Abstract
Background:
Patients positive for hepatitis B core antibody (anti-HBc) may experience hepatitis B reactivation during cancer chemotherapy. Intravenous immunoglobulin (IVIG) used in supportive care can produce passive transfer of anti-HBc. We estimated the probability of passive transfer of anti-HBc after IVIG infusion in cancer patients.
Methods:
Institutional databases were reviewed to identify adult patients who received outpatient chemotherapy during 1/1/2004 – 12/31/2011 at MD Anderson Cancer Center. We estimated the proportion with anti-HBc passive transfer and identified correlates using Wilcoxon rank-sum test, Fisher’s exact test, and repeated-measures logistic regression.
Findings:
Of 18,874 chemotherapy patients, 950 received IVIG, and 870 had pre-IVIG anti-HBc testing. One hundred ninety-nine patients who were anti-HBc-negative before receiving IVIG were retested after receiving IVIG; 29 (15%, 95% CI, 10%−20%) became anti-HBc-positive. Predicted probabilities of anti-HBc positivity one and 12 weeks after IVIG were 34% (95% CI, 22%−48%) and 4% (95% CI, 2%−7%), respectively. Of the 29 anti-HBc-positive patients, none became hepatitis B surface antigen positive, and none of 17 tested for HBV DNA had detectable HBV DNA.
Interpretation:
Conversion from anti-HBc-negative to anti-HBc-positive status was common after IVIG administration. Probability of positive tests decreased with time since IVIG administration. Testing for anti-HBc shortly after IVIG administration may identify passive transfer; results of such testing should be interpreted with caution.
Introduction
Intravenous immunoglobulin (IVIG) is used to address antibody deficiencies in patients with hematologic malignancies, autoimmune conditions, or infectious diseases.1–5 IVIG therapy results in passive transfer of antibodies, which may include hepatitis B core antibody (anti-HBc).6–8 Patients with cancer anticipated to undergo therapies associated with a high risk of reactivation of hepatitis B virus (HBV) replication, such as anti-CD20 monoclonal antibody therapy or stem cell transplant (SCT),9 are routinely screened for HBV with tests for hepatitis B surface antigen (HBsAg), and anti-HBc (past HBV infection HBsAg+/anti-HBc- or chronic HBV infection HBsAg+/anti-HBc+). Patients with hematologic malignancies who are HBsAg-negative but anti-HBc-positive are recommended to receive anti-HBV prophylaxis before chemotherapies associated with high HBV reactivation risk. A positive anti-HBc test result due to passive transfer rather than chronic or past infection is a false positive test result and could lead to inappropriate use of anti-HBV prophylaxis.7,10 In this study, we aimed to estimate the false-positive rate and timing of anti-HBc passive transfer in patients with cancer receiving IVIG therapy.
Methods
Study design and participants
After receiving Institutional Review Board approval, we conducted a retrospective chart review of institutional databases to identify adult cancer outpatients who received chemotherapy during 1/1/2004 – 12/31/2011 at The University of Texas MD Anderson Cancer Center; received IVIG therapy; were anti-HBc-negative and HBsAg-negative before IVIG infusion; and had anti-HBc testing after IVIG infusion (Figure 1).
Figure 1.
