Abstract
There is no standardization on the timing of the best approach to treat a non-functioning renal graft.
We reviewed the literature and performed a proportional meta-analysis of case series of transplantectomy and embolization for a non-functioning renal graft. The groups were compared for mortality and morbidity outcomes.
A total of 2421 patients were included in this review. Of these, 2232 patients underwent transplantectomy and 189 underwent percutaneous embolization. The mortality rate in the nephrectomy group was 4% [95% confidence interval [CI], 2–7%; I2=87%] as compared with 0.1% [95% CI, 0.1–0.5%; I2=0%] in the embolization group. The rates of common morbidities were 18% [95% CI, 13–26%, I2=79.7%] for nephrectomy compared with 1.2% [95% CI, 0.7–2.1%, I2=26.4%] for embolization. The incidence of post-embolization syndrome was 68%, and 20% of patients needed post-embolization nephrectomy.
Percutaneous embolization was associated with lower mortality and morbidity rates but also with a high rate of post-embolization syndrome. However, in most cases this complication had easily manageable symptoms. Embolization is a new and attractive technique that can be considered in treating non-functioning renal grafts.
MeSH Keywords: Kidney Transplantation; Nephrectomy; Radiography; Radiology, Interventional
Background
Advances in immunosuppressive therapy have improved kidney graft survival [1–5]. Even so, the rates of kidney graft dysfunction remain high, at about 10% in the first year and from 3% to 5% in the following years [6]. Most kidney allografts will fail during the lifetime of the recipient, resulting in a non-functioning renal graft. There is no consensus on the management of a non-functioning renal graft, and some patients whose graft remains may develop graft intolerance syndrome [7,8], which is characterized by pain, hematuria, fever, hypertension, and difficulty controlling anemia. Anti-inflammatory drugs and the maintenance of decreasing doses of immunosuppressants are used to manage this syndrome. Despite clinical treatment with concomitant minimization of immunosuppression, some patients require graft removal because of the persistence of graft intolerance syndrome, a chronic inflammatory state, or rejection [9].
A non-functioning graft can be treated by 2 different procedures: nephrectomy (transplantectomy) and percutaneous embolization. Transplantectomy is a surgical procedure with high mortality rates [10–12]. Percutaneous embolization [13] is a minimally invasive alternative to treat dysfunctional grafts.
To date, no randomized controlled studies have compared transplantectomy with embolization for non-functioning renal grafts. The primary objective of this study was to determine the best therapeutic approach for dysfunctional kidney grafts: transplantectomy or percutaneous embolization.
Material and Methods
We performed a systematic review with proportional meta-analysis of all published case series on transplantectomy and/or embolization for non-functioning renal grafts. Studies were identified from MEDLINE (1966 until May 2016), EMBASE (1980 until May 2016), and LILACS (1982 until May 2016) using MeSH (Medical Subject Heading) terms, text words, and a list of synonyms: “nephrectomy”, “embolization”, and “renal transplantation patients” (see Attachment 1 for the full search strategy). The search strategy was adapted for each database in order to maximize the ability to identify eligible studies. There were no language restrictions.
The following inclusion criteria were used: (a) randomized controlled trials and case series with more than 5 reported patients, (b) renal transplantation patients with non-functioning renal graft, and (c) reported 30-day all-cause mortality as the primary outcome. We excluded narrative reviews and original articles that did not report mortality.
Data extraction
Two independent reviewers selected all of the titles and abstracts identified by the bibliographic research. The publications considered potentially relevant were obtained in their full version to verify their eligibility. Possible divergences of opinion were resolved by consulting a third reviewer and by discussing such divergences to assure the quality of the process. A standard form was used to extract the following data: characteristics of the study design, patient characteristics (average age, sex, baseline disease, and cause of graft loss), interventions in groups, all-cause mortality and morbidity rates, and duration of follow-up.
We used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [14] and MOOSE (Meta-analysis Of Observational Studies in Epidemiology) [15] recommendations to prepare the protocol of this study and to describe the findings.
Patient outcomes
The primary outcome analyzed was all-cause mortality in the first 30 days. Secondary outcomes were morbidities, separated into 2 categories: those that are common to both transplantectomy and embolization, and those that are specific to embolization. The common morbidities were bleeding, wound infection, septicemia, lung infection, abscesses, and aneurysm. The morbidities specific to embolization were post-embolization syndrome and need for transplantectomy after embolization. The post-embolization syndrome was defined as low fever after the embolization procedure (either associated or not associated with local pain), non-specific malaise, increase in graft size, and hematuria [13,16–18].
Statistical analysis
The proportional meta-analysis of the case series studies was conducted by using R software [19] with the metaphor package [20]. The Mantel-Haenszel method was used with the mixed-effects model (Hedges estimator) to perform the analysis. We added 0.1 to the event number when no event occurred in the study. The outcomes of interest were treated as dichotomous variables, with their respective reliability intervals of 95% (95% confidence interval [CI]). Because of clear differences between the included studies and other non-controllable variables, a random-effects model was used to perform the pooled analysis of proportions [21]. The grouped analysis of case series proportions was conducted as described by El Dib et al. [22] and is described in detail below.
