Abstract
Combining gene therapy approaches with tissue engineering procedures is an active area of translational research for the effective treatment of articular cartilage lesions, especially to target chondrogenic progenitor cells such as those derived from the bone marrow. This study evaluated the effect of genetically modifying concentrated human mesenchymal stem cells from bone marrow to induce chondrogenesis by recombinant adeno-associated virus (rAAV) vector gene transfer of the sex-determining region Y-type high-mobility group box 9 (SOX9) factor upon seeding in three-dimensional-woven poly(ɛ-caprolactone; PCL) scaffolds that provide mechanical properties mimicking those of native articular cartilage. Prolonged, effective SOX9 expression was reported in the constructs for at least 21 days, the longest time point evaluated, leading to enhanced metabolic and chondrogenic activities relative to the control conditions (reporter lacZ gene transfer or absence of vector treatment) but without affecting the proliferative activities in the samples. The application of the rAAV SOX9 vector also prevented undesirable hypertrophic and terminal differentiation in the seeded concentrates. As bone marrow is readily accessible during surgery, such findings reveal the therapeutic potential of providing rAAV-modified marrow concentrates within three-dimensional-woven PCL scaffolds for repair of focal cartilage lesions.
Keywords: : cartilage repair, human bone-marrow aspirates, chondrogenesis, rAAV, SOX9, 3D-woven poly(ɛ-caprolactone) scaffolds
Introduction
Adult articular cartilage is an avascular tissue that provides a low-friction weight-bearing surface in the joints. As a consequence of its lack of vascularization, the cartilage does not have direct access to potentially chondroregenerative cells that may allow for repair or restoration of the original hyaline structure with native mechanical function and integrity following injury.1,2 While a variety of surgical approaches are used to enhance cartilage repair, none permit the complete and long-term regeneration of the articular cartilage,3,4 showing the critical need for novel, effective therapeutic options. Mesenchymal stem cells (MSCs) that are present in the subchondral bone marrow are an attractive source of chondrogenic progenitors5–7 and have shown promise in treating focal cartilage defects and osteoarthritic lesions.8–15 However, there is no evidence showing complete functional restoration of the original hyaline cartilage in treated patients, rather leading to the production of a fibrotic tissue that is not capable of withstanding mechanical loads over time.16,17
Gene therapy offers powerful tools that may be combined with tissue engineering strategies as a means to stimulate and improve the chondroregenerative properties of bone marrow–derived MSCs in supportive matrixes for enhanced, adapted cartilage repair.18–20 Among the various agents known for their chondrogenic activities (e.g., bone morphogenetic proteins, transforming growth factor beta [TGF-β], basic fibroblast growth factor, insulin-like growth factor I, zinc finger protein 145, Indian hedgehog, and cartilage oligomeric matrix protein),21–30 the sex-determining region Y-type high-mobility group box 9 (SOX9) factor is a key transcription factor involved in MSC chondrodifferentiation and cartilage formation31 while inhibiting terminal differentiation and hypertrophy.32,33 Delivery of SOX9 in MSCs thus far has been attempted with classical gene transfer techniques such as nonviral vectors,34,35 adenovirus vectors (AAVs),36,37 and retro-/lentiviral vectors.38 However, these approaches may have low and/or short-term efficacy (nonviral vectors and AAVs) or the risk for insertional mutagenesis (retro-/lentiviral vectors). In contrast, the use of highly efficient, clinically adapted recombinant vehicles derived from the AAV is particularly suited for translational approaches,39 as recombinant AAV (rAAV) vectors do not carry any viral coding sequences and are maintained over extended periods of time under stable episomal forms in their targets, which include MSCs.22,24,29,30,40 Of specific interest, it was previously reported that this vector class could be used for highly efficient genetic modification of isolated human MSCs (hMSCs) but proved even more effective in concentrated hMSCs within their native microenvironment (concentrates).40,41 Accordingly, the overexpression of a SOX9 gene sequence in hMSC concentrates was demonstrated to promote chondrogenic differentiation in vitro to levels significantly superior to those achieved in the absence of therapeutic treatment.41
The goal of the present study was to combine the transfer of the rAAV SOX9 candidate in human bone-marrow concentrates containing MSCs with a delivery procedure using three-dimensional (3D), bio-/immunocompatible, slowly degrading woven poly(ɛ-caprolactone; PCL) scaffolds42 that can mimic the anisotropic, nonlinear, and viscoelastic biomechanical characteristics of native cartilage.42 Such a combined scaffold-/gene-associated approach may provide extra beneficial cues within the cell microenvironment43 relative to the previously tested conventional scaffold-free gene transfer strategy40,41 and may further support and improve cartilage reparative processes versus single genetic treatment while being well adapted in clinical setups.42 The data show that rAAV SOX9-treated human bone marrow concentrates successfully undergo chondrogenic differentiation when seeded on 3D-woven PCL scaffolds compared to control treatment (rAAV lacZ transduction, lack of vector application) with significantly reduced levels of hypertrophic and terminal differentiation, thus providing new and effective combined strategies for future translational applications in treating cartilage defects in patients.
Methods
Reagents
Reagents were from Sigma–Aldrich (Munich, Germany) unless otherwise indicated. Recombinant TGF-β was purchased at R&D Systems (Wiesbaden-Nordenstadt, Germany). The dimethylmethylene blue dye was from Serva (Heidelberg, Germany). The anti-SOX9 (C-20) and anti-FLAG (BioM2) antibodies were from Santa Cruz Biotechnology (Heidelberg, Germany), the anti-type-II collagen (AF-5710) and anti-type-I collagen (AF-5610) antibodies from Acris (Hiddenhausen, Germany), the anti-type-X collagen (COL-10) antibody from Sigma–Aldrich, and biotinylated secondary antibodies with ABC reagent from Vector Laboratories (Alexis Deutschland GmbH, Grünberg, Germany). The type-II collagen enzyme-linked immunosorbent assay (ELISA; Arthrogen-CIA Capture ELISA kit) was from Chondrex (Redmond, WA).