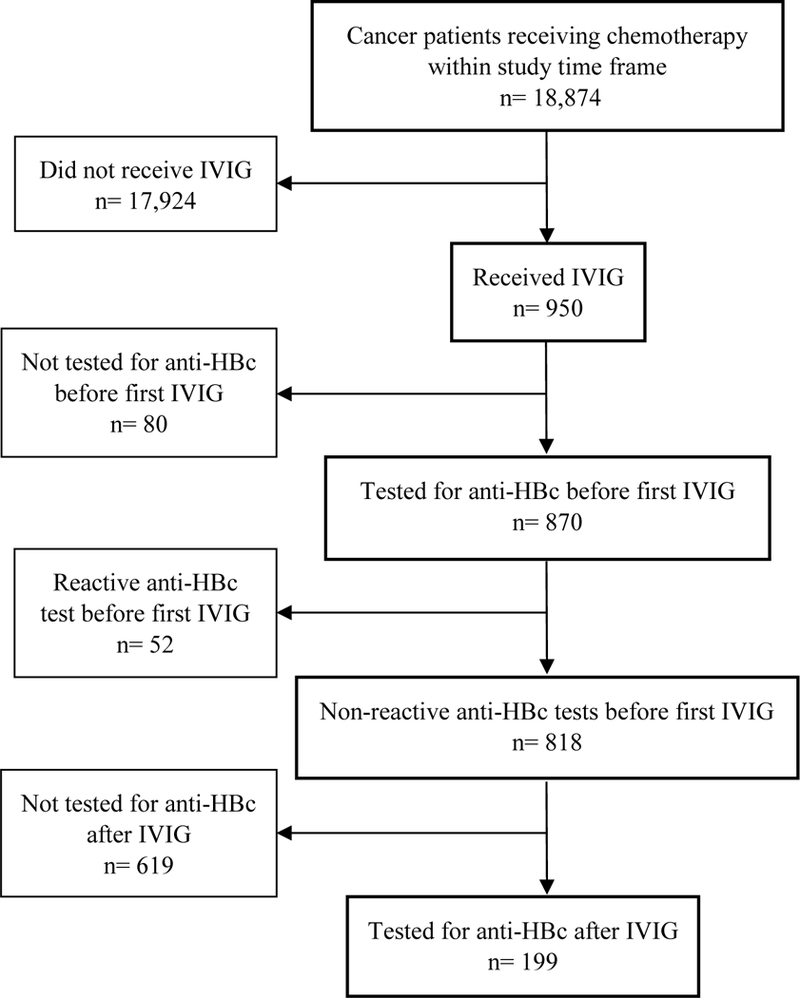
Diagram of analytic sample selection from all patients receiving chemotherapy from 2004–2011
Procedures and Outcomes
The baseline anti-HBc test was defined as the most recent test before initiation of IVIG therapy. We reviewed patient records for demographic and clinical variables (listed in the next paragraph) and information about anti-HBc positivity after IVIG infusion, subsequent HBV DNA testing, and hepatitis flare (alanine aminotransferase level ≥100 U/L and ≥3 times baseline) after anticancer therapy.
Statistical analysis
The percentage of patients who became anti-HBc-positive after IVIG administration was reported with a corresponding exact binomial confidence interval. We used Fisher’s exact test and the Wilcoxon rank-sum test to examine associations between anti-HBc passive transfer and demographic and clinical variables, including age at baseline anti-HBc testing, sex, race/ethnicity, cancer type, receipt of rituximab or SCT, hepatitis flare, year of first IVIG administration, and days between IVIG infusion and subsequent anti-HBc test. Some patients had multiple IVIG administrations and multiple corresponding anti-HBc tests, resulting in multiple observations per individual. To account for within-subject correlations, we used generalized estimating equations (GEE) to fit a repeated-measures logistic regression model (PROC GENMOD, SAS v. 9·4, SAS Institute Inc., Cary, NC) with a binomial distribution and logit link function to evaluate the odds of a positive anti-HBc test based on the log-transformed number of days between IVIG receipt and testing. We used the quasi-likelihood under the independence model criterion (QIC) to choose the best correlation structure and found that an independent working correlation structure best fit the data.11 The repeated measures multiple logistic regression analysis considered time from IVIG administration, cancer type, rituximab therapy, SCT, and patient age, race, and sex as potential independent variables. A backward stepwise selection process was applied to identify significant variables in the multivariate setting. All statistical tests were 2-sided with α=·05. As a sensitivity analysis, modeling was repeated while excluding the 63 participants with baseline testing conducted more than three months before their first IVIG administration. Analyses were conducted using SAS for Windows version 9·4 (Cary, NC).
Results and Discussion
Sample characteristics
Of 18,874 patients undergoing chemotherapy during 2004–2011, 950 received IVIG. Of these, 870 had anti-HBc testing before IVIG administration and 52 had a reactive test. Of the 818 with nonreactive baseline anti-HBc tests, 619 did not have record of an anti-HBc test after receipt of IVIG. The remaining 199 patients were anti-HBc-negative and HBsAg-negative before IVIG infusion and were tested for anti-HBc after IVIG infusion; these patients constituted the study sample. The median interval between the baseline anti-HBc and IVIG infusion for the study sample was 26 days (range, one day to 4·8 years). Post-IVIG HBsAg test results were available for all but two patients in the study sample, and all test results were negative. The study sample baseline characteristics are summarized in Table 1. Median patient age at baseline anti-HBc testing was 51 years (range, 20–79 years). Sixty-five percent (n=130) of the patients were non-Hispanic white, 94% had hematologic malignancies (n=188; 68/188 had Non-Hodgkin’s lymphoma and 66/188 with acute leukemia), 42% (n=83) were treated with rituximab, and 77% (n=153) underwent SCT. No patient received anti-HBV therapy as prophylaxis or treatment.
