Summary
A ubiquitous feature of active (REM) sleep in mammals and birds is its relative abundance in early development. In rat pups across the first two postnatal weeks, active sleep promotes the expression of synchronized oscillatory activity within and between cortical and subcortical sensorimotor structures. Sensory feedback from self-generated myoclonic twitches—which are produced exclusively during active sleep—also triggers neural oscillations in those structures. We have proposed that one of the functions of active sleep in early infancy is to provide a context for synchronizing developing structures. Specifically, neural oscillations contribute to a variety of neurodevelopmental processes, including synapse formation, neuronal differentiation and migration, apoptosis, and the refinement of topographic maps. In addition, synchronized oscillations promote functional connectivity between distant brain areas. Consequently, any condition or manipulation that restricts active sleep can, in turn, deprive the infant animal of substantial sensory experience, resulting in atypical developmental trajectories.
Keywords: sleep, neural circuit, connectivity, LFP, EEG, neurodevelopment, brain rhythms, red nucleus, hippocampus, cortex
1. Introduction
Complex behavioral and cognitive skills are supported by precisely timed interactions between distant neural networks. These long-range interactions depend on the temporal coupling of neural oscillations, or brain rhythms[1,2]. Neural oscillations are the result of synchronized activity within large populations of neurons and exhibit specific spatiotemporal features depending on brain area, behavioral state, and age.
The functional roles of neural oscillations have been predominantly examined in the adult forebrain, although brain rhythms are present throughout the neuraxis and emerge in early development[3-17]. In the cerebral cortex of humans and rodents, the earliest neural activity is characterized by the presence of brief oscillatory bursts interposed with periods of silence; this pattern of fragmented activity is referred to as discontinuous. Although different from the continuous oscillatory patterns of adults, these early oscillations appear to have important functions for the development of the nervous system[3,18-20]. In addition, temporal coupling or coactivation of brief oscillatory events is indicative of connectivity among functionally related neural structures[3,6,7,9,21].
Early oscillatory dynamics in rats have often been described using in vitro preparations or under anesthetic conditions in vivo that preclude the normal expression of sleep-wake cycles. Based on such studies, one might conclude that the expression of brain rhythms is independent of experience or behavioral state. Within the developing sensorimotor system, however, early neural oscillations are largely triggered by sensory input arising from external stimulation (exafference) or sensory feedback arising from self-generated movements (reafference)[9,22].
The opportunities for reafferent stimulation in the developing sensorimotor system are not restricted to periods of wakefulness. In the perinatal period when sleep is the predominant behavioral state, myoclonic twitching during active sleep is one of the most abundant of all behaviors[23-25]. Twitches are brief, discrete jerky movements that occur against a background of muscle atonia and are generated by brainstem motor structures, including the red nucleus[26,27]. Unlike wake-related movements, twitches are ideally suited for the efficient transmission of peripheral sensory feedback and provide a major source of neural activation to the infant’s plastic brain (for review, see [28,29]). Moreover, sensory feedback from twitches reliably triggers synchronized neural oscillations across the neuraxis, suggesting that these oscillations also contribute to the development of long-range network connectivity. More broadly, independently of twitching, active sleep may provide a context that promotes functional connectivity in the sensorimotor system including, as recently shown in 12-day-old rats, highly synchronized oscillatory activity between the hippocampus and red nucleus[9].
These and other findings regarding active sleep and the early expression of coupled oscillatory activity have important methodological and clinical implications. From a methodological perspective, any testing condition (e.g., anesthesia) that interferes with the normal expression of sleep-wake cycles will also interfere with the normal expression of oscillatory activity. From a clinical standpoint, disruption or deprivation of active sleep in early infancy is likely to interfere with the typical development of network interactions in the sensorimotor system; indeed, prolonged disruptions of perinatal sleep may help to explain the sensorimotor deficits that characterize such neurodevelopmental disorders as autism and schizophrenia.
2. Synchronized oscillations orchestrate the development of neuronal networks
Early oscillatory activity in vivo has been most extensively—but not exclusively [9,14]—described in the cortex and hippocampus[3,4,6-8,10-12,15,17,30-32]. In these forebrain structures, fragmented oscillatory activity is thought to assist in the development of local neuronal networks, with effects on synapse formation, neuronal differentiation and migration, programmed cell death (apoptosis), and formation and refinement of topographic maps[3,18-20,33-35].
