Abstract
The aim of this work was to explore the relationship between intracranial pressure (ICP)-derived indices of cerebrovascular reactivity and the lower limit of autoregulation (LLA) during arterial hypotension. We retrospectively reviewed recorded physiological data from piglets that underwent controlled hypotension. Hypotension was induced by inflation of a balloon catheter in the inferior vena cava. ICP, cortical laser Doppler flowmetry (LDF), and arterial blood pressure (ABP) monitoring was conducted. ICP-derived indices were calculated: pressure reactivity index (PRx; correlation between ICP and mean arterial pressure [MAP]); pulse amplitude index (PAx; correlation between pulse amplitude of ICP [AMP] and MAP); and RAC (correlation between AMP and cerebral perfusion pressure [CPP]). LLA was estimated by piece-wise linear regression of CPP versus LDF. We produced error bar plots for PRx, PAx, and RAC against 5-mm Hg bins of CPP, displaying the relationship with the LLA. We compared CPP values at clinically relevant thresholds of PRx, PAx, and RAC to CPP measured at the LLA. Receiver operating curve (ROC) analysis was performed for each index across the LLA using 5-mm Hg bins for CPP. Mean LLA was 36.2 ± 10.5 mm Hg. Error bar plots demonstrated that PRx, PAx, and RAC increased, with CPP decreasing below the LLA. CPP at clinically relevant thresholds for PRx, PAx, and RAC displayed weak associations with the LLA, indicating that thresholds defined in TBI may not apply to a model of arterial hypotension. ROC analysis indicated that PRx, PAx, and RAC predicted the LLA, with AUCs of: 0.806 (95% confidence interval [CI], 0.750–0.863; p < 0.0001), 0.726 (95% CI, 0.664–0.789; p < 0.0001), and 0.710 (95% CI, 0.646–0.775; p < 0.0001), respectively. Three ICP-derived continuous indices of cerebrovascular reactivity, PRx, PAx, and RAC, were validated against the LLA within this experimental model of arterial hypotension, with PRx being superior.
Keywords: : arterial hypotension, autoregulation, ICP indices, validation
Introduction
Continuous monitoring of cerebrovascular reactivity is becoming increasing common within the management of the critically ill neurological patient.1 The largest volume of literature for continuous monitoring of this aspect of cerebral physiology exists in adult traumatic brain injury (TBI).1,2 Various signals derived from multi-modal monitoring can be used as surrogate markers of cerebral blood volume (CBV) of slow or pulsatile (heart) frequency or cerebral blood flow (CBF), such as intracranial pressure (ICP) or transcranial Doppler (TCD)-based cerebral blood flow velocity (CBFV).2 These signals are low-pass filtered and correlated with a driving pressure, such as mean arterial pressure (MAP) or cerebral perfusion pressure (CPP), using moving Pearson correlation coefficients between slow waves of these signals. The concept behind these indices is that slow-wave ICP fluctuations are a surrogate for CBV/CBF, and they are changing in response to slow-wave changes (slower than 0.05 Hz; i.e., 0.05 cycle per sec or a period of 20 sec)3 in a driving pressure, MAP or CPP. They are therefore capable of providing information regarding cerebrovascular reactivity and thus autoregulatory capacity.1
To date, numerous indices of cerebrovascular reactivity have been derived.1 Given ICP monitoring is common within critically ill neurological patients, ICP-derived indices have received the most attention. Pressure reactivity index (PRx) is the most widely cited index and is the correlation between slow waves recorded in ICP and MAP.4 Numerous studies link PRx to patient outcome in TBI,1,4 with critical thresholds associated with morbidity and mortality defined within the literature.5 Further, PRx has been validated in a piglet model against the lower limit of autoregulation (LLA) during arterial hypotension, one of only three indices to be validated in this type of model (the other being near infrared spectroscopy [NIRS]-derived COx and HVx).6
Aside from PRx, two other ICP-derived indices of cerebrovascular reactivity exist. Pulse amplitude index (PAx), the correlation between pulse amplitude of ICP (AMP) and MAP, has been demonstrated to be comparable to PRx in outcome prediction for TBI patients. In cases with low ICP (e.g., after decompressive craniectomy), PAx is probably more useful than PRx.7,8 However, limited literature exists in the application of PAx clinically. Similarly, RAC, the correlation (R) between AMP (A) and CPP (C), has been recently described within the TBI population, with limited literature to date.9,10 It remains currently unknown whether PAx or RAC respect the LLA, similarly to PRx.
Part I of this article series provided support for the validation of PRx and PAx against the LLA in a rabbit model of sustained intracranial hypertension. Given that PAx and RAC have not been assessed against the LLA in a model of pure arterial hypotension, we elected to explore this within archived experimental piglet data, thus producing part II of the article series on validating ICP-derived indices of cerebrovascular reactivity in experimental models. The aim of this article, part II of the two-part series, was to determine whether PAx and RAC respect the LLA during arterial hypotension.