Table 1.
Baseline demographic and clinical characteristics of patients with and without conversion from anti-HBc- to anti-HBc+
| Characteristic | Conversion (n = 29) |
No Conversion (n = 170) |
Total (n = 199) |
p |
|||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Age at baseline anti-HBc test, median (range) | 52 (20–73) | 51 (20–79) | 51 (20–79) | 0.99* | |||
| Race/ethnicity, n (%) | |||||||
| White | 19 | (66) | 111 | (65) | 130 | (65) | 0.90 |
| Hispanic | 6 | (21) | 32 | (19) | 38 | (19) | |
| Black | 4 | (13) | 18 | (11) | 22 | (11) | |
| Asian | 0 | (0) | 3 | (2) | 3 | (2) | |
| Other | 0 | (0) | 6 | (4) | 6 | (3) | |
| Sex, n (%) | |||||||
| Female | 9 | (31) | 65 | (38) | 74 | (37) | 0.54 |
| Male | 20 | (69) | 105 | (62) | 125 | (63) | |
| Cancer type, n (%) | |||||||
| Solid tumor (not HCC) | 1 | (3) | 10 | (6) | 11 | (6) | 1.00 |
| Hematologic malignancy | 28 | (97) | 160 | (94) | 188 | (94) | |
| Rituximab, n (%) | |||||||
| No | 15 | (52) | 101 | (59) | 116 | (58) | 0.54 |
| Yes | 14 | (48) | 69 | (41) | 83 | (42) | |
| SCT, n (%) | |||||||
| Allogeneic | 16 | (55) | 95 | (56) | 111 | (56) | 0.77 |
| Autologous | 5 | (17) | 37 | (22) | 42 | (21) | |
| No SCT | 8 | (28) | 38 | (22) | 46 | (23) | |
NOTE: HBV: hepatitis B virus. SCT: stem cell transplant. IVIG: intravenous immunoglobulin. HCC: hepatocellular cancer. anti-HBc: hepatitis B core antibody.
P-values are from Fisher’s exact test, with the exception of the test for the association between anti-HBc status and age, which was from a Wilcoxon rank sum test.
Conversion from anti-HBc-negative to anti-HBc-positive
Patients received a median of four IVIG doses (range, 1–53) between baseline and first reactive anti-HBc test or last test among those who remained anti-HBc-negative. Twenty-nine of the 199 patients (15%; 95% CI, 10%−20·0%) converted from anti-HBc-negative at baseline to anti-HBc-positive at some point after IVIG. The interval between IVIG receipt and testing ranged from one to 60 days, with an additional patient retested on day 366. Seventeen of 29 (59%) had HBV DNA tested after the first anti-HBc-positive test result, and all had undetectable HBV DNA. All 29 had HBsAg tests performed after the first anti-HBc-positive test result, and all tests were negative. The rate of hepatitis flare after anticancer therapy did not differ between patients who converted to anti-HBc positivity (62%, n=18/29) and those who did not (68%, n=115/170), p=0·67. After the last positive anti-HBc test, ten of 29 patients were nonreactive on a subsequent anti-HBc test, and 19 were not retested for anti-HBc.
Associations with conversion from anti-HBc-negative to anti-HBc-positive
Median year of first IVIG administration did not differ significantly between patients with and without passive transfer. No significant associations were observed between passive transfer and age, race/ethnicity, sex, cancer type, rituximab use, or SCT. All 361 available anti-HBc tests that were taken after the first IVIG administration were included in the repeated-measures analysis. Patients had one to nine (median, 1) post-baseline anti-HBc tests. Thirty-four tests were reactive, and 327 were nonreactive. Eighty-eight percent (30/34) of reactive tests versus 12% (39/327) of nonreactive tests were performed within four weeks after IVIG administration. During the first week after IVIG administration, 35 patients were tested for anti-HBc, and 17 (49%) tested positive. (One patient was tested twice and found positive both times.)