During the first postnatal week in rats, sensory feedback from whiskers drives two distinct oscillatory patterns of activity in the whisker “barrel” cortex: spindle bursts (5-30 Hz with components in the theta, alpha, and beta bands) and early gamma oscillations (EGOs; 30-50 Hz)[10,36]. Both spindle bursts and EGOs originate in cortical layer 4 and are highly dependent on thalamic input. Between birth and 3 days of age, spindle bursts and EGOs are both enabled by gap junctions; by the end of the first postnatal week, such oscillatory patterns are primarily generated by glutamatergic currents and involve the recruitment of cortical interneurons. In the hippocampus by the end of the first postnatal week, brief oscillatory events at theta (4-14 Hz) and gamma (~20-100 Hz) frequencies become apparent[11,15], likely reflecting the proliferation of hippocampal interneurons[37]. In addition, the emergence of theta oscillations reflects the strengthening of cholinergic and GABAergic projections from the medial septum[38].
Perhaps the most direct, causal evidence for a role of oscillations in brain development comes from studies assessing their involvement in apoptosis [18,34]. Apoptosis is the physiological process through which neurons and other cells die. This death is a normal process that complements the over-production of neurons that characterizes early development; accordingly, apoptosis is a key contributor to the anatomical and functional development of the nervous system[39]. Notably, in rat pups, oscillatory activity appears to determine the rate of apoptosis in sensorimotor cortex [18]. Specifically, area-specific levels of neuronal activity inversely correlate with the number of apoptotic neurons detected in local networks. Importantly, selective manipulation of neuronal activity affects the rate of apoptosis in sensory and motor cortices. In support of this finding, the amount of alcohol-induced suppression of early oscillatory bursts correlates with the amount of apoptosis detected in somatosensory cortex[34]. These findings demonstrate that alteration of oscillatory activity in early development is detrimental to typical cortical development and can mediate the detrimental effects associated with early exposure to teratogens. These findings may have translational value: In premature human infants, the amount of early oscillatory cortical activity correlates with subsequent brain growth[40] and later mental development[41].
As noted above, most of our knowledge about early brain oscillations in vivo comes from studies that have not examined oscillatory dynamics across the sleep-wake cycle. When behavioral state is taken into consideration, it is evident that neural oscillations in the developing sensorimotor system are predominantly expressed during active sleep[9,11,12,32].
3. Spontaneous motor activity during active sleep drives early oscillatory activity within local cortical and subcortical sensorimotor networks
Rodents rely heavily on the whisker system to navigate and explore their world[42]. For this reason, the whisker system has proven extremely valuable for understanding how complex sensorimotor systems develop[43,44]. In rat pups, deprivation of whisker-related sensory experience during sensitive periods—including the first postnatal week—disrupts anatomical and functional development of whisker-related brain areas, including somatosensory cortex[45-47]. Such studies demonstrate how typical development of this system relies heavily on sensory experience.
In developing brain areas that process whisker-related sensory information (such as the ventral posterolateral thalamus and barrel cortex), twitching of the whiskers during active sleep is a prominent source of sensory experience[43]. Sensory feedback from twitching elsewhere in the body, such as the limbs, drives neural activity in somatotopically related brain areas (i.e., areas that specifically map to parts of the body; see Table 1 and Figure 1). In rat pups through the first two postnatal weeks, twitches drive oscillatory events with distinct spatiotemporal features depending on the brain area. For example, in the newborn rat sensorimotor cortex, twitch-related reafference triggers spindle bursts[12,13,48]. Spindle bursts are thought to contribute to the anatomical and functional development of the cortex[33,36,49,50]. Although spindle bursts can be generated endogenously, their occurrence decreases threefold (i.e., only ~30% of spindles remain) in the absence of peripheral sensory input [13]. Moreover, the occurrence of spindle bursts in intact rat pups is markedly higher during periods of twitching in relation to wakefulness[12,48]. In the neonatal rat hippocampus, periods of twitching are also associated with the emergence and maximal expression of two prominent rhythms that synchronize hippocampal networks: theta and gamma[9,11,32]. All together, these results highlight the contribution of active sleep and twitch-related sensory feedback to the generation of early oscillatory activity within local circuits in the neonatal cortex and hippocampus.
Table 1.