Methods
Animal model
The neonatal piglet data described within were retrospectively amalgamated from three separate experiments. Inclusion criteria for the current study were normothermic, sham control piglets that had complete and time-synchronized data for arterial blood pressure, laser Doppler flowmetry (LDF), and ICP from our previously published studies.6,11,12 Twenty-two piglets met the inclusion criteria: 1) control animals from a study on LLA6 (n = 8; age, 5–10 days; weight, 2.2–3.9 kg); 2) sham controls for a model of cardiac arrest11 (n = 7; age, 3–5 days; weight, 1–2.5 kg); and 3) sham normothermic controls for a model of cardiac arrest with hypothermic therapy12 (n = 7; age, 3–5 days; weight, 1–2.5 kg). All procedures were approved by the Animal Care and Use Committee at Johns Hopkins University and complied with the United States Public Health Service Policy on Human Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals. Further, animal care was in accord with National Institutes of Health Guidelines and ensured animals' comfort.
Anesthesia and surgical preparation
We previously published detailed methodology of these experiments.6,11,12 Briefly, male piglets were intubated and mechanically ventilated to maintain normocapnea. General anesthesia was provided with isoflurane in a 50%/50% nitrous oxide/oxygen mixture, fentanyl infusion and, as needed, boluses, and pancuronium or vecuronium infusions. Fentanyl and neuromuscular blockade were given through a femoral venous catheter. The isoflurane dose was held constant for the duration of the experiment. Arterial blood pressure was continuously monitored by an indwelling femoral artery catheter. A 5-F esophageal balloon catheter (Cooper Surgical, Trundall, CT) was placed into the contralateral femoral vein and advanced into the inferior vena cava for later induction of hypotension. A ventricular ICP monitor and a cortical LDF probe (model DRT4; 60 Hz; Moor Instruments, Devon, UK) to measure CBF were placed through small cranial burr holes. ICP and LDF were monitored in the same cerebral hemisphere. For all animals, pCO2 was targeted to maintain eucarbia and a pH of 7.35–7.456 or a pCO2 of 40 ± 5 mm Hg11 and 40 ± 7 mm Hg.12
Controlled hypotension
The balloon catheter was slowly inflated in the inferior vena cava using a saline syringe pump. Hypotension was induced from baseline to near-zero blood pressure over 2–3 h. This slow induction of hypotension permitted capture of slow-wave ICP fluctuations for analysis of cerebrovascular reactivity.
Signal acquisition and processing
All signals from the combined above monitoring modalities were recorded and archived for future retrospective use. All recorded signals were digitized by an A/D converter (DT9804; Data Translation, Marlboro, MA), sampled at a frequency of 50 Hz or higher, using ICM+ software (Cambridge Enterprise Ltd, Cambridge, UK; http://icmplus.neurosurg.cam.ac.uk). Signal artifacts, such as transducer adjustments, were removed before further processing or analysis using tools available in ICM+.
CPP was determined as: MAP – ICP. AMP was determined by calculating the fundamental Fourier amplitude of the ICP signal over a 10-sec window, updated every 10 sec. This was done over the range consistent with the normal range for piglet heart rate (i.e., 100–350 beats per minute). Finally, 10-sec moving averages (without data overlap) were calculated for all recorded signals: ICP, AMP, ABP (i.e., producing MAP), CPP, and LDF measurement of CBF (LDF-CBF). Piglets' archived signals were retrospectively interrogated and analyzed.
The following continuous indices of cerebrovascular reactivity were derived: PRx, PAx, RAC, and LDF-derived Lx (correlation between LDF-CBF and CPP). All indices were derived by moving Pearson correlation coefficients between 30 consecutive 10-sec average values of relevant signals and their parameters (i.e., 5 min of data), updated every minute.
Statistical analysis
All statistical analysis was conducted utilizing R statistical software (R Core Team [2016]; R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). The following packages were used: ggplot2, dplyr, tidyverse, lubridate, segmented, and pROC. Where significance is reported, alpha was set at 0.05. The following analysis described is similar to that performed within previous studies on the LLA.6 This was done so as to allow comparison between the results and potentially provide validation of the results observed within that study.6
Finding the lower limit of autoregulation
In order to determine the LLA of autoregulation in the 22 animals, we used piece-wise linear regression of the CPP versus LDF-CBF plots. The LDF-CBF signal was standardized against the individual animal's baseline LDF-CBF signal, producing “% change of LDF-CBF from baseline.” This is similar to other studies evaluating LDF-CBF.6,11,12
The piece-wise regression process used a starting point for estimation of the break point in either LDF-CBF. This starting point was visually estimated from the ICM+ plots of CPP versus LDF-CBF. The piece-wise regression process used a starting point for estimation of the break point in either LDF-CBF. This starting point was visually estimated from the ICM+ plots of CPP versus LDF-CBF versus CPP, described above. Despite this initial visual inspection, the automated piece-wise linear regression was conducted by the “segmented” computational package within R statistical software. The “start point” is only a starting reference for the automated algorithm to perform the piece-wise regression, with the full range of available CPP values tested during the process. This process functions on the assumption of continuity in data, splitting the data into two distinct linear segments. The intersection point of these two linear segments is considered the break point in the piece-wise function. The break point identified by the piece-wise regression process is one in which minimized the sum residual square error (SSE) of the two linear segments, above and below this point. This break point represents the LLA, with this method being described previously for the determination of the LLA in experimental models. This was conducted for each animal, with piece-wise regression plots produced denoting the 95% confidence interval (CI) for each fitted linear segment. Finally, the mean LLA for the cohort of 22 piglets was determined by averaging all 22 LLA values obtained.