Woven PCL scaffolds
Three-dimensional-woven textile scaffolds were produced by arranging multifilament PCL yarns (∼150 μm in diameter; EMS-Griltech, Domat, Switzerland) in three orthogonal directions.42 An overall scaffold thickness of 0.75 mm was achieved by stacking a total of nine layers of yarns in alternating x (0°) and y (90°) directions, and held together by a series of interwoven z-direction yarns. After weaving was complete and the material was removed from the loom, it was soaked in 4 M of NaOH for 15–16 h to remove surface contaminants and increase hydrophilicity of the constituent PCL. Scaffolds 6 mm in diameter were then punched from the flat material using a biopsy punch and sterilized using ethylene oxide gas.
Plasmids and rAAV vectors
The constructs were all derived from the same parental AAV-2 genomic clone, pSSV9.44,45 rAAV-lacZ is an AAV-2-based vector plasmid carrying the lacZ gene encoding β-galactosidase (β-gal) under the control of the cytomegalovirus immediate-early (CMV-IE) promoter.40,41 rAAV-RFP carries the Discosoma sp. red fluorescent protein gene (RFP) and rAAV-FLAG-hsox9 a FLAG-tagged sox9 sequence (1.7 kb) instead of lacZ.40,41 All vectors were packaged as conventional (not self-complementary) vectors in the 293 cell line, an adenovirus-transformed human embryonic kidney cell line, by using Adenovirus 5 to provide helper functions in combination with the transacting AAV-2 factors for replication and encapsidation functions supplied by the pAd8 helper plasmid. The vector preparations were purified, dialyzed, and titered by real-time polymerase chain reaction (PCR),40,41 averaging 1011 functional units/mL.
Human bone-marrow aspirates
Bone-marrow aspirates (∼15 mL; 0.5–1.3 × 109 cells/mL) were obtained from the distal femurs of patients undergoing total knee arthroplasty (n = 14). The study was approved by the Ethics Committee of the Saarland Physicians Council. All patients provided informed consent before inclusion in the study. All procedures were in accordance with the Declaration of Helsinki.
rAAV-mediated gene transfer
Aspirates were immediately aliquoted in a volume of 150 μL/well in 96-well plates and transduced with 100 μL of vector (2 × 106 functional recombinant viral particles; multiplicity of infection = 15 ± 5).41 Next, a mixture of fibrinogen (17 mg/mL)/thrombin (5 IU/mL; Baxter, Volketswil, Switzerland) was added to the samples that were then immediately seeded in 3D-woven PCL scaffolds in new 96-well plates. Conditions using fibrinogen/thrombin alone or PCL scaffolds alone were not included here, as they have been largely tested previously.42,43 The samples were incubated over time in defined chondrogenic differentiation medium (DMEM; 0.1 μM of dexamethasone, 50 μg/mL of ascorbic acid, 40 μg/mL of proline, 110 μ/mL of pyruvate, 6.25 μg/mL of insulin, 6.25 μg/mL of transferrin, 6.25 μg/mL of selenious acid, 1.25 mg/mL of bovine serum albumin, 5.55 μg/mL of linoleic acid, and 10 ng/m TGF-β3)40,41 for further evaluations at the denoted time points.
Transgene expression
Expression of the lacZ transgene was analyzed by X-Gal staining and visualization under light microscopy (Olympus BX45; Olympus, Hamburg, Germany).41 RFP was detected by live fluorescence using a fluorescent microscopy with a 568 nm filter (Olympus CKX41).41 SOX9 expression was monitored by immunohistochemistry using a specific SOX9 antibody, a biotinylated secondary antibody, and diaminobenzidine (DAB) as a chromogen (ABC method).40,41 A control condition with omission of the primary antibody was included to check for secondary immunoglobulins. All sections were examined under light microscopy (Olympus BX45).
Histology, immunocytochemistry, and immunohistochemistry
The samples were harvested, fixed in 4% formalin with subsequent dehydration in graded alcohols, paraffin embedded, and sectioned at 3 μm.41 Samples were processed for immunohistochemical analyses, and sections were also stained with safranin O (matrix proteoglycans), hematoxylin and eosin (H&E; cellularity), and alizarin red (matrix mineralization) according to routine protocols.40,41 Expression of type-II, -I, and -X collagen was detected by immunohistochemistry using specific antibodies (1:200), biotinylated secondary antibodies (1:200), and the ABC method with DAB as the chromogen.40,41
Histomorphometry
The transduction efficiencies (ratio of X-Gal-stained surface to the total surface evaluated) and the cell densities (cell numbers per standardized area on H&E-stained sections) were measured on histological sections from samples.40,41 The immunohistochemical and histological grading scores were measured using four histological sections for each condition with the SIS AnalySIS program. SOX9 immunostaining, safranin O staining, type-II collagen immunostaining, alizarin red staining, and type-I and -X collagen immunostaining were scored for uniformity and intensity according to a modified Bern Score grading system46 as follows: 0 (no staining), 1 (heterogeneous and/or weak staining), 2 (homogeneous and/or moderate staining), 3 (homogeneous and/or intense staining), and 4 (very intense staining). Sections were scored by two individuals who were blind to the conditions.