Twitch-related neural activity in the developing nervous system.
| Area | Species | Age | MUA | Oscillatory Events (LFP/EEG) | Refs. | |
|---|---|---|---|---|---|---|
| Event Features | Description | |||||
| Cortex | ||||||
| Somatosensory cortex | Rat | P1-12 | Twitch-following Somatotopic | Spindle Burst ~5-30 Hz 0.1-3 s. | Twitch-following Somatotopic | [12,16,48,74-77*] |
| Human | PCA 29-31 | N/A | Delta Brush ~8-25 Hz 1-2 s. | Twitch-following Somatotopic | [79] | |
| Primary motor cortex | Rat | P4-12 | Twitch-following Somatotopic AS-On | Spindle Burst ~5-30 Hz | Twitch-following Somatotopic AS-On | [12,80] |
| Gamma oscillation ~30-40 Hz 0.1-0.3 s. | Twitch-following | [80] | ||||
| Hippocampus | ||||||
| CA1 | Rat | P1-9 | Twitch-following Twitch-active AS-On | Theta oscillation (>P7) ~4-14 Hz | Twitch-following Twitch-active | [11,55] |
| Gamma oscillation ~ 20-100 Hz | Twitch-active | |||||
| P11-13 | Theta (continuous) ~4-14 Hz; 4-7 Hz | Twitch-following Twitch-active AS-On | [9,11] | |||
| DG | Rat | P1-12 | Twitch-following Twitch-active AS-On | Theta oscillation ~4-14 Hz | Twitch-following Twitch-active | [11] |
| Gamma oscillation ~20-100 Hz | Twitch-following Twitch-active | |||||
| Cerebellum | ||||||
| Cortex (Purkinje Cell) | Rat | P4-P12 | Twitch-following AS-On | ? | N/A | [81,82] |
| Interposed Nucleus | Rat | P8-13 | Twitch-following Somatotopic | ? | N/A | [83] |
| Thalamus | ||||||
| Somatosensory | Rat | P1-8 | Twitch-following Somatotopic | ? | N/A | [12,13] |
| Midbrain/Brainstem | ||||||
| Red Nucleus | Rat | P7-9 | Twitch-following Twitch-preceding Somatotopic | Theta oscillation ~4-7 Hz | Twitch-following | [9,26,83] |
| P11-13 | “ “ | Theta (continuous) ~4-7 Hz | Twitch-active Twitch-following AS-On | [9] | ||
| External cuneate | Rat | Twitch-following Somatotopic AS-On | ? | N/A | [48] | |
| Medulla (e.g., Gi, LC) | Rat | P6-10 | Twitch-active AS-On | ? | N/A | |
| Spinal Cord | ||||||
| Rat | P5-17 | Twitch-following Twitch-preceding Somatotopic | Negative deflection <1 Hz | Twitch-following Somatotopic | [14,53] | |
MUA = multi-unit activity;LFP = local field potential;EEG = electroencephalogram;P = postnatal day;PCA = post-conceptional age (in weeks);CA = cornus amonius;DG = dentate gyrus;Gi: nucleus gigantocellularis;LC: locus coeruleus.
this study also uses voltage-sensitive dye imaging.Twitch-following: neural activity increases immediately after twitch events.Twitch-active: neural activity increases duringperiods of twitching.Twitch-preceding: neural activity increases immediately before twitch events.AS-On: Neural activity increases during active sleep in relation to other behavioral states.N/A = not applicable.
Figure 1. Diagram illustrating anatomical pathways conveying twitch-related sensory feedback in neonatal rats.
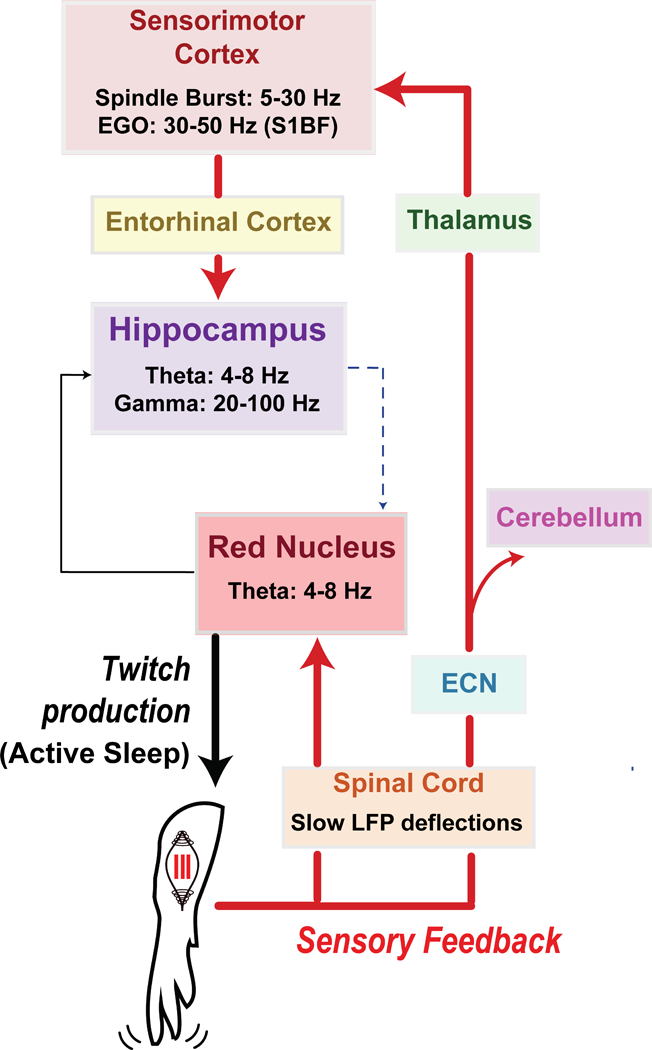
A twitch of the forelimb is generated in the red nucleus during active sleep. Sensory feedback (or reafference) from the twitch causes a cascade of precisely timed neural responses in sensorimotor structures across the neuraxis, including spinal cord, red nucleus, sensorimotor cortex, and hippocampus. Through repeated activation of these structures, twitches provide a unique opportunity for the synchronization of oscillatory activity. See text for further discussion.