Binned cohort data and plot
After delineating the mean LLA for the cohort, we then produced cohort-wide plots to inspect the population trend of various physiological measures against the LLA. We first binned all data across 5-mm Hg bins of CPP, using R statistical software. The following error bar plots were then produced: CPP versus % change in LDF-CBF from baseline, CPP versus PRx, CPP versus PAx, CPP versus RAC, and CPP versus Lx.
Comparing CPP for various clinical thresholds of PRx, PAx, and RAC to LLA
We wished to conduct a rough comparison of the CPP for clinically defined thresholds of PRx, PAx, and RAC to the CPP at the LLA, defined by piece-wise regression in each animal. To do so, we used a simplified piece-wise linear regression of CPP versus PRx and CPP versus PAx, using these models to determine the CPP in each animal for the following thresholds of PRx and PAx defined in TBI patients. For PRx, the thresholds of 0, +0.25, and +0.35 were tested, based on previous work in TBI4 and currently unpublished data from our lab.13 For PAx, the thresholds of 0 and +0.25 were tested, based on unpublished work from our lab. Finally, for RAC, the thresholds of −0.10 and −0.05 were tested, again based on unpublished work from our lab. We then compared the CPP values at each threshold for PRx, PAx, and RAC, with the CPP values at the LLA using a Pearson correlation coefficient and Bland–Altman analysis. The Bland–Altman analysis was only conducted for those thresholds reaching statistically significant correlations with the LLA (i.e., PRx 0, PRx +0.25, and PRx +0.35).
Prediction of continuous indices for impaired autoregulation
As done in previous studies,6 we performed receiver operating curve (ROC) analysis of PRx, PAx, RAC, and Lx across the cohort-defined LLA. This was conducted in order to determine the ability of these indices to predict being either above or below the LLA. For each piglet, one mean value for each variable was obtained at each 5-mm Hg bin of CPP (i.e., CPP = 40 mm Hg, 45 mm Hg, etc.). We utilized 5-mm Hg bins of CPP for the ROC analysis, given that this was what was conducted within the previous study by Brady and colleagues.6
Data were then given the binary designation of being above the LLA, or below the LLA, based on the LLA defined previously. Data from all 22 piglets was then used for the ROC analysis. Area under the curve (AUC) for the ROCs was reported and 95% CI reported by Delong's method. Significance values (i.e., p values) for the AUCs were derived from univariate logistic regression analysis. Comparison between AUCs was conducted using Delong's test.
We elected to use the ROC analysis based on population LLA, given that this is how the original studies were conducted to validate PRx- and NIRS-based indices in experimental models.6 In order to allow comparison across all studies (i.e., the previous work, and both part I and II of this article series), we maintained the analysis as such. Individual animal-specific LLA ROC analysis would lead to the inability to compare results to before and further limit the ability to extrapolate results to other animal models or human TBI data.