In multivariable analysis, time between IVIG administration and anti-HBc testing was the only independent variable that was significantly associated with anti-HBc positivity. Table 2 displays the parameter estimates based on this single-effect model. The predicted probability of conversion to anti-HBc positivity was higher with a shorter interval between IVIG administration and anti-HBc testing (Appendix Table S1). The predicted probability of an anti-HBc-positive result was 34% (95% CI, 22%−48%) within seven days after IVIG administration and decreased to 12% (95% CI, 7%−18%) at one month and 4% (95% CI, 2%−7%) at three months (Figure 2). Results were similar in a sensitivity analysis that excluded records for 63 participants who had baseline testing conducted more than three months before they received their first IVIG.
Table 2.
Parameter estimates of repeated logistic regression analysis (GEE) to predict conversion from anti-HBc- to anti-HBc+ based on the natural log of the time interval from IVIG administration to anti-HBc testing
| Parameter | Estimate | Standard Error |
95% Confidence Limits | Z | Pr > |Z| | |
|---|---|---|---|---|---|---|
| Intercept | 1.2286 | 0.4715 | 0.3044 | 2.1529 | 2.61 | 0.0092 |
| Ln (days from IVIG to anti-HBc test) | −0.9774 | 0.1264 | −1.2251 | −0.7296 | −7.73 | <.0001 |
Figure 2.
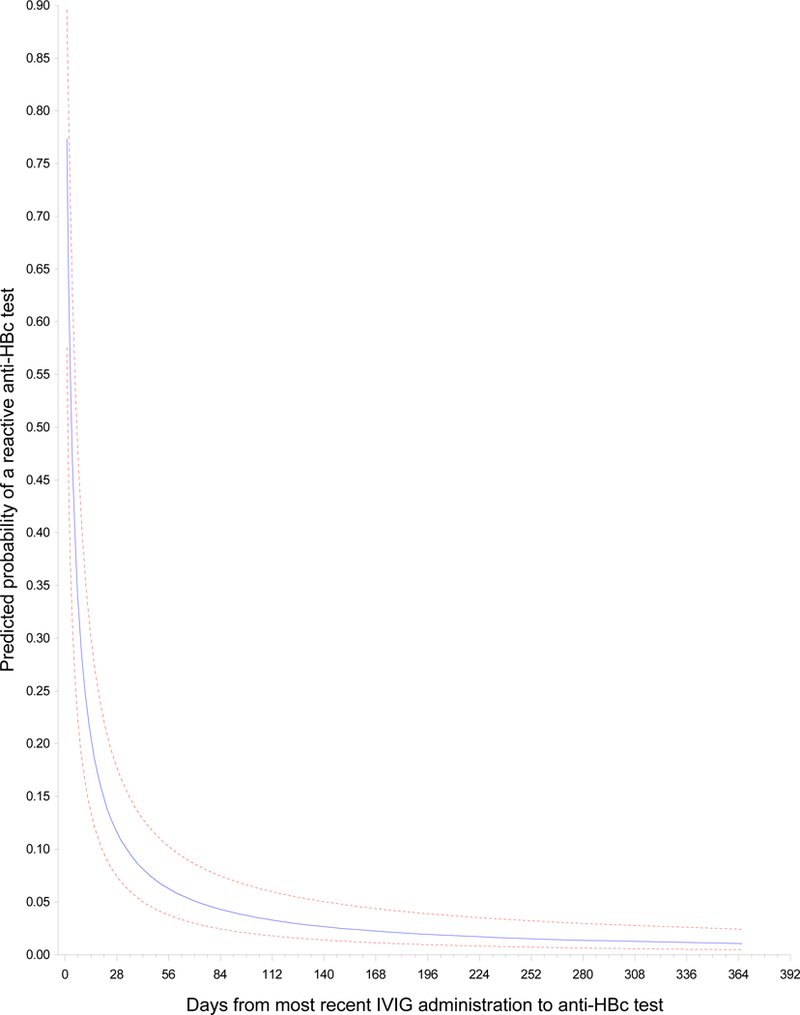
Predicted probability (95% confidence bands) of passive transfer of anti-HBc By days between IVIG infusion and anti-HBc test
Discussion
In this study, we found that conversion from anti-HBc-negative to anti-HBc-positive status after IVIG infusion was common. We also found that the predicted probability of anti-HBc positivity was 34% (95% CI, 22%−48%) for tests performed within the first week after IVIG administration but only 4% (95% CI, 2%−7%) for tests performed at three months. This finding, together with the observed loss of anti-HBc over time in patients with follow-up testing and the absence of conversion to HBsAg positivity/HBV DNA positivity in the small subset evaluated for HBV infection, supports our notion that conversion to anti-HBc positivity after IVIG administration is a result of passive transfer.