As illustrated in Table 1, the role of twitch-related sensory feedback in the generation of brain rhythms is not restricted to cortical and hippocampal circuits. Using rat pups, we showed that the red nucleus, a brainstem structure that plays an important role in the production of infant motor behavior[26], exhibits theta oscillations predominantly during active sleep[9]. Indeed, in 8-day-old rats, brief, discontinuous theta oscillations were most prominently associated with twitches. Just four days later, theta oscillations in the red nucleus were expressed continuously during active sleep while also exhibiting enhanced amplitude during periods of twitching. The discovery of state-dependent theta oscillations in the developing brainstem raises a host of new questions. For example, do oscillations play a similar developmental role in the brainstem as they do in the forebrain? What are the mechanisms that promote sleep-dependent oscillations in the brainstem? What cognitive and behavioral functions are supported by oscillatory coupling between brainstem and forebrain areas? These and other questions can be answered now that we have overcome the technical barriers to recording brain activity in the neonatal brainstem[51].
4. Oscillations during active sleep promote neuronal synchrony across distant sensorimotor structures in early development
So far, we have discussed the role of twitching during active sleep in the generation of early oscillatory activity within local sensorimotor networks. In addition, coupled oscillatory activity across neuronal structures is a hallmark of long-range functional connectivity in both the infant and adult nervous system[1,6,7,9,52]. In the developing prefrontal-hippocampal network, for instance, synchronized oscillatory activity in the ventral hippocampus drives oscillatory activity in prefrontal cortex[7]. Because twitches drive precisely timed oscillatory activity across the neuraxis, including the hippocampus (Figure 1), twitching may optimize the probability of early synchronization of sensorimotor networks during active sleep. Indeed, even within the neonatal spinal cord, reafference from spontaneous self-generated movements (including wake-related movements and twitches) coordinates neuronal activity between motor and sensory zones[14,53]. Dorsal rhizotomy, which prevents sensory input to the spinal cord, uncouples this neural activity, thus demonstrating a role for early sensory feedback in neuronal synchrony in intrasegmental spinal circuits[14]. Such disruptions of spinal processing can have functional consequences; for example, genetically altered newborn mice that lack muscle spindles—which provide proprioceptive input to the spinal cord—fail to develop the monosynaptic stretch reflex [54].
Future research will assess the relative role of twitch-related sensory feedback in the coupling of oscillatory activity in higher-order sensorimotor networks. For example, twitch-related activation of hippocampal circuits depends on sensory input from somatosensory cortex via entorhinal pathways[55]. In support of a role for twitching in promoting such cortico-hippocampal communication, twitching of the whiskers during active sleep specifically promotes oscillatory coupling between barrel cortex and dorsal CA1 in hippocampus[56]. Figure 2 provides a representative example of the oscillatory dynamics in the barrel field and hippocampus of an 8-day-old rat following whisker twitches during active sleep. As shown in Figure 2A, sensory feedback from whisker twitches reliably triggers 20-30 Hz oscillations in both structures. Spectral coherence (a measure of synchrony) across behavioral states reveals enhanced cortico-hippocampal synchrony at 20-30 Hz following twitches but not during active wake (AW) or behavioral quiescence (BQ; Figure 2B).
Figure 2. Twitches during active sleep promote coherent oscillations in developing sensorimotor networks.