Forest plots are presented to summarize the data. Each horizontal line in the plot represents a case series included in the meta-analysis. The estimated effect on the outcome analyzed is represented by a black solid square, and the size of the square represents the weight of the corresponding study in the meta-analysis. The total combined estimate of the studies is given by an unfilled diamond at the bottom of the forest plot. The combined proportions of each intervention and their 95% CIs were then compared between each other; the presence of an overlap in the 95% CI for interventions suggests that they have similar effects on the outcome. Nevertheless, non-overlapping 95% CIs suggest different effects of the interventions studied. Therefore, a statistically significant difference between the treatment and control groups was established when their 95% CIs did do not overlap [22].
We evaluated publication bias by visually inspecting the funnel plots for each outcome for which we identified 8 or more eligible studies. In addition to the visual inspection of the funnel plots, we used the Egger test to assess the asymmetry of the chart. Statistical heterogeneity was assessed by using the I2 statistical test, and significance was assumed when the I2 value exceeded 50%. This measure illustrates the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling errors [23] and may be interpreted as follows: 0% to 40%, heterogeneity may not be important; 30% to 60%, may represent moderate heterogeneity; 50% to 90%, may represent substantial heterogeneity; 75% to 100%, considerable heterogeneity present.
The results of the underlying disease, indication for the procedure, and causes of death and morbidities are presented in percentages by using the following calculation: number of events divided by the total number of events reported. The papers that did not report the event were not considered in the calculation.
In the indications for post-embolization nephrectomy, the percentage was calculated based on the total number of all papers reporting the event.
Results
Study Selection
The bibliographic search conducted until May 2016 identified 16 320 titles. After screening per title and abstract, we obtained copies of the complete texts of 130 studies on transplantectomy and embolization that were potentially eligible to be included in the review. However, most of those studies were off-topic because they did not assess all-cause mortality or post-procedure complications. Finally, a total of 26 case series that satisfied all the methodological requisites (Figure 1) were selected [7,8,12,13,16–18,24–42].
Figure 1.
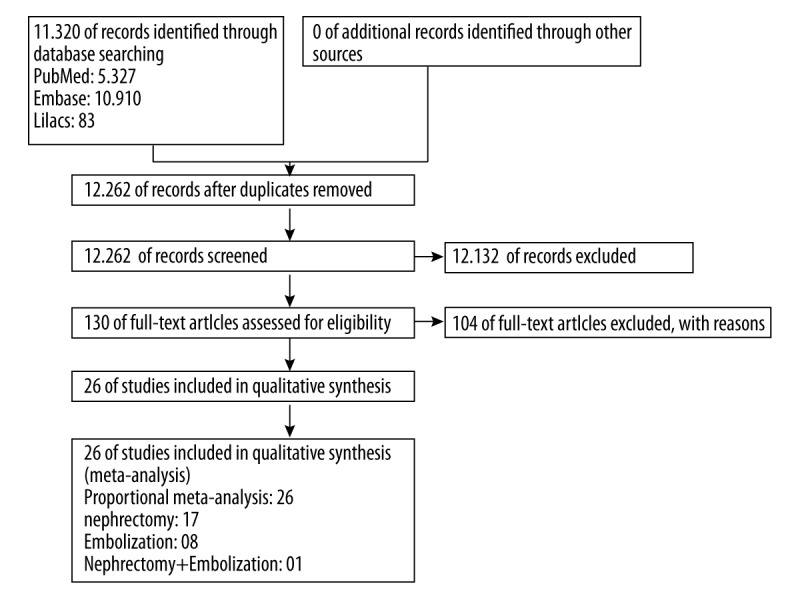
Flowchart of the bibliographic search and selection of the articles identified and evaluated during the review process.
Study characteristics
Twenty-six case series, with a total of 2421 patients, were included in this review. A total of 2232 patients (18 case series) [12,18,24–27,29,30,32–41] underwent transplantectomy, and the other 189 (9 case series) [7,8,13,16–18,28,31,42] underwent percutaneous embolization. Only 1 study reported on a pediatric patient [8] (Table 1).
Table 1.
Characteristics of the renal transplantation patients of non-functioning renal graft: comparison between transplantectomy and embolization.
| Total | Nephrectomy | Embolization | |
|---|---|---|---|
| Total case series (n) | 26* | 18 [12,18,24–27,29,30,32–41] | 9 [7,8,13,16–18,28,31,42] |
| Total number of patients (n) | 2421 | 2232 | 189 |
| Average age | 40.5 | 41 [12,18,24–26,32–35,38] | 39.4 [8,17,18,31,42] |
| Males, n (%) | 63.3 | 63.5 [12,24–26,32,34–36,38,39] | 63.6 [8,16,17,31,42] |
| Caucasian (%) | 54 | 54 [32] | nr |
| Mean time to nephrectomy (months) | 11.3 | 48. 2 | |
| Cause of graft loss | |||
| Acute | |||
| Hyperacute rejection (%) | 5 | 5 [29,35] | nr |
| Acute rejection (%) | 21.5 | 22.6 [29,32,35,38,39] | 9.3 [18] |
| Irreversible acute rejection (%) | 24.4 | 22.5 [39] | 28.5% [42] |
| Bleeding (%) | 3.2 | 3.2 [29,39] | nr |
| Non-function (%) | 1.9 | 1.8 [32,38,39] | 3.1 [18] |
| Thrombosis (%) | 14.5 | 14.5 [29,32,35,38,39] | nr |
| Infection (%) | 11.2 | 11.2 [29,39] | nr |
| Acute vasculopathy (%) | 7.3 | 7.3 [29,32] | nr |
| Graft rupture (%) | 7.3 | 7.3 [29,32] | nr |
| Chronic | |||
| Relapse of glomerulopathy (%) | 5.8 | 5.6 [29,32,38] | 6.8 [18,31,42] |
| Chronic nephropathy (%) | 44.2 | 44.2 [32] | nr |
| Chronic rejection (%) | 43 | 36.1 [29,35,38,39] | 77.5 [18,31,42] |
| Other (%) | 1 | 1 [29] | nr |
| Indication of the procedure | |||
| Intolerance Syndrome (%) | 71.2 | 47.5 [24,34] | 100 [7,8,13,16,17] |
| Acute rejection (%) | 40.2 | 40.2 [30,33,34,40] | nr |
| Chronic inflammatory state (%) | 16.6 | 16.6 [26,30,34] | nr |
| Graft rupture (%) | 1.4 | 1.4 [34] | nr |
| Hematuria (%) | 19.7 | 19.7 [34] | nr |
| Infection (%) | 8.9 | 8.9 [26,34,36] | nr |
| Thrombosis (%) | 12.9 | 12.9 [25,26,34,40] | nr |
| Non-function (%) | 25.2 | 25.2 [25,27,30,34] | nr |
| Bleeding (%) | 3.8 | 3.8 [26] | nr |
| Chronic rejection (%) | 27.3 | 27.3 [25,30,36,40] | nr |
| Other (%) | 7 | 7 [34] | nr |
| Country | |||
| North America (Canada and the United States of America) (n) | 2 | 2 [33,41] | |
| Asia (China, India, Israel and Korea) (n) | 6 | 4 [29,35,36,39] | 2 [8,28] |
| Europe (France, Ireland, Italy, The Netherlands, Portugal, Spain and the United Kingdom) (n) | 16* | 10 [18,24–27,30,37,38,40] | 7 [7,13,16–18,31,42] |
| Eurasia (Turkey) (n) | 1 | 1 [34] | |
| South America (Brazil) (n) | 1 | 1 [32] | |
nr – not reported;
The study of González-Satué C.et al. [18] was cited in both groups.
Of the 26 studies, 2 were conducted in North America (Canada [33] and the United States [41]); 6 in Asia (China [28,29], India [35,39], Israel [8], and South Korea [36]); 16 in Europe (France [37], The Netherlands [12,27], Italy [16,31], Ireland [30], Portugal [26], Spain [7,13,17,18,24,38,42], and the United Kingdom [25,40]); 1 in Eurasia (Turkey [34]); and 1 in South America (Brazil [32]) (Table 1).
The main cause of post-transplantation renal function loss, considering both groups, was chronic rejection with 43% of the cases according to 7 studies [18,29,31,35,38,39,42], followed by chronic graft nephropathy with 44.2%, as reported in 1 series [32], and acute rejections with 21.5% according to 6 studies [18,29,32,35,38,39]. In the transplantectomy group, the main cause was chronic graft nephropathy at 44.2% [32]; while in the embolization group, it was chronic rejection, at 77.5% [18,31,42] (Table 1).
Intolerance syndrome was the main indication for nephrectomy and embolization, at 71.2%, according to 7 studies, [8,13,16,17,24,34]. The other causes were acute rejection, at 40.2% according to 6 papers [30,33,34,40], and chronic rejection, at 27.3% according to 4 studies [25,30,36,40] (Table 1).
For embolization, the following materials were used in an isolated or combined manner: absolute alcohol [8], polyvinyl alcohol [8,42], springs [43], steel springs and polyvinyl alcohol [31], absolute alcohol and steel springs [13, 28], triacyl gelatin and saline solution [16], Tris-acryl gelatin and springs [7], and polyvinyl alcohol and tungsten springs [17].
Outcomes
All-cause mortality
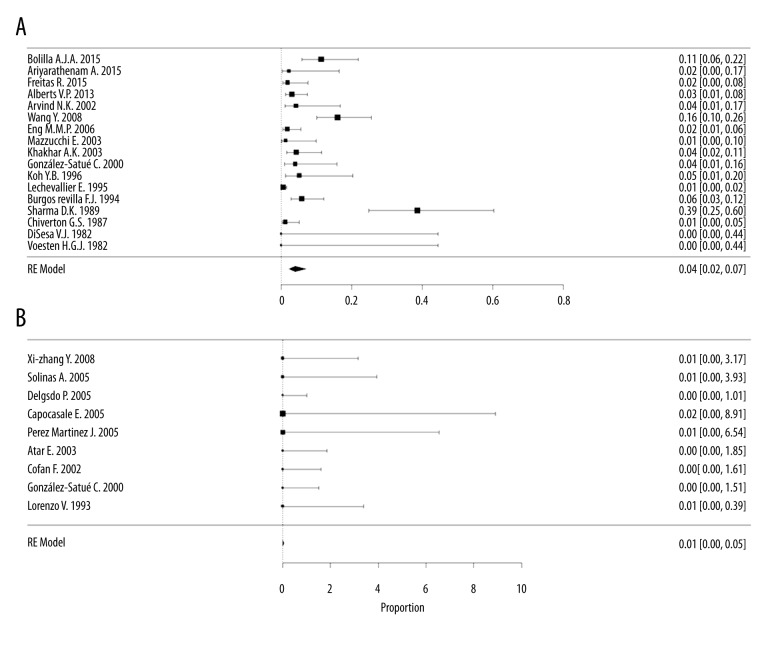
The mortality rate for transplantectomy was 4% [95% CI, 2–7%; I2=87%] in 17 case series [12,18,24–27,29,30,32,33,35–41], with a total of 2175 participants, compared with 0.1% [95% CI, 0.1–0.5%; I2=0%] in the embolization group, with 9 case series [7,8,13,16–18,28,31,42] and 189 participants (Figure 2). Mortality significantly differed between the 2 groups studied, with a lower mortality in the embolization group (no overlap of the 95% CIs) (Figure 3).
Figure 2.
Proportional meta-analysis forest plot of case series regarding mortality after a non-functioning renal graft. (A) Mortality in the transplantectomy group (scale ×100); (B) Mortality in the embolization group (scale ×10).
Figure 3.
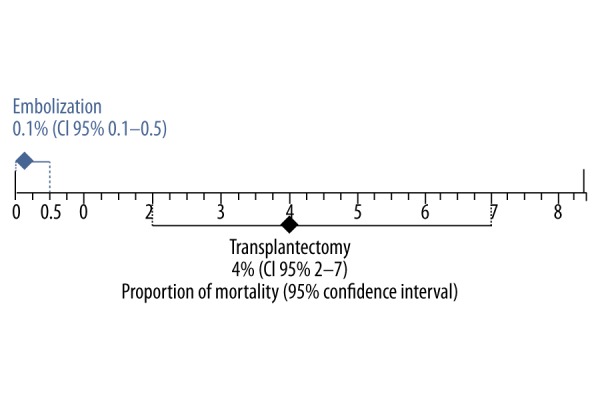
Combined mortality rate with 95% confidence interval of studies of non-functioning renal grafts in the transplantectomy and embolization groups. There are significant differences between groups due to no overlap of the 95% confidence intervals.
Evaluation of publication bias and heterogeneity showed great heterogeneity (I2=87%) and possible publication bias (funnel plot showed asymmetry) in the transplantectomy group. We found a more homogenous result in the embolization group, with low heterogeneity (I2=0%) and low risk of publication bias (a symmetrical inverted funnel plot) (Attachment 2). When we divided the analysis between acute and chronic causes, nephrectomy was the acute cause of mortality in 4 studies (9%) [29,32,35,38,39] [95% CI 3–27%, I2=90.9%] compared with 0% [95% CI 0.1–0.37%; I2=0%] in embolization in 2 studies [18,42].
Infection was the main cause of mortality in the transplantectomy group, at 64.2%, according to 8 studies [24,26,27,29,30,38–40]; multiple-organ failure resulted in a 53.3% mortality as reported in 1 study [29] (Table 2). There were no deaths in the embolization group.
Table 2.
Causes of mortality in the nephrectomy group in absolute numbers.
| Autor | Total mortality | Infection | Cerebrovascular | Cardiac | Pulmonary | Cardio-Pulmonary | Multiple-organ failure | Gastrointe-stinal | Bledding | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Bolilla AJA 2015 [24] | 8 | 4 | 2 | 2 | ||||||
| Ariyarathenam A 2015 [25] | 1 | 1 | ||||||||
| Freitas R 2015 [26] | 2 | 2 | ||||||||
| Alberts VP 2013 [27] | 5 | 2 | 1 | 2 | ||||||
| Wang Y 2008 [29] | 15 | 4 | 1 | 8 | 2 | |||||
| Eng MMP 2006 [30] | 3 | 3 | ||||||||
| Mazzucchi E 2003 [32] | 1 | 1 | ||||||||
| Khakhar AK 2003 [33] | 4 | 2 | 1 | 1 | ||||||
| Arvind NK 2002 [35] | 2 | 2 | ||||||||
| González-Satué C 2000 [18] | 2 | 1 | 1 | |||||||
| Koh YB 1996 [36] | 2 | 1 | 1 | |||||||
| Lechevallier E 1995 [37] | 5 | 5 | ||||||||
| Burgos Revilla FJB 1994 [38] | 7 | 3 | 1 | 2 | 2 | |||||
| Sharma DK 1989 [39] | 12 | 8 | 2 | 2 | ||||||
| Chiverton GS 1987 [40] | 2 | 1 | 1 | |||||||
| DiSesa VJ 1982 [41] | 0 | |||||||||
| Voesten HGJ 1982 [12] | 0 |
Morbidity
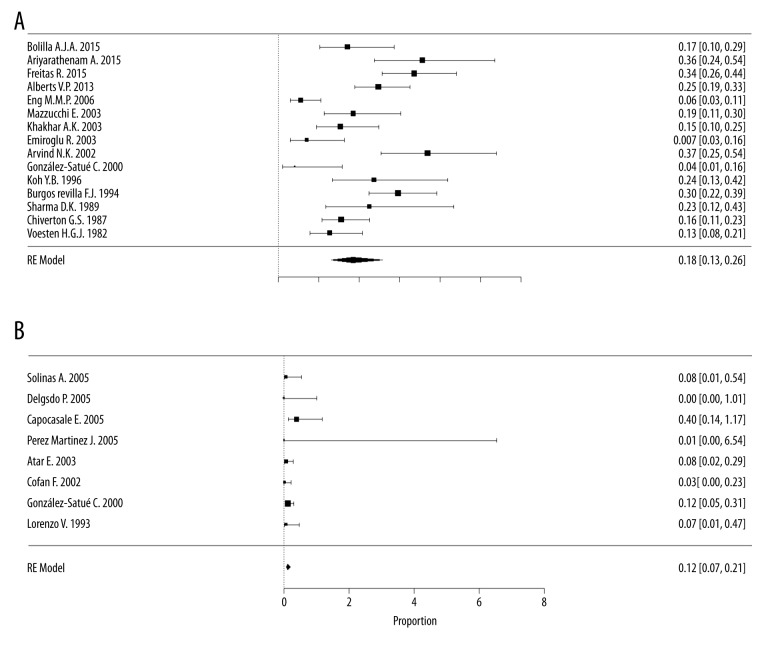
The incidence of morbidity with transplantectomy was 18% [95% CI, 13–26%, I2=79.7%] in 15 case series [12,18,24–27,30, 32–36,38–40], with a total of 1312 participants, compared with an incidence of 1.2% [95% CI, 0.7–2.1%, I2=26.4%] in the embolization group with 9 case series [7,8,13,16–18,31,42] in a sample of 174 participants (Figure 4). The CIs did not overlap, indicating that embolization is associated with less morbidity compared with nephrectomy (Figure 5).
Figure 4.
Proportional meta-analysis forest plot of case series regarding morbidities after a non-functioning renal graft. (A) Morbidity in the transplantectomy group (scale ×100); (B) Morbidity in the embolization group (scale ×10).
Figure 5.
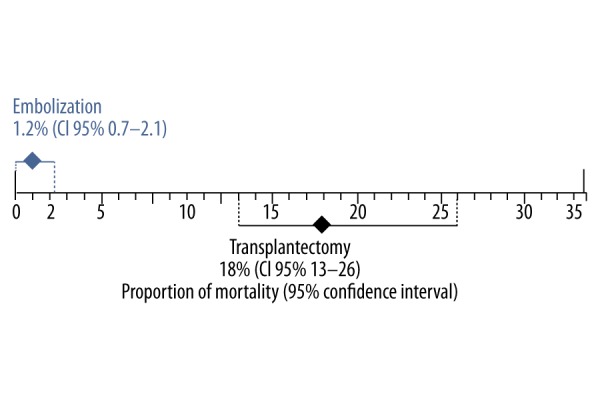
Combined morbidity rate with 95% confidence interval of studies of a non-functioning renal graft in the transplantectomy and embolization groups. There are significant differences between groups due to no overlap of the 95% confidence intervals.
In the evaluation of publication bias and heterogeneity for morbidities, we found a great heterogeneity (I2=79.7%) and a possible publication bias in the transplantectomy group. The result was more homogenous in the embolization group, with low heterogeneity (I2=26.4%) and low risk of publication bias (a symmetrical inverted funnel plot) (Attachment 3).
When we divided the analysis between acute and chronic causes, mortality from nephrectomy in acute phase was 28% [95% CI, 21–36%; I2= 42.9%] with 4 studies [9,32,35,38] compared with 11% [95% CI, 5–26%; I2=0%] in the embolization group with 2 studies [18,42].
The main morbidity in the transplantectomy group was abscess/collection in 56% of cases reported in 2 studies [24,32], followed by bleeding in 35.8% of the cases in 11 studies [12,25,27,30,32–36,38,40], and surgical wound infection in 34.4% of cases in 9 studies [12,25,27,33–36,39,40].
In the embolization group, the main morbidities were abscesses/collections in 83% of cases reported in 3 studies [8,18,42], followed by infectious diseases, at 66% of cases in 2 studies [8,13,16].
Specific morbidities of percutaneous embolization
Post-embolization syndrome had an incidence of 68% in the embolization group [95% CI, 57–82%, I2=62.5%], as reported in 7 case series [7,8,13,16–18,42], with a total of 85 participants.
Post-embolization nephrectomy was needed in 20% (95% CI 11–38%, I2=67.7%) of patients reported in 6 case series [8,13,16–18,42], with a total of 85 participants. Among the causes reported, 61% of the indications were the persistence or the re-emergence of graft intolerance syndrome; 15% were due to graft infections, reported in 3 studies [13,17,43], and 15% were due to abscesses, reported in 2 studies [8,13].
Discussion
There is no standardization for the timing of the best approach to treat a non-functioning renal graft. Surgical removal of the graft (transplantectomy) occurs in cases of intolerance syndrome or persistence of the chronic inflammatory state [13,16,17]. Generally, in cases of graft loss in the first year of transplantation or vascular complications, such as thrombosis, surgical removal of the graft is preferred. In later cases, after 1 year of transplantation, the surgical team can choose to retain the graft with subsequent reduction or even complete withdrawal of immunosuppressants [44]. However, the level of evidence for this approach is low because it is based on the opinion of specialists [45].
Thus, when renal graft removal is indicated, the classic technique is nephrectomy [46]. Recently, the percutaneous embolization technique [17,18] has been described as a less invasive approach to treat a non-functioning graft, with potentially lower morbidity and mortality. The present study is the first review of case series in the literature to compare these 2 approaches for dysfunctional renal grafts.
The first point to be discussed is the mortality rates for the 2 procedures. The mortality rate for surgical nephrectomy is not described in the literature; however, removal of the dysfunctional graft is technically more difficult than transplantation because of inflammatory responses and fibrosis resulting from rejection, as well as the time since transplantation [45]. Our review shows that the mortality rate for nephrectomy is high, reaching 4%. When we considered only cases with an acute indication, this rate increases to 9%. The data were very heterogeneous, possibly because of publication bias, which may suggest an even greater mortality for this procedure. This possible heterogeneity is common in case series, which are less likely to report negative results [47]. Data from our study show that mortality rates for nephrectomy are higher than the rate of admissions for surgical emergencies in the United States (1.23%) and lower than the rate of admissions for emergencies requiring laparotomy (23.76%) [48]. Compared with renal transplant surgery, mortality data for transplantectomy after a non-functioning graft are also higher [1].
In contrast, the mortality for embolization was lower, with the greater homogeneity of the data suggesting absence of publication bias. Thus, the mortality for embolization in this series was low, which is in line with findings for minimally invasive percutaneous procedures. We must, however, consider that most studies of nephrectomy are older than studies on embolization, a fact that can lead to unfavorable results of nephrectomy. Similarly, embolization cannot be performed for indications such as renal rupture or thrombosis, and this reduces the comparability of the groups.
The second point to address is the morbidities common to both procedures. High morbidity has been reported after transplantectomy, with complications such as bleeding and infection [45]. The morbidity data for transplantectomy in our study reached 18%. These rates are similar to those reported for surgical emergencies in the United States (15%) [48]. In contrast, the rate of complications for percutaneous embolization was lower (1.2%) and the results were more homogeneous.
The third point is the morbidity specific to embolization. Post-embolization syndrome is still frequent with this type of procedure, occurring in 68% of cases. Similar data have been reported in cases of renal artery embolization due to trauma or tumors [49, 50]. Despite the frequency of the post-embolization syndrome, it is easily managed with analgesics and antipyretics.
The weaknesses of this review are in the indication for procedures used to remove a non-functioning renal graft. Because of the lack of prospective studies, it is not possible to establish the percentage of cases that were successfully treated with clinical management and the percentage of indications for graft removal. Prospective studies are especially lacking for patients with early vascular graft loss, who may not have had an elective indication for embolization.
The strengths of this study are the compilation of all the results available in the literature on transplantectomy and embolization, allowing us to establish mortality and morbidity rates for these procedures not yet reported. In the absence of clinical trials, case series reviews provide the best available evidence.
Conclusions
Our review found that transplantectomy has higher mortality and morbidity rates than renal transplantation, possibly because of the surgical difficulties posed by graft fibrosis. Embolization is a less invasive technique and may be an alternative with fewer complications, but its outcomes in terms of specific morbidities, such as post-embolization syndrome, are still not fully known. This is a specific complication of the therapy, but in most cases it is symptomatic and easily managed.
Attachments
The full search strategy.
Funnel plot of case series studies that reported mortality after non-functioning renal graft. (A) Nephrectomy group; (B) Embolization group.
Funnel plot of case series studies that reported morbidities after non-functioning renal graft. (A) Nephrectomy group; (B) Embolization group.
Footnotes
Source of support: Departmental sources
Conflict of Interest
None.
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859–65. doi: 10.1681/ASN.2004121092. [DOI] [PubMed] [Google Scholar]
- 3.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917–22. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 5.McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transplant. 2002;17:2212–19. doi: 10.1093/ndt/17.12.2212. [DOI] [PubMed] [Google Scholar]
- 6.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: Have we made signifi cant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289–95. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 7.Perez Martinez J, Gallego E, Julia E, et al. Embolization of non-functioning renal allograft: Efficacy and control of systemic inflammation. Nefrologia. 2005;25:422–27. [PubMed] [Google Scholar]
- 8.Atar E, Belenky A, Neuman-Levin M, Yussim A, et al. Nonfunctioning renal allograft embolization as an alternative to graft nephrectomy: Report on seven years’ experience. Cardiovasc Intervent Radiol. 2003;26:37–39. doi: 10.1007/s00270-002-1976-z. [DOI] [PubMed] [Google Scholar]
- 9.Noel C, Hazzan M, Boukelmoune M, et al. Indication for allograft nephrectomy after irreversible rejection: Is there an ideal delay? Transplant Proc. 1997;29:145–46. doi: 10.1016/s0041-1345(96)00041-3. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland DER, Simmons RL, Howard RJ, Najarian JS. Intracapsular technique of transplant nephrectomy. Surg Gynecol Obstet. 1978;146:950–52. [PubMed] [Google Scholar]
- 11.Silberman H, Fitzgibbons TJ, Butler J, Berne TV. Renal allografts retained in situ after failure. Arch Surg. 1980;115:42–43. doi: 10.1001/archsurg.1980.01380010034006. [DOI] [PubMed] [Google Scholar]
- 12.Voesten HGJ, Slooff MJH, Hooykaas JAP. Safe removal of failed transplanted kidneys. Br J Surg. 1982;69:480–81. doi: 10.1002/bjs.1800690818. [DOI] [PubMed] [Google Scholar]
- 13.Delgado P, Diaz F, Gonzalez A, et al. Intolerance syndrome in failed renal allografts: Incidence and efficacy of percutaneous embolization. Am J Kidney Dis. 2005;46:339–44. doi: 10.1053/j.ajkd.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–69. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Capocasale E, Larini P, Mazzoni MP, et al. Percutaneous renal artery embolization of nonfunctioning allograft: Preliminary experience. Transplant Proc. 2005;37:2523–24. doi: 10.1016/j.transproceed.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Cofan F, Real MI, Vilardell J, et al. Percutaneous renal artery embolisation of non-functioning renal allografts with clinical intolerance. Transpl Int. 2002;15:149–55. doi: 10.1007/s00147-002-0390-4. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Satue C, Riera L, Franco E, et al. Percutaneous embolization of non-functioning renal graft as therapeutic alternative to surgical transplantectomy. Actas Urol Esp. 2000;24:319–24. doi: 10.1016/s0210-4806(00)72455-7. [DOI] [PubMed] [Google Scholar]
- 19.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 20.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.El Dib R, Nascimento P, Jr, Kapoor A. An alternative approach to deal with the absence of clinical trials: A proportional meta-analysis of case series studies. Acta Cir Bras. 2013;28:870–76. doi: 10.1590/s0102-86502013001200010. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonilla AJA, Alfaro AG, Henandez JPC, et al. Review of a transplantectomy series. Transplant Proc. 2015;47:81–83. doi: 10.1016/j.transproceed.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Ariyarathenam A, Bamford A, Akoh JA. Transplant nephrectomy – A single-center experience. Saudi J Kidney Dis Transpl. 2015;26:1108–12. doi: 10.4103/1319-2442.168557. [DOI] [PubMed] [Google Scholar]
- 26.Freitas R, Malheiro J, Santos C, et al. Allograft nephrectomy: A single-institution, 10-year experience. Transplant Proc. 2015;47:992–95. doi: 10.1016/j.transproceed.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Alberts VP, Minnee RC, Bemelman FJ, et al. Transplant nephrectomy: What are the surgical risks? Ann Transplant. 2013;18:174–81. doi: 10.12659/AOT.883887. [DOI] [PubMed] [Google Scholar]
- 28.Yang XZ, Yang L, Chen ZQ, Yang YY. Arterial embolization for treating post-transplanted renal failure. J Interv Radiol. 2008;17:484–85. [Google Scholar]
- 29.Wang Y, Li XB, Hu XP, et al. [Operation opportunity for nephrectomy of transplanted kidney]. National Medical Journal of China. 2008;88:1093–95. [in Chinese] [Google Scholar]
- 30.Eng MMP, Power RE, Hickey DP, Little DM. Vascular complications of allograft nephrectomy. Eur J Vasc Endovasc Surg. 2006;32:212–16. doi: 10.1016/j.ejvs.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Solinas A, De Giorgi F, Frongia M. Ablation of non functioning renal allograft by embolization: A valid alternative to graft nephrectomy? Arch Ital Urol Nefrol Androl. 2005;77:99–102. [PubMed] [Google Scholar]
- 32.Mazzucchi E, Nahas WC, Antonopoulos IM, et al. Surgical complications of graft nephrectomy in the modern transplant era. J Urol. 2003;170:734–37. doi: 10.1097/01.ju.0000080566.42381.94. [DOI] [PubMed] [Google Scholar]
- 33.Khakhar AK, Shahinian VB, House AA, et al. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc. 2003;35:862–63. doi: 10.1016/s0041-1345(02)04031-9. [DOI] [PubMed] [Google Scholar]
- 34.Emiroglu R, Sevmis S, Moray G, et al. One center’s experience with allograft nephrectomy. Transplant Proc. 2003;35:2668–69. doi: 10.1016/j.transproceed.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 35.Arvind NK, Srivastava A, Kumar A, Das SK. Graft nephrectomy: The SGPGI experience. Indian J Urol. 2002;19:68–72. [Google Scholar]
- 36.Koh YB, Lee KH, Moon IS, et al. Transplant nephrectomy in 927 kidney transplantations. Transplant Proc. 1996;28:1470–71. [PubMed] [Google Scholar]
- 37.Lechevallier E. [Kidney transplantectomy: A multicenter study of the Committee of Transplantation of the French Urology Association]. Prog Urol. 1995;5:204–10. [in French] [PubMed] [Google Scholar]
- 38.Burgos Revilla FJ, Orofino Azcue L, del Hoyo Campos J, et al. Nephrectomy of the transplanted kidney. Arch Esp Urol. 1994;47:255–61. [PubMed] [Google Scholar]
- 39.Sharma DK, Pandey AP, Nath V, Gopalakrishnan G. Allograft nephrectomy – A 16-year experience. Br J Urol. 1989;64:122–24. doi: 10.1111/j.1464-410x.1989.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 40.Chiverton SG, Murie JA, Allen RD, Morris PJ. Renal transplant nephrectomy. Surg Gynecol Obstet. 1987;164:324–28. [PubMed] [Google Scholar]
- 41.DiSesa VJ, Tilney NL. Conservative management of the failed renal allograft: Indications for transplant nephrectomy. Curr Surg. 1982;39:417–18. [PubMed] [Google Scholar]
- 42.Lorenzo V, Diaz F, Perez L, et al. Ablation of irreversibly rejected renal allograft by embolization with absolute ethanol: A new clinical application. Am J Kidney Dis. 1993;22:592–95. doi: 10.1016/s0272-6386(12)80934-6. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Satue C, Riera L, Franco E, et al. Percutaneous embolization of the failed renal allograft in patients with graft intolerance syndrome. BJU Int. 2000;86:610–12. doi: 10.1046/j.1464-410x.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Gomez JM, Perez-Flores I, Jofre R, et al. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol. 2004;15:2494–501. doi: 10.1097/01.ASN.0000137879.97445.6E. [DOI] [PubMed] [Google Scholar]
- 45.Danovitch GM. Handbook of kidney transplantation. 5th Edition. Lippincott Williams & Wilkins; Philadelphia, PA USA: 2010. p. 194. [Google Scholar]
- 46.Fernandez Aparicio T, Minana Lopez B, Fraile Gomez B, et al. Renal transplantectomy. Arch Esp Urol. 1996;49:1079–91. [PubMed] [Google Scholar]
- 47.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 48.Scott JW, Olufajo OA, Brat GA, et al. Use of national burden to define operative emergency general surgery. JAMA Surg. 2016;151:e160480. doi: 10.1001/jamasurg.2016.0480. [DOI] [PubMed] [Google Scholar]
- 49.Guzinski M, Kurcz J, Tupikowski K, et al. The role of transarterial embolization in the treatment of renal tumors. Adv Clin Exp Med. 2015;24:837–43. doi: 10.17219/acem/29143. [DOI] [PubMed] [Google Scholar]
- 50.Thorlund MG, Wennevik GE, Andersen M, Andersen PE, Lund L. High success rate after arterial renal embolisation. Dan Med J. 2015;62 pii: A5061. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full search strategy.
Funnel plot of case series studies that reported mortality after non-functioning renal graft. (A) Nephrectomy group; (B) Embolization group.
Funnel plot of case series studies that reported morbidities after non-functioning renal graft. (A) Nephrectomy group; (B) Embolization group.