration (n=4/group). COX-1 and COX-2 protein levels are plotted as the ratio of protein levels over α-tubulin levels across treatment groups. (D)Typical immunoband. *Denotes significant difference to respective control (P<.05). [For: formalin; OVX: ovariectomized; Int: intact.]
Table 1.
Progesterone, estrogen, corticosterone, and prostaglandin E2 serum levels in OVX vs. intact female rats
| Treatment | Group | Progesterone (ng/mL) | Estradiol (pg/mL) | Corticosterone (ng/mL) | PGE2 (pg/mL) |
|---|---|---|---|---|---|
| Vehicle | Intact | 12.24 ± 2.26 | 39.17 ± 18.84 | 59.71 ± 6.23 | 224.85 ± 50.89 |
| OVX | 2.44 ± 1.23* | 9.55 ± 30.79* | 58.14 ± 12.44 | 57.96 ± 8.16* | |
| Formalin | Intact | 22.90 ± 0.82 | 77.58 ± 58.92 | 713.47 ± 28.26 † | 196.16 ± 108.28 |
| OVX | 6.74 ± 1.61* | 23.67 ± 6.83* | 552.73 ± 58.57† | 71.74 ± 15.60* |
Indicates main effect of gonadectomy.
Indicates main effect of formalin treatment.
Bold indicates significant differences to intact-formalin treated rats (P<.05).
Table 2.
Correlation of flinching behavior and PGE2, corticosterone, progesterone and estradiol serum levels
| Phase | PGE2 | Corticosterone | Progesterone | Estradiol |
|---|---|---|---|---|
| I | R2=.004; m=.004 ± .018 | R2=.269; m=−1.817 ± .799 | R2=.343; m=−.066 ± .024 | R2=.023; m=.190 ± .327 |
| II | R2=.006; m=.001 ± .003 | R2=0.075; m=−.192 ± .179 | R2=.029; m =−.003 ± .005 | R2=.021; m=.036 ± .065 |
Bold indicates significant correlations (P<.05); m=slope.
Table 3.
Correlation of PGE2 and endogenous steroid serum levels
| Treatment | Corticosterone | Progesterone | Estradiol |
|---|---|---|---|
| Vehicle | R2=.025; m=−1.443 ± 2.388 | R2=.028; m=.195 ± .331 | R2=.011; m=1.109 ± 2.717 |
| Formalin | R2=.088; m=26.79 ± 22.93 | R2=.187; m=1.260 ± .701 | R2=.039; m=6.337 ± 8.355 |
m=slope.
Discussion
Our results demonstrated that endogenous gonadal hormones alter flinching responses to 5% formalin. However, the OVX-induced flinching responses were seen only during Phase I. Previous studies have demonstrated no effect or an increase in behavioral responses after ovariectomy in rats. Although ovariectomy had no effect on behavioral responses to 2% formalin during Phase I and II,4 it significantly increased subject’s time spent licking its injected paw, but not the number of flinching responses, after 10% formalin.3 However, female rats in that study had been ovariectomized for 6 months before formalin testing,3 whereas in this study they had been ovariectomized for only 2 weeks. Thus, it is feasible that the rat’s age and/or length of time since ovariectomy may contribute to the observed differences. Alternatively, it is feasible that the direction of effects from gonadectomy is affected, in part, by the intensity of the painful stimuli and/or pain model used.
Gonadectomy decreased corticosterone levels in females, a finding consistent with previous reports.13 Corticosterone is known to exert anti-inflammatory actions, and evidence indicates that inflammation contributes to the nociceptive responses in the formalin test.15 For example, the inflammation associated with the injection of complete Freund’s adjuvant was significantly attenuated with the administration of the glucocorticoid dexamethasone. More recently, Zhang et al demonstrated that endogenous glucocorticoids exert a powerful suppressive effect on complete Freund’s adjuvant-induced inflammatory hyperalgesia.20 Indeed, we found that OVX females displayed a higher nociceptive response than intact females during Phase I, and they had significantly lower corticosterone levels. Furthermore, a significant negative correlation was found between flinching in Phase I and corticosterone. These data further suggest that corticosterone may play a significant role in mediating nociceptive responses to formalin administration.
Although both isoforms of COX were present in the lumbosacral region of the spinal cord and, as expected, COX-1 levels were higher than COX-2, the pattern of expression was affected by ovariectomy. This finding suggests that the ovariectomy effects in formalin-induced behavior were not the result of either basal or formalin effects on COX-1 or COX-2 protein levels. Thus, endogenous gonadal hormone regulation of COX-protein levels may play a limited role in the regulation of sex differences in inflammatory nociceptive responses. However, COX-2 protein synthesis has been shown to remain unaltered up to 4 hours after formalin administration, and this may explain, in part, the lack of a significant finding.7,14
Furthermore, observed changes in PGE2 levels after ovariectomy suggest activation, and not change in protein levels, of COX-1 and COX-2 may in part mediate the effects of gonadectomy on formalin-induced behavioral responses. Indeed, activation of COX-1/COX-2 has been shown to be an important step in the cascade of intra-cellular responses to formalin. For example, Tegeder et al demonstrated that after 5% formalin administration, PGE2 release coincided with nociceptive behavioral responses, which returned to baseline levels approximately 2 hours after formalin injection.14 Moreover, formalin-induced PGE2 increases and nociceptive behavioral responses were reduced after administration of a COX-1 inhibitor, SC560, whereas the administration of a COX-2 inhibitor, celecoxib, affected neither PGE2 release nor nociceptive behaviors.14 Previous studies, however, have shown the effectiveness of COX-2 inhibitors in both formalin and other nociceptive models.14,16,18 It is important to note that in this study, levels of PGE2 were analyzed 60 minutes after formalin administration rather than during the Phase I or Phase II behavioral responses, when behavioral sex differences were observed. Thus, a more detailed time course determination is needed to further elucidate if sex differences in PGE2 release occur at those times during inflammation-induced behavioral responses.