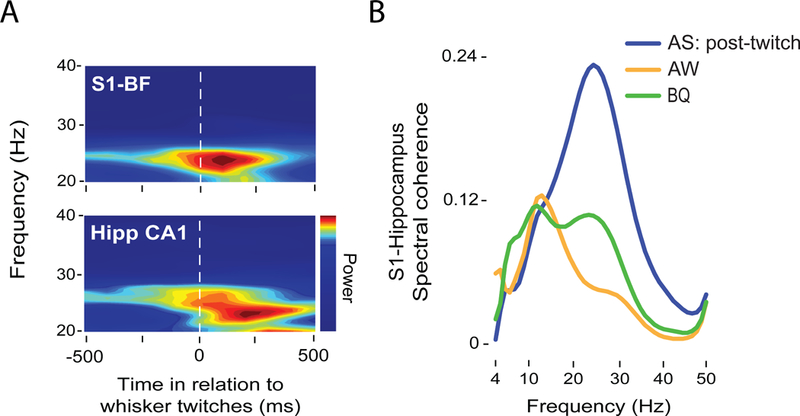
A: Representative twitch-triggered time-frequency spectrogram in the barrel field of somatosensory cortex (S1-BF, top) and hippocampal CA1 (Hipp CA1, bottom) of an 8-day-old rat. Note the increase in power at ~25 Hz in both structures after twitch onset. B: Representative coherence spectra between S1-BF and Hipp CA1 following twitches during active sleep (restricted to the 500-ms period after twitch onset; blue), active wake (AW; orange), and behavioral quiescence (BQ; green).
Independent of twitching, the state of active sleep facilitates the emergence and expression of early functional connectivity in the sensorimotor system. Using P11-13 rats, we addressed this question by characterizing state-dependent oscillatory coupling between the red nucleus and the hippocampus[9]. When continuous theta oscillations in the red nucleus emerged around P11, they were coherent (i.e., synchronous) and co-modulated (i.e., their amplitudes vary in lockstep) with theta oscillations in the hippocampus. Crucially, synchronization of theta rhythms between the hippocampus and red nucleus occurred almost exclusively during periods of active sleep; twitching enhanced the amplitude of these oscillations. Whereas pharmacological inactivation of the medial septum abolished sleep-dependent theta activity in both structures, twitch-dependent theta was still preserved, suggesting that these two forms of theta arise from two independent pathways. Altogether, these results indicate that active sleep is necessary for the expression of functional connectivity upon the emergence of continuous oscillatory activity in networks involving both forebrain and brainstem structures.
5. State-dependent functional connectivity and the origins of neurodevelopmental disorders
Aberrant network connectivity can be detected very early in development and appears to presage cognitive and sensorimotor impairments[57]. In humans, atypical connectivity patterns are present in individuals diagnosed with ADHD [58], autism [59], and schizophrenia[60].
Importantly, connectivity and neural synchrony in healthy infants and adults varies across the sleep-wake cycle[9,61-63]. Unfortunately, the majority of studies assessing early functional connectivity in neurodevelopmental disorders have not examined state-dependent network interactions. When behavioral state is taken into account, however, it becomes clear that sleep affects the expression of synchronized oscillatory activity in young and adult individuals diagnosed with autism[64,65]. Thus, analyses of state-dependent connectivity may provide novel insights into the etiology and mechanisms underlying a variety of neurocognitive symptoms, and could be used as an early marker for atypical development[66].
6. A causal role of active-sleep disruption in atypical development
Early perturbations to the sensorimotor system, specifically during sensitive periods, can trigger irreversible developmental consequences[45-47]. Given the role of active sleep in brain development, early active-sleep disruption or deprivation can be one such perturbation. Consistent with this idea, sleep deprivation affects synaptic plasticity in such sensorimotor structures as motor cortex[67], hippocampus[68], and cerebellum[69]. In addition, because sensory feedback from twitching is a major source of stimulation to the neonatal brain, active sleep restriction or deprivation can be also conceptualized as a form of sensory deprivation during critical periods for brain development. If active sleep indeed contributes to the early expression of coupled oscillatory activity between distant sensorimotor structures, active-sleep restriction may underlie the sensorimotor deficits present in a variety of neurodevelopmental disorders. Indeed, decreased active sleep is a prevalent symptom in neurodevelopmental disorders characterized by sensorimotor deficits, including autism, Rett Syndrome, Fragile X Syndrome, Angelman Syndrome, Williams Syndrome, and Down Syndrome[70].