Results
Defining the lower limit of autoregulation
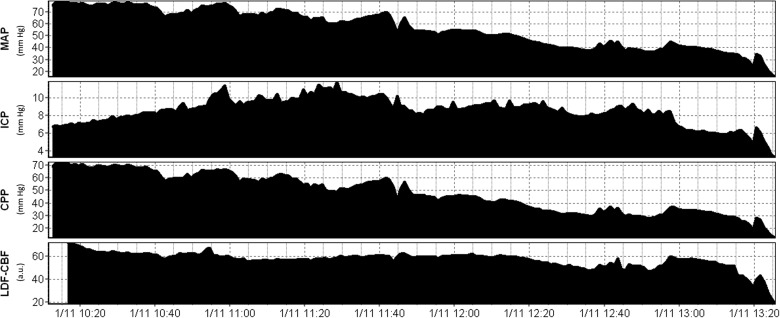
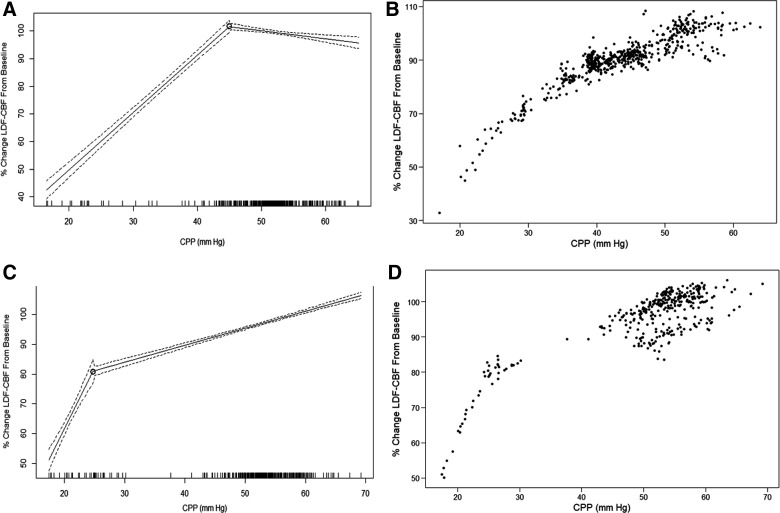
Through piece-wise linear regression analysis of each piglet, we obtained the LLA for each animal. Figure 1 displays an example of the recorded MAP, ICP, CPP, and LDF-CBF signal during the hypotension experiment, whereas Figure 2 displays two examples of piece-wise linear regression analysis of the LLA. Mean LLA was 36.2 ± 10.5 mm Hg. Appendix A (see online supplementary material at http://www.liebertpub.com) displays all of the piece-wise linear regression plots and scatter plots for each of the 22 piglets.
FIG. 1.
Example of physiological signal changes during hypotension. a.u., arbitrary units; CPP, cerebral perfusion pressure; ICP, intracranial pressure; LDF-CBF, laser Doppler flowmetry cerebral blood flow; MAP, mean arterial pressure; mm Hg, millimeters of mercury.
FIG. 2.
Examples of piece-wise linear regression analysis of LLA. (A and B) Piece-wise linear regression and scatter plot for 1 patient. (C and D) Piece-wise linear regression and scatter plot for 1 patient. Note: Dashed line on piecewise linear regression plots represents the 95% confidence intervals for the fitted lines. CPP, cerebral perfusion pressure; LDF-CBF, laser Doppler flowmetry cerebral blood flow; LLA, lower limit of autoregulation; mm Hg, millimeters of mercury.
Population-wide trends
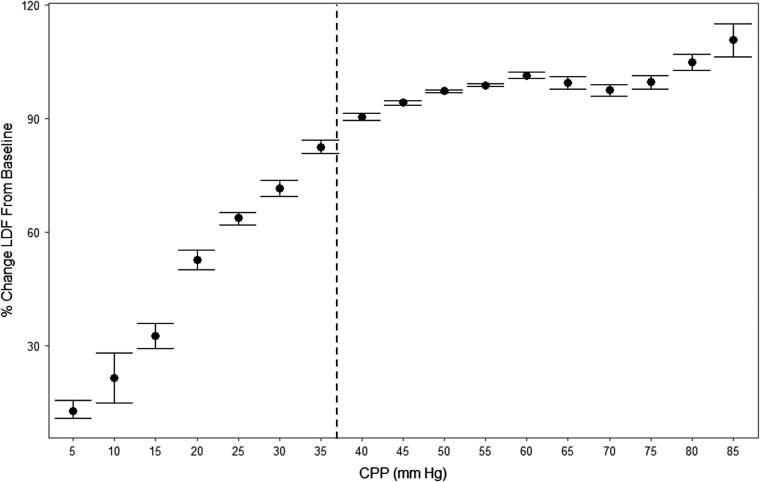
In order to provide a population-wide assessment of % change in LDF-CBF and the ICP-derived indices during changes in CPP, we produced various error bar plots. Figure 3 displays the plot of CPP versus % change in LDF-CBF from baseline, with the vertical dashed line indicating the approximate mean LLA, derived above. This demonstrates that there is a precipitous drop in LDF-CBF below the LLA.
FIG. 3.
Population-wide CPP versus % change in LDF from baseline. Note: Vertical dashed line represents the approximate mean LLA for the population defined through piece-wise linear regression in each animal. CPP, cerebral perfusion pressure; LDF, laser Doppler flowmetry; LLA, lower limit of autoregulation; mm Hg = millimeters of mercury.