Biochemical assays
The samples were harvested with selective papain digestion to monitor the proteoglycan content by binding to dimethylmethylene blue dye, the DNA content using a fluorometric assay based on Hoechst 33258, and the type-II collagen contents by ELISA.40 Values were normalized to total cellular proteins monitored via Pierce Thermo Scientific Protein Assay (Thermo Fisher Scientific, Schwerte, Germany). All measurements were performed by using a GENios spectrophotometer/fluorometer (Tecan, Crailsheim, Germany).
Real-time reverse transcription PCR analysis
Total cellular RNA was extracted from the samples using the RNeasy Protect Mini Kit with an on-column RNase-free DNase treatment (Qiagen, Hilden, Germany). RNA was eluted in 30 μL of RNase-free water. Reverse transcription was carried out with 8 μL of eluate by using the 1st Strand cDNA Synthesis kit for reverse transcription (RT)-PCR (AMV; Roche Applied Science, Penzberg, Germany). An aliquot of the cDNA product (3 μL) was amplified with real-time PCR by using the Brilliant SYBR Green QPCR Master Mix (Stratagene; Agilent Technologies, Waldbronn, Germany)40 on an Mx3000P qPCR operator system (Stratagene) as follows: (95°C for 10 min), amplification by 55 cycles (denaturation at 95°C for 30 s; annealing at 55°C for 1 min; extension at 72°C for 30 s), denaturation (95°C for 1 min), and final incubation (55°C for 30 s). The primers (Invitrogen GmbH, Darmstadt, Germany) used were SOX9 (chondrogenic marker; forward 5′-ACACACAGCTCACTCGACCTTG-3′; reverse 5′-GGGAATTCTGGTTGGTCCTCT-3′), aggrecan (ACAN; chondrogenic marker; forward 5′-GAGATGGAGGGTGAGGTC-3′; reverse 5′-ACGCTGCCTCGGGCTTC-3′), type-II collagen (COL2A1; chondrogenic marker; forward 5′-GGACTTTTCTCCCCTCTCT-3′; reverse 5′-GACCCGAAGGTCTTACAGGA-3′), type-I collagen (COL1A1; osteogenic marker; forward 5′-ACGTCCTGGTGAAGTTGGTC-3′; reverse 5′-ACCAGGGAAGCCTCTCTCTC-3′), type-X collagen (COL10A1; marker of hypertrophy; forward 5′-CCCTCTTGTTAGTGCCAACC-3′; reverse 5′-AGATTCCAGTCCTTGGGTCA-3′), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; housekeeping gene and internal control; forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; reverse, 5′-GAAGATGGTGATGGGATTTC-3′; all 150 nM final concentration).40 Control conditions included reactions using water and non-reverse-transcribed mRNA. Specificity of the products was confirmed by melting curve analysis and agarose gel electrophoresis. The threshold cycle (Ct) value for each gene of interest was measured for each amplified sample using MxPro QPCR software (Stratagene), and values were normalized to GAPDH expression by using the 2–ΔΔCt method, as described previously.40
Statistical analysis
Data are expressed as means ± standard deviation (SD) of separate experiments. Each treatment condition was performed in triplicate in three independent experiments for each patient. Data were obtained by two individuals who were blind respect to the treatment groups. The t-test and Mann–Whitney's rank-sum test were used where appropriate. p-Values of <0.05 was considered statistically significant.
Results
rAAV-mediated transgene expression in human bone-marrow aspirates seeded in 3D-woven PCL scaffolds
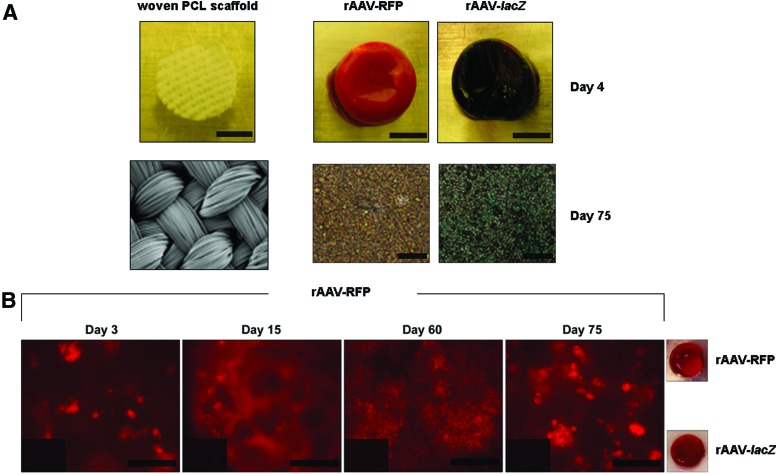
Human bone-marrow aspirates were first transduced with rAAV reporter gene vectors (rAAV-lacZ, rAAV-RFP) and seeded in 3D-woven PCL scaffolds to monitor the ability of this vector class to promote transgene expression over time in these biomechanically functional scaffolds under conditions of chondrogenic induction. Sustained lacZ expression was observed when applying rAAV-lacZ versus control treatment (rAAV-RFP) starting on day 4 after gene transfer and for at least 75 days (the longest time point evaluated), with transduction efficiencies approaching 100%, as seen on histological sections from rAAV-lacZ-treated samples (Fig. 1A). Similar results were noted when evaluating live fluorescence in rAAV-RFP-treated samples versus control (rAAV-lacZ) transduction (Fig. 1B). Reporter gene expression was undetectable in untreated samples (data not shown).
Figure 1.