7. Conclusions and future directions
Synchronized neural oscillations are a hallmark of coordinated activity in developing and adult cortical and subcortical networks[1,3,6-9,19,52]. We have reviewed evidence here that one of the functions of active sleep in early infancy is to facilitate the expression of neuronal oscillations and oscillatory coupling in the sensorimotor system. Specifically, the evidence thus far indicates that sensory feedback from sleep-related twitches drives precisely timed oscillatory activity across the sensorimotor system, thereby contributing to that system’s activity-dependent development.
Until now, the importance of active sleep in development and plasticity has been supported by showing the negative impact of sleep deprivation[71,72]. The methods used to deprive animals of sleep, however, often entail nonspecific side-effects that make it difficult to identify the specific contributions of sleep to developmental outcomes. Also, the motor centers that generate twitching in early development also support wake-related movements [26]; because methods that disable these motor structures affect all motor activity, they cannot distinguish the relative contributions of twitching to brain development. Recent methodological advances with optogenetics, however, now enable the rapid expression of opsins during the first two postnatal weeks, which allows direct manipulation of neonatal networks with high temporal precision and cell specificity[73,74]. In addition, as recently demonstrated in adult mice[75], optogenetics can be used to selectively manipulate active-sleep-dependent oscillations and assess their specific roles in behavior and cognition. Accordingly, it may soon be possible to selectively enhance or block sensory input and motor output to evaluate the role of twitch-related reafference in the activity-dependent development of sensorimotor structures.
Acknowledgments
We thank Greta Sokoloff and Jimmy Dooley for helpful suggestions. This research was supported by National Institute of Child Health and Human Development Grant R37-HD081168 to M. S. Blumberg. C. Del Rio-Bermudez was supported by the Fulbright Foreign Student Program.
REFERENCES
- [1].Buzsáki G, Draguhn A, Science 2004, 304, 1926. [DOI] [PubMed] [Google Scholar]
- [2].Buzsáki G, Rhythms of the Brain, Oxford University Press, 2006. [Google Scholar]
- [3].Khazipov R, Luhmann HJ, Trends Neurosci. 2006, 29, 414. [DOI] [PubMed] [Google Scholar]
- [4].Yang J-W, Hanganu-Opatz IL, Sun J-J, Luhmann HJ,J. Neurosci. 2009, 29, 9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vanhatalo S, Kaila K, Semin. Fetal Neonatal Med. 2006, 11, 471. [DOI] [PubMed] [Google Scholar]
- [6].Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W, Trends Cogn. Sci. 2010, 14, 72. [DOI] [PubMed] [Google Scholar]
- [7].Brockmann MD, Poschel B, Cichon N, Hanganu-Opatz IL, Neuron 2011, 71, 332. [DOI] [PubMed] [Google Scholar]
- [8].Bitzenhofer SH, Ahlbeck J, Wolff A, Wiegert JS, Gee CE, Oertner TG, Hanganu-Opatz IL, Nat. Commun. 2017, 8, 14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Del Rio-Bemudez C, Kim J, Sokoloff G, Blumberg MS, Curr. Biol. 2017, 27, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luhmann HJ, Khazipov R, Neuroscience 2017, DOI 10.1016/j.neuroscience.2017.05.025. [Google Scholar]
- [11].Mohns EJ, Blumberg MS, J. Neurosci. 2008, 28, 10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tiriac A, Del Rio-Bermudez C, Blumberg MS, Curr. Biol. 2014, 24, 2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G, Nature 2004, 432, 758. [DOI] [PubMed] [Google Scholar]
- [14].Inácio AR, Nasretdinov A, Lebedeva J, Khazipov R, Nat. Commun. 2016, 7, 13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsáki G, Science 2002, 296, 2049. [DOI] [PubMed] [Google Scholar]
- [16].An S, Kilb W, Luhmann HJ, J. Neurosci. 2014, 34, 10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Buhl DL, Buzsáki G, Neuroscience 2005, 134, 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Blanquie O, Kilb W, Sinning A, Luhmann HJ, Neuroscience 2017, DOI 10.1016/j.neuroscience.2017.06.030. [DOI] [PubMed] [Google Scholar]