We observed an overall conversion rate of 15% (95% CI, 10%−20·0%, n=29/199). A previous cross-sectional study reported a high prevalence (46%) of anti-HBc positivity after IVIG administration6 but did not include the pre-IVIG anti-HBc test results required to differentiate passive transfer from pre-existing presence of anti-HBc. Our study did not detect differences in the number of anti-HBc tests or cumulative doses of IVIG received between those who did and those who did not have passive transfer of anti-HBc.
Our results have several clinical implications. First, anti-HBc testing should be performed before any IVIG administration to ascertain whether false-positive results have occurred due to passive transfer. Second, given the expected half-life of IVIG of approximately three to four weeks,8 if anti-HBc testing is performed soon after IVIG infusion and results are positive, we recommend retesting at least three to four months after IVIG in patients with unknown pre-IVIG HBV status, to determine whether the result is secondary to passive transfer. If immediate clarification is needed, e.g., in a patient requiring initiation of anti-cancer therapy or experiencing liver enzyme elevation, additional testing for HBsAg and HBV DNA and evaluation by hepatitis specialists should be considered. Since hepatitis B surface antibody in IVIG may neutralize HBsAg, making testing for HBsAg less reliable, HBV DNA testing for confirmation of acute HBV infection in patients previously anti-HBc-negative who become anti-HBc-positive after IVIG is warranted.
This study has limitations that primarily relate to the retrospective study design and the absence of a standardized protocol for anti-HBc testing in patients receiving IVIG supportive care. Our analysis included patients who underwent baseline anti-HBc testing at varying times, some well before IVIG administration; however, our results did not change when we included only those patients who had baseline anti-HBc testing within three months before receiving their first IVIG infusion. The observed rate of conversion to anti-HBc-positive status, 15%, is likely an underestimate owing to the limited retesting after IVIG infusion and the range of times from IVIG infusion to retesting. On the other hand, in the absence of a standard protocol, anti-HBc retesting after an IVIG infusion was based on provider discretion, potentially introducing a source of bias that could increase the estimated conversion rate if patients who were retested had a greater conversion risk compared to those who were not retested. Another limitation is that not all patients who became anti-HBc positive had HBV DNA or liver chemistry testing to rule out the possibility of an actual infection. Finally, our institution does not routinely test for anti-HBc in IVIG products; thus, we cannot verify that the source of anti-HBc was the infused IVIG.
In conclusion, our results suggest that passive transfer of anti-HBc is common after IVIG infusion, a finding with important implications for cancer patients who receive IVIG and may require immunosuppressive therapy. Therefore, anti-HBc testing should be considered prior to each IVIG administration in such patients.
Research in context
Evidence before this study
The review of the literature was conducted by a medical librarian using Ovid MEDLINE, PubMed and EMBASE through June 2018. Cited reference searching was completed in Scopus for highly relevant studies. There were no date or language restrictions on the search. The earliest start date was 1946, the year in which Ovid MEDLINE was initiated. Search terms included MeSH subject headings as well as keywords to augment the search which focused on hepatitis B and intravenous immunoglobulin.
Added value of this study
Patients with cancer who receive intravenous immunoglobulin (IVIG) during anti-cancer care are at risk for passive transfer of antibodies, including hepatitis B core antibody. These patients may also be at risk for reactivation of hepatitis B infection due to highly immunosuppressive therapies such anti-CD20 agents and stem cell transplant. The rate and risk factors for passive transfer among patients with cancer receiving IVIG is unknown. In this paper, we determined that the hepatitis B core antibody conversion was common after IVIG administration, at a rate of 15% overall, and highest in the first week after IVIG administration (49%, n=17/35), decreasing rapidly after one month.