Detection of reporter gene expression in recombinant adeno-associated virus (rAAV)-transduced human bone-marrow aspirates seeded in three-dimensional (3D)-woven poly(ɛ-caprolactone; PCL) scaffolds. Aspirates (150 mL) were transduced with rAAV-lacZ or rAAV-RFP (100 mL each vector) or left untreated and seeded in the scaffolds in fibrinogen/thrombin using chondrogenic medium, as described in the Methods. The samples were processed at the denoted time points to detect (A) lacZ expression by X-Gal staining (macroscopic views and magnification 40 × ; all representative data) and (B) RFP expression by live fluorescence (inserts: rAAV-lacZ control treatment; magnification 40 × and macroscopic views at day 75; all representative data). Scale bars: (A) 3 mm (scaffold), 1 cm (aspirates), and 100 mm (histological sections); (B) 200 mm. Color images available online at www.liebertpub.com/hum
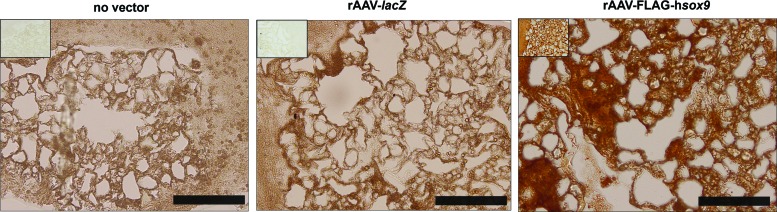
The ability of rAAV to overexpress the candidate SOX9 gene sequence in human bone-marrow aspirates seeded in 3D-woven PCL scaffolds was then evaluated by comparison to control treatments (rAAV-lacZ application, absence of vector treatment) over a period of 21 days, which is adequate for chondrogenic induction in such samples.41 Sustained, elevated SOX9 production levels were achieved in the SOX9-treated aspirates within the scaffolds relative to the controls (Fig. 2), with significantly higher histomorphometric grading scores of SOX9 expression when providing rAAV-FLAG-hsox9 versus rAAV-lacZ or the no vector condition (sevenfold difference; p ≤ 0.001; Table 1). High FLAG expression was confined to the SOX9-treated aspirates (Fig. 2).
Figure 2.
Detection of FLAG SOX9 expression in rAAV-transduced human bone-marrow aspirates seeded in 3D-woven PCL scaffolds. Aspirates were transduced with rAAV-lacZ or rAAV-FLAG-hsox9 or left untreated and seeded in the scaffolds in fibrinogen/thrombin using chondrogenic medium, as described in Fig. 1 and in the Methods. The samples were processed after 21 days to detect SOX9 (magnification 10 × ) and FLAG expression (inserts: magnification 20 × ) by immunohistochemistry (all representative data). Scale bars: 200 mm. Color images available online at www.liebertpub.com/hum
Table 1.
Histomorphometric analyses in rAAV-transduced human bone-marrow aspirates seeded in 3D-woven PCL scaffolds
| Parameter | No vector | lacZ | SOX9 |
|---|---|---|---|
| SOX9 | 0.5 (0.4) | 0.5 (0.6) | 3.5 (0.6)*,† |
| Safranin O | 1.8 (0.3) | 1.9 (0.4) | 3.4 (0.2)*,† |
| Type-II collagen | 1.5 (0.6) | 1.5 (0.5) | 3.8 (0.4)*,† |
| Cell densities | 2358 (56) | 2422 (39) | 2463 (44) |
| Alizarin red | 3.6 (0.4) | 3.4 (0.5) | 1.3 (0.3)*,† |
| Type I collagen | 2.8 (0.5) | 2.8 (0.4) | 1.1 (0.4)*,† |
| Type X collagen | 3.6 (0.7) | 3.4 (0.4) | 1.1 (0.1)*,† |
SOX9 immunostaining, safranin O staining, type-II collagen immunostaining, alizarin red staining, and types I and X collagen immunostaining were scored for uniformity and intensity as: 0, no staining; 1, heterogeneous and/or weak staining; 2, homogeneous and/or moderate staining; 3, homogeneous and/or intense staining; and 4, very intense staining.46 The cell densities measured on H&E-stained histological sections are given in cells/mm2. Values are given as means (SD; n = 3). Statistically significant compared with *no vector treatment and with †rAAV-lacZ.
rAAV, recombinant adeno-associated virus; 3D, three-dimensional; PCL, poly(ɛ-caprolactone); H&E, hematoxylin and eosin; SD, standard deviation.
Effects of SOX9 overexpression upon the biological and chondrogenic activities in human bone-marrow aspirates seeded in 3D-woven PCL scaffolds
The candidate SOX9 vector was provided to human bone-marrow aspirates seeded in 3D-woven PCL scaffolds to monitor the effects of the transcription factor via rAAV application on biological and differentiation activities. These constructs were maintained in chondrogenic culture conditions for 21 days and compared to control treatments (rAAV-lacZ, lack of vector administration).