- [19].Hanganu-Opatz IL, Brain Res. Rev. 2010, 64, 160. [DOI] [PubMed] [Google Scholar]
- [20].McVea DA, Murphy TH, Mohajerani MH, Front. Neural Circuits 2016, 10, DOI 10.3389/fncir.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bitzenhofer SH, Ahlbeck J, Wolff A, Wiegert JS, Gee CE, Oertner TG, Hanganu-Opatz IL, Nat. Commun. 2017, 8, 14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Akhmetshina D, Nasretdinov A, Zakharov A, Valeeva G, Khazipov R,J. Neurosci 2016, 36, 9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roffwarg HP, Muzio JN, Dement WC, Science. 1966, 152, 604. [DOI] [PubMed] [Google Scholar]
- [24].Jouvet-Mounier D, Astic L, Lacote D, Dev. Psychobiol. 1970, 2, 216. [DOI] [PubMed] [Google Scholar]
- [25].Blumberg MS, Seelke AMH, Oxford Univ. Press; 2010, 391. [Google Scholar]
- [26].Del Rio-Bermudez C, Sokoloff G, Blumberg MS, J. Neurosci. 2015, 35, 8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gassel MM, Marchiafava PL, Pompeiano O, Nature 1966, 209, 1218. [DOI] [PubMed] [Google Scholar]
- [28].Blumberg MS, Marques HG, Iida F, Curr. Biol. 2013, 23, R532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blumberg MS, Curr. Dir. Psychol. Sci. 2015, 24, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kilb W, Kirischuk S, Luhmann HJ, Eur. J. Neurosci. 2011, 34, 1677. [DOI] [PubMed] [Google Scholar]
- [31].Vanhatalo S, Kaila K, Semin. Fetal Neonatal Med. 2006, 11, 471. [DOI] [PubMed] [Google Scholar]
- [32].Lahtinen H, Palva JM, Sumanen S, Voipio J, Kaila K, Taira T,J. Neurophysiol 2002, 88, 1469. [DOI] [PubMed] [Google Scholar]
- [33].Yang J-W, Reyes-Puerta V, Kilb W, Luhmann HJ, Neural Plast. 2016, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lebedeva J, Zakharov A, Ogievetsky E, Minlebaeva A, Kurbanov R, Gerasimova E, Sitdikova G, Khazipov R, Cereb. Cortex 2015, 1. [DOI] [PubMed] [Google Scholar]
- [35].Blanquie O, Yang J-W, Kilb W, Sharopov S, Sinning A, Luhmann HJ, Elife 2017, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Suchkov D, Sharipzyanova L, Minlebaev M, Front. Cell. Neurosci. 2018, 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Danglot L, Triller A, Marty S, Hippocampus 2006, 16, 1032. [DOI] [PubMed] [Google Scholar]
- [38].Bender R, Plaschke M, Naumann T, Wahle P, Frotscher M, J. Comp. Neurol. 1996, 372, 204. [DOI] [PubMed] [Google Scholar]
- [39].Meier P, Finch A, Evan G, Nature 2000, 407, 796. [DOI] [PubMed] [Google Scholar]
- [40].Benders MJ, Palmu K, Menache C, Borradori-Tolsa C, Lazeyras F, Sizonenko S, Dubois J, Vanhatalo S, Hüppi PS, Cereb. Cortex 2015, 25, 3014. [DOI] [PubMed] [Google Scholar]
- [41].Wikstro S, Iyer KK, Roberts JA, Hellstro L, Pupp IH, Ley D, Vanhatalo S, Breakspear M, Brain 2015, 2206. [DOI] [PubMed] [Google Scholar]
- [42].Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E, Nat. Rev. Neurosci. 2008, 9, 601. [DOI] [PubMed] [Google Scholar]
- [43].Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS, Curr. Biol. 2012, 22, 2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Erzurumlu RS, Gaspar P, Eur. J. Neurosci. 2012, 35, 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fox K,J. Neurosci 1992, 12, 1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Simons DJ, Land PW, Nature 1987, 326, 694. [DOI] [PubMed] [Google Scholar]
- [47].Crocker-Buque A, Brown SM, Kind PC, Isaac JTR, Daw MI, Cereb. Cortex 2015, 25, 2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tiriac A, Blumberg MS, Elife 2016, 5, DOI 10.7554/eLife.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McVea DA, Murphy TH, Mohajerani MH, Front. Neural Circuits 2016, 10, DOI 10.3389/fncir.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Blanquie O, Yang J-W, Kilb W, Sharopov S, Sinning A, Luhmann HJ, Elife 2017, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Blumberg MS, Sokoloff G, Tiriac A, Del Rio-Bermudez C, Dev. Psychobiol. 2015, 57, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Karalis N, Dejean C, Chaudun F, Khoder S, Rozeske RR, Wurtz H, Bagur S, Benchenane K, Sirota A, Courtin J, Herry C, Nat. Neurosci. 2016, 19, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Petersson P, Waldenström A, Fåhraeus C, Schouenborg J, Nature 2003, 424, 72. [DOI] [PubMed] [Google Scholar]