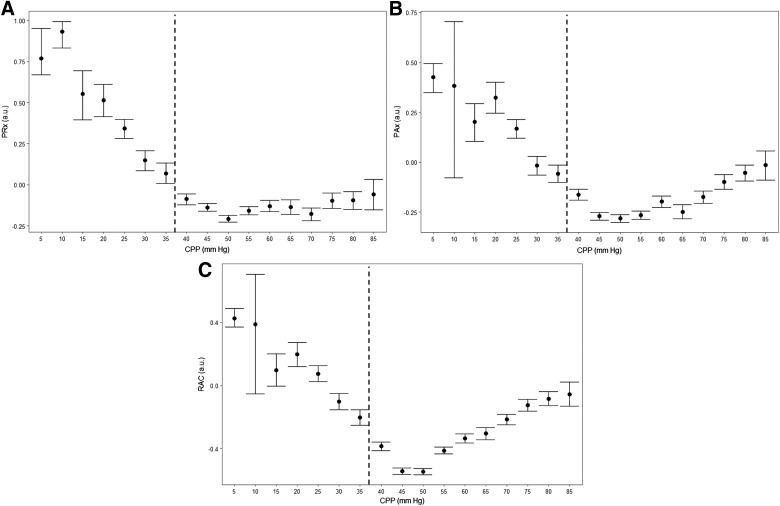
Similarly, we plotted the ICP indices across 5-mm Hg bins of CPP, producing error bar plots. Figure 4 displays these plots. It can be seen that PRx, PAx, and RAC all correlated with the LLA, denoted by the vertical dashed line. Appendix B (see online supplementary material at http://www.liebertpub.com) displays the error bar plot for CPP versus Lx.
FIG. 4.
Population-wide error bar plots: CPP versus PRx, CPP versus PAx, and CPP versus RAC. AMP, pulse amplitude of ICP; a.u., arbitrary unit; CPP, cerebral perfusion pressure; ICP, intracranial pressure; PAx, pulse amplitude index (correlation between AMP and MAP); PRx, pressure reactivity index (correlation between ICP and MAP); RAC, correlation between AMP and CPP. Note: Vertical dashed line represents the approximate mean LLA for the population, derived through piece-wise linear regression in each animal.
Comparing CPP for various clinical thresholds of PRx and PAx to LLA
For each animal, the CPP at each threshold for PRx, PAx, and RAC was roughly derived through a simplified piecewise linear model of CPP versus PRx, CPP versus PAx, and CPP versus RAC in each individual animal. These CPP values were compared to the CPP for the LLA derived in each animal, as described above. Table 1 displays the results for Pearson correlation between the CPP at TBI-defined critical thresholds and the LLA within the cohort of piglets. Only the PRx thresholds appeared to produce statistically significant correlations, though they were weak in strength. Bland–Altman analysis comparing the CPP values at these PRx thresholds, and the CPP at the LLA, can be found in Appendix C of the Supplementary Materials (see online supplementary material at http://www.liebertpub.com). This analysis displays poor agreement between the threshold CPP values and the CPP at the LLA.
Table 1.
Comparison of CPP at Index Threshold to LLA: Pearson Correlation
| Index threshold tested against LLA | Pearson correlation coefficient with LLA | p value for Pearson coefficient |
|---|---|---|
| PRx = 0 | 0.538 | 0.014 |
| PRx = +0.25 | 0.571 | 0.008 |
| PRx = +0.35 | 0.512 | 0.021 |
| PAx = 0 | −0.078 | 0.745 |
| PAx = +0.25 | 0.294 | 0.2079 |
| RAC = −0.10 | 0.394 | 0.077 |
| RAC = −0.05 | 0.350 | 0.120 |
Bolded values are those which reached statistical significance.
AMP, pulse amplitude of ICP; CPP, cerebral perfusion pressure; ICP, intracranial pressure; LLA, lower limit of autoregulation; PAx, pulse amplitude index (correlation between AMP and MAP); PRx, pressure reactivity index (correlation between ICP and MAP); RAC, correlation between AMP and CPP.
Lower limit of autoregulation receiver operating curve analysis
Through ROC analysis across the LLA, using the data from the 22 piglets, we were able to identify the AUCs for each continuous index. The AUC for PRx, PAx, and RAC was: 0.806 (95% CI, 0.750–0.863; p < 0.0001), 0.726 (95% CI, 0.664–0.789; p < 0.0001), and 0.710 (95% CI, 0.646–0.775; p < 0.0001), respectively. Finally, the AUC for Lx was 0.809 (95% CI, 0.754–0.863; p < 0.0001). Comparing AUCs by Delong's test, there was a statistically significant difference between the AUCs generated, when comparing PRx to PAx (p = 0.0004) and PRx to RAC (p < 0.0001). However, the AUCs for PAx and RAC were not statistically different (p = 0.214). ROC curves can be seen within Appendix D of the Supplementary Materials (see online supplementary material at http://www.liebertpub.com).
Discussion
Through retrospective analysis of archived experimental piglet data, we have been able to find a cohort of animals subjected to pure arterial hypotension, allowing for the assessment of ICP-derived continuous indices against the LLA. A few important points deserve highlighting.