Implications of all the available evidence
We found that cancer patients who receive intravenous immunoglobulin (IVIG) may be at risk for passive transfer of hepatitis B core antibody (anti-HBc). Our study supports that anti-HBc testing should be done before IVIG infusion to avoid false-positive results due to passive transfer. Hepatitis B testing with hepatitis B surface antigen (HBsAg) and anti-HBc should be performed in all patients before IVIG is initiated to identify the viral status of patients receiving anti-cancer therapies and clarify the risk of hepatitis B reactivation. In patients known to have hypogammaglobulinemia and prior hepatitis B infection, anti-HBc may be negative. HBV DNA testing may be considered; however, HBV DNA would be undetectable in the serum of most patients with past hepatitis B infection.
Supplementary Material
Acknowledgements
Sources of funding This study was supported by the National Cancer Institute under grants K07CA132955 (Hwang) and R21CA167202 (Hwang). Dr. Suarez-Almazor was a recipient of a K24 career award from the National Institute for Musculoskeletal and Skin Disorders (K24 AR053593) during the study. The University of Texas MD Anderson Cancer Center is supported by the National Cancer Institute under award number P30CA016672.
We are thankful for the assistance from the following individuals at the University of Texas MD Anderson Cancer Center: Andrea Barbo for data collection, Laurissa Gann for literature review, and Stephanie Deming for editing.
Funding: This study was supported by The National Cancer Institute grants K07CA132955 (Hwang) and R21CA167202 (Hwang). The National Institute for Musculoskeletal and Skin Disorders career award, K24 AR053593 (Suarez-Almazor). The University of Texas MD Anderson Cancer Center is supported by the National Cancer Institute award P30CA016672.
Prior Presentation: Abstract presented at the annual meeting of the American Association for the Study of Liver Diseases, Washington, DC, October 23, 2017
Role of the funding source
The funding sources had no role in this study. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Footnotes
Declaration of interests The authors report the following potential conflicts of interest: Hwang: Gilead, Merck; Lok: Bristol-Myers Squibb, Gilead; Suarez-Almazor: Pfizer, Endo Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly; Torres: Gilead, Merck. All other authors report no conflicts of interest.
Full professors
References
- 1.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. The Cochrane database of systematic reviews. 2008(4):Cd006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueda M, Berger M, Gale RP, Lazarus HM. Immunoglobulin therapy in hematologic neoplasms and after hematopoietic cell transplantation. Blood Rev. 2017. [DOI] [PubMed] [Google Scholar]
- 3.Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT. Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2012;78(13):1009–1015. [DOI] [PubMed] [Google Scholar]
- 4.Shehata N, Palda V, Bowen T, et al. The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfus. Med. Rev 2010;24 Suppl 1:S28–50. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed AR, Dahl MV. Consensus statement on the use of intravenous immunoglobulin therapy in the treatment of autoimmune mucocutaneous blistering diseases. Arch. Dermatol. 2003;139(8):1051–1059. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay I, Gorton RL, Patel M, et al. Transmission of Hepatitis B Core Antibody and Galactomannan Enzyme Immunoassay Positivity via Immunoglobulin Products: A Comprehensive Analysis. Clin. Infect. Dis 2016;63(1):57–63. [DOI] [PubMed] [Google Scholar]
- 7.Benwell N, Boan P, Raby E, McGettigan B. False positive hepatitis B virus core and surface antibodies due to intravenous immunoglobulin. Intern. Med. J. 2017;47(1):119–120. [DOI] [PubMed] [Google Scholar]
- 8.Thibault V, Pinte L, Vergez J, Leger JM, Liou A. Too Often Forgotten: Passive Transfer of Antibodies. Clin. Infect. Dis. 2016;63(5):709–710. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology provisional clinical opinion update. J. Clin. Oncol 2015;33(19):2212–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold DM, Crowther MA, Meyer RM, et al. Misleading hepatitis B test results due to intravenous immunoglobulin administration: implications for a clinical trial of rituximab in immune thrombocytopenia. Transfusion (Paris). 2010;50(12):2577–2581. [DOI] [PubMed] [Google Scholar]
- 11.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


