Abstract
Background
As end-stage liver disease progresses, renal blood flow linearly correlates with mean arterial blood pressure (MBP) due to impaired autoregulation. We investigated whether the lower degree of postoperative MBP would predict the occurrence of postoperative acute kidney injury (AKI) after liver transplantation.
Material/Methods
This retrospective study enrolled 1,136 recipients with normal preoperative kidney function. Patients were categorized into two groups according to the averaged postoperative MBP: <90 mmHg (MBPbelow90) and ≥90 mmHg (MBPover90). The primary endpoint was occurrence of postoperative AKI, defined by the creatinine criteria of the Kidney Disease Improving Global Outcomes. The logistic regression model with inverse probability treatment weighting (IPTW) of propensity score was used to compare the risk of postoperative AKI between two groups.
Results
MBPbelow90 group (83.0±5.1 mmHg) showed higher prevalence and risk of postoperative AKI (74.2% versus 62.6%, p<0.001; IPTW-OR 1.34 [1.12–1.61], p=0.001) compared with MBPover90 group (97.3±5.2 mmHg). When stratified by quartiles of baseline cystatin C glomerular filtration ratio (GFR), the association between MBPbelow90 and postoperative AKI remained significant only with the lowest quartile (cystatin C GFR ≤85 mL/min/1.73 m2; IPTW-OR 2.24 [1.53–3.28], p<0.001), but not with 2nd–4th quartiles.
Conclusions
Our results suggest that maintaining supranormal MBP over 90 mmHg may be beneficial to reduce the risk of post-LT AKI, especially for liver transplant recipients with cystatin C GFR ≤85 mL/min/1.73 m2.
MeSH Keywords: Acute Kidney Injury, Arterial Pressure, Liver Transplantation, Renal Circulation
Background
Postoperative acute kidney injury (AKI) is a major cause of morbidity and mortality following surgery [1,2]. AKI occurrence depends on pre-existing renal function [3,4], the complexity of the surgery [1,5,6]. and perioperative management, including hemodynamic optimization [7,8]. Avoiding perioperative hypotension and optimizing mean arterial blood pressure (MBP) have been known to prevent injury to the kidney, which is the most sensitive organ to these conditions [7,9]. Studies have suggested an MBP of 55–60 mmHg as a threshold point for the autoregulation plateau of renal blood flow to prevent postoperative AKI [4,8,10,11], however, those findings were extrapolated from patients undergoing noncardiac surgery and healthy animals. For critically ill patients (e.g., patients with sepsis or septic shock, especially those with chronic hypertension), a target MBP up to 80–95 mmHg has been associated with favorable outcomes, including a reduction in renal failure and mortality [12,13], presumably because renal autoregulation is impaired in those patients [14–16].
Patients with end-stage liver disease (ESLD) also experience impaired renal autoregulation. Although the mechanisms leading to autoregulation failure are not entirely clear, evidences from both animal and human studies have shown that the renal autoregulation curve shifts to the right and becomes linearized as ESLD progresses [17,18]. Hence, at a given MBP level, renal blood flow might be markedly lower than normal. Even small changes in MBP may directly affect renal blood flow, thus making patients with ESLD vulnerable to kidney injury during hemodynamic fluctuation.
In this regard, postoperative AKI occurs in up to 80% of patients after liver transplantation (LT), with an adverse impact on the outcome of LT [19,20]. In addition, clinical studies have demonstrated that raising MBP up to “supranormal” levels was associated with a reversal of hepatorenal syndrome (HRS) regardless of the type of vasoactive therapy [18,21–25]. Thus, we hypothesized that raising MBP would also have a favorable impact on the occurrence of postoperative AKI following LT in the same manner, as it did for septic shock and HRS. However, studies on how MBP influences kidney outcomes after LT are scarce. Thus, we aimed to determine the association between postoperative MBP and occurrence of postoperative AKI after LT using population data from our large-volume center.
Material and Methods
This retrospective observational study was approved by our institutional review board. We reviewed consecutive patients who underwent living-donor LT between July 2011 and October 2015. Patients who were older than 20 years of age were included and a total of 1,292 patients were identified. As to include the patients only with normal kidney functions, we excluded patients who had chronic kidney disease (CKD, n=15). CKD was defined as positive when patients were already diagnosed by nephrologist prior to LT candidacy. To exclude the patients who had any possibility of being diagnosed as HRS, those with preoperative creatinine >1.5 mg/dL, which was adapted from HRS diagnostic criteria [26], were excluded (n=69). We also excluded patients who did not have preoperative cystatin C glomerular filtration rate (GFR) data (n=72). In all, 1,136 patients were enrolled.
Anesthesia and hemodynamic monitoring were performed in accordance with our institutional standard [27]. Briefly, we maintained anesthesia with sevoflurane or desflurane, a mixture of 50% O2 and 50% air, and a continuous infusion of fentanyl. Arterial pressure was monitored using radial and femoral arterial catheters. For advanced hemodynamic monitoring, a pulmonary arterial catheter was inserted and connected to a Vigilance device (Vigilance II, Edwards Lifesciences LLC). According to the patient’s blood pressure and hemodynamics, either fluid or vasoactive drugs were given by the attending anesthesiologist. In cases of low systemic vascular resistance, a continuous infusion of norepinephrine, vasopressin, or terlipressin was used to maintain MBP. We attempted to maintain intraoperative MBP above the preoperative level. After surgery, patients were transferred to the intensive care unit in an intubated state. Patients were sedated with remifentanil and usually extubated on postoperative day 1.
We collected the patients’ preoperative and early postoperative MBP values. MBP was calculated using the following equation: MBP=(systolic blood pressure +2×diastolic blood pressure)/3. Preoperative MBP was defined as the averaged blood pressure value measured during the week before surgery. Early postoperative MBP was defined as the averaged MBP value until the postoperative day 1. Because a single measurement of MBP would not accurately represent MBP status, we derived the MBP automatically. Using our institution’s electronic medical record system (Asan Biomedical Research Environment), a total of 35,467 preoperative MBP and 33,185 early postoperative MBP values were collected and then averaged for each patient to represent preoperative and early postoperative MBP variables.
The preoperative characteristics and perioperative variables were also collected. Preoperative characteristics included age, sex, body mass index (BMI), Model for End-Stage Liver Disease (MELD) score, heart rate, comorbidities (hypertension, diabetes mellitus), hypertension medication, etiology of liver cirrhosis, and donor age. Preoperative laboratory variables included serum creatinine, cystatin C GFR, serum albumin, and total bilirubin. Intraoperative variables included units of transfused packed red blood cell, severe post-reperfusion syndrome, graft-to-recipient body weight ratio, and total ischemic time. Severe post-reperfusion syndrome was defined as the need for more than 30 μg of epinephrine during the reperfusion period.
The primary outcome in our study was the occurrence of postoperative AKI. The Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria were used to determine AKI [28]. KDIGO classification diagnosis of AKI is based on changes in serum creatinine within 1–7 postoperative days (POD) from baseline creatinine. KDIGO classification is further divided into three stages; stage 1 as an increase in creatinine ≥0.3 mg/dL within 48 hours or 1.5–1.9 times baseline within 7 days; stage 2 as an increase in creatinine of 2.0–2.9 times baseline within 7 days; stage 3 as an increase in creatinine of 3.0 times baseline or ≥4.0 mg/dL, with an acute increase of at least 0.5 mg/dL, or the need for renal replacement therapy within 7 days. For consecutive creatinine data in the same POD, the highest level of each day was selected for data collection. The secondary outcome was development of stage 3 or higher CKD, which was defined when the estimated GFR decreased to <60 mL/min/1.73 m2 in two consecutive occasions at least three months apart [29].
Variables are expressed as numbers and percentages, means ± standard deviation, or medians with interquartile ranges (IQR) if skewed. We categorized all patients into two groups based on the early postoperative MBP: less than 90 mmHg (MBPbelow90) and greater than 90 mmHg (MBPover90). Intergroup comparisons were performed using the chi-squared test or Fisher’s exact test for categorical variables, and the Student’s t-test or Mann-Whitney U test for continuous variables as appropriate. Multivariate logistic regression was applied to determine whether the association of early postoperative MBP with postoperative AKI was independent of confounding variables. Variables with p values of <0.1 in the univariate analysis were included for multivariable analysis. To further decrease the effect of potential confounding in this observational study, we also rigorously adjusted for significantly different characteristics by performing weighted logistic regression modeling with inverse probability treatment weighting (IPTW) [30]. Weights for MBPover90 group were the inverse of 1 – propensity score, and weights for MBPbelow90 were the inverse of propensity score. We calculated propensity scores without regard to results from multivariate logistic regression modeling. All preoperative and perioperative covariates represented in Table 1 were included in a full non-parsimonious model. Discrimination of the model was assessed by C-statistics and calibration was evaluated with the Hosmer-Lemeshow statistics. We stratified patients according to the baseline cystatin C GFR (quartile 1 [Q1] versus quartiles 2–4 [Q2–4]) to examine the association between baseline kidney function and the effect of early postoperative MBP on postoperative AKI. IPTW-weighted logistic regression modeling was applied for each subgroup. To present the relationship between early postoperative MBP and postoperative AKI, we conducted multivariable generalized logistic regression analysis with logit model using significant independent risk factors from multivariable analysis to plot predicted probability and relative risk. We considered p values <0.05 to be statistically significant. Statistical analyses were performing using SPSS version 22 (IBM, New York, NY, USA) or R version 3.3.2 (http://www.R-project.org/).
Table 1.
Perioperative variables according to the development of postoperative acute kidney injury.
| AKI (n=777, 68.4%) | No AKI (n=359) | Total (n=1136) | P value | |
|---|---|---|---|---|
| Perioperative MBP | ||||
| Preoperative MBP | 81.6±9.4 | 84.2±9.4 | 82.4±9.5 | <0.001 |
| Early postoperative MBP | 89.3±8.6 | 92.0±8.8 | 90.2±8.8 | <0.001 |
| Increase in MBP after surgery | 8.2 [0.9; 14.7] | 8.4 [0.9; 14.7] | 8.3 [0.9; 14.7] | 0.998 |
| Demographics | ||||
| Age (years) | 53.2±8.3 | 52.7±7.4 | 53.0±8.0 | 0.311 |
| Sex, male | 575 (74.0%) | 284 (79.1%) | 859 (75.6%) | 0.074 |
| Body mass index (kg/m2) | 24.4±3.5 | 23.6±2.8 | 24.1±3.4 | <0.001 |
| Diabetes mellitus | 161 (20.7%) | 68 (18.9%) | 229 (20.2%) | 0.538 |
| Hypertension | 78 (10.0%) | 48 (13.4%) | 126 (11.1%) | 0.119 |
| Heart rate | 72.9±11.8 | 72.3±10.9 | 72.7±11.5 | 0.393 |
| MELD score | 14.1±6.3 | 12.4±6.8 | 13.6±6.5 | <0.001 |
| Preoperative vasopressor use | 32 (4.1%) | 12 (3.3%) | 44 (3.9%) | 0.765 |
| Donor age | 28.4±8.6 | 27.2 ±8.1 | 28.0±8.4 | 0.031 |
| Hypertension medication | ||||
| Angiotensin II receptor antagonist | 21 (5.9%) | 47 (6.1%) | 68 (6.0%) | 1.000 |
| Angiotensin-converting-enzyme inhibitor | 1 (0.3%) | 2 (0.3%) | 3 (0.3%) | 1.000 |
| Calcium channel blocker | 34 (9.5%) | 80 (10.3) | 114 (10.0) | 0.746 |
| Beta blockers | 50 (14.0%) | 119 (15.3%) | 169 (14.9%) | 0.602 |
| Etiology of liver cirrhosis | ||||
| Hepatitis B virus | 445 (57.3%) | 245 (68.2%) | 690 (60.7%) | 0.001 |
| Hepatitis C virus | 64 (8.2%) | 23 (6.4%) | 87 (7.7%) | 0.338 |
| Alcohol abuse | 161 (20.7%) | 46 (12.8%) | 207 (18.2%) | 0.002 |
| Biliary cirrhosis | 29 (3.7%) | 12 (3.3%) | 41 (3.6%) | 0.876 |
| Others | 97 (12.5%) | 38 (10.6%) | 135 (11.9%) | 0.412 |
| Preoperative laboratory variables | ||||
| Creatinine (mg/dL) | 0.7±0.2 | 0.7±0.2 | 0.7±0.2 | 0.471 |
| Cystatin C GFR (ml/min/1.73 m2) | 102.7±28.5 | 112.7±37.4 | 105.9±31.9 | <0.001 |
| Albumin (g/dL) | 3.1±0.5 | 3.3±0.5 | 3.2±0.6 | <0.001 |
| Total bilirubin (mg/dL) | 4.7±7.6 | 4.3±8.1 | 4.5±7.8 | 0.469 |
| Perioperative variables | ||||
| Transfused pRBC (units) | 6 [2; 12] | 4 [0; 8] | 5 [1; 10] | 0.001 |
| Intraoperative vasopressor uses | 724 (93.2%) | 327 (91.1%) | 1051 (92.5%) | 0.317 |
| Severe post-reperfusion syndrome | 90 (11.6%) | 35 (9.8%) | 125 (11.0%) | 0.424 |
| Graft-to-recipient weight ratio | 1.1±0.2 | 1.2±0.2 | 1.1±0.2 | 0.006 |
| Total ischemic time (min) | 129.8±30.8 | 130.0±51.9 | 129.9±38.7 | 0.941 |
| Postoperative vasopressor use (days) | 0 [0; 1] | 0 [0; 0] | 0 [0; 1] | <0.001 |
| Use of FK506 | 696 (89.6%) | 329 (91.6%) | 1025 (90.2%) | 0.333 |
| Trough level of FK506 (ng/mL) | 6.2±2.5 | 6.2±2.4 | 6.2±2.5 | 0.737 |
| Outcome variables | ||||
| Renal replacement therapy | 8 (2.2%) | 50 (6.4%) | 58 (5.1%) | 0.004 |
| 3-month CKD | 30 (3.7%) | 7 (2.0%) | 37 (3.3%) | 0.132 |
| Overall CKD | 190 (24.5%) | 47 (13.1%) | 237 (20.9%) | <0.001 |
| Hospital stay (days) | 28 [23; 38] | 32 [24; 47] | 30 [24; 44] | <0.001 |
| Intensive care unit stay (days) | 2 [2; 4] | 3 [2; 5] | 3 [2; 5] | <0.001 |
Values are expressed as mean±SD, median [IQR], or numbers (percent). AKI – acute kidney injury; MBP – mean arterial blood pressure; MELD – model for end-stage liver disease; GFR – glomerular filtration rate; pRBC – packed red blood cells; CKD – chronic kidney injury.
Results
A total of 1,136 patients were evaluated. Table 1 lists the preoperative characteristics and perioperative variables for these patients according to the development of AKI. Postoperative AKI occurred in 777 (68.4%) patients. Of them, 466 patients (41.0%) were classified with stage 1 AKI, 233 patients (20.5%) with stage 2 AKI, and 78 patients (6.9%) with stage 3 AKI. Patients who developed postoperative AKI had lower early postoperative MBPs (89.3±8.6 versus 92.0±8.8, p<0.001; Figure 1). Other variables, including BMI, MELD score, donor age, hepatitis B cirrhosis, alcoholic cirrhosis, cystatin C GFR, serum albumin concentration, units of transfused packed red blood cells, and graft-to-recipient body weight ratio differed between patients who developed postoperative AKI and patients who did not (p<0.05).
Figure 1.
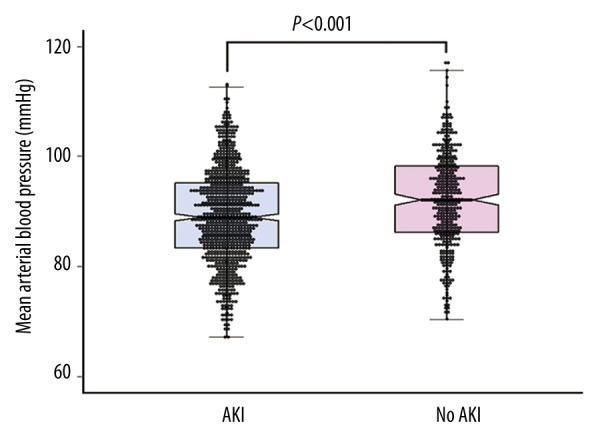
Comparison of early postoperative mean arterial blood pressure according to the occurrence of postoperative AKI. Boxes represent median with interquartile range and the whiskers the 95th percentile. AKI – acute kidney injury.
To compare the incidence of postoperative AKI according to early postoperative MBP, patients were divided into two groups by their early postoperative MBP: MBPover90 and MBPbelow90. Table 2 shows the perioperative variables according to these groups. The optimum cutoff value of 90 mmHg was derived from receiver operating curve analysis (area under the curve=0.588, p<0.001). Additionally, other studies of patients with HRS [18,21–25], septic shock [13,31], and cardiac surgery [32] recommended a value of 90 mmHg for the prevention of kidney injury. The average early postoperative MBP values for the MBPover90 and MBPbelow90 groups were 97.3 ± 5.2 mmHg (range: 90–117 mmHg) and 83.0 ± 5.1 mmHg (range: 67–89 mmHg), respectively. When MBP was compared with preoperative and early postoperative MBP values, the degrees of the increases in MBP after surgery were 12.7 mmHg (IQR: 5.2–18.9 mmHg) and 4.9 mmHg (IQR: −1.9–10.3 mmHg) for the MBPover90 and MBPbelow90 groups, respectively (p<0.001).
Table 2.
Perioperative variables according to early postoperative mean arterial blood pressure.
| MBPover90 (n=567) | MBPbelow90 (n=569) | Total (n=1136) | P value | |
|---|---|---|---|---|
| Perioperative MBP | ||||
| Preoperative MBP | 85.6±9.4 | 79.2±8.4 | 82.4±9.5 | <0.001 |
| Early postoperative MBP | 97.3±5.2 | 83.0±5.1 | 90.2±8.8 | <0.001 |
| Increase in MBP after surgery | 12.7 [5.2; 18.9] | 4.9 [–1.9; 10.3] | 8.3 [0.9; 14.7] | <0.001 |
| Demographics | ||||
| Age (years) | 51.8±8.1 | 54.2±7.8 | 53.0±8.0 | <0.001 |
| Sex, male | 453 (79.9%) | 406 (71.4%) | 859 (75.6%) | 0.001 |
| Body mass index (kg/m2) | 24.2±3.1 | 24.0±3.6 | 24.1±3.4 | 0.100 |
| Diabetes mellitus | 98 (17.3%) | 131 (23.0%) | 229 (20.2%) | 0.019 |
| Hypertension | 79 (13.9%) | 47 (8.3%) | 126 (11.1%) | 0.003 |
| Heart rate | 72.5±11.2 | 72.9±11.8 | 72.7±11.5 | 0.636 |
| MELD score | 13.1±6.6 | 14.0±6.3 | 13.6±6.5 | <0.001 |
| Preoperative vasopressor use | 24 (4.2%) | 20 (3.5%) | 44 (3.9%) | 0.636 |
| Donor age | 27.4±8.6 | 28.6 ±8.3 | 28.0±8.4 | 0.023 |
| Hypertension medication | ||||
| Angiotensin II receptor antagonist | 40 (7.1%) | 28 (4.9%) | 68 (6.0%) | 0.164 |
| Angiotensin-converting-enzyme inhibitor | 2 (0.4%) | 1 (0.2%) | 3 (0.3%) | 0.998 |
| Calcium channel blocker | 67 (11.8%) | 47 (8.3%) | 114 (10.0%) | 0.058 |
| Beta blockers | 80 (14.1%) | 89 (15.6%) | 169 (14.9%) | 0.521 |
| Etiology of liver cirrhosis | ||||
| Hepatitis B virus | 361 (63.7%) | 329 (57.8%) | 690 (60.7%) | 0.050 |
| Hepatitis C virus | 38 (6.7%) | 49 (8.6%) | 87 (7.7%) | 0.272 |
| Alcohol abuse | 105 (18.5%) | 102 (17.9%) | 207 (18.2%) | 0.856 |
| Biliary cirrhosis | 12 (2.3%) | 28 (4.9%) | 41 (3.6%) | 0.027 |
| Others | 61 (10.8%) | 74 (13.0%) | 135 (11.9%) | 0.281 |
| Preoperative laboratory variables | ||||
| Creatinine (mg/dL) | 0.7±0.2 | 0.7±0.2 | 0.7±0.2 | 0.017 |
| Cystatin C GFR (ml/min/1.73 m2) | 107.9±31.2 | 103.9±32.5 | 105.9±31.9 | 0.011 |
| Albumin (g/dL) | 3.2±0.6 | 3.1±0.5 | 3.2±0.6 | <0.001 |
| Total bilirubin (mg/dL) | 4.4±7.8 | 4.7±7.8 | 4.5±7.8 | 0.022 |
| Perioperative variables | ||||
| Transfused pRBC (Units) | 5 [0; 10] | 6 [2; 12] | 5 [1; 10] | 0.006 |
| Intraoperative vasopressor uses | 510 (89.9%) | 541 (95.1%) | 1051 (92.5%) | 0.002 |
| Severe post-reperfusion syndrome | 50 (8.8%) | 75 (13.2%) | 125 (11.0%) | 0.024 |
| Graft-to-recipient weight ratio | 1.1±0.2 | 1.1±0.3 | 1.1±0.2 | 0.381 |
| Total ischemic time (min) | 128.9±45.2 | 130.9±30.9 | 129.9±38.7 | 0.071 |
| Postoperative vasopressor use (days) | 0 [0; 0] | 0 [0; 1] | 0 [0; 1] | <0.001 |
| Use of FK506 | 518 (91.4%) | 4.5±39.4 | 1025 (90.2%) | 0.120 |
| Trough level of FK506 (ng/mL) | 6.2±2.4 | 507 (89.1%) | 6.2±2.5 | 0.238 |
| Outcome variables | ||||
| Acute kidney injury | 355 (62.6%) | 422 (74.2%) | 777 (68.4%) | <0.001 |
| Grade 1 | 227 (40.0%) | 239 (42.05) | 466 (41.0%) | 0.539 |
| Grade 2 | 95 (16.8%) | 138 (24.3%) | 233 (20.5%) | 0.002 |
| Grade 3 | 33 (5.8%) | 45 (7.9%) | 78 (6.9%) | 0.202 |
| 3-month CKD | 18 (3.2%) | 19 (3.3%) | 37 (3.3%) | 1.000 |
| Overall CKD | 115 (20.3%) | 122 (21.4%) | 237 (20.9%) | 0.684 |
| Hospital stay (days) | 30 [24;41] | 31 [24;47] | 30 [24;44] | 0.108 |
| Intensive care unit stay (days) | 3 [2; 4] | 3 [2; 5] | 3 [2; 5] | 0.002 |
Values are expressed as mean±SD, median [IQR], or numbers (percent). MBP – mean arterial blood pressure; MELD – model for end-stage liver disease; GFR – glomerular filtration rate; pRBC – packed red blood cells; CKD – chronic kidney injury.
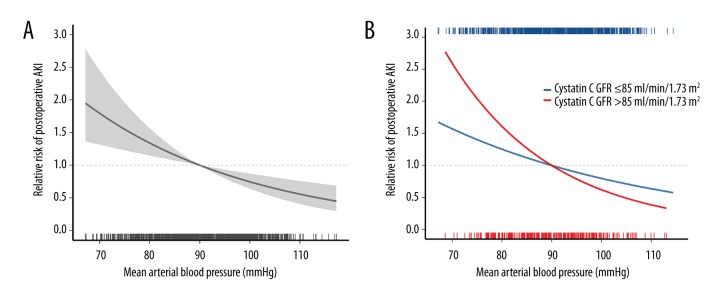
The incidence of postoperative AKI was significantly different between the two groups: 62.6% (355/567) for the MBPover90 group and 74.2% (422/569) for the MBPbelow90 group (p < 0.001). The odds ratio (OR) and IPTW-adjusted OR of MBPbelow90 for predicting postoperative AKI were 1.71 (95% confidence interval [CI]: 1.33–2.21, p<0.001) and 1.34 (95% CI: 1.12–1.61, p=0.001) when compared with MBPover90 (Table 3). Multivariable logistic regression with backward selection showed the significance of early postoperative MBP for predicting postoperative AKI (OR: 1.36; 95% CI: 1.16–1.59 by a decrease of 10 mmHg, p<0.001; Table 4). Other independent compounders of the final model were BMI, cystatin C GFR, serum albumin concentration, and units of transfused packed red blood cells. Figure 2A shows the relative risk of postoperative AKI after adjustment for independent confounders.
Table 3.
Prevalence and odds ratio of acute kidney injury according to the early postoperative mean arterial blood pressure.
| Incidence of acute kidney injury | Odds ratio | P value | IPTW adjusted odds ratio | P value | |
|---|---|---|---|---|---|
| Overall (n=1136) | |||||
| Early postoperative MBP ≥90 mmHg | 355/567 (62.6%) | 1 (reference) | < 0.001 | 1 (reference) | 0.001 |
| Early postoperative MBP <90 mmHg | 422/569 (74.2%) | 1.71 (1.33–2.21) | 1.34 (1.12–1.61) | ||
| Cystatin C GFR ≤85 ml/min/1.73m2 (n=290) | |||||
| Early postoperative MBP ≥90 mmHg | 84/127 (66.1%) | 1 (reference) | 0.007 | 1 (reference) | < 0.001 |
| Early postoperative MBP <90 mmHg | 131/163 (80.4%) | 2.10 (1.23–3.59) | 2.24 (1.53–3.28) | ||
| Cystatin C GFR >85 ml/min/1.73 m2 (n=846) | |||||
| Early postoperative MBP ≥90 mmHg | 271/440 (61.6%) | 1 (reference) | 0.002 | 1 (reference) | 0.157 |
| Early postoperative MBP <90 mmHg | 291/406 (71.7%) | 1.58 (1.18–2.11) | 1.16 (0.94–1.43) | ||
Values are expressed as numbers (present) or odds ratio (95% confidence interval). Model discrimination was assessed with C-statistics (overall, C=0.7169; GFR ≤85, C=0.7521; GFR >85, C=0.7309), and model calibration was assessed with Hosmer-Lemeshow statistics (overall, χ2=6.0478, df=8, p=0.6419; GFR ≤85, χ2=5.458, df=8, p=0.7077; GFR >85, χ2=5.2234, df=8, p=0.7335). OR – odds ratio; CI – confidence interval; MBP – mean blood pressure; GFR – glomerular filtration rate; MBP – mean arterial blood pressure; GFR – glomerular filtration rate; IPTW – inverse probability of treatment weighing.
Table 4.
Multivariable analysis of risk factors associated with postoperative acute kidney injury after liver transplantation defined by KDIGO criteria.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Male sex | 0.75 | 0.56–1.02 | 0.063 | |||
| Body mass index | 1.08 | 1.04–1.12 | <0.001 | 1.09 | 1.04–1.13 | <0.001 |
| Hypertension | 0.72 | 0.49–1.06 | 0.097 | |||
| Early postoperative MBP (by decrease of 10 mmHg) | 1.44 | 1.24–1.66 | <0.001 | 1.36 | 1.16–1.59 | <0.001 |
| MELD score | 1.04 | 1.02–1.07 | <0.001 | |||
| Cystatin C GFR (by decrease of 10 ml/min/1.73 m2) | 1.10 | 1.06–1.15 | <0.001 | 1.05 | 1.01–1.10 | 0.016 |
| Albumin | 0.42 | 0.33–0.54 | <0.001 | 0.52 | 0.40–0.68 | <0.001 |
| Hepatitis B cirrhosis | 0.61 | 0.47–0.79 | <0.001 | |||
| Alcoholic cirrhosis | 1.77 | 1.25–2.55 | 0.002 | 1.44 | 0.99–2.12 | 0.062 |
| Donor age | 1.02 | 1.00–1.03 | 0.039 | |||
| Transfused packed red blood cell (by unit) | 1.04 | 1.02–1.05 | <0.001 | 1.02 | 1.00–1.04 | 0.021 |
| Graft-to-recipient weight ratio | 0.50 | 0.30–0.84 | 0.008 | |||
KDIGO – the Kidney Disease: Improving Global Outcomes; OR – odds ratio; CI – confidence interval; MBP – mean arterial blood pressure; MELD – model for end-stage liver disease; GFR – glomerular filtration rate.
Figure 2.
Relative risk plot showing the relationship between early postoperative mean blood pressure and postoperative acute kidney injury (AKI) of overall patients (A). When stratified by the baseline kidney function, a steeper relative risk gradient is observed in patients with lower baseline kidney function (cystatin C GFR ≤85 mL/min/1.73 m2) than patients with higher baseline kidney function (cystatin C GFR >85 mL/min/1.73 m2, (B). Estimates are adjusted for independent confounders from multivariable generalized logistic regression model. The solid lines and translucent band depict relative risk and 95% confidence intervals of those estimates. AKI – acute kidney injury; GFR – glomerular filtration ratio.
To examine the association between baseline kidney function and the effect of MBP on AKI, we stratified patients according to cystatin C GFR (Q1 versus Q2–4). The quartiles of cystatin C GFR were 70.5±11.6 mL/min/1.73 m2 (Q1), 95.1±5.3 mL/min/1.73 m2 (Q2), 112.3±5.4 mL/min/1.73 m2 (Q3), and 146.1±28.9 mL/min/1.73 m2 (Q4). Among patients with the lowest quartile (Q1, cystatin C GFR ≤85 mL/min/1.73 m2), MBPbelow90 was still significantly associated with postoperative AKI (IPTW-adjusted OR: 2.24, 95% CI: 1.53–3.28, p<0.001; Table 3). However, among patients with higher baseline kidney function (Q2–4: cystatin C GFR >85 mL/min/1.73 m2), MBPbelow90 was not significantly associated with postoperative AKI (IPTW-adjusted OR: 1.16, 95% CI: 0.94–1.43, p=0.157). In multivariate-adjusted relative risk plot, patients with lower baseline kidney function (Q1) had a steeper relative risk gradient than patient with higher baseline kidney function (Q2–4, Figure 2B).
Three-month CKD and overall CKD after surgery were not different between MBPover90 and MBPbelow90 (18/567 [3.2%] versus 19/569 [3.3%], p=1.000 and 115/567 [20.3%] versus 122/569 [21.4%], p=0.684, respectively).
Discussion
In this retrospective observational study, we found that the degree of early postoperative MBP was independently associated with postoperative AKI after living-donor LT even after adjusted by IPTW of propensity score. The IPTW-adjusted odd ratio of MBPbelow90 for predicting postoperative AKI was 1.34 (95% CI: 1.12–1.61, p=0.001) when compared with MBPover90. In multivariate analysis, a 10 mmHg decrease of MBP was associated with 1.36-fold increased risk of postoperative AKI. Notably, despite MBP was maintained at a relatively high level (90.2±8.8 mmHg), the risk of AKI was still affected by the degree of MBP. This association between MBP and postoperative AKI remained significant only in patients with lower baseline cystatin C GFR ≤85 mL/min/1.73 m2.
In patients with ESLD, renal blood flow is known to be critically dependent on blood pressure [18]. Normal renal blood flow autoregulation curve has a sigmoid shape with a plateau, which operates at a renal perfusion pressure greater than 55–60 mmHg [4,8]. However, the autoregulation curve is altered and even lost in patients with ESLD. Although the mechanisms leading to autoregulation failure are not entirely clear, several factors have been identified, including an activated sympathetic nervous system [17,18], decreased myogenic responses of the renal vascular smooth muscle [33], and an activated renin-angiotensin system [11,34]. As a result, autoregulation curve shifts to the right and becomes linearized as ESLD progresses [17,18]. Therefore, the renal blood flow of a patient with ESLD is markedly lower at the same given level of MBP when compared with normal patients. Moreover, because MBP is linearly correlated with renal blood flow, even a small change of MBP can directly affect renal blood flow [17]. A previous study by Stadlbauer et al. demonstrated that the renal blood flow of normal patients was approximately 1,000 mL/min/g at an MBP of 70 mmHg, whereas renal blood flow for patients with ESLD was approximately 200–800 mL/min/g at the same MBP level [18]. Hence, for patients with ESLD who are scheduled for LT, renal hypoperfusion and ischemic kidney injury may not be prevented unless the MBP is kept at a high level.
An MBP increase is tightly correlated with an improvement in renal function for patients with HRS, which is the most severe form of renal dysfunction in patients with ESLD. Clinical studies on HRS have documented the clinical significance of increasing MBP. A previous randomized trial by Sharma et al., which was originally designed to observe the effect of different types of vasoconstrictors, eventually demonstrated that high MBP (83.0±8.5 mmHg versus 76.7±8.3 mmHg, p=0.023) is a strong predictor of therapeutic response regardless of the type of vasoconstrictor used [23]. In another similar randomized trial, patients in the highest quartile according to MBP increase (15.9–20.9 mmHg from baseline values) showed a greater treatment response to vasoconstrictor therapy regardless of therapy type [24]. A meta-analysis confirmed those parallel associations between MBP and improvement of kidney function in ESLD patients with HRS [25]. Among patients with a baseline MBP of ≥77 mmHg, there was also significant correlation between an increase of MBP and a change of creatinine (ρ=−0.83, p<0.001). The author stated that raising MBP might be required to maximize renal flow optimization and thus protect renal function in ESLD patients with HRS.
Our study indicated that the importance of increasing MBP is not only limited to the setting of HRS but is also applicable to post-LT status. According to our data, MBP has a significant impact on postoperative AKI in patients with normal preoperative creatinine levels as well as in patients with HRS. Similar results may have been found for both ESLD patients with normal creatinine and patients with HRS because the renal autoregulation curve has already started to alter in the early stages of ESLD, even before developing HRS [18]. Hence, factors affecting renal perfusion pressure, such as MBP and BMI, may have been independently associated with postoperative AKI in our study, as well as studies regarding HRS [21,24]. In addition, even if LT is performed successfully, renal autoregulation seems to return to normal only after a few weeks [35]. In a previous study of ESLD patients with and without HRS, renal function declined even during first week after LT and recovered to preoperative levels after more than a week [36]. Thus, perioperative MBP needs to be tightly regulated to maintain proper renal blood flow because the return of normal renal autoregulation cannot be guaranteed until weeks after LT.
Another remarkable aspect of our results is that supranormal levels of MBP helped to prevent the occurrence of postoperative AKI. This result is also in line with previous studies regarding MBP and HRS [21–24]. A meta-analysis demonstrated that improvement in renal function were observed not only when MBP increased from low to normal, but also when MBP increased from normal to “supranormal” [25]. Those previous studies stated that an MBP of >90 mmHg might be needed to minimize renal injury in patients with ESLD. Supranormal MBP is also recommended for patients with septic shock and for cardiac surgery, when renal autoregulation impairment frequently develops [13,31,32]. Our receiver operating curve analysis showed the best MBP cutoff value for predicting postoperative AKI to be 90 mmHg, which is a supranormal level. Also, the degree of MBP increase from preoperative values was highest in our MBPover90 group (12.7 mmHg, IQR: 5.2–18.9 mmHg), which is similar to the value in a previous study regarding MBP increases and HRS [24]. In that previous study, the group with the greatest MBP increase showed an MBP increase from 15.9 mmHg to 20.9 mmHg; this group also demonstrated the greatest creatinine reduction. Other previous studies showed that an increase of approximately 10 mmHg – from normal to supranormal – was needed to demonstrate a clinical difference in patients with HRS [21,22].
The effect of MBP on postoperative AKI was more prominent in patients with lower cystatin C GFR values. Considering that our study cohort did not have HRS, our result suggests a possibility that not only the patients with HRS but also the patients with even mild renal impairment (cystatin C GFR ≤85 mL/min/1.73 m2), consequently much wider spectrum of cirrhotic patients, may benefit from maintaining adequate MBP to avoid further injuries to the kidney. In addition, we demonstrated that cystatin C GFR was an independent risk factor for postoperative AKI. We included cystatin C GFR in the multivariable analysis instead of creatinine because cystatin C GFR is known to better reflect the renal function of patients with ESLD [37]. In this regard, our study showed that preoperative creatinine levels were not different between patients with and without postoperative AKI (0.7±0.2 mg/dL for both groups, p=0.471). However, cystatin C GFR was significantly higher in patients who developed postoperative AKI (112.7±37.4 mL/min/1.73 m2 versus 102.7±28.5 mL/min/1.73 m2, p<0.001). Moreover, cystatin C GFR was significantly correlated with preoperative MBP (r=0.184, p<0.001). We speculated that preoperative MBP within a week before LT may affect preoperative baseline renal function as measured by cystatin C GFR. BMI and albumin levels were also found to be independent risk factors for postoperative AKI. This finding agrees with previous results: the increased intra-abdominal pressure of obese patients was found to negatively affect renal perfusion pressure [38], and preoperative and postoperative low albumin levels were found to be independent risk factors for AKI after LT [39,40].
There are several limitations to our study. First, although we conducted an observational analysis of over 1,000 patients, causality could not be determined. Thus, a randomized study is warranted to confirm our observations. Second, we did not collect intraoperative MBP. We have intraoperative hand-written medical record. But, due to the lack of accuracy in demonstrating precise blood pressure, which might be very important in studies like ours, we decide to not to use those hand-written intraoperative blood pressures. Rather, we included units of packed red blood cells and intraoperative use of vasopressor as intraoperative variables, because episodes of hypotension mostly occurred due to massive bleeding. Third, because this was a single-center study, our institutional perioperative strategy might influence the occurrence of postoperative AKI. Finally, because we only included patients who underwent living-donor LT and had normal preoperative creatinine levels, our results cannot be generalized to other populations.
Conclusions
Our results demonstrate that lower postoperative MBP values may increase the likelihood of postoperative AKI in liver transplant recipients with normal baseline kidney function. Even when MBP is maintained at a relatively high level, the risk of AKI is still affected by the degree of MBP increase. Maintaining a supranormal MBP may be beneficial to reduce the risk of postoperative AKI after LT especially in patients with baseline cystatin C GFR ≤85 mL/min/1.73 m2.
Abbreviations
- ESLD
end-stage liver disease
- MBP
mean arterial blood pressure
- AKI
acute kidney injury
- LT
liver transplantation
- IPTW
inverse probability treatment weighting
- HRS
hepatorenal syndrome
- POD
postoperative day
- aOR
adjusted odds ratio
- KDIGO
The Kidney Disease: Improving Global Outcomes
- GFR
glomerular filtration rate
- BMI
body mass index
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg. 2014;207:53–59. doi: 10.1016/j.amjsurg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Borthwick E, Ferguson A. Perioperative acute kidney injury: Risk factors, recognition, management, and outcomes. BMJ. 2010;341:c3365. doi: 10.1136/bmj.c3365. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–84. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 4.Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: Results from a national data set. Anesthesiology. 2009;110:505–15. doi: 10.1097/ALN.0b013e3181979440. [DOI] [PubMed] [Google Scholar]
- 5.Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: Role of perioperative management. Ann Thorac Surg. 2012;93:584–91. doi: 10.1016/j.athoracsur.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 6.Shaw A. Update on acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2012;143:676–81. doi: 10.1016/j.jtcvs.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 7.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–90. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- 8.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 9.Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet. 2008;371:1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 10.Bullivant M. Autoregulation of plasma flow in the isolated perfused rat kidney. J Physiol. 1978;280:141–53. doi: 10.1113/jphysiol.1978.sp012377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirchheim HR, Ehmke H, Hackenthal E, et al. Autoregulation of renal blood flow, glomerular filtration rate and renin release in conscious dogs. Pflugers Arch. 1987;410:441–49. doi: 10.1007/BF00586523. [DOI] [PubMed] [Google Scholar]
- 12.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 13.Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–93. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 14.Hatib F, Jansen JR, Pinsky MR. Peripheral vascular decoupling in porcine endotoxic shock. J Appl Physiol (1985) 2011;111:853–60. doi: 10.1152/japplphysiol.00066.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiltebrand LB, Krejci V, Banic A, et al. Dynamic study of the distribution of microcirculatory blood flow in multiple splanchnic organs in septic shock. Crit Care Med. 2000;28:3233–41. doi: 10.1097/00003246-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Albanese J, Leone M, Garnier F, et al. Renal effects of norepinephrine in septic and nonseptic patients. Chest. 2004;126:534–39. doi: 10.1378/chest.126.2.534. [DOI] [PubMed] [Google Scholar]
- 17.Persson PB, Ehmke H, Nafz B, Kirchheim HR. Sympathetic modulation of renal autoregulation by carotid occlusion in conscious dogs. Am J Physiol. 1990;258:F364–70. doi: 10.1152/ajprenal.1990.258.2.F364. [DOI] [PubMed] [Google Scholar]
- 18.Stadlbauer V, Wright GA, Banaji M, et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111–19. doi: 10.1053/j.gastro.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 19.Barri YM, Sanchez EQ, Jennings LW, et al. Acute kidney injury following liver transplantation: definition and outcome. Liver Transpl. 2009;15:475–83. doi: 10.1002/lt.21682. [DOI] [PubMed] [Google Scholar]
- 20.Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth. 2015;114:919–26. doi: 10.1093/bja/aeu556. [DOI] [PubMed] [Google Scholar]
- 21.Maddukuri G, Cai CX, Munigala S, et al. Targeting an early and substantial increase in mean arterial pressure is critical in the management of type 1 hepatorenal syndrome: A combined retrospective and pilot study. Dig Dis Sci. 2014;59:471–81. doi: 10.1007/s10620-013-2899-z. [DOI] [PubMed] [Google Scholar]
- 22.Nazar A, Pereira GH, Guevara M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219–26. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689–97. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 24.Velez JC, Kadian M, Taburyanskaya M, et al. Hepatorenal acute kidney injury and the importance of raising mean arterial pressure. Nephron. 2015;131:191–201. doi: 10.1159/000441151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: A pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928–38. doi: 10.1053/j.ajkd.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salerno F, Gerbes A, Gines P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–18. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YK, Shin WJ, Song JG, et al. Effect of right ventricular dysfunction on dynamic preload indices to predict a decrease in cardiac output after inferior vena cava clamping during liver transplantation. Transplant Proc. 2010;42:2585–89. doi: 10.1016/j.transproceed.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 30.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Leone M, Asfar P, Radermacher P, et al. Optimizing mean arterial pressure in septic shock: A critical reappraisal of the literature. Crit Care. 2015;19:101. doi: 10.1186/s13054-015-0794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464–71. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: New perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–67. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadowski J, Badzynska B. Specific features and roles of renal circulation: Angiotensin II revisited. J Physiol Pharmacol. 2006;57(Suppl 11):169–78. [PubMed] [Google Scholar]
- 35.Iwatsuki S, Popovtzer MM, Corman JL, et al. Recovery from “hepatorenal syndrome” after orthotopic liver transplantation. N Engl J Med. 1973;289:1155–59. doi: 10.1056/NEJM197311292892201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonwa TA, Morris CA, Goldstein RM, et al. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome – experience in 300 patients. Transplantation. 1991;51:428–30. doi: 10.1097/00007890-199102000-00030. [DOI] [PubMed] [Google Scholar]
- 37.Poge U, Gerhardt T, Stoffel-Wagner B, et al. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21:660–64. doi: 10.1093/ndt/gfi305. [DOI] [PubMed] [Google Scholar]
- 38.Sugrue M, Buist MD, Hourihan F, et al. Prospective study of intra-abdominal hypertension and renal function after laparotomy. Br J Surg. 1995;82:235–38. doi: 10.1002/bjs.1800820234. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Singhapricha T, Hu KQ, et al. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: a matched study. Transplantation. 2011;91:348–53. doi: 10.1097/TP.0b013e31820437da. [DOI] [PubMed] [Google Scholar]
- 40.Sang BH, Bang JY, Song JG, Hwang GS. Hypoalbuminemia within two postoperative days is an independent risk factor for acute kidney injury following living donor liver transplantation: A propensity score analysis of 998 consecutive patients. Crit Care Med. 2015;43:2552–6. doi: 10.1097/CCM.0000000000001279. [DOI] [PubMed] [Google Scholar]