First we have been able to provide confirmatory evidence that PRx correlates with the LLA within a model of hypotension. This was conducted using both the animal data from the initial publication documenting this relationship, plus another 14 sham control animals from other experiments. We have also been able to demonstrate a similar AUC (0.806; p < 0.0001) in the prediction of the LLA using PRx. Finally, evaluating clinically relevant thresholds for PRx, we have been able to show that all PRx thresholds fail to produce strong correlations with the LLA within a model of arterial hypotension. This was confirmed by poor agreement with the LLA on Bland–Altman analysis for all PRx clinical thresholds tested. This is likely because these thresholds have been defined within adult TBI populations, and thus the underlying disease and influence of ICP elevation post-injury may produce these thresholds that are disease specific. It is therefore not surprising that these thresholds do not necessarily respect the LLA in a model of pure hypotension. As with PAx and RAC, it must be stated that given the small numbers of animals within the current study, strong conclusions regarding these clinical thresholds cannot be made at this time. This work remains preliminary.
Second, for the first time, we have provided some evidence validating PAx and RAC against the LLA within a model of hypotension. This suggests that both indices provide information regarding cerebral autoregulatory capacity with a moderate accuracy. However, we were unable to provide conclusive evidence that the index threshold values, as defined in a TBI population, respect the LLA within this current model. The Pearson correlations between the LLA and the CPP at these thresholds were poor and not statistically significant. It remains uncertain as to whether the TBI-defined critical thresholds for PAx and RAC can be applied outside of the TBI population, given the poor performance of these thresholds within this model of arterial hypotension. Further, it remains unclear as to whether these thresholds represent relevant aspects of cerebral autoregulation, aside from associations with patient outcome in TBI. It is also likely that threshold values for reactivity indices may vary by individual. It must be acknowledged these results are preliminary.
Third, the “old” way of viewing these continuous indices is likely too simplistic (i.e., positive is “bad” and negative is “good”). As was observed with the results of this study, even below the LLA, PAx and RAC remained in the negative range until extremely low values for CPP. We do believe they still respect and measure the LLA, given that the analysis demonstrates that they both become progressively more positive as CPP decreases. However, each index is clearly different and requires detailed evaluation on its own, in specific pathologies, in order to identify what index value may indicate “impaired” versus “intact” cerebrovascular reactivity. This can be seen in our recent publication on thresholds for ICP indices and for TCD-derived systolic flow index (Sx)/Sx-a.13,14 The thresholds associated with clinical outcome for some indices can be negative, indicating that the cut-off point for “impaired” reactivity may, in fact, be a negative value for some indices. This is also likely true for the threshold associated with the LLA in humans for some indices, though has yet to be proven. Thus, using a blanket rule for all indices (i.e., positive is “bad” and negative is “good”) should probably be avoided.
Finally, the clinically defined thresholds for PRx, PAx, and RAC tested are defined within a TBI population. Thus, exploring how they relate to the LLA in an animal model of arterial hypotension may explain why many of the thresholds for PRx, PAx, and RAC do not appear to be related to the LLA. As mentioned above, the results of this analysis are preliminary, and thus strong conclusions about the relationship between the clinical thresholds and the LLA cannot be made. Further validation of our results is required. Further to this, as mentioned within the limitations of part I of this article series, even though the CPP at some of the clinically defined index thresholds appeared to be related to the LLA within this piglet model, one must interpret this with caution. Given that the LLA represents the point at which cerebral autoregulation becomes impaired (i.e., not the point at which vascular reactivity is completely lost), the lack of strong associations with CPP at thresholds defined by clinical outcome is not surprising. These thresholds for the ICP-defined indices were derived from TBI patient outcome at 6 months post-injury. As a result, these index thresholds may represent the severe end of the autoregulation spectrum, the point of complete failure of vascular reactivity. Hence, the relationship between the CPP at thresholds and the LLA may not be robust, given that they could be representing different aspects of impaired cerebrovascular reactivity. As well, one must assume that there are individual animal-based differences in vascular reactivity, introducing the influence of potential random effects. Much further interrogation of these clinically defined index thresholds is required, with the current analysis providing some preliminary insight.
One may question, why compare animal to human data for these indices (i.e., using human derived outcome thresholds)? The reason for doing this was to highlight the difficulties in extrapolating results of such animal studies directly to human TBI monitoring and care, emphasizing the need for future work on defining PRx, PAx, and RAC thresholds for the LLA (i.e., not clinical global outcome) in humans. We address potential future research directions within the “Future Directions” subsection in this discussion.