- [54].Blumberg MS, Coleman CM, Sokoloff G, Weiner JA, Fritszch B, McMurray B, Curr. Biol. 2015, 25, 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mohns EJ, Blumberg MS,J. Neurosci 2010, 30, 3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Del Rio-Bermudez C, Kim J, Sokoloff G, Blumberg MS, in Neurosci. Meet. Plan., Society For Neuroscience, Washington, DC, 2017. [Google Scholar]
- [57].Hartung H, Cichon N, De Feo V, Riemann S, Schildt S, Lindemann C, Mulert C, Gogos JA, Hanganu-Opatz IL, Cereb. Cortex 2016, 26, 4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP, J. Neurosci. Methods 2008, 169, 249. [DOI] [PubMed] [Google Scholar]
- [59].Kennedy DP, Redcay E, Courchesne E, Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Whitfield-Gabrieli AS, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, Mccarley RW, Shenton ME, Green AI, Nieto A, Laviolette P, Wojcik J, Gabrieli JDE, Seidman LJ, Raichle E, Thermenosbcd HW, Milanovicbce S, Tsuangdf MT, Whitfield-gabrieli S, Mccarleybh RW, Shentonhl ME, Nieto-castanona A, Laviolettes P, Proc. Natl. Acad. Sci. U. S. A 2013, 106, 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Watanabe T, Kan S, Koike T, Misaki M, Konishi S, Miyauchi S, Miyahsita Y, Masuda N, Neuroimage 2014, 98, 1. [DOI] [PubMed] [Google Scholar]
- [62].Montgomery SM, Sirota A, Buzsáki G, J. Neurosci 2008, 28, 6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Vijayan S, Lepage KQ, Kopell NJ, Cash SS, Elife 2017, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Leveille C, Barbeau EB, Bolduc C, Limoges E, Berthiaume C, Chevrier E, Mottron L, Godbout R, Autism Res. 2010, 3, 280. [DOI] [PubMed] [Google Scholar]
- [65].Daoust AM, Limoges É, Bolduc C, Mottron L, Godbout R, Clin. Neurophysiol. 2004, 115, 1368. [DOI] [PubMed] [Google Scholar]
- [66].S. Kurth, B. A. Riedner, D. C. Dean, J. O. Muircheartaigh, R. Huber, O. G. Jenni, S. C. L. Deoni, M. K. Lebourgeois, n.d., 10.
- [67].Li W, Ma L, Yang G, Gan W-B, Nat. Neurosci. 2017, 20, DOI 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Abel T, Havekes R, Saletin JM, Walker MP, Curr. Biol. 2013, 23, DOI 10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sei H, Saitoh D, Yamamoto K, Morita K, Morita Y, Brain Res. 2000, 877, 387. [DOI] [PubMed] [Google Scholar]
- [70].Picchioni D, Reith R, Nadel J, Smith C, Brain Sci. 2014, 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Frank MG, Prog. Brain Res. 2011, 193, 221. [DOI] [PubMed] [Google Scholar]
- [72].Kayser MS, Biron D, Genetics 2016, 203, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bitzenhofer SH, Ahlbeck J, Hanganu-Opatz IL, Front. Cell. Neurosci. 2017, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Murata Y, Colonnese MT, Elife 2016, 5, DOI 10.7554/eLife.18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Boyce R, Glasgow SD, Williams S, Adamantidis AR, Science. 2016, 352, 812. [DOI] [PubMed] [Google Scholar]
- [76].Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G, Nature 2004, 432, 758. [DOI] [PubMed] [Google Scholar]
- [77].Akhmetshina D, Nasretdinov A, Zakharov A, Valeeva G, Khazipov R, J. Neurosci 2016, 36, 9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].McVea DA, Mohajerani MH, Murphy TH, J. Neurosci 2012, 32, 10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R, Cereb. Cortex 2007, 17, 1582. [DOI] [PubMed] [Google Scholar]
- [80].An S, Kilb W, Luhmann HJ, J. Neurosci 2014, 34, 10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sokoloff G, Plumeau AM, Mukherjee D, Blumberg MS, J. Neurophysiol 2015, 114, 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sokoloff G, Uitermarkt BD, Blumberg MS, Dev. Neurobiol. 2015, 75, 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Del Rio-Bermudez C, Plumeau AM, Sattler NJ, Sokoloff G, Blumberg MS, J. Neurophysiol 2016, jn. 004612016. [DOI] [PMC free article] [PubMed] [Google Scholar]