Endogenous hormones have been shown to directly alter the synthesis of prostaglandins in non-central nervous system tissue, eg, progesterone increased but estrogen decreased PGE2 synthesis.9 Our study failed to find a significant correlation between PGE2 and either estradiol or progesterone (PGE2 and progesterone correlated negatively with a significance of .08). However, behavioral responses during Phase I displayed a significant negative correlation with serum levels of progesterone. The same negative correlation was found between behavioral responses during Phase I and levels of corticosterone. Since corticosterone is derived from progesterone,12 these results further suggest a possible correlation of HPA activation and formalin-behavioral responses.
Implications for Improving Health Disparities
Female rats display greater nociceptive responses to persistent and inflammatory pain stimuli than male rats.1,5 On the basis of our findings and those of others, we postulate that sex differences in response to inflammatory pain are, in part, mediated through influences of gonadal hormones on inflammation-mediated responses.
Acknowledgments
We are grateful to Dr. Patricia Stephens for her editorial comments. This work was supported by RR-03037, NF39534, and DA 12136.
References
- 1.Aloisi AM, Ceccarelli I, Fiorenzani P. Gonadectomy affects hormonal and behavioral responses to repetitive nociceptive stimulation in male rats. Ann N Y Acad Sci. 2003;1007: 232–237. [DOI] [PubMed] [Google Scholar]
- 2.Barrett AC, Smith ES, Picker MJ. Capsaicin-induced hyperalgesia and mu-opioid-induced antihyperalgesia in male and female fischer 344 rats. J Pharmacol Exp Ther. 2003; 307(1):237–245. [DOI] [PubMed] [Google Scholar]
- 3.Ceccarelli I, Fiorenani P, Massafra C, et al. Long-term ovariectomy changes formalin-induced licking in female rats; the role of estrogens. Reprod Biol Endocrinol. 2003;1: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002; 958(1):139–145. [DOI] [PubMed] [Google Scholar]
- 5.Kuba T, Wu HB, Nazarian A, et al. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm Behav. 2006;49(4):441–9. [DOI] [PubMed] [Google Scholar]
- 6.Kuba T, Quinones-Jenab V. The role of female gonadal hormones in behavioral sex differences in persistent and chronic pain: clinical vs. preclinical studies. Brain Res Bull. 2005;66(3):179–188. [DOI] [PubMed] [Google Scholar]
- 7.Maihofner C, Tegeder I, Euchenhofer C, et al. Localization and regulation of cyclooxygenase-1 and −2 and neuronal nitric oxide synthase in mouse spinal cord. Neuroscience. 2000;101(4): 1093–1108. [DOI] [PubMed] [Google Scholar]
- 8.Malmberg AB, Yaksh TL. The effect of morphine on formaline-evoked behaviour and spinal release of excitatory amino acids and prostaglandin E2 using microdialysis in concious rats. Br J Pharmacol. 1995;114(5): 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann GE. Hormone control of prostaglandin F(2 alpha) production and oxytocin receptor concentrations in bovine endometrium in explant culture. Domest Anim Endocrinol. 2001;20(3):217–226. [DOI] [PubMed] [Google Scholar]
- 10.Mannino CA, South SM, Quinones-Jenab V, et al. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8(4):334–342. [DOI] [PubMed] [Google Scholar]
- 11.Mannino CA, South SM, Inturrisi CE, et al. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6(12):809–816. [DOI] [PubMed] [Google Scholar]
- 12.McShane PM, Fencl MD. Conversion of progesterone to corticosteroids by the midterm fetal adrenal and kidney. Steroids. 1983;42(3): 299–310. [DOI] [PubMed] [Google Scholar]
- 13.Seale JV, Wood SA, Atkinson HC, et al. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol. 2004; 16(6):516–524. [DOI] [PubMed] [Google Scholar]
- 14.Tegeder I, Niederberger E, Vetter G, et al. Effects of selective COX-1 and −2 inhibition on formalin-evoked nociceptive behaviour and prostaglandin E2 release in the spinal cord. J Neurochem. 2001;79(4):777–786. [DOI] [PubMed] [Google Scholar]
- 15.Tjolsen A, Berge OG, Hunskaar, et al. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. [DOI] [PubMed] [Google Scholar]
- 16.Torres-Lopez JE, Ortiz MI, Castaneda-Hernandez G, et al. Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test. Life Sci. 2002;70(14):1669–1676. [DOI] [PubMed] [Google Scholar]
- 17.Vane JR, Bakhle YS. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. [DOI] [PubMed] [Google Scholar]
- 18.Veiga APC, Duarte IDG, Avila MN, et al. Prevention by celecoxib of secondary hyperalgesia induced by formalin in rats. Life Sci. 2004;75(23):2807–2817. [DOI] [PubMed] [Google Scholar]
- 19.Yaksh TL, Ozaki G, McCumber D, et al. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001;90(6):2386–2402. [DOI] [PubMed] [Google Scholar]
- 20.Zhang RX, Lao L, Qiao JT, et al. Endogenous and exogenous glucocorticoid suppresses up-regulation of preprodynorphin mRNA and hyperalgesia in rats with peripheral inflammation. Neurosci Lett. 2004;359(1–2):85–88. [DOI] [PubMed] [Google Scholar]