Abstract
Background
The prognosis of the patients of acute liver failure (ALF) with onset of hepatic coma is often dismal. ALF is a well-accepted indication for liver transplantation (LT) and has markedly improved the prognosis of these patients. However, its role in ALF patients with onset of hepatic coma has never been elucidated before. The aim of our study was to analyze the outcome in patients of ALF with hepatic coma who underwent LT.
Material/Methods
From January 2002 to December 2015, a total of 726 liver transplantations were done at China Medical University Hospital, Taiwan. The hospital database of 59 recipients that underwent LT for ALF was analyzed. Eleven ALF patients with the onset of hepatic coma (grade IV encephalopathy) requiring mechanical ventilatory support were retrospectively analyzed. The patients were sub-grouped in 2 groups depending on the timing of LT after the onset of hepatic coma: Group A had LT within 48 h of onset of coma (n=7) and Group B had LT after 48 h of onset of coma (n=4).
Results
The study cohort (group A and B) comprised 8 males and 3 females, with an average age of 39.63±13.95 years (range, 13 to 63). Ten patients received living donor liver transplantation (LDLT) and deceased donor liver transplantation (DDLT) was done in 1 recipient. All the patients in group A had complete neurological recovery and were extubated within 48 h after LT, whereas extubation was delayed for various reasons for group B patients. At a mean follow up of 36 months (range, 20 to 76 months), the overall survival of all the recipients (group A and B) was 72%. Three-year survival for Group A (n=7) was 85% and for Group B (n=4) it was 50%. There were no acute rejection episodes.
Conclusions
LT is an acceptable modality of treatment for patients of ALF with new onset of hepatic coma. Neurological recovery is expected in all patients if LT can be done within 48 h of onset of hepatic coma without increasing the risk of morbidity. Due to shortage of deceased donor organs in Asia, LDLT can be used proactively, with a success rate comparable to that of non-ALF patients undergoing LT.
MeSH Keywords: Brain Edema; Hepatic Encephalopathy; Liver Failure, Acute
Background
ALF is a life-threatening condition leading to rapid deterioration of clinical condition. Untreated, the prognosis of ALF remains poor; therefore, timely diagnosis and surgical treatment by LT is crucial and has markedly improved the prognosis of ALF patients [1]. Prior to the application of LT as a salvage therapy, reported mortality rates secondary to ALF were as high as 85% [2]. However, in the past 2 decades, ALF management, both pre- and post-LT, has been improved due to increased experience in the field of LT, better understanding of the pathophysiology of the ALF disease process, and application of multidisciplinary management with reported post-LT survival rates of 60–80% [3,4]. Although spontaneous recovery has been reported to occur in up to 48% of patients with ALF, they are often gravely ill and spontaneous recovery is not seen across all the spectrum of conditions causing ALF. Spontaneous recovery is most apparent in paracetamol-related ALF [5]. The assessment of ALF patients for spontaneous recovery, therefore, is critical in considering the option of LT as soon as possible.
The clinical course of patients with ALF is unpredictable. All patients with acute onset of moderate or severe hepatitis with an elevated coagulation profile and altered sensorium are diagnosed with ALF; hence, early transfer to the intensive care unit (ICU) is recommended [6]. Hepatic encephalopathy (HE), which is a defining characteristic of ALF, is considered the most lethal complication of ALF. Progressive HE is associated with progressive cerebral edema, which may lead to brain ischemia and hypoxia, as well as brainstem herniation, which are the most common causes of death in these patients [7]. Cerebral edema and intracranial hypertension (ICH) in ALF patients indicate the underlying severity of HE. However, grade I–II HE is seldom associated with cerebral edema. The risk of edema increases to 25–35% with progression to HE grade III. Cerebral edema is present in more than 75% of the patients with hepatic coma (Grade IV HE), requiring mechanical ventilation [8,9]. Acute hepatitis with hepatic coma is an ominous sign and although LT can be the only definitive treatment at this clinical stage, an uncontrolled elevated ICH and hepatic coma are traditionally considered as contraindications for LT. ALF requiring mechanical ventilation prior to LT is considered as an independent risk factor leading to decreased survival in ALF patients [10].
