Abstract
Background
Bridging treatments are employed in liver transplant waitlist patients with hepatocellular carcinoma (HCC) because of the risk of tumor progression during the waiting time. Radioembolization is mostly employed in the control of large or multifocal HCCs when other locoregional treatment modalities cannot be applied because of the number or size of lesions. The purpose of this study was to evaluate our experience with the use of radioembolization as a bridge to transplantation and its effect on tumor recurrence and survival after liver transplantation.
Material/Methods
A retrospective review of 40 consecutive patients with HCC who underwent liver transplantation after radioembolization bridging treatment between January 2007 and December 2015 at the University Hospital Essen, Germany, was performed. Patients’ characteristics, alpha-fetoprotein (AFP) levels, pathologic tumor response, tumor recurrence rate, and survival rates were examined through chart review.
Results
Histopathological examination of the explanted liver specimen revealed complete tumor necrosis in 17 specimens, partial necrosis in 18 specimens, and no significant necrosis in five specimens. Median overall survival was 46 months. Nine patients developed recurrent HCC. Median time from liver transplantation to diagnosis of tumor recurrence was 15 months. There was a trend towards a lower risk of tumor recurrence for patients with complete necrosis on explant specimens. Patients with tumor recurrence demonstrated statistically significantly higher pre- and post-treatment AFP levels (p=0.0234 and p=0.0236) and statistically significantly more frequently microvascular invasion (p=0.0163).
Conclusions
Histopathological assessment of explanted livers revealed at least partial necrosis in 87.5% of patients. Patients with successful bridging treatment, i.e. complete necrosis of explant specimens, demonstrate a trend towards a lower risk of tumor recurrence.
MeSH Keywords: Carcinoma, Hepatocellular; Liver Transplantation; Recurrence; Survival Rate; Yttrium Isotopes
Background
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the second most frequent cause of cancer-related mortality [1,2]. Liver transplantation remains the best treatment option for selected HCC of the cirrhotic liver; and in addition to removal of tumor tissue, liver tissue with multifocal HCC potential is also removed [3]. Expansion of the Milan criteria continues to be discussed because selected patients with larger tumors may achieve similar survival rates after liver transplantation [4,5]. The increasing shortage of donor organs, however, prolongs waiting time and therefore increases the risk of tumor progression [6].
As a bridge to transplantation, locoregional therapies are employed to prevent waitlist dropout because of tumor progression [5]. Treatment strategies intended to control tumor growth include transarterial chemoembolization (TACE), radiofrequency ablation (RFA) or radioembolization, among other strategies.
The acceptable safety profile and the efficacy of radioembolization in controlling tumor progression have been acknowledged in several international guidelines, and it has been approved for treatment of HCC with or without portal vein thrombosis [7,8]. Radioembolization is mostly employed in the control of large or multifocal HCCs, particularly in the case of portal vein thrombosis, when other locoregional treatment modalities cannot be applied because of the number and/or size of lesions or portal vein thrombosis [9,10]. In a prospective phase 2 study, radioembolization was shown to have the potential for an effective local tumor control rate with low morbidity in patients with intermediate to advanced HCC [11]. Since there is only minimal alteration in vascularity without compromising the blood flow to the hepatic parenchyma, radioembolization can be utilized even in patients with compromised liver function [12]. In a recent phase 2 prospective randomized study of chemoembolization versus radioembolization in patients with early to intermediate HCC, radioembolization provided better local tumor control [13].
In our center, radioembolization treatment is applied using nonbiodegradable glass microspheres containing the radionuclide yttrium-90 (TheraSphere®, BTG International, London, United Kingdom) [14,15]. Yttrium-90 is a pure beta-emitter with a half-life of 64.2 hours and an average and maximum tissue penetration of 2.5 mm and 10 mm, respectively. The microspheres are predominantly taken up by the tumor capillary bed and exert a local radiation effect leading to significant tumor necrosis and sparing of the surrounding non-tumorous liver parenchyma [16,17]. The majority of the radiation dose is delivered over ten days. To date, several thousands of patients have been treated with radioembolization but there is little data on radioembolization used as bridging treatment before liver transplantation [18–23]. In the largest published prospective dataset of 291 patients, 32 were able to undergo liver transplantation [24].
Liver transplantation provides the unique opportunity to study the treated lesions histopathologically. Riaz et al. demonstrated that radioembolization led to complete necrosis in 61% of the treated lesions. The length of time from first treatment to liver transplantation predicted the degree of necrosis. All of the tumor lesions that were explanted <3 months after radioembolization had <50% necrosis [22]. Still, the effect of ablative therapy on survival and tumor recurrence after liver transplantation is not yet clear.
We here report pathologic response, tumor recurrence, and survival in 40 patients treated with radioembolization as a bridge to transplantation for HCC at our transplant center. The aim of this study was to evaluate the effect of radioembolization as a bridge to transplantation on tumor recurrence and survival after liver transplantation.
Material and Methods
We retrospectively analyzed prospectively collected data on 40 patients who underwent liver transplantation after bridging treatment consisting of radioembolization with yttrium-90 microspheres at our transplant center between January 2007 and December 2015. The study was approved by the local ethics committee and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008.
All patients were reviewed at a multidisciplinary transplant conference and a multidisciplinary radioembolization conference. A consensus was reached that each of these patients would be treated with radioembolization as a bridge to transplantation to avoid tumor progression during waiting time. Baseline diagnostic imaging consisted of contrast-enhanced abdominal computed tomography (CT) scan or magnetic resonance imaging (MRI). All patients underwent mapping angiography of the liver to determine vascular anatomy and arterial variant anatomy. Technetium-99 macroaggregated albumin (99Tc-MAA) scanning quantified potential gastrointestinal and pulmonary shunting. Prophylactic embolization of nontarget vessels was performed in order to avoid nontarget deposition of microspheres.
Yttrium-90 microspheres were administered into the tumor via a catheter placed within the right or the left hepatic artery at the lobar or segmental level [12]. Sequential bilobar treatment was performed within four weeks as indicated. Follow-up CT scans one month after treatment and then every three months assessed tumor response according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria [25].
Alpha-fetoprotein (AFP) levels were obtained before treatment and monthly afterwards. Retrospectively, patients with baseline AFP levels of >200 IU/mL were studied for changes in pre-treatment and post-treatment levels. AFP response was defined as >50% decrease from baseline [26].
When a suitable organ offer became available, patients underwent orthotopic liver transplantation without veno-venous bypass. Initial immunosuppression consisted of tacrolimus 0.1 mg/kg adjusted to a trough level of 5–7 ng/mL and prednisolone 20 mg, tapered and withdrawn over six weeks.
Tumor response was determined using explant pathology. One centimeter sections of the explanted liver were performed and representative samples of the lesions were stained with routine hematoxylin-eosin stains for histologic examination. The target lesions were carefully examined for the presence of viable tumor cells. The percentage of necrosis of the treated lesions was judged as follows: complete necrosis (100% necrosis, absence of any viable tumor cells), partial necrosis (>50% necrosis, clusters of viable tissue), and viable tumor cells (<50% necrosis) [22].
Data collection and statistical analysis were performed using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism version 6.07 for Windows (GraphPad Software, San Diego, CA, USA). All data were tested for normality using the method of Kolmogorov-Smirnov. Data are presented as median (range) for continuous variables unless stated otherwise. Differences in continuous variables were tested using t-tests or Mann-Whitney tests as appropriate. For comparison of pre- and post-treatment AFP levels, Wilcoxon matched pairs test was performed. Differences between categorical variables were tested using Fisher’s exact test or chi-square test as appropriate. A p value ≤ 0.05 (two-tailed) was considered to be significant. Least-squares linear regression analysis was performed to analyze the association between the time elapsed from first radioembolization treatment to liver transplantation and the rate of tumor necrosis, as well as the association between maximum tumor size on explant specimen and the rate of tumor necrosis. Patient survival was evaluated using the Kaplan-Meier method and compared with the log-rank test. The reference point for all calculations of survival was the day of liver transplantation. In order to provide conclusions focusing on the oncological issue, i.e., tumor recurrence, patients who died within 30 days after liver transplantation were excluded from further survival analysis.
Results
Study population
Thirty-two patients were male and eight patients were female. The patients had a mean age of 59±6 years. The etiology of HCC was alcoholic liver cirrhosis (n=12), chronic hepatitis B (n=8), chronic hepatitis C (n=9), non-alcoholic steatohepatitis (NASH) (n=6), cryptogenic cirrhosis (n=2), hemochromatosis (n=2), and one patient had developed HCC in the absence of cirrhosis. Non-tumoral portal vein thrombosis was present in one patient.
Prior to bridging treatment, severity of liver disease as measured by true Model for End-Stage Liver Disease (MELD) score was 12 (range 6–40). Pre-treatment radiological imaging showed a maximum tumor size of 3.5 cm (range 0.5–11.0). In 15 patients, ≤3 nodules were found, whereas in 25 patients, tumor extent was multifocal. According to Eurotransplant International Foundation (Eurotransplant), 10 patients received an upgraded MELD score as they met Milan criteria.
Bridging treatment
Radioembolization was applied to the right liver lobe in 18 patients and to the left liver lobe in two patients. In 20 patients, radioembolization of both lobes was performed in two consecutive treatment sessions. The mean dose administered to target site was 114±12 Gy in the right lobe and 108±17 Gy in the left lobe. The mean activity delivered to treatment site was 2.9±1.1 GBq in the right lobe and 1.3±0.7 GBq in the left lobe.
Pre- and post-bridging treatment AFP levels were similar among all patients (22.5 IU/mL (range 1.0–13926.0) vs. 24.4 IU/mL (range 3.1–6373.0). In nine patients, pre-treatment AFP was elevated above 200 IU/mL and five of those demonstrated an AFP response, i.e., >50% decline in baseline AFP after bridging treatment, resulting in a statistically significant decrease in AFP levels in this subgroup (p=0.0039).
None of the patients demonstrated progressive tumor disease or deteriorating liver function after bridging treatment and none of the patients dropped out from the waitlist. MELD score before and after radioembolization was without significant alterations (9 (range 5–17) vs. 10 (range 5–22), p=0.1144). Patients underwent liver transplantation 129 (range 13–658) days after radioembolization.
Tumor necrosis
Histopathological examination of the explanted liver specimen revealed a maximum tumor size of 2.6 cm (range 0.7–10.5). Complete tumor necrosis was detected in 17 (42.5%) specimens, partial necrosis in 18 (45.0%) specimens and in five (12.5%) specimens no significant necrosis was detected.
Linear regression analysis demonstrated that the time from radioembolization to liver transplantation does not predict the rate of tumor necrosis in the explanted liver specimen (r2=0.0002143). Yet, there is a trend that when liver transplantation is performed at least three months post radioembolization bridging treatment, there will be a higher rate of complete tumor necrosis (Figure 1). Maximum tumor size on explant specimen does not predict the rate of tumor necrosis either (r2=0.07685); there is a trend that tumor nodules of less than 3 cm present tumor necrosis more frequently, however (Figure 2).
Figure 1.
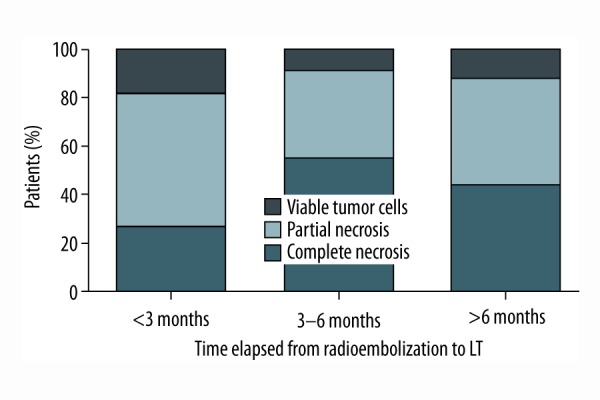
Rate of histologic necrosis stratified according to time period between first radioembolization bridging treatment and liver transplantation.
Figure 2.
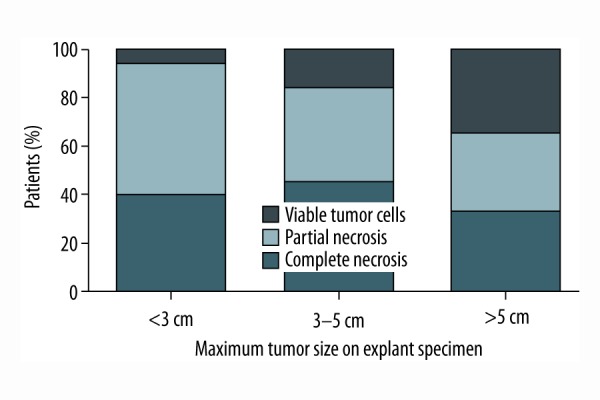
Rate of histologic necrosis stratified according to maximum tumor size on explant specimen.
Patients with viable tumor on explant specimen demonstrated significantly higher AFP levels prior to bridging treatment as well as significantly more frequently microvascular invasion (p=0.0425 and p=0.0041, respectively) (Table 1). Patients with AFP response did not present a higher rate of tumor necrosis on explant specimen.
Table 1.
Comparison of patients’ und tumor characteristics according to rate of necrosis on explant specimen.
| Complete necrosis (n=17) | Partial necrosis (n=18) | Viable tumor (n=5) | p | |
|---|---|---|---|---|
| Milan in pre-treatment (%) | 5.9 | 28.9 | 40.0 | 0.0560 |
| AFP pre-treatment (U/mL) | 8.3 [1.0–1262.0] | 76.0 [3.7–13926.0] | 176.1 [4.7–7454.0] | 0.0425 |
| AFP post-treatment (U/mL) | 15.8 [3.1–113.4] | 36.6 [4.9–6373.0] | 54.7 [6.2–5496.0] | 0.2885 |
| Time to LT after bridging (days) | 180 [26–658] | 146 [13–528] | 95 [37–473] | 0.8450 |
| Maximum tumor size (cm) | 2.7 [0.7–8.0] | 2.4 [1.3–10.5] | 4.5 [2.4–9.0] | 0.1465 |
| Tumor grading >G2 (%) | 0.0 | 22.2 | 20.0 | 0.1200 |
| Microvascular invasion V1 (%) | 0.0 | 16.7 | 60.0 | 0.0041 |
| Tumor recurrence (%) | 11.8 | 27.8 | 40.0 | 0.3183 |
LT – liver transplantation.
Survival
Median overall survival was 46 months (Figure 3). Perioperatively, i.e., within the first 30 days after liver transplantation, six patients died, three due to infectious complications (bacterial sepsis), two due to cardiovascular complications (cirrhotic cardiomyopathy), and one from primary non-function of the graft. Within one year after liver transplantation, three patients died from infectious complications (sepsis, after three months; pneumonia, after four months; HCV reinfection, after 12 months) and one patient died from cardiovascular complications (right heart failure, eight months after liver transplantation).
Figure 3.
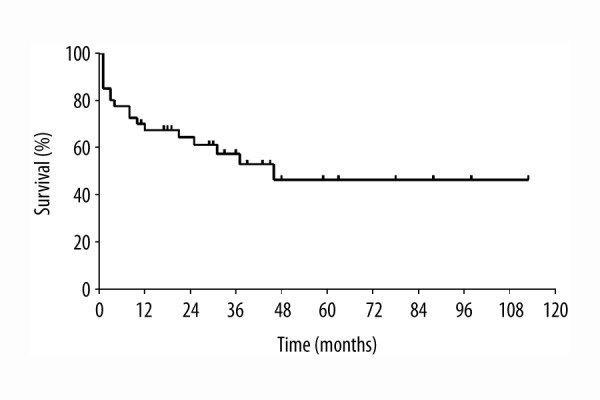
Overall survival after radioembolization bridging treatment and liver transplantation.
Tumor recurrence
Nine patients were diagnosed with HCC recurrence. Time to diagnosis of HCC recurrence was 13 months (range 4–56) after liver transplantation. A comparison of patients with and without recurrent hepatocellular carcinoma is given in Table 2. There were no statistically significant differences in age, gender, underlying liver disease, true MELD score, and Milan criteria. Pre- and post-treatment AFP levels were statistically significantly higher among patients with tumor recurrence (p=0.0234 and p=0.0236, respectively) while patients without recurrence expressed stable AFP levels <20 IU/mL pre- and post-treatment.
Table 2.
Comparison of patients with and without recurring hepatocellular carcinoma.
| Tumor recurrence (n=9) | Recurrence-free (n=31) | p | |
|---|---|---|---|
| Age (years) | 56±4 | 60±7 | 0.1216 |
|
| |||
| Male gender (%) | 55.6 | 87.1 | 0.0594 |
|
| |||
| Underlying disease | |||
| Hepatitis B/C | 6 | 11 | 0.3773 |
| Alcoholic cirrhosis | 1 | 11 | |
| Other | 2 | 9 | |
|
| |||
| True MELD score | 10 [8–40] | 13 [6–37] | 0.9093 |
|
| |||
| Milan in (%) | 33.3 | 22.6 | 0.6650 |
|
| |||
| AFP pre-treatment (U/mL) | 129.3 [4.5–7454.0] | 11.8 [1.0–13926.0] | 0.0234 |
|
| |||
| AFP post-treatment (U/mL) | 82.3 [11.3–5496.0] | 17.3 [3.1–6373.0] | 0.0236 |
|
| |||
| Time to LT after bridging (days) | 108 [21–526] | 180 [13–658] | 0.8090 |
|
| |||
| Maximum tumor size (cm) | 4.0 [0.7–8.0] | 2.4 [1.3–10.5] | 0.5904 |
|
| |||
| Tumor grading >G2 (%) | 22.2 | 9.7 | 0.3114 |
|
| |||
| Microvascular invasion V1 (%) | 44.4 | 6.5 | 0.0163 |
|
| |||
| Tumor necrosis | |||
| Complete necrosis | 2 | 15 | 0.3183 |
| Partial necrosis | 5 | 13 | |
| Viable tumor cells | 2 | 3 | |
LT – liver transplantation.
There were no statistically significant differences in waiting time from radioembolization bridging treatment to liver transplantation nor in maximum tumor size, rate of tumor necrosis, and tumor grading on explant specimen. Microvascular invasion was statistically significantly more frequently present in patients with recurrent HCC (p=0.0163). There is a trend towards a lower risk of tumor recurrence among patients with complete necrosis on explant specimen; however, this did not reach statistical significance (Figure 4).
Figure 4.
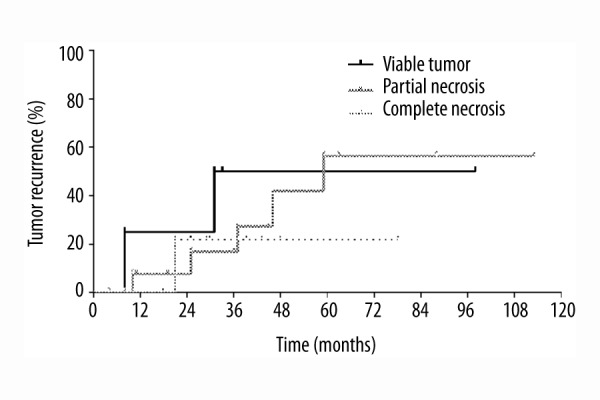
Risk of tumor recurrence stratified according to rate of tumor necrosis on explant specimen.
Patients with pre-treatment AFP values of <20 IU/mL demonstrate a statistically significant lower risk for tumor recurrence (p=0.0266; Figure 5). Remarkably, patients with recurrent HCC presented an AFP response after radioembolization bridging treatment (129.3 IU/mL (range 4.5–7454.0) vs. 82.3 IU/mL (range 11.3–5496.0, p=0.0234) as opposed to patients without recurrence (11.8 IU/mL (range 1.0–13926.0) vs. 17.3 IU/mL (range 3.1–6373.0), p=0.7585).
Figure 5.
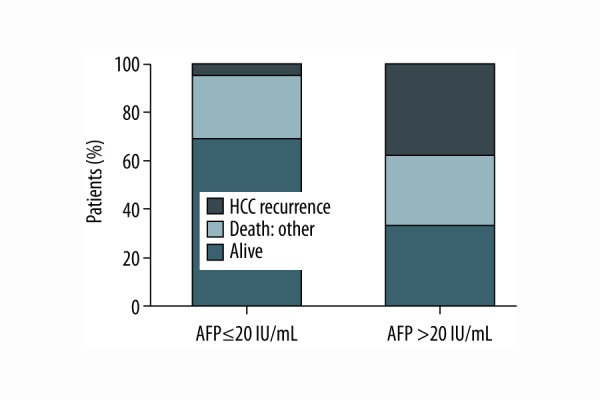
Risk for tumor recurrence stratified according to pre-treatment AFP (p=0.0266).
Survival of recurrence-free patients was statistically significantly longer than among patients with tumor recurrence as depicted in Figure 6 (p=0.0193).
Figure 6.
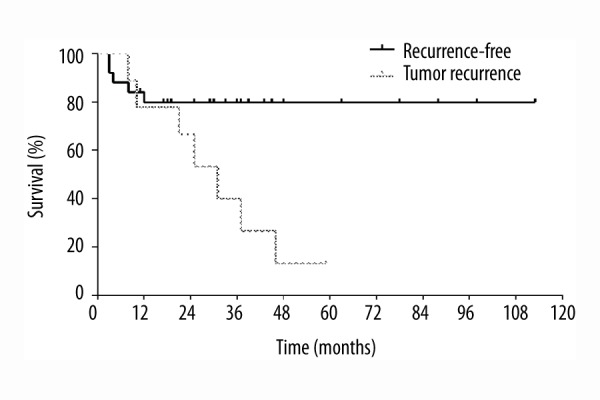
Survival stratified according to tumor recurrence (p=0.0193).
Discussion
Waiting time to liver transplantation has progressively increased due to critical donor organ shortage. Therefore, there is a substantial need for bridging procedures that provide prolonged disease control in HCC patients during waiting time. Furthermore, bridging treatment may help to exclude high-risk patients who have tumors with an unfavorable tumor biology resulting in early tumor progression after locoregional treatment. The aim of this study was to provide evidence on the safety and efficacy of radioembolization bridging treatment and to determine long-term survival in HCC patients after bridging treatment and liver transplantation.
Compared with TACE, radioembolization has been reported to be more effective in downstaging, better tolerated, and associated with less hospitalization and fewer treatment sessions [13,27–29]. Patients who sustain tumor response after bridging treatment may demonstrate a favorable tumor biology contributing to recurrence-free survival. Otto et al. showed that the response to pre-transplant TACE influences the outcome after liver transplantation significantly [30,31]. Similar data concerning radioembolization are not available so far.
Although locoregional treatments have proven to be effective in tumor growth control, viable residual tumor cells are often identified on explant specimens [32]. In our study, patients demonstrated an overall favorable radiologic response to radioembolization bridging treatment without significant alterations in MELD score. The favorable tumor response was confirmed by the histopathological assessment of the explanted livers providing evidence of at least partial necrosis in 87.5% of patients treated. Riaz et al. observed a trend for smaller lesions to exhibit better response to radioembolization and that the length of time from first treatment to liver transplantation predicted the degree of necrosis on explant specimen [22]. Nalesnik et al. showed no obvious correlation between the date of radioembolization and the extent of necrosis [16]. In our study, no influence of tumor size or waiting time to liver transplantation on rate of tumor necrosis was evident.
Pre-treatment AFP level has been identified as a risk factor for HCC recurrence [5,33]. In our study, patients with pre-treatment AFP levels <20 IU/mL were less likely to develop recurrent HCC. Previous studies have shown some degree of correlation between the decline in AFP after bridging treatment and patient outcome [26,34,35]. Unfortunately, we detected AFP response particularly in patients with tumor recurrence. AFP response to locoregional treatment seems to be sufficiently heterogeneous and we cannot conclude that AFP response may be a surrogate marker of tumor biology.
Despite refined selection criteria, recurrent HCC is the rate-limiting factor for long-term survival in approximately 20% of patients undergoing liver transplantation for HCC [36,37]. In our study, 23% of patients developed recurrent HCC. There is a trend towards a lower risk of tumor recurrence among patients with complete necrosis on explant specimen. Yet, we cannot conclude that successful radioembolization bridging treatment resulting in tumor necrosis on explant specimen decreases risk of tumor recurrence.
Conclusions
Optimal measures to prevent tumor progression and drop-out as patients await liver transplantation still need to be defined. The combination of locoregional treatment and liver transplantation may improve long-term recurrence-free survival in HCC patients. In our study, histopathological assessment of the explanted livers revealed at least partial necrosis in 87.5% of patients. Patients with successful bridging treatment, i.e., complete necrosis on explant specimen, demonstrate a trend towards a lower risk of tumor recurrence. Future studies will need to further examine the effect of bridging treatment on the biological behavior of HCC and overall survival in HCC patients.
Footnotes
Source of support: Departmental sources
References
- 1.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–99. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 4.Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012;13:e11–22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bittermann T, Niu B, Hoteit MA, Goldberg D. Waitlist priority for hepatocellular carcinoma beyond milan criteria: A potentially appropriate decision without a structured approach. Am J Transplant. 2014;14:79–87. doi: 10.1111/ajt.12530. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–22. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: Interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107–10. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 10.Lau WY, Sangro B, Chen PJ, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: The emerging role for radioembolization using yttrium-90. Oncology. 2013;84:311–18. doi: 10.1159/000348325. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: A phase 2 study. Hepatology. 2013;57:1826–37. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 12.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–49. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–63.e2. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salem R, Thurston KG, Carr BI, et al. Yttrium-90 microspheres: Radiation therapy for unresectable liver cancer. J Vasc Interv Radiol. 2002;13:S223–29. doi: 10.1016/s1051-0443(07)61790-4. [DOI] [PubMed] [Google Scholar]
- 15.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–78. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 16.Nalesnik MA, Federle M, Buck D, et al. Hepatobiliary effects of 90yttrium microsphere therapy for unresectable hepatocellular carcinoma. Hum Pathol. 2009;40:125–34. doi: 10.1016/j.humpath.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Salem R, Lewandowski RJ. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013;11:604–11. doi: 10.1016/j.cgh.2012.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulik LM, Mulcahy MF, Hunter RD, et al. Use of yttrium-90 microspheres (TheraSphere) in a patient with unresectable hepatocellular carcinoma leading to liver transplantation: A case report. Liver Transpl. 2005;11:1127–31. doi: 10.1002/lt.20514. [DOI] [PubMed] [Google Scholar]
- 19.Kim DY, Kwon DS, Salem R, et al. Successful embolization of hepatocelluar carcinoma with yttrium-90 glass microspheres prior to liver transplantation. J Gastrointest Surg. 2006;10:413–16. doi: 10.1016/j.gassur.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Khalaf H, Alsuhaibani H, Al-Sugair A, et al. Use of yttrium-90 microsphere radioembolization of hepatocellular carcinoma as downstaging and bridge before liver transplantation: A case report. Transplant Proc. 2010;42:994–98. doi: 10.1016/j.transproceed.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Sotiropoulos GC, Hilgard P, Antoch G, et al. Liver transplantation for hepatocellular carcinoma after yttrium therapy: A case report. Transplant Proc. 2008;40:3804–5. doi: 10.1016/j.transproceed.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Riaz A, Kulik L, Lewandowski RJ, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49:1185–93. doi: 10.1002/hep.22747. [DOI] [PubMed] [Google Scholar]
- 23.Ettorre GM, Levi Sandri GB, Laurenzi A, et al. Yttrium-90 radioembolization for hepatocellular carcinoma prior to liver transplantation. World J Surg. 2017;41(1):241–49. doi: 10.1007/s00268-016-3682-z. [DOI] [PubMed] [Google Scholar]
- 24.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Riaz A, Ryu RK, Kulik LM, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: Oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–42. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 27.El Fouly A, Ertle J, El Dorry A, et al. In intermediate stage hepatocellular carcinoma: Radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627–35. doi: 10.1111/liv.12637. [DOI] [PubMed] [Google Scholar]
- 28.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: Chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–28. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 29.Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35:1715–21. doi: 10.1111/liv.12750. [DOI] [PubMed] [Google Scholar]
- 30.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–67. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 31.Otto G, Schuchmann M, Hoppe-Lotichius M, et al. How to decide about liver transplantation in patients with hepatocellular carcinoma: Size and number of lesions or response to TACE? J Hepatol. 2013;59:279–84. doi: 10.1016/j.jhep.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Tohme S, Sukato D, Chen HW, et al. Yttrium-90 radioembolization as a bridge to liver transplantation: A single-institution experience. J Vasc Interv Radiol. 2013;24:1632–38. doi: 10.1016/j.jvir.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 33.Ciccarelli O, Lai Q, Goffette P, et al. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int. 2012;25:867–75. doi: 10.1111/j.1432-2277.2012.01512.x. [DOI] [PubMed] [Google Scholar]
- 34.Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: Serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–52. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 35.Chen LT, Shiah HS, Chao Y, et al. Alpha-fetoprotein response in advanced hepatocellular carcinoma receiving cytostatic agent. J Clin Oncol. 2009;27:e271. doi: 10.1200/JCO.2009.23.8311. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: A review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–88. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 37.Kornberg A, Kupper B, Tannapfel A, et al. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: Clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275–80. doi: 10.1016/j.ejso.2009.10.001. [DOI] [PubMed] [Google Scholar]