Limitations
Despite the interesting and promising results, a few limitations deserve emphasis. First, this is a retrospective analysis of an amalgamated cohort of piglets from three separate experiments. Though the anesthetic, procedures, and experimental hypotension techniques were similar for all animals, the cohorts were not exactly identical. For example, 8 of the animals were a little older (i.e., 5–10 days, vs. 3–5 days), with slightly higher weights. This could influence the cerebrovascular response slightly. Second, despite have a sizable cohort of piglets for this retrospective analysis, it is still a relatively small number of animals, thus the conclusions drawn must be taken with caution. Further to this, it must be acknowledged that given fluctuations in both ICP and MAP commonly observed in clinical care of human TBI, the results found in this study mainly apply to animal models of normal ICP more directly, thus limiting the ability to extrapolate these results to human TBI care.
Future directions
Based on the results from part I and II of this article series, exploring the ability of ICP-derived indices to measure the LLA in experimental models, some confidence exists in the ability of these indices to measure autoregulation. Directly extrapolating the results from these two animal studies to human TBI is difficult, given species-related differences and the relatively controlled settings of these models. Specific thresholds for the LLA in these two animal cohorts cannot be translated to humans; however, the ability of the ICP-derived indices to measure the LLA provides confidence for their ability to do so in humans. However, further analysis is required to confirm these results and validate these indices in other circumstances.
First, rarely are ICP or MAP altered in isolation within the clinical TBI setting. Thus, further models evaluating fluctuations in MAP, toward the LLA, during episodes of sustained ICP elevation, are required. These models could evaluate ICP sustained at various levels (i.e., 20–30 mm Hg, 30–40 mm Hg, etc.) while driving MAP toward and below the LLA. Similar analysis of the ICP-derived indices could then occur. The ability of these indices to measure the LLA during these circumstances would provide further confidence in their use in clinical TBI monitoring.
Second, the upper limit of autoregulation (ULA) also needs investigation. No studies to date have confirmed the ability of any continuous measure of cerebrovascular reactivity to accurately measure the ULA. This could be done again both with and without various ICP elevations, mimicking the physiological variation observed in clinical TBI practice. MAP would then be driven toward and above the ULA using potentially a combination of vasopressors and/or intraaortic balloon. Through these experiments, if the ability to measure the ULA is confirmed, this would assuredly solidify these ICP indices as true measures of autoregulation.
Third, the impact of decompressive craniectomy (DC) is also important to evaluate. We know that DC impacts ICP and PRx values in clinical practice.15,16 Only through experimental models will we truly understand the impact on autoregulation that DC has. Further, producing experimental models in which ICP and MAP are altered post-DC would allow us to evaluate the ability of these ICP indices to measure both the LLA and ULA post-craniectomy. It is currently unknown as to whether index values post-craniectomy are reliable measures of cerebrovascular reactivity.
Fourth, our current understanding of “thresholds” of ICP-based indices in adult TBI are based on global clinical outcomes, not actual thresholds of the LLA or ULA. This is the current main limitation to this type of monitoring in clinical practice. Derivation of ICP index thresholds for the LLA and ULA in humans is difficult, given that clinical care is directed at avoiding these extremes of MAP, and the design of a clinical study to assess this is fraught with potential dangers to the subjects. We believe these threshold studies will require two types of data. First, multi-center widespread data collection for all critically ill patients with cranial monitoring, so that cerebrovascular reactivity indices during physiological extremes may be captured in a large number of patients. This would allow stratification by age, sex, and pathology, creating a potential normative value range for index thresholds. Second, larger mammal/primate models, evaluating the LLA and ULA, may provide the closest controlled evaluation of index thresholds. This may allow extrapolation of these experimental derived thresholds to human clinical practice. It is acknowledged that such animal models are costly and would require multi-center coordination to be conducted successfully.
Finally, aside from ICP-derived indices of cerebrovascular reactivity, other invasive and noninvasive multi-modal monitoring devices can provide indices of vascular reactivity. These, too, require investigation within the types of future animal models described above, before widespread implementation and confidence in their ability to measure cerebral autoregulation.
Conclusions
The three ICP-derived continuous indices of cerebrovascular reactivity, PRx, PAx, and RAC, were evaluated against the LLA within this experimental model of arterial hypotension. All three indices appear to respect the LLA within this model of pure arterial hypotension, with PRx being superior.
Supplementary Material
Acknowledgments
This work was made possible through salary support through the Cambridge Commonwealth Trust Scholarship, the Royal College of Surgeons of Canada–Harry S. Morton Travelling Fellowship in Surgery, and the University of Manitoba Clinician Investigator Program.
M.C. is supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C1790).
Author Disclosure Statement
F.A.Z. has received salary support for dedicated research time, during which this project was partially completed. Such salary support came from: the Cambridge Commonwealth Trust Scholarship, the Royal College of Surgeons of Canada–Harry S. Morton Travelling Fellowship in Surgery, and the University of Manitoba Clinician Investigator Program.
J.K.L. received research support from the National Institutes of Health, Bethesda, Maryland (K08NS080984).