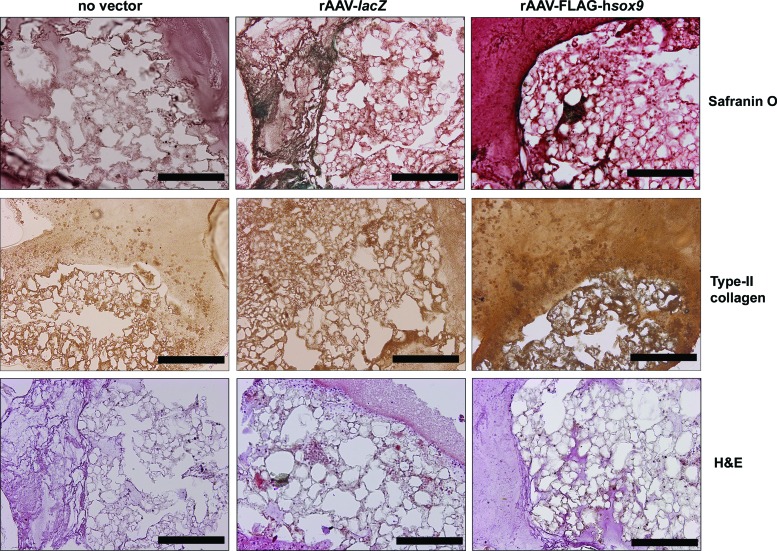
Successful chondrogenic differentiation was noted in all the samples after 21 days, as seen by effective safranin O staining and type-II collagen immunostaining, but with higher staining intensities in the presence of rAAV-FLAG-hsox9 (Fig. 3). A histomorphometric analysis performed using a system that grades the intensity of safranin O staining and of type-II collagen immunostaining revealed significantly higher scores of cartilage matrix deposition with SOX9 versus control conditions (1.9- and 2.5-fold difference, respectively; p ≤ 0.001; Table 1). Administration of rAAV-FLAG-hsox9 also significantly increased the proteoglycan and type-II collagen content in the samples relative to the controls (up to 3- and 1.9-fold, respectively; p ≤ 0.001; Table 2). Of note, no effects of SOX9 treatment were observed upon the DNA content relative to the control condition (p ≥ 0.181; Table 2), a finding confirmed by an estimation of the cell densities on H&E-stained histological sections (p ≥ 0.108; Fig. 3 and Table 1).
Figure 3.
Chondrogenic differentiation in rAAV-transduced human bone-marrow aspirates seeded in 3D-woven PCL scaffolds. Aspirates were transduced with rAAV-lacZ or rAAV-FLAG-hsox9 or left untreated and seeded in the scaffolds in fibrinogen/thrombin using chondrogenic medium, as described in Figs. 1 and 2 and in the Methods. The samples were processed after 21 days for histological staining with safranin O and hematoxylin and eosin and to detect immunoreactivity to type II collagen (magnification 10 × ; all representative data). Scale bars: 200 mm. H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/hum
Table 2.
Biochemical assays in rAAV-transduced human bone-marrow aspirates seeded in 3D-woven PCL scaffolds
| Parameter | No vector | lacZ | SOX9 |
|---|---|---|---|
| Proteoglycans (mg/mg total proteins) | 1.8 (0.1) | 1.8 (0.2) | 4.1 (0.3)*,† |
| Type II collagen (ng/mg total proteins) | 2.3 (0.2) | 2.5 (0.2) | 3.8 (0.3)*,† |
| DNA (ng/mg total proteins) | 15.8 (0.2) | 14.3 (0.3) | 13.4 (0.2) |
| Proteoglycans/DNA (mg/ng) | 0.1 (0.1) | 0.1 (0.1) | 0.3 (0.1)*,† |
| Type II collagen/DNA (ng/ng) | 0.15 (0.02) | 0.17 (0.02) | 0.28 (0.02)*,† |
Values are given as means (SD; n = 3). Statistically significant compared with *no vector treatment and with †rAAV-lacZ.
Effects of SOX9 overexpression upon the hypertrophic and terminal differentiation processes in human bone-marrow aspirates seeded in 3D-woven PCL scaffolds
Human bone-marrow aspirates were treated with the candidate SOX9 vector and seeded onto 3D-woven PCL scaffolds to examine the effects of the transcription factor via rAAV application upon the hypertrophic and terminal differentiation activities. Constructs were cultured in chondrogenic conditions and compared to control treatments (rAAV-lacZ, absence of vector administration).
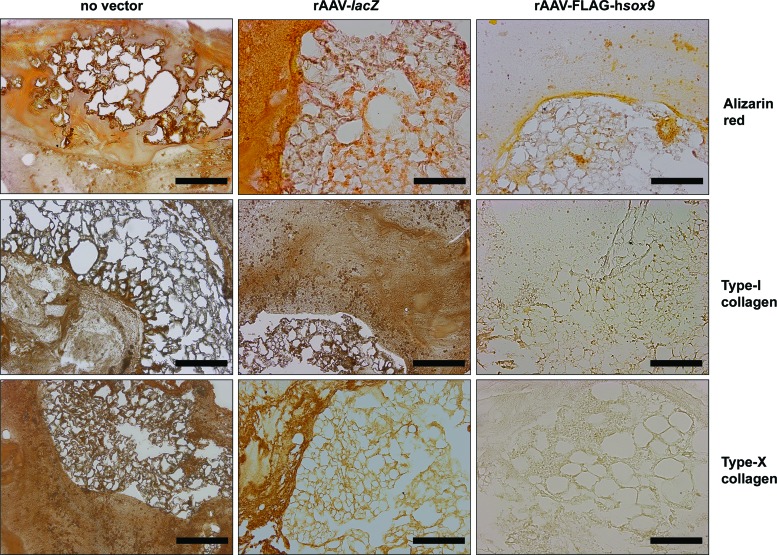
Gene transfer via rAAV-FLAG-hsox9 reduced both matrix mineralization and the production of type-I and -X collagen, as seen by lower alizarin red staining and type-I and -X collagen immunostaining relative to the controls (Fig. 4). This observation was corroborated by results of a histomorphometric analysis using the similar grading system as employed for cartilage matrix components (2.8-, 2.5-, and 3.3-fold decrease in alizarin red staining, type-I and -X collagen immunostaining, respectively; p ≤ 0.001; Table 1).
Figure 4.
Hypertrophic and terminal differentiation in rAAV-transduced human bone-marrow aspirates seeded in 3D-woven PCL scaffolds. Aspirates were transduced with rAAV-lacZ or rAAV-FLAG-hsox9 or left untreated and seeded in the scaffolds in fibrinogen/thrombin using chondrogenic medium, as described in Figs. 1–3 and in the Methods. The samples were processed after 21 days for histological staining with alizarin red and to detect immunoreactivity to type I and X collagen (magnification 10 × ; all representative data). Scale bars: 200 mm. Color images available online at www.liebertpub.com/hum
Real-time RT-PCR gene expression analyses in human bone-marrow aspirates seeded in 3D-woven PCL scaffolds
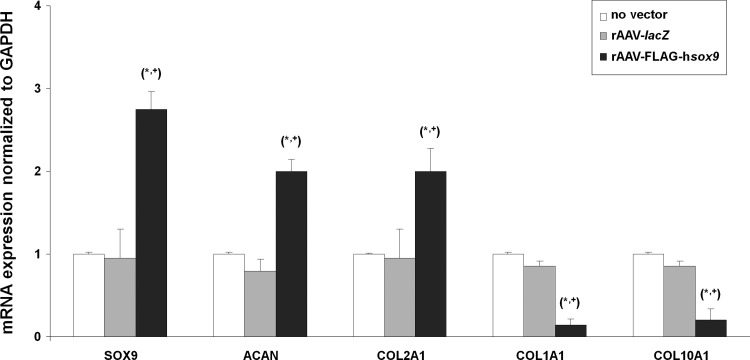
Overall, the previous findings were corroborated by results of a real-time RT-PCR analysis that evaluated the gene expression profiles in the samples over the period of chondrogenic induction. Specifically, enhanced chondrogenic differentiation was noted in the samples when applying rAAV-FLAG-hsox9 relative to the controls (2.9-, 2.5-, and 2.1-fold increased SOX9, ACAN, and type-II collagen expression levels, respectively; p ≤ 0.001; Fig. 5). Instead, reduced osteogenic/hypertrophic differentiation was observed upon SOX9 gene transfer compared with the controls (6.7- and 5-fold decreased type-I and type-X collagen expression levels, respectively; p ≤ 0.001; Fig. 5).
Figure 5.
Real-time reverse transcription polymerase chain reaction analysis in rAAV-transduced human bone-marrow aspirates seeded in 3D-woven PCL scaffolds. Aspirates were transduced with rAAV-lacZ or rAAV-FLAG-hsox9 or left untreated and seeded in the scaffolds in fibrinogen/thrombin using chondrogenic medium, as described in Figs. 1–4 and in the Methods. The samples were processed after 21 days to monitor the gene expression profiles of SOX9, aggrecan (ACAN), type-II (COL2A1), type-I (COL1A1), and type-X collagen (COL10A1), with GAPDH serving as a housekeeping gene and internal control (all primers are listed in the Methods). Ct values were obtained for each target and for GAPDH as a control for normalization, and fold inductions (relative to the untreated samples) were measured by using the 2–ΔΔCt method. Statistically significant compared with *no vector treatment and with +rAAV-lacZ.
Discussion
The use of gene therapy combined with tissue engineering strategies has strong potential for the development of novel treatments capable of effectively enhancing the repair of damaged articular cartilage by targeting bone-marrow chondroregenerative MSCs.18–20 The present study tested the feasibility of delivering the cartilage-specific and highly chondrogenic transcription factor SOX931 to chondrogenically competent human bone-marrow concentrates9 via clinically relevant rAAV gene transfer vectors.39 These bone-marrow aspirates were seeded onto 3D-woven PCL scaffolds that provide a cartilage-like environment in terms of their mechanical properties42 relative to a more classical, less stable, scaffold-free gene delivery system.41 The study findings indicate that cells within bone-marrow aspirates, which are easily accessible during surgery, can be readily transduced via rAAV vectors to overexpress SOX9, and that this expression significantly enhances chondrogenesis of bone-marrow aspirates within mechanically functional 3D-woven scaffolds.
The results first indicate that human bone-marrow aspirates can be effectively modified by rAAV vectors within 3D-woven PCL scaffolds with elevated transduction efficiencies (∼100% with reporter genes) over an extended period of time (at least 75 days, the longest time point evaluated), showing the stability of rAAV-mediated gene transfer afforded using this class of biomaterial. Successful SOX9 overexpression via rAAV was also significantly achieved in the constructs compared with control treatments (reporter rAAV-lacZ gene transfer, lack of vector application) for at least 21 days, a time point suitable to appraise chondrogenic differentiation events in marrow concentrates,41 in good agreement with the findings in scaffold-free gene transfer conditions.41 While initially all cell populations forming the aspirates may be transduced by the vectors (MSCs, fibroblasts, hematopoietic cells), under stable and prolonged chondrogenic induction as performed here, only the MSCs with a specific ability to differentiate in chondrocytes in this environment41,47,48 may overexpress the various rAAV transgenes in the samples after 21 days.
The data further show that effective, prolonged rAAV-mediated SOX9 overexpression significantly enhanced the anabolic and chondrogenic activities in PCL-seeded aspirates (deposition of matrix proteoglycans and type II collagen) for at least 21 days relative to the controls, without affecting the indexes of proliferation, again concordant with the observations in a scaffold-free system41 and with the properties of the transcription factor.31,34,35,37,40 Again, after 21 days of continuous chondrogenic induction, the cells in the aspirates responsible for these effects may mostly be the MSCs that may specifically commit toward the chondrogenic phenotype. Most notably, administration of the rAAV SOX9 vector led to a significant, beneficial decrease in the levels of undesirable hypertrophic and osteogenic differentiation versus control treatments in the constructs over time (matrix mineralization, deposition of type-I and -X collagen), also consistent with the properties of SOX932–34,40 and with the previous findings using scaffold-free cultures.41
For comparison, and in marked contrast with the present findings using rAAV, chondrogenic effects of SOX9 gene transfer have been reported using other types of biocompatible materials such as alginates,35 polyglycolic acid,37 polylactic-co-glycolic acid,49 and a fibrin-polyurethane composite scaffold36 in isolated MSCs either over short-term periods of time using less effective (nonviral vectors) or more toxic/immunogenic gene vehicles (adenoviral vectors; 7–14 days),35,37,49 or solely upon additional mechanical loading.36
The present evaluation provides evidence of effective and prolonged SOX9 gene transfer and overexpression in concentrated MSCs seeded in functional scaffolds as a convenient strategy to enhance the processes of chondrogenic differentiation adapted for cartilage repair using rAAV vectors suited for human regenerative medicine.39 Work is currently ongoing to test the benefits of implanting such constructs within clinically relevant, experimental orthotopic cartilage lesions in vivo37,50 as a step toward clinical translation. In conclusion, this study demonstrates the value of combining rAAV-mediated gene transfer with a scaffold-based strategy to modify bone-marrow aspirates as a workable platform for the treatment of cartilage injury and disease.
Acknowledgments
This research was funded by a grant from the Collaborative Research Partner Acute Cartilage Injury Program of AO Foundation, Davos, Switzerland (M.C., H.M., and F.G.) and NIH grant AR66439. We thank R.J. Samulski (The Gene Therapy Center, University of North Carolina, Chapel Hill, NC), X. Xiao (The Gene Therapy Center, University of Pittsburgh, Pittsburgh, PA), and E.F. Terwilliger (Division of Experimental Medicine, Harvard Institutes of Medicine and Beth Israel Deaconess Medical Center, Boston, MA) for providing the genomic AAV-2 plasmid clones and the 293 cell line, and G. Scherer (Institute for Human Genetics and Anthropology, Albert-Ludwig University, Freiburg, Germany) for the human sox9 cDNA.
Author Disclosure
F.T.M., B.T.E., and F.G. are employees of Cytex Therapeutics, Inc. No competing financial interests exist for the remaining authors.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum 1998;41:1331–1342 [DOI] [PubMed] [Google Scholar]
- 2.O'Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am 1998;80:1795–1812 [PubMed] [Google Scholar]
- 3.Safran MR, Seiber K. The evidence for surgical repair of articular cartilage in the knee. J Am Acad Orthop Surg 2010;18:259–266 [DOI] [PubMed] [Google Scholar]
- 4.Cucchiarini M, Madry H, Guilak F, et al. . A vision on the future of articular cartilage repair. Eur Cell Mater 2014;27:12–16 [DOI] [PubMed] [Google Scholar]
- 5.Mackay AM, Beck SC, Murphy JM, et al. . Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 1998;4:415–428 [DOI] [PubMed] [Google Scholar]
- 6.Yoo JU, Barthel TS, Nishimura K, et al. . The chondrogenic potential of human bone marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am 1998;80:1745–1757 [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, et al. . Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 [DOI] [PubMed] [Google Scholar]
- 8.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 2004;36:568–584 [DOI] [PubMed] [Google Scholar]
- 9.Orth P, Cucchiarini M, Wagenpfeil S, et al. . PTH [1–34]-induced alterations of the subchondral bone provoke early osteoarthritis. Osteoarthritis Cartilage 2014;22:813–821 [DOI] [PubMed] [Google Scholar]
- 10.Wakitani S, Mitsuoka T, Nakamura N, et al. . Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant 2004;13:595–600 [DOI] [PubMed] [Google Scholar]
- 11.Slynarski K, Deszczynski J, Karpinski J. Fresh bone marrow and periosteum transplantation for cartilage defects of the knee. Transplant Proc 2006;38:318–319 [DOI] [PubMed] [Google Scholar]
- 12.Wakitani S, Okabe T, Horibe S, et al. . Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Reg Med 2011;5:146–150 [DOI] [PubMed] [Google Scholar]
- 13.Gigante A, Cecconi S, Calcagno S, et al. . Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc Tech 2012;1:e175–e180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orozco L, Munar A, Soler R, et al. . Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation 2013;95:1535–1541 [DOI] [PubMed] [Google Scholar]
- 15.Kim JD, Lee GW, Jung GH, et al. . Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol 2014;24:1505–1511 [DOI] [PubMed] [Google Scholar]
- 16.Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am 2010;92:994–1009 [DOI] [PubMed] [Google Scholar]
- 17.Kalson NS, Gikas PD, Briggs Current strategies for knee cartilage repair. Int J Clin Pract 2010;64:1444–1452 [DOI] [PubMed] [Google Scholar]
- 18.Johnstone B, Alini M, Cucchiarini M, et al. . Tissue engineering for articular cartilage repair—the state of the art. Eur Cell Mater 2013;25:248–267 [DOI] [PubMed] [Google Scholar]
- 19.Cucchiarini M, Madry H. Use of tissue engineering strategies to repair joint tissues in osteoarthritis: viral gene transfer approaches. Curr Rheumatol Rep 2014;16:449. [DOI] [PubMed] [Google Scholar]
- 20.Frisch J, Venkatesan JK, Rey-Rico A, et al. . Current progress in stem cell-based gene therapy for articular cartilage repair. Curr Stem Cell Res Ther 2015;10:121–131 [DOI] [PubMed] [Google Scholar]
- 21.Kawamura K, Chu CR, Sobajima S, et al. . Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol 2005;33:865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagnotto MR, Wang Z, Karpie JC, et al. . Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther 2007;14:804–813 [DOI] [PubMed] [Google Scholar]
- 23.Steinert AF, Palmer GD, Pilapil C, et al. . Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer. Tissue Eng Part A 2009;15:1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cucchiarini M, Ekici M, Schetting S, et al. . Metabolic activities and chondrogenic differentiation of human mesenchymal stem cells following recombinant adeno-associated virus-mediated gene transfer and overexpression of fibroblast growth factor 2. Tissue Eng Part A 2011;17:1921–1933 [DOI] [PubMed] [Google Scholar]
- 25.Liu TM, Guo XM, Tan HS, et al. . Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum 2011;63:2711–2720 [DOI] [PubMed] [Google Scholar]
- 26.Haleem-Smith H, Calderon R, Song Y, et al. . Cartilage oligomeric matrix protein enhances matrix assembly during chondrogenesis of human mesenchymal stem cells. J Cell Biochem 2012;113:1245–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinert AF, Weissenberger M, Kunz M, et al. . Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther 2012;14:R168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann AJ, Alini M, Archer CW, et al. . Chondrogenesis of human bone marrow–derived mesenchymal stem cells is modulated by complex mechanical stimulation and adenoviral-mediated overexpression of bone morphogenetic protein 2. Tissue Eng Part A 2013;19:1285–1294 [DOI] [PubMed] [Google Scholar]
- 29.Frisch J, Venkatesan JK, Rey-Rico A, et al. . Influence of insulin-like growth factor I overexpression via recombinant adeno-associated vector gene transfer upon the biological activities and differentiation potential of human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther 2014;5:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frisch J, Venkatesan JK, Rey-Rico A, et al. . Determination of the chondrogenic differentiation processes in human bone marrow-derived mesenchymal stem cells genetically modified to overexpress transforming growth factor-beta via recombinant adeno-associated viral vectors. Hum Gene Ther 2014;25:1050–1060 [DOI] [PubMed] [Google Scholar]
- 31.Bi W, Deng JM, Zhang Z, et al. . Sox9 is required for cartilage formation. Nat Genet 1999;22:85–89 [DOI] [PubMed] [Google Scholar]
- 32.Akiyama H, Lyons JP, Mori-Akiyama Y, et al. . Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 2004;18:1072–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung VY, Gao B, Leung KK, et al. . SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet 2011;7:e1002356-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchiya H, Kitoh H, Sugiura F, et al. . Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Comm 2003;301:338–343 [DOI] [PubMed] [Google Scholar]
- 35.Babister JC, Tare RS, Green DW, et al. . Genetic manipulation of human mesenchymal progenitors to promote chondrogenesis using “bead-in-bead” polysaccharide capsules. Biomaterials 2008;29:58–65 [DOI] [PubMed] [Google Scholar]
- 36.Kupcsik L, Stoddart MJ, Li Z, et al. . Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A 2010;16:1845–1855 [DOI] [PubMed] [Google Scholar]
- 37.Cao L, Yang F, Liu G, et al. . The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials 2011;32:3910–3920 [DOI] [PubMed] [Google Scholar]
- 38.Li H, Haudenschild DR, Posey KL, et al. . Comparative analysis with collagen type II distinguishes cartilage oligomeric matrix protein as a primary TGFβ-responsive gene. Osteoarthritis Cartilage 2011;19:1246–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cucchiarini M. Human gene therapy: novel approaches to improve the current gene delivery systems. Discov Med 2016;21:495–506 [PubMed] [Google Scholar]
- 40.Venkatesan JK, Ekici M, Madry H, et al. . SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res Ther 2012;3: 22–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rey-Rico A, Frisch J, Venkatesan JK, et al. . Determination of effective rAAV-mediated gene transfer conditions to support chondrogenic differentiation processes in human primary bone marrow aspirates. Gene Ther 2015;22:50–57 [DOI] [PubMed] [Google Scholar]
- 42.Moutos FT, Guilak F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng Part A 2010;16:1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Recha-Sancho L, Moutos FT, Abellà J, et al. . Dedifferentiated human articular chondrocytes redifferentiate to a cartilage-like tissue phenotype in a poly(ɛ-caprolactone)/self-assembling peptide composite scaffold. Materials 2016;9:472–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samulski RJ, Chang LS, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol 1987;61:3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol 1989;63:3822–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rey-Rico A, Venkatesan JK, Sohier J, et al. . Adapted chondrogenic differentiation of human mesenchymal stem cells via controlled release of TGF-beta1 from poly(ethylene oxide)-terephtalate/poly(butylene terepthalate) multiblock scaffolds. J Biomed Mater Res Part A 2015;103:371–383 [DOI] [PubMed] [Google Scholar]
- 47.Lennon DP, Haynesworth SE, Arm DM, et al. . Dilution of human mesenchymal stem cells with dermal fibroblasts and the effects on in vitro and in vivo osteochondrogenesis. Dev Dyn 2000;219:50–62 [DOI] [PubMed] [Google Scholar]
- 48.Anam K, Davis TA. Comparative analysis of gene transcripts for cell signaling receptors in bone marrow-derived hematopoietic stem/progenitor cell and mesenchymal stromal cell populations. Stem Cell Res Ther 2013;4:112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Li J, Wang E, et al. . Dynamic compression combined with SOX-9 overexpression in rabbit adipose-derived mesenchymal stem cells cultured in a three-dimensional gradual porous PLGA composite scaffold upregulates HIF-1alpha expression. J Biomed Mater Res Part A 2015;103:3886–3895 [DOI] [PubMed] [Google Scholar]
- 50.Cucchiarini M, Orth P, Madry H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J Mol Med 2013;91:625–636 [DOI] [PubMed] [Google Scholar]