Abstract
Background
Liver transplantation (LT) using extended criteria donor (ECD) grafts is frequently associated with a high flush fluid potassium concentration (FFK) and acute hyperkalemia after reperfusion, which puts patients at greater risk of postreperfusion cardiac arrest (PRCA).
Case Report
Herein, we present a case with an extremely high FFK that was successfully pretreated to avoid the risk of PRCA. A 3-year-old boy with biliary atresia underwent LT from a 623-g donation after brain death liver graft with localized frostbite on the right lobe surface. The FFK was 18.8 mmol/L after flushing with 1000 mL of 5% albumin. To prevent PRCA due to acute hyperkalemia, further portal vein (PV) flush, retrograde reperfusion via the inferior vena cava, and antegrade reperfusion via the PV were adopted to remove the excessive potassium ions. Ultimately, the liver graft was reperfused when the perfused blood potassium concentration was 7.5 mmol/L without subsequent development of PRCA during the immediate reperfusion period. Nevertheless, the patient still experienced vasoplegic syndrome during the late reperfusion period.
Conclusions
Our case illustrates that the FFK measurement is helpful for identifying ECD-related hyperkalemia and for providing advance warning of PRCA. Future investigations are warranted to confirm the relationship between high FFK and PRCA and to observe the effectiveness of other interventions to prevent PRCA due to ECD-related hyperkalemia.
MeSH Keywords: Heart Arrest, Induced; Hyperkalemia; Liver Transplantation; Pediatrics
Background
Postreperfusion cardiac arrest (PRCA) is a dreaded complication of liver transplantation (LT) for both transplant surgeons and anesthesiologists and has been considered the most severe form of postreperfusion syndrome (PRS) with various causes [1–10]. Hyperkalemic preservation solution released from the liver allograft after reperfusion is an acknowledged and well-controlled risk factor for PRCA. However, extended criteria donor (ECD)-related hyperkalemia is usually unheeded in the development of PRCA in LT. Predicting and preventing PRCA during LT using ECD liver grafts remains a challenge. We provide an effective approach for preventing potential PRCA before reperfusion of a high-risk liver graft under the guidance of flushed fluid potassium concentration (FFK) measurements.
Case Report
A 3-year-old boy (height 93 cm, weight 14 kg) with end-stage liver disease (ESLD) from biliary atresia and a pediatric end-stage liver disease score of 7 was listed for LT. Recurrent esophageal variceal hemorrhage, ascites, and splenomegaly had complicated his ESLD. His medical history was significant only for a previous portoenterostomy procedure, and showed no history of congenital heart disease, diabetes mellitus, pneumonia, or acute upper respiratory tract infection. After the patient was transferred to the operating room, routine monitors, including electrocardiogram, pulse oximetry, and non-invasive blood pressure monitors, were established. General anesthesia was induced with titrated midazolam, fentanyl, cisatracurium and propofol and was maintained with sevoflurane in oxygen, remifentanil, and cisatracurium infusions. The trachea was orally intubated without any difficulty, and the patient was mechanically ventilated using pressure control ventilation. Mechanical ventilation was adjusted to maintain the end-tidal carbon dioxide concentration between 35 and 45 mmHg using a 60% oxygen/air mixture. Additional vascular access for invasive monitoring and transfusion was obtained with an arterial line in the left radial artery and a 5.5 French triple-lumen central venous catheter in the right internal jugular vein. The patient was protected from hypothermia by forced-air warming systems (Bair Hugger, 3 M, St. Paul, MN, USA) throughout the procedure. Additionally, a cell saver (CATS, Fresenius Kabi, Bad Homburg, Germany) was used to remove potassium from banked red blood cells (RBCs) and salvaged blood from surgical bleeding.
The donor liver was procured from a 7-year-old girl who died of cerebral trauma through standard donation after brain death process. The graft weighed 623 g and had a normal color, and the cold ischemia time (CIT) was 7 hours upon arrival at our center. However, we considered that localized frostbite might have occurred during transport due to an ice block in the preservation solution, and the right lobe was noted to have a rubbery feel. Because the frostbitten region was relatively small and returned to normal a few minutes later, the operation was cleared to proceed by the surgical team. The recipient hepatectomy was uneventful, and the graft was transplanted using a standard inferior vena cava (IVC) replacement technique without venovenous bypass. The patient remained hemodynamically stable after the portal vein (PV), infrahepatic vena cava (IHVC), and suprahepatic vena cava (SHVC) were clamped. The IHVC anastomosis was initiated when the SHVC anastomosis was completed, and the graft was flushed via the PV with a room temperature albumin solution [11–13]. Unfortunately, the appearance of the flush fluid from the IHVC was slightly turbid (Figure 1) after flushing with 600 mL of 5% albumin. Consequently, an additional 400 mL of 5% albumin was flushed, and the flush fluid from the graft gradually cleared. Soon afterward, the FFK was measured as 18.8 mmol/L, which likely would have resulted in acute hyperkalemia and PRCA after reperfusion.
Figure 1.
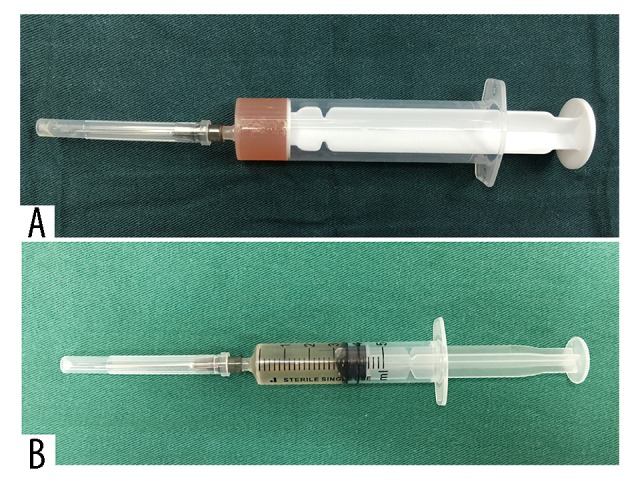
(A) The abnormal flush fluid appears turbid. (B) The normal flush fluid appears clear.
After an urgent consult with the surgical and anesthesia teams, the FFK decreased to 9.4 mmol/L after further PV flush with 1200 mL of normal saline. Subsequently, the IHVC anastomosis was completed. During the PV anastomosis, the graft was retrograde-perfused via the IVC with autologous blood by declamping the SHVC and IHVC [14,15]. When the PV anastomosis was nearly completed, the perfused blood potassium concentration was measured. The result was 16.6 mmol/L of potassium after perfusion with 100 mL of autologous blood. As a consequence, the PV was not declamped, and antegrade reperfusion [12,13] via the PV appeared to be the only effective option to remove the potassium from the graft. After the IHVC and SHVC were clamped again and 3 stitches were removed from the anterior wall of the IHVC, the PV was declamped for reperfusion, and the hyperkalemic blood from the graft was drained from the IHVC and salvaged by the cell saver instead of being returned to the patient’s heart. The perfused blood potassium concentration was gradually decreased to 7.5 mmol/L after perfusion with 600 mL of autologous blood (Table 1). The PV was then reclamped, and the anterior wall of the IHVC was resutured. The graft was reperfused again when the residual IHVC anastomosis was completed.
Table 1.
Evolution of the potassium level of the perfused fluid after different treatment strategies.
| PV flush | PV flush | Retrograde reperfusion | Antegrade reperfusion | Antegrade reperfusion | Antegrade reperfusion | |
|---|---|---|---|---|---|---|
| Perfused fluid | 5% albumin | Normal saline | Autologous blood | Autologous blood | Autologous blood | Autologous blood |
| Volume (mL) | 1000 | 1200 | 100 | 50 | 350 | 200 |
| Sampling location | IHVC | IHVC | PV | IHVC | IHVC | IHVC |
| K (mmol/L) | 18.8 | 9.4 | 16.6 | 18.5 | 11.8 | 7.5 |
PV – portal vein; IHVC – infrahepatic vena cava; K – serum potassium concentration.
The patient did not experience a PRCA during the immediate reperfusion period. However, the patient’s arterial blood pressure gradually decreased from 76/38 mmHg to 62/31 mmHg and was effectively treated with boluses of 3 μg of epinephrine, 20 μg of phenylephrine, and 100 mg of calcium chloride boluses. Simultaneously, the arterial blood gas analysis showed an increase in the serum potassium level from 3.6 to 4.3 mmol/L without a noteworthy widening of the QRS waves, bradycardia, or PRCA. Subsequently, the patient experienced persistent severe hypotension and anuria despite an increase in the dose of norepinephrine from 0.03 to 0.50 μg/kg/min in the late reperfusion period. Therefore, an infusion of pituitrin was started as a consequence of the suspected diagnosis of vasoplegic syndrome. Approximately 5 minutes later, the blood pressure gradually increased from 67/34 mmHg to 101/46 mmHg. Soon afterward, the patient’s urine output gradually increased to more than 1 mL/kg/min, and the requirement for norepinephrine was reduced to 0.20 μg/kg/min until the end of the surgery (Table 2).
Table 2.
The hemodynamics, temperature, electrolyte, and blood gas measurements during the procedure.
| Preanhepatic phase | Anhepatic phase | Neohepatic phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before incision | Before PV clamp | 1 minute | 35 minutes | 70 minutes | 1 minute | 60 minutes | 120 minutes | 225 minutes | |
| HR (bpm) | 107 | 107 | 114 | 102 | 109 | 109 | 103 | 105 | 109 |
| MAP (mmHg) | 68 | 63 | 61 | 62 | 51 | 41 | 45 | 57 | 63 |
| CVP (mmHg) | 8 | 10 | 8 | 7 | 6 | 9 | 8 | 10 | 9 |
| T (°C) | 36.0 | 35.6 | 35.5 | 34.8 | 35.4 | 35.4 | 35.9 | 36.0 | 37.0 |
| pH | 7.38 | 7.34 | 7.40 | 7.43 | 7.47 | 7.32 | 7.37 | 7.35 | 7.38 |
| PaCO2 (mmHg) | 36 | 35 | 35 | 32 | 35 | 37 | 39 | 43 | 43 |
| PaO2 (mmHg) | 568 | 323 | 349 | 520 | 511 | 509 | 349 | 330 | 475 |
| BE (mmol/L) | −3.5 | −6.2 | −2.7 | −2.7 | 1.8 | −6.4 | −2.5 | −1.8 | 0.2 |
| Na (mmol/L) | 134 | 137 | 142 | 142 | 146 | 142 | 145 | 146 | 146 |
| K (mmol/L) | 3.3 | 3.7 | 3.5 | 3.8 | 3.6 | 4.3 | 2.8 | 3.4 | 3.5 |
| Ca (mmol/L) | 1.19 | 1.13 | 1.06 | 1.21 | 1.16 | 1.42 | 1.04 | 1.05 | 1.11 |
| GLU (mmol/L) | 5.1 | 3.9 | 3.9 | 2.8 | 3.2 | 5.2 | 7.9 | 7.8 | 7.5 |
| LAC (mmol/L) | 2.3 | 2.5 | 3.6 | 4.1 | 4.3 | 5.1 | 6.1 | 5.2 | 3.5 |
| HCT (%) | 20 | 25 | 26 | 24 | 22 | 31 | 28 | 25 | 23 |
| NE (μg/kg/min) | 0.06 | 0.03 | 0.03 | 0.03 | 0.20 | 0.50 | 0.20 | 0.20 | None |
| Pituitrin (U/h) | None | None | None | None | None | None | 1 | 1 | None |
BE – base excess; Ca – serum calcium concentration; CVP – central venous pressure; GLU – blood glucose concentration; HCT – hematocrit; HR – heart rate; K – serum potassium concentration; LAC – blood lactate concentration; MAP – mean arterial pressure; Na – serum sodium concentration; NE – norepinephrine; PaCO2 – arterial carbon dioxide pressure; PaO2 – arterial oxygen pressure; PV – portal vein; T – body temperature.
The remainder of the surgery was completed without any incident. The total duration of the surgery was 435 minutes, and the estimated blood loss was approximately 1100 mL. The patient received 800 mL of packed RBCs, 200 mL of salvaged RBCs, and 400 mL of fresh frozen plasma during the procedure. After surgery, the patient was admitted to the intensive care unit (ICU) without the use of vasopressors and was extubated 1.5 hours later. However, the patient experienced early allograft dysfunction with a temporary increase in the aspartate aminotransferase level to 9339 IU/L. The patient was shifted from the ICU on postoperative day (POD) 5 and was discharged on POD 78. The reason for the prolonged hospitalization was the occurrence of a cytomegalovirus infection. Currently, 16 months postsurgery, the patient is well and shows normal liver function.
Discussion
PRCA is a rare but catastrophic intraoperative complication during LT, with a reported incidence of 0.5–3.2% [1–4]. The occurrence of a PRCA is unpredictable, and the underlying etiology, which includes hyperkalemia, pulmonary embolism, anaphylaxis, intracardiac thrombus, and acute coronary events, is complex and not well understood [1–10]. Recently, considerable attention has been focused on the high incidence of PRCA during LT using ECD liver grafts, and ECD-related hyperkalemia might play a crucial role [16,17]. A previous study [18] demonstrated that high FFK was associated with both severe hypotension and significant arrhythmia during the immediate reperfusion period in LT. In this paper, we report the successful prevention of a case of PRCA with an extremely high FFK when an ECD liver graft was implanted.
Because both PRCA and PRS occur within a few minutes after reperfusion of the donor liver, the causes and manifestations of PRS and PRCA are sometimes confused with one another. PRCA is commonly defined by loss of a spontaneous heartbeat and requires either external or direct cardiac massage after reperfusion of the liver graft [2], while PRS is typically denoted as cardiovascular collapse following liver graft reperfusion [19]. The etiologies of PRCA and PRS remain unclear; the latter is a multifactorial complication [10–23], whereas the former is generally caused by a specific cause, such as PRS with and without hyperkalemia and pulmonary embolism [5–10]. Additionally, PRCA is much less common than PRS and is usually regarded as the most serious form of PRS in LT.
Recently, LTs from donation after circulatory death (DCD) liver grafts have increased notably every year [24]. However, DCD liver grafts are always regarded as marginal donors or ECDs. Other ECDs include liver grafts with an older donor age, a higher donor serum sodium level, a higher degree of steatosis, and a prolonged CIT or warm ischemia time [20,22,23], while a frostbitten liver graft is an infrequent kind of ECD and may result in liver allograft primary nonfunction [25]. We currently encounter PRS and PRCA more frequently in LT using ECD liver grafts, despite the routine application of the PV flush and speed control reperfusion techniques [23,26]. Moreover, we occasionally encounter extremely high serum potassium levels after successful resuscitation of PRS and PRCA [16–18], and the release of intracellular potassium from the damaged hepatocytes of ECD liver grafts are the most likely cause (Table 3).
Table 3.
Etiology, treatments, and outcomes of reported cases of PRCA during liver transplantation.
| Refs. | Age/gender | Diagnosis | Co-existing diseases | Donor condition | ECD liver graft | Suspected cause of PRCA | Rise in K/hyperkalemia | Treatments | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Shi et al. [4] | 52/NA | Cirrhosis | NA | Deceased | NA | PE | Yes/No | CPR (undescribed) | Survived |
| 49/NA | HCC | NA | Deceased | NA | NA | Yes/No | CPR (undescribed) | Survived | |
| 45/NA | Hepatitis B cirrhosis | NA | Deceased | NA | NA | No/No | CPR (undescribed) | Death in ICU | |
| 51/NA | HCC, cirrhosis | NA | Deceased | NA | Hyperkalemia | Yes/Yes | CPR (undescribed) | Death in OR | |
| 49/NA | HCC | NA | Deceased | NA | Hyperkalemia | Yes/Yes | CPR (undescribed) | Death in OR | |
| Aufhauser et al. [1] | NA/NA | NA/NA | 3/16 DM, 5/16 PPHTN, 7/16 CRRT | Deceased, 2/16 DCD and 1/16 required ECMO | 3/16 | 14/16 PRS and 2/16 PE | NA/4/16 | 2/16 ECCM+ ED 14/16 TDOCM (7/14 CPB) |
Death in OR (3/16) Death in ICU (3/16) |
| Ulukaya et al. [7] | 66/M | Alcoholic cirrhosis | ST-T changes | Deceased, mild steatosis | Yes | PRS (hyperkalemia) | NA/NA | TDOCM | Survived |
| Vannucci et al. [8] | 74/F | Primary biliary cirrhosis | Hypothyroidism | Deceased | NA | Hyperkalemia | Yes/Yes | ECCM, TDCM, ED | Death in ICU |
| Tejani et al. [30] | 61/M | HCC, hepatitis B cirrhosis | No | Deceased | NA | PRS | Yes/No | ECCM, TDOCM, ID, ECMO | Survived |
| Lee et al. [3] | 53/F | Hepatitis B cirrhosis | DM, HRS | Deceased | NA | PRS (hyperkalemia) | Yes/Yes | CPR (undescribed) | Survived |
| 38/M | Hepatitis B cirrhosis | No | Deceased | NA | PRS | NA/NA | CPR (undescribed) | Death in ICU | |
| 32/F | HCC, hepatitis B cirrhosis | HE | Deceased | NA | PRS (hyperkalemia) | Yes/Yes | CPR (undescribed) | Survived | |
| 47/M | HCC, hepatitis B cirrhosis | No | Deceased, mild steatosis | Yes | PRS (hyperkalemia) | Yes/Yes | CPR (undescribed) | Survived | |
| 48/M | Alcoholic, hepatitis B cirrhosis | HE, HRS | Deceased, mild steatosis | Yes | PRS | NA/NA | CPR (undescribed) | Death in ICU | |
| Schnüriger et al. [5] | 44/M | Hepatitis C cirrhosis | NA | NA | NA | Hyperkalemia | Yes/Yes | TDOCM, ECMO | Survived |
| 63/M | Alpha-1 antitrypsin deficiency | NA | NA | NA | Hyperkalemia | Yes/Yes | TDOCM, ED or ID | Death in OR | |
| 67/M | Nonalcoholic steatohepatitis | NA | NA | NA | Hyperkalemia | Yes/Yes | TDOCM, ED or ID | Death in OR | |
| 64/F | Hepatic echinococcosis | NA | NA | NA | Hyperkalemia | Yes/Yes | TDOCM | Death in OR | |
| Andjelić et al. [10] | 54/M | Hepatitis B cirrhosis | No | Deceased | NA | Anaphylaxis | NA/NA | ECCM, ED | NA |
| Wang et al. [32] | 54/M | Hepatitis B cirrhosis | No | Living, right lobe | NA | VF, MI | NA/NA | ECCM, ED, TDOCM, ID, IABP | Death in ICU |
| Kim et al. [9] | 61/M | HCC, hepatitis C cirrhosis | No | Deceased, 2 h of hypoxemia | Yes | Intracardiac thrombus | Yes/No | TDCM, ED | Survived |
| Shah et al. [29] | 54/M | Cirrhosis | No | Deceased, required ECMO | Yes | PRS (hyperkalemia) | Yes/Yes | ECCM, TDOCM, ED, CPB | Survived |
CPB – cardiopulmonary bypass; CPR – cardiopulmonary resuscitation; CRRT – continuous renal replacement therapy; DM – diabetes mellitus; ECCM – external chest cardiac compression; ECD – expanded criteria donor; ECMO – extracorporeal membrane oxygenation; ED – external defibrillation; HCC – hepatocellular carcinoma; HE – hepatoencephalopathy; HRS – hepatorenal syndrome; IABP – intra-aortic balloon pump; ICU – Intensive Care Unit; ID – internal defibrillation; K – serum potassium concentration; MI – myocardial infarction; NA – data not available; OR – operating room; PE – pulmonary embolism; PPHT – portopulmonary hypertension; PRCA – postreperfusion cardiac arrest; PRS – postreperfusion syndrome; TDCM – transdiaphragmatic cardiac compression; TDOCM – transdiaphragmatic open cardiac massage; VF – ventricular fibrillation.
Hyperkalemia in LT usually results from the transfusion of banked RBCs, which have a potassium concentration of 76 mmol/L after 35 days of cold storage [27]. This problem has been solved in our center through the use of a cell saver to remove potassium from the banked RBCs. Another important source of potassium might be derived from the University of Wisconsin (UW) preservation solution [28]. However, graft washout techniques, including the most widely used techniques (PV flush, retrograde reperfusion, and antegrade reperfusion), have been shown to reduce the incidences of PRS and PRCA [11–15,21]. Importantly, all the washout techniques remove not only the preservation solution but also the necrotic elements of the damaged hepatocytes. A previous study [11] found that PV flush with 500 mL of 5% albumin is sufficient to remove 90% of the UW preservation solution and to avoid hyperkalemia after reperfusion in the adult population. The type, volume, and temperature of the flush fluid vary based on center-specific experiences. A recent study [18] found that FFK ≥6.75 mmol/L is associated with significant arrhythmias and persistent severe hypotension in adult LT. The most likely reason for the extremely high FFK is hepatocyte injury of the ECD liver grafts. Therefore, the flush fluid volume might be adjusted when an ECD liver graft is used, and the FFK should be routinely monitored in LT using ECD liver grafts.
Some institutes employ a speed-control reperfusion technique by manually controlling the reperfusion speed, which has been proposed as an effective method for the prevention of PRS and PRCA [23,26]. The results are in accordance with the changes in the serum potassium concentration after reperfusion. A transient 2- to 3-fold increase in the potassium level has been reported to occur immediately after reperfusion; the potassium level then returns to normal in 1 to 2 minutes due to uptake by the new liver [27,29]. The speed-control reperfusion technique avoids the sudden rise in the serum potassium level, thus preventing PRS and PRCA due to acute hyperkalemia.
Over the past decade, many studies have focused on the treatment of PRCA during LT. Newly developed methods include standard cardiopulmonary resuscitation with external chest compression or direct cardiac massage, external or internal defibrillation, cardiopulmonary bypass, and extracorporeal membrane oxygenation support [5–10,30–32]. However, very few studies have focused on the prediction and prevention of PRCA during LT [33]. Here, we report the first successfully pretreated case of PRCA due to ECD-related hyperkalemia. To the best of our knowledge, the quality of the donor liver might play a crucial role in the development of PRCA during the reperfusion phase of LT, and the main reason for PRCA during LT using ECD liver grafts might be the hyperkalemic blood released from the damaged hepatocytes during organ retrieval, preservation, and transport.
Conclusions
Our case illustrates that FFK measurement is quite helpful for identifying ECD-related hyperkalemia and should be routinely monitored in LTs using ECD liver grafts.
Abbreviations
- CIT
cold ischemia time
- DCD
donation after circulatory death
- ECD
extended criteria donor
- ESLD
end-stage liver disease
- FFK
flush fluid potassium concentration
- ICU
intensive care unit
- IHVC
infrahepatic vena cava
- IVC
inferior vena cava
- LT
liver transplantation
- POD
postoperative day
- PRCA
postreperfusion cardiac arrest
- PRS
postreperfusion syndrome
- PV
portal vein
- RBC
red blood cell
- SHVC
suprahepatic vena cava
- UW
University of Wisconsin
Footnotes
Source of support: The study was supported by Beijing Municipal Administration of Hospitals Ascent Plan (Code: DFL20150101) and Scientific Research Key Program of Beijing Municipal Commission of Education (NO.KZ201510025026)
References
- 1.Aufhauser DD, Jr, Rose T, Levine M, et al. Cardiac arrest associated with reperfusion of the liver during transplantation: Incidence and proposal for a management algorithm. Clin Transplant. 2013;27(2):185–92. doi: 10.1111/ctr.12052. [DOI] [PubMed] [Google Scholar]
- 2.Matsusaki T, Hilmi IA, Planinsic RM, et al. Cardiac arrest during adult liver transplantation: A single institution’s experience with 1238 deceased donor transplants. Liver Transpl. 2013;19(11):1262–71. doi: 10.1002/lt.23723. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Gwak MS, Choi SJ, et al. Intra-operative cardiac arrests during liver transplantation – a retrospective review of the first 15 yr in Asian population. Clin Transplant. 2013;27(2):E126–36. doi: 10.1111/ctr.12085. [DOI] [PubMed] [Google Scholar]
- 4.Shi XY, Xu ZD, Xu HT, et al. Cardiac arrest after graft reperfusion during liver transplantation. Hepatobiliary Pancreat Dis Int. 2006;5(2):185–89. [PubMed] [Google Scholar]
- 5.Schnüriger B, Studer P, Candinas D, et al. Transdiaphragmatic resuscitative open cardiac massage: Description of the technique and a first case-series of an alternative approach to the heart. World J Surg. 2014;38(7):1726–29. doi: 10.1007/s00268-013-2432-8. [DOI] [PubMed] [Google Scholar]
- 6.Kazanci D, Turan S, Bostanci B, et al. Cardiac arrest that developed during liver transplantation and intervention with cardiopulmonary bypass: case report. Transplant Proc. 2012;44(6):1759–60. doi: 10.1016/j.transproceed.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Ulukaya S, Alper I, Aydin U, et al. Successful resuscitation of cardiac arrest due to postreperfusion syndrome during orthotopic liver transplantation: A case report. Transplant Proc. 2007;39(10):3527–29. doi: 10.1016/j.transproceed.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Vannucci A, Burykin A, Krejci V, et al. Postreperfusion cardiac arrest and resuscitation during orthotopic liver transplantation: Dynamic visualization and analysis of physiologic recordings. Shock. 2012;37(1):34–38. doi: 10.1097/SHK.0b013e318239b128. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, DeMaria S, Jr, Cohen E, et al. Prolonged intraoperative cardiac resuscitation complicated by intracardiac thrombus in a patient undergoing orthotopic liver transplantation. Semin Cardiothorac Vasc Anesth. 2016;20(3):246–51. doi: 10.1177/1089253216652223. [DOI] [PubMed] [Google Scholar]
- 10.Andjelić N, Erdeljan S, Popović R, et al. Anaphylaxis on graft reperfusion during orthotopic liver transplantation: A case study. Srp Arh Celok Lek. 2015;143(7–8):467–70. doi: 10.2298/sarh1508467a. [DOI] [PubMed] [Google Scholar]
- 11.Homvises B, Sirivatanauksorn Y, Limsrichamrern S, et al. The minimal flush volume for washout of preservation fluid in liver transplantation. Transplant Proc. 2008;40(7):2123–26. doi: 10.1016/j.transproceed.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Fukazawa K, Nishida S, Hibi T, et al. Crystalloid flush with backward unclamping may decrease post-reperfusion cardiac arrest and improve short-term graft function when compared to portal blood flush with forward unclamping during liver transplantation. Clin Transplant. 2013;27(4):492–502. doi: 10.1111/ctr.12130. [DOI] [PubMed] [Google Scholar]
- 13.Gruttadauria S, Cintorino D, Musumeci A, et al. Comparison of two different techniques of reperfusion in adult orthotopic liver transplantation. Clin Transplant. 2006;20(2):159–62. doi: 10.1111/j.1399-0012.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 14.Daniela K, Michael Z, Florian I, et al. Influence of retrograde flushing via the caval vein on the post-reperfusion syndrome in liver transplantation. Clin Transplant. 2004;18(6):638–41. doi: 10.1111/j.1399-0012.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 15.Kniepeiss D, Iberer F, Grasser B, et al. A single-center experience with retrograde reperfusion in liver transplantation. Transpl Int. 2003;16(10):730–35. doi: 10.1007/s00147-003-0621-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang WJ, Xia WL, Pan HY, et al. Postreperfusion hyperkalemia in liver transplantation using donation after cardiac death grafts with pathological changes. Hepatobiliary Pancreat Dis Int. 2016;15(5):487–92. doi: 10.1016/s1499-3872(16)60116-9. [DOI] [PubMed] [Google Scholar]
- 17.Pan X, Apinyachon W, Xia W, et al. Perioperative complications in liver transplantation using donation after cardiac death grafts: A propensity-matched study. Liver Transpl. 2014;20(7):823–30. doi: 10.1002/lt.23888. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Tian M, Sun L, et al. Association between flushed fluid potassium concentration and severe postreperfusion syndrome in deceased donor liver transplantation. Med Sci Monit. 2017;23:5158–67. doi: 10.12659/MSM.907132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal S, Kang Y, Freeman JA, et al. Postreperfusion syndrome: Cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19(4 Suppl 3):54–55. [PubMed] [Google Scholar]
- 20.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14(4):504–8. doi: 10.1002/lt.21381. [DOI] [PubMed] [Google Scholar]
- 21.Siniscalchi A, Gamberini L, Laici C, et al. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J Gastroenterol. 2016;22(4):1551–69. doi: 10.3748/wjg.v22.i4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukazawa K, Yamada Y, Gologorsky E, et al. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J Cardiothorac Vasc Anesth. 2014;28(4):994–1002. doi: 10.1053/j.jvca.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Fiegel M, Cheng S, Zimmerman M, et al. Postreperfusion syndrome during liver transplantation. Semin Cardiothorac Vasc Anesth. 2012;16(2):106–13. doi: 10.1177/1089253212444791. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Zeng L, Gao X, et al. Transformation of organ donation in China. Transpl Int. 2015;28(4):410–15. doi: 10.1111/tri.12467. [DOI] [PubMed] [Google Scholar]
- 25.Potanos K, Vakili K, Weldon C, et al. The frostbitten liver: Can subzero cold preservation be a cause of primary nonfunction? Pediatric Transplantation. 2011;15:61. [Google Scholar]
- 26.Cordoví de Armas L, Jiménez Paneque RE, Gala López B, et al. Rapid and homogeneous reperfusion as a risk factor for postreperfusion syndrome during orthotopic liver transplantation. Rev Bras Anestesiol. 2010;60(2):154–61. 88–92. [PubMed] [Google Scholar]
- 27.Scott VL, Wahl KM, Soltys K, et al. Anesthesia for organ transplantation. In: Davis PJ, Cladis FP, Motoyama EK, editors. Smith’s anesthesia for infants and children. 8th ed. Philadelphia, PA: Mosby/Elsevier; 2011. p. 924. [Google Scholar]
- 28.García-Gil FA, Serrano MT, Fuentes-Broto L, et al. Celsior versus University of Wisconsin preserving solutions for liver transplantation: Postreperfusion syndrome and outcome of a 5-year prospective randomized controlled study. World J Surg. 2011;35(7):1598–607. doi: 10.1007/s00268-011-1078-7. [DOI] [PubMed] [Google Scholar]
- 29.Acosta F, Sansano T, Contreras RF, et al. Changes in serum potassium during reperfusion in liver transplantation. Transplant Proc. 1999;31(6):2382–83. doi: 10.1016/s0041-1345(99)00391-7. [DOI] [PubMed] [Google Scholar]
- 30.Shah R, Gutsche JT, Patel PA, et al. Cardiopulmonary bypass as a bridge to clinical recovery from cardiovascular collapse during graft reperfusion in liver transplantation. J Cardiothorac Vasc Anesth. 2016;30(3):809–15. doi: 10.1053/j.jvca.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Tejani M, Yi SY, Eudailey KW, et al. Extracorporeal membrane oxygenation as a rescue device for postreperfusion cardiac arrest during liver transplantation. Liver Transpl. 2015;21(3):410–14. doi: 10.1002/lt.24056. [DOI] [PubMed] [Google Scholar]
- 32.Ulukaya S, Alper I, Aydin U, et al. Successful resuscitation of cardiac arrest due to postreperfusion syndrome during orthotopic liver transplantation: A case report. Transplant Proc. 2007;39(10):3527–29. doi: 10.1016/j.transproceed.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 33.Wang CH, Cheng KW, Chen CL, et al. The effectiveness of prophylactic attachment of adhesive defibrillation pads in adult living donor liver transplantation. Ann Transplant. 2015;20:97–102. doi: 10.12659/AOT.892639. [DOI] [PubMed] [Google Scholar]