M.C. and P.S. have financial interest in a part of licensing fee for ICM+ software (Cambridge Enterprise Ltd, UK).
References
- 1.Zeiler F.A., Donnelly J., Calviello L., Smielewski P., Menon D.K., and Czosnyka M. (2017). Pressure autoregulation measurement techniques in adult traumatic brain injury, part II: a scoping review of continuous methods. J. Neurotrauma 34, 3224–3237 [DOI] [PubMed] [Google Scholar]
- 2.Zeiler F.A., Donnelly J., Calviello L., Menon D.K., Smielewski P., and Czosnyka M. (2017). Pressure autoregulation measurement techniques in adult traumatic brain injury, part I: a scoping review of intermittent/semi-intermittent methods. J. Neurotrauma 34, 3207–3223 [DOI] [PubMed] [Google Scholar]
- 3.Fraser C.D., III, Brady K.M., Rhee C.J., Easley R.B., Kibler K., Smielewski P., Czosnyka M., Kaczka D.W., Andropoulos D.B., and Rusin C. (1985). The frequency response of cerebral autoregulation. J. Appl. Physiol. 115, 52–56 [DOI] [PubMed] [Google Scholar]
- 4.Czosnyka M., Smielewski P., Kirkpatrick P., Laing R.J., Menon D., and Pickard J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41, 11–17 [DOI] [PubMed] [Google Scholar]
- 5.Sorrentino E., Diedler J., Kasprowicz M., Budohoski K.P., Haubrich C., Smielewski P., Outtrim J.G., Manktelow A., Hutchinson P.J., Pickard J.D., Menon D.K., and Czosnyka M. (2012). Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care 16, 258–266 [DOI] [PubMed] [Google Scholar]
- 6.Brady K.M., Lee J.K., Kibler K.K., Easley R.B., Koehler R.C., and Shaffner D.H. (2008). Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 39, 2531–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aries M.J., Czosnyka M., Budohoski K.P., Kolias A.G., Radolovich D.K., Lavinio A., Pickard J.D., and Smielewski P. (2012). Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit. Care 17, 67–76 [DOI] [PubMed] [Google Scholar]
- 8.Radolovich D.K., Aries M.J., Castellani G., Corona A., Lavinio A., Smielewski P., Pickard J.D., and Czosnyka M. (2011). Pulsatile intracranial pressure and cerebral autoregulation after traumatic brain injury. Neurocrit. Care 15, 379–386 [DOI] [PubMed] [Google Scholar]
- 9.Zeiler F.A., Cardim D., Donnelly J., Menon D.K., Czosnyka M., and Smielewski P. (2018). Transcranial Doppler systolic flow index and ICP derived cerebrovascular reactivity indices in TBI. J. Neurotrauma 35, 314–322 [DOI] [PubMed] [Google Scholar]
- 10.Zeiler F.A., Donnelly J., Cardim D., Menon D.K., Smielewski P., and Czosnyka M. (2018). ICP versus laser Doppler cerebrovascular reactivity indices to assess brain autoregulatory capacity. Neurocrit. Care 28, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.K., Yang Z.J., Wang B., Larson A.C., Jamrogowicz J.L., Kulikowicz E., Kibler K.K., Mytar J.O., Carter EL., Burman H.T., Brady K.M., Smielewski P., Czosnyka M., Koehler R.C., and Shaffner D.H. (2012). Noninvasive autoregulation monitoring in a swine model of pediatric cardiac arrest. Anesth Analg 114, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.K., Brady K.M., Mytar J.O., Kibler K.K., Carter E.L., Hirsch K.G., Hogue C.W., Easley R.B., Jordan L.C., Smielewski P., Czosnyka M., Shaffner D.H., and Koehler R.C. (2011). Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit. Care Med. 39, 2337–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeiler F.A., Donnelly J., Smielewski P., Menon D.K., Hutchinson P.J., and Czosnyka M. (2018). Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J. Neurotrauma 35, 1107–1115 [DOI] [PubMed] [Google Scholar]
- 14.Zeiler F.A., Cardim D., Donnelly J., Menon D.K., Czosnyka M., and Smielewski P. (2018). Transcranial Doppler systolic flow index and ICP-derived cerebrovascular reactivity indices in traumatic brain injury. J. Neurotrauma 35, 314–322 [DOI] [PubMed] [Google Scholar]
- 15.Timofeev I., Czosnyka M., Nortje J., Smielewski P., Kirkpatrick P., Gupta A., and Hutchinson P. (2008). Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J. Neurosurg. 108, 66–73 [DOI] [PubMed] [Google Scholar]
- 16.Zweifel C., Lavinio A., Steiner L.A., Radolovich D., Smielewski P., Timofeev I., Hiler M., Balestreri M., Kirkpatrick P.J., Pickard J.D., Hutchinson P., and Czosnyka M. (2008). Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg. Focus 25, E2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.