In general, about 15–30% of patients die before LT can be performed, usually due to hepatic coma leading to irreversible neurological damage, which is often accompanied by other complications of ALF, such as sepsis, hemodynamic instability, multiorgan failure, and gastrointestinal bleeding. Experience of LT in ALF patients with deep hepatic coma is scarce and limited to only few institutional reports [11–13]. However, as the timing of LT has been an important determining factor to enlist ALF patients with hepatic coma before an irreversible neurological damage occurs, LT can still be successfully performed. As deceased donation rates remain low in Asia, LDLT is the only rapid source of donor liver allografts. However, LT should be arranged at earliest because rapidly worsening clinical condition of the recipient offers a very narrow therapeutic window. Herein, we describe successful application of LDLT for treatment of ALF patients with hepatic coma who required mechanical ventilation.
Material and Methods
From January 2002 to December 2015, a total of 726 liver transplantations were done at China Medical University Hospital, Taiwan. The hospital database was analyzed retrospectively for the 59 patients who underwent LT for ALF. Eleven ALF patients (n=11) with onset of hepatic coma requiring mechanical ventilatory support in an ICU setting were included in the study. The demographic data, perioperative details, and postoperative follow up records were studied.
The patients were further classified into 2 sub-groups based on timing of LT after the onset of hepatic coma: Group A was LT within 48 h of onset of coma (n=7) and Group B was LT after 48 h of onset of coma (n=4).
Institutional review board (IRB) permission was obtained to conduct this study. All the patients were treated in an ICU setting prior to transplantation.
ICU management protocol in ALF patients
Patients with ALF and worsening encephalopathy in this study cohort were managed in an ICU setting prior to LDLT, and all patients required mechanical ventilation. Computed tomography (CT) scan of brain was performed to evaluate cerebral edema and any structural changes. The management of HE in the critically ill patients primarily involved correction of precipitating factors and supportive care. Supportive management involved restoring electrolyte balance, fluid maintenance, and respiratory care. Lactulose enemas (300 mL in 1 liter of water, retained for 1 h were given for all the patients. Plasma ammonia levels were strictly monitored. Prophylactic antibiotics were administered in all the patients which were later changed according to blood culture sensitivity study, if available. We do not perform invasive monitoring of intra-cranial pressure (ICP). Coagulopathy associated with ALF was aggressively managed by administration of plasma products.
Fast-track living donor evaluation protocol for emergency LDLT
Once the diagnosis of hepatic coma due to ALF was confirmed, and we were certain there was no contraindication for LT, the workup for potential living donor was initiated as soon as possible with simultaneous listing of the patient for DDLT as a priority according to the current criteria of the donor allocation system in Taiwan.
If a deceased liver was available, DDLT was performed. However, in most cases a suitable donor was selected, and imaging study was performed adhering to institutional protocol described earlier [14]. After the living-donor workup, documents, including operation and anesthesia consent of donor and recipient, were sent to the hospital IRB for approval of emergent LDLT. The average fast-track living door workup time was 8–12 h.
Post-LDLT management protocol
The patients continued to be managed in the ICU in the post-transplant period. Blood products were administered strictly according to operative blood loss and hematocrit levels. Suggested hemodynamic goals were a mean arterial pressure of 65 mm Hg, central venous pressure 8–12 mm Hg, urine output >0.5 mL/kg/h, and central venous oxygen saturation of 70% or greater. Weaning from mechanical ventilation was attempted, and patients were eventually extubated if they fulfilled the extubation criteria. Immunosuppressive therapy was given according to our institutional protocol [15]. In patients with low albumin and normal liver enzymes with deranged renal functions, the immunosuppressive therapy was temporarily withheld and such patients were closely followed for graft functions.
Statistical analysis
Descriptive statistics were computed for age, MELD score, graft weight, and laboratory data. The results of the cohort population are presented as mean values ± standard deviation (SD). Survival was assessed using Kaplan-Meier analysis and the curve was plotted.
Results
The study cohort comprised 11 recipients (Male: Female, 8: 3) with an average age of 40±14 years (range, 13–63 years). Ten patients underwent LDLT whereas one patient received DDLT. Preoperative disease of patients comprised 3 patients with fulminant hepatic failure without any discernible cause, 1 patient with alcoholic liver cirrhosis who developed acute decompensation of hepatic functions, and 7 patients with ALF secondary to HBV flare-up.
The pre-transplant mean ammonia level was 179.62 μg/dL (range, 111–242 μg/dL) that normalized gradually in immediate postoperative period. All recipients required mechanical ventilatory support and were treated in an intensive care unit prior to liver transplantation. Mild cerebral edema was present in 4 recipients. In the LDLT subgroup, 9 patients received right liver allografts and 1 patient received a left liver allograft, with a 0.95% average graft-to-recipient weight ratio (GRWR). During anesthesia, noninvasive monitoring of cerebral oxygenation was done for all patients.
In the post-transplant period, immunosuppressive therapy was delayed and/or withheld in 2 patients for 1 month due to poor nutritional status. The liver allograft functions remained normal without evidence of acute rejection.
All patients in group A had complete neurological recovery and were extubated within 48 h after LT, whereas extubation was delayed for various reasons for group B patients. Only 1 patient in group B died in the perioperative period, due to failure of neurological recovery (Table 1). Two patients in group A showed acute renal failure in the post-transplant period (patients 2 and 4, as shown in Table 1). However, no hemodialysis was required, and the renal dysfunction normalized in 2nd postoperative month. One patient from group A died in the 30th month post-LDLT due to biliary sepsis secondary to severe biliary stricture. In another patient, post-LDLT recovery was delayed and required tracheostomy due to poor cough reflex (Table 1). The 2-year survival achieved in group A was 100%. The aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, serum creatinine, and prothrombin time at the 7th postoperative day, 1st postoperative month, and 6th postoperative months are as shown in Table 2.
Table 1.
Demographic details, characteristics and complications of the study cohort.
| Diagnosis | Age/sex | Encephalopathy | Mechanical ventilation | Timing of LT after onset of hepatic coma | Follow up and survival | Complications after LT |
|---|---|---|---|---|---|---|
| Group A | ||||||
| FHF | 12/F | Grade IV | Yes | <48 hours | Expired/ 30 months | Biliary stricture and biliary sepsis |
| ALF due to HBV flare up | 31/M | Grade IV | Yes | <48 hours | Alive/ 70 month | Acute renal failure-recovered |
| FHF | 39/M | Grade IV | Yes | <48 hours | Alive/ 38 months | Delayed recovery |
| ALF due to HBV flare up | 54/F | Grade IV | Yes | <48 hours | Alive/ 26 months | Acute renal failure-recovered |
| ALF due to HBV flare up | 35/M | Grade IV | Yes | <48 hours | Alive/ 36 month | – |
| Alcoholic ESLD with acute decompensation | 39/M | Grade IV | Yes | <48 hours | Alive/ 39 months | – |
| ALF due to HBV flare up | 48/M | Grade IV | Yes | <48 hours | Alive/ 68 months | – |
| Group B | ||||||
| ALF due to HBV flare up | 35/M | Grade IV | Yes | 72 hours | Alive/ 36 month | – |
| ALF due to HBV flare up | 29/M | Grade IV | Yes | 96 hours | Expired/ 3 days | Irreversible neurological damage |
| ALF due to HBV flare up | 63/M | Grade IV | Yes | 72 hours | Alive/ 36 months | – |
| FHF | 51/F | Grade IV | Yes | >48 hours | Expired/ 23 months | Sepsis |
ALF – acute liver failure; FHF – fulminant hepatic failure; HBV – hepatitis B virus; ESLD – end-stage liver disease.
Table 2.
Postoperative laboratory tests.
| At admission | Before liver transplantation | 7th Post-operative day | 30th Post-operative day | 6th Post-operative month | |
|---|---|---|---|---|---|
| Hb (gm/dL) | 11.85±3.32 (Range, 7.5–16) | 10.67±2.17 (8.3–13.2) | 10.16±0.51 (9.8–11.3) | 12.10±1.40 (9.9–13.6) | 13.41±1.68 (11.4–15.4) |
| WBC | 10.0±4.86 (Range, 3.3–16.8) | 10.13±2.81 (7.15–15.50) | 11.62±6.12 (4.1±21.8) | 8.07±6.12 (4.1–21.8) | 7.26±2.92 (4.69–12.2) |
| INR | 2.75±0.91 (Range, 1.32–4.42) | 2.63±0.78 (1.42–3.99) | 1.15±0.19 (0.87–1.41) | 1.07±0.10 (0.97–1.24) | 1.02±0.04 (0.99–1.11) |
| AST (IU/L) | 904.71±1140.3 (Range, 50–2733) | 105±33.48 (45–148) | 108.5±95.33 (56–312) | 43.37±18.32 (20–80) | 30.5±17.27 (19–71) |
| ALT (IU/L) | 814±770.08 (Range, 34–1771) | 163.14±151.20 (36–449) | 175±130.16 (39–326) | 58±27.51 (17–103) | 37.75±31.95 (19–116) |
| T. Bilirubin (mg/dL) | 23.65±9.15 (Range, 10.6–31.59) | 23.37±6.02 (13.7–34.2) | 5.46±2.80 (1.2–9.5) | 1.52±0.53 (1–2.39) | 0.76±0.21 (0.35–1) |
| S. Creat (mg/dL) | 1.10±0.73 (Range, 0.51–2.58) | 1.39±1.07 (0.4–3.31) | 0.89±0.27 (0.57–1.32) | 1.06±0.18 (0.7–1.28) | 1.16±0.16 (0.9–1.27) |
| Albumin | 2.98±0.47 (Range, 2.1–3.4) | 2.92±0.24 (2.6–3.3) | 3.23±0.51 (2.6–4) | 3.74±0.77 (2.6–4.6) |
Hb – hemoglobin; WBC – white blood cells; INR – international normalized ratio; AST – aspartate aminotransferase; ALT – alanine aminotransferase.
There were no acute rejection episodes. Infectious complications were present in 5 of the recipients in the cohort prior to surgery, without any adverse sequalae in the postoperative period.
At mean follow-up of 36 months (range, 20–76 months), the overall survival for all recipients (groups A and B) was 72%. For group A (n=7), 3-year survival was 85.71%, whereas for group B (n=4) it was 50% (Figure 1).
Figure 1.
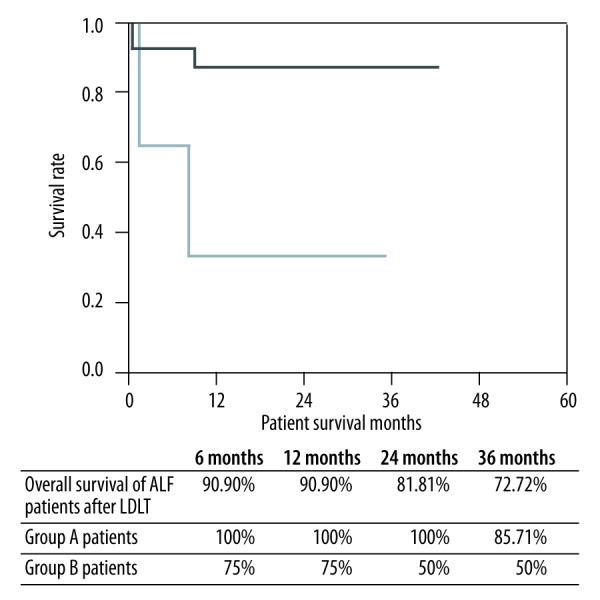
Overall survival of ALF patients with hepatic coma after LT.
Discussion
The role of LDLT in ALF patient with hepatic coma has been sporadically reported. Our successful experience shows the safety and effectiveness of LDLT in patients ALF, even with grade 4 HE. In this retrospective analysis, the timing of LT from hepatic coma onset was a determining factor. If LT can be arranged within 48 h of coma onset, mortality directly related to coma due to irreversible brain damage can be averted. Although a few reports have mentioned the feasibility of LT for ALF patients with grade 4 HE, the impact of coma on outcome and long-term follow-up of such patients was not described [11,12].
Cerebral edema, coagulopathy, renal failure, metabolic disturbances, and infection are the main clinical sequelae in patients with ALF, which carry substantially increased risk of mortality [16]. HE in ALF progressing to grade IV is universally fatal if immediate LT cannot be arranged. The severity of HE cannot be predicted before transplantation and, hence, the LDLT remains controversial in such a situation. However, in ALF patients, the presence of severe cerebral edema prior to the LT is the major cause of neurological deficit and irreversible brain damage [12,17]. In our series, 4 patients had mild cerebral edema and none of them progressed to severe brain edema. Hence, the accompanying hepatic coma can be attributed to elevation of blood concentrations of toxins, mainly ammonia secondary to ALF rather than cerebral edema. The mean serum ammonia level was 179.62 μg/dL (range, 111–242 μg/dL), which normalized after LT surgery.
Hepatic coma secondary to severe brain edema implies organic failure in the brain, which is irreversible [18]. Some researchers have suggested ICP monitoring for patients with hepatic coma, as irreversibly elevated ICP is associated with poor neurological outcome in every case [19]. As cerebral edema evolves, treatment should focus on preservation of cerebral perfusion to prevent ischemia, which mainly comprises sedation, hypertonic saline, and mannitol [20]. However, the procedures of invasive ICP monitoring can be hazardous and even fatal in patients with advanced hepatic failure associated with severe coagulopathy. Furthermore, patients with ALF with rising ICP and hepatic coma have almost certain mortality without LT. Even after LT in such patients, authors have reported a high mortality rate due to severe cerebral edema with inability to recover from the hepatic coma due to irreversible brain damage. The increasing cerebral edema impairs cerebral autoregulation and, subsequently, reduced cerebral arterial flow that results in deepening of the coma [21]. Hence, the decision to perform LT should be made as soon as possible. As deceased donation remains rare in Taiwan, LDLT is the only rapid means for donor liver organs and can be arranged by a fast-track donor screening protocol. Although LDLT in treating ALF patients with coma is considered controversial by some researchers, and the reversibility of the hepatic coma cannot be predicted before transplantation, complete recovery is possible if transplantation can be arranged on a priority basis. In this retrospective analysis, all 8 patients who underwent LDLT within 48 h of hepatic coma onset had complete neurological recovery, whereas only 2 patients recovered who underwent LT after 48 h. One patient in group B died of extensive brain edema without recovering consciousness even after LT. The transplantation was done after 96 h of hepatic coma onset. However, there was no cognitive abnormality in any of the surviving transplant recipients after LT, which suggests that the absence of severe brain edema prior to LT can help in complete recovery after LT if done before deepening of the coma.
For DDLT, current policy is to prioritize the patients for transplantation based on risk of waitlist death defined by the MELD score system. Although controversial and not universally practiced, many centers regard ALF with hepatic coma requiring mechanical ventilation as a contraindication [22]. The argument in this regard is often the relative survival benefit after LT in such patients and ethical use of the public resource of donor organs. In the absence of an ideal number, a likelihood of 50% survival at 5 years is often used as a cutoff for rational use of a scarce deceased donor liver [23]. Furthermore, better understanding of the disease process, multidisciplinary treatment of ALF, and technical advances in LT surgery have led an excellent outcome and survival comparable to the elective LT surgeries done for ESLD patients. However, due to the shortage of deceased donor organs, LDLT was adapted and later become the most common modality of LT in Asia. LDLT is an alternative and applicable option for the treatment of ALF [24]. When transplantation is performed in critically ill patients such as those with ALF with hepatic coma, pressure on the living-related donor is high and concerns regarding informed consent may be greater than for elective LDLT. However, our high success rate and lack of donor risk justify making the option of emergency LDLT available for patients with grade IV encephalopathy. All the patients in this study cohort that underwent LDLT within 48 hours of onset of hepatic coma showed a favorable outcome with complete neurological recovery. Earlier, successful LDLTs were reported in fulminant hepatic failure patients with electroencephalogram showing almost flat waveforms [12,18].
The most important aspects before transplantation are timely diagnosis of ALF patients with progressive encephalopathy, and multidisciplinary treatment support in the ICU. The LT team should be informed, and the need for and urgency of LT surgery should be discussed with the patient’s relatives. As circulatory imbalance is a frequent occurrence, fluid management must be cautious and hemodynamics should be carefully assessed [25]. Coagulation parameters, complete blood cell count, and metabolic parameters should be checked frequently. The coagulopathy associated with ALF should be carefully corrected, and prophylactic administration of fresh frozen plasma (FFP) in absence of bleeding is unadvised because it lowers the prothrombin time and decreases the accuracy of prognosis. In addition, this can present as a significant volume shift, thus exacerbating already existing renal dysfunction and contributing to a rise in ICP. The worsening encephalopathy is sometimes difficult to assess for mechanically ventilated patients. Hence, any ALF patient with hepatic coma should be subjected to LT as early as possible. Bioartificial liver support systems have recently been used in clinical trials for ALF patients, which can provide metabolic support and act as a bridge to LT until a suitable donor becomes available. However, currently available liver support systems are not routinely recommended outside of clinical trials, and data in support of such treatment modalities in ALF with coma are scant [26,27].
Conclusions
LT is a feasible option even for mechanically ventilated ALF patients with grade IV encephalopathy. Success rates increase if the transplantation is performed within 48 h of onset of hepatic coma. Due to scarcity of deceased donor organs, LDLT can be used proactively, with a success rate comparable to non-ALF patients undergoing LT.
Footnotes
Source of support: Departmental sources
Conflicts of interest.
None.
References
- 1.Mendizabal M, Silva MO. Liver transplantation in acute liver failure: A challenging scenario. World J Gastroenterol. 2016;22:1523–31. doi: 10.3748/wjg.v22.i4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liou IW, Larson AM. Role of liver transplantation in acute liver failure. Semin Liver Dis. 2008;28:201–9. doi: 10.1055/s-2008-1073119. [DOI] [PubMed] [Google Scholar]
- 3.Germani G, Theocharidou E, Adam R, et al. Liver transplantation for acute liver failure in Europe: Outcomes over 20 years from the ELTR database. J Hepatol. 2012;57:288–96. doi: 10.1016/j.jhep.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 5.O’Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663–70. doi: 10.1016/j.jhep.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Polson J, Lee WM. AASLD position paper: The management of acute liver failure. Hepatology. 2005;41:1179–97. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 7.Castaldo ET, Chari RS. Liver transplantation for acute hepatic failure. HPB (Oxford) 2006;8:29–34. doi: 10.1080/13651820500465741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz SJ. Difficult management problems in fulminant hepatic failure. Semin Liver Dis. 1993;13:395–413. doi: 10.1055/s-2007-1007368. [DOI] [PubMed] [Google Scholar]
- 9.Stravitz RT, Kramer AH, Davern T, et al. Intensive care of patients with acute liver failure: Recommendations of the U.S. Acute Liver Failure Study Group. Critical Care Med. 2007;35:2498–508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 10.O’Mahony C, Patel S, Suarez J. Have U.S. orthotopic liver transplant (OLT) outcomes for acute liver failure (ALF) improved in the last decade? Hepatology. 2007;46:492A. [Google Scholar]
- 11.Takeichi T, Asonuma K, Kim I, et al. Compressed spectral arrays of patients with fulminant hepatic failure in hepatic coma undergoing liver transplantation. Clin Transplant. 2002;16:273–79. doi: 10.1034/j.1399-0012.2002.01138.x. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Ochiai T, Hori S, et al. Complete recovery from fulminant hepatic failure with severe coma by living donor liver transplantation. Hepatogastroenterology. 2003;50:515–18. [PubMed] [Google Scholar]
- 13.Kumamoto T, Takeda K, Ishibe A, et al. Complete neurological recovery from fulminant hepatic failure with subarachnoid hemorrhage by living donor liver transplantation: A case report. Transplant Proc. 2009;4:1982–86. doi: 10.1016/j.transproceed.2009.01.108. [DOI] [PubMed] [Google Scholar]
- 14.Jeng LB, Thorat A, Yang HR, et al. “Rooftop and skeletonization technique” of hepatic transection to include or exclude the middle hepatic vein during donor hepatectomy in living donor liver transplantation: solving the middle hepatic vein controversy – experience in 397 sequential live donors. Med Sci Tech. 2016;57:6–15. [Google Scholar]
- 15.Jeng LB, Thorat A, Hsieh YW, et al. Experience of using everolimus in the early stage of living donor liver transplantation. Transplant Proc. 2014;46:744–48. doi: 10.1016/j.transproceed.2013.11.068. [DOI] [PubMed] [Google Scholar]
- 16.Thiel J, Brems J, Nadir A, et al. Liver transplantation for fulminant hepatic failure. J Gastroenterol. 2001;36:1–4. doi: 10.1007/s005350170146. [DOI] [PubMed] [Google Scholar]
- 17.Hattori H, Higuchi Y, Tsuji M, et al. Living-related liver transplantation and neurological outcome in the children with fulminant hepatic failure. Transplantation. 1998;65:686–92. doi: 10.1097/00007890-199803150-00015. [DOI] [PubMed] [Google Scholar]
- 18.Kubota T, Sekido H, Takeda D, et al. Acute hepatic failure with deep hepatic coma treated successfully by high-flow continuous hemodiafiltration and living-donor liver transplantation: A case report. Transplant Proc. 2003;35:394–96. doi: 10.1016/s0041-1345(02)03832-0. [DOI] [PubMed] [Google Scholar]
- 19.Punch JD. Bridges to transplantation. Anesthesiol Clin North Am. 2004;22:863–69. doi: 10.1016/j.atc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Tofteng F, Larsen FS. Management of patients with fulminant hepatic failure and brain edema. Metab Brain Dis. 2004;19:207–14. doi: 10.1023/b:mebr.0000043970.34533.04. [DOI] [PubMed] [Google Scholar]
- 21.Guevara M, Bru C, Gines P, et al. Increased cerebrovascular resistance in cirrhotic patients with ascites. Hepatology. 1998;28:39–44. doi: 10.1002/hep.510280107. [DOI] [PubMed] [Google Scholar]
- 22.Vaquero J, Fontana RJ, Larson AM, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–89. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 23.Barr ML, Belghiti J, Villamil FG, et al. A report of the Vancouver Forum on the care of the live organ donor: Lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81:1373–85. doi: 10.1097/01.tp.0000216825.56841.cd. [DOI] [PubMed] [Google Scholar]
- 24.Fuchinoue W, Tanaka K, Takasaki E, et al. Living-related liver transplantation for fulminant hepatic failure. Transplant Proc. 1997;29:424. doi: 10.1016/s0041-1345(96)00162-5. [DOI] [PubMed] [Google Scholar]
- 25.Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG clinical guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109:950–66. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 26.Van de Kerkhove MP, Hoekstra R, Chamuleau RA, et al. Clinical application of bioartificial liver support systems. Ann Surg. 2004;240:216–30. doi: 10.1097/01.sla.0000132986.75257.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demetriou AA, Brown RS, Jr, Busuttil RW, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–67. doi: 10.1097/01.sla.0000124298.74199.e5. discussion 667